Abstract
Understanding germination of seeds constituting a persistent soil seed bank is important because the ability to predict longevity of a seed in soil and a particular combination of environmental conditions supporting dormancy break and germination can be critical for restoration of threatened species having poor regeneration. We studied the pattern of germination under experimentally manipulated environmental conditions in several threatened iris species of section Oncocyclus. In the first experiment, spanning 11 years, we manipulated soil type and precipitation regime, and in the second experiment, spanning 7 years, we tested an effect of cumulative amount of rainfall during a season on germination. In all studied species the germination process was intermittent, with distinct germination pulses separated by years with no or very low germination. High innate dormancy appears to be a general property of newly produced seeds of Oncocyclus irises, but there are exceptions. Although in three tested Oncocyclus species considerable (above 25%) germination fraction was observed only in the second and subsequent seasons regardless of the soil moisture experienced by a seed, in Iris atropurpurea it occurred in the first season under watering equivalent or exceeding cumulative seasonal precipitation of 100 mm. In the three other species, although high cumulative germination percentage was observed by year four under all experimental conditions a substantial fraction of seeds was still ungerminated and viable. Our results have important conservation implications. We recommend for mass seed germination creation of a soil seed bank under natural conditions with minimal modifications, monitored for more than two years. Supplementary watering (if feasible) will speed up the germination process and shading will have a similar effect but neither of these two is a requirement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed persistence is the prolonged survival of seeds through time following their maturation on the parent plant and dispersal. Seed persistence avoids germination in conditions not supporting seedling survival and development (Cohen 1966; Long et al. 2015). The duration of seed persistence in the soil, and the propensity of seeds to exit the soil seed bank by germinating, depends on the physical and physiological characteristics of seeds and how they respond to the biotic and abiotic environment. Often seeds persistent in the soil for years possess seed dormancy, a characteristic that prevents germination under conditions that are favorable for germination but not for seedling survival (Vleeshouwers et al. 1995; Fenner and Thompson 2005). In species that possess seed dormancy, a point in time when dormancy breaks is determined by either mechanical changes in a seed coat, balance of endogenous hormones preventing germination, rate of embryo development or a combination of these factors (Baskin and Baskin 1998, 2004; Finch-Savage and Leubner-Metzger 2006). Disentangling the role of environmental factors in breaking dormancy (e.g., timing and amount of rainfall) from effect of seed coat and germination-preventing substances, and understanding how they interact over time requires controlled experiments. In these experiments manipulating the environment experienced by a seed over time and duration of an experiment can be crucial, because changes in seed coat permeability and hormonal changes can be slow processes taking many years. Understanding germination of seeds constituting a persistent soil seed bank has not only theoretical but also practical merits (Meyer 2009). The ability to predict longevity of a seed in soil and a particular combination of environmental conditions supporting dormancy break and germination can be critical in conservation projects, because poor regeneration with no seedlings emerging for years is a common phenomenon in many populations of threatened species (Volis 2019).
Iris species of the section Oncocyclus are characterized by seeds with a very hard seed coat that develops during the first summer, a period when a dispersed seed dries and shrinks (Avishai 1977). These seeds have strong dormancy and a slow rate of germination in natural environmental settings, usually no more than 30% in the first few years (Shimshi 1967). According to Avishai (1977), up to 15% of seeds germinate in the first year, while the rest of the seeds germinate throughout the subsequent 5–6 years. Oncocyclus species grow in herbaceous plant communities of the Near East, Turkey, and Caucasus, and experience a semi-arid or arid climate (Avishai 1977). Because precipitation is both low and unpredictable in these environments, slow and intermittent germination over many years is traditionally considered to be an adaptive strategy increasing the proportion of successfully established seedlings by spreading risk of germination failure (Blumenthal et al. 1986). Blumenthal et al. (1986) showed that germination rate in Oncocyclus is species-specific. In I. lortetii Barbey, 1% of seeds germinated in the first year while in I. atropurpurea Dinsmore the majority of seeds germinated in the first year (60%).
Thus far, the role of environment in germination of Oncocyclus seeds has never been tested. To understand how environmental factors affect germination of Oncocyclus seeds, we set up two experiments. In the first experiment, we manipulated soil type and soil moisture and used two Israeli species representing the arid part of the Oncocyclus irises' distribution, Iris mariae Barbey and I. atrofusca Baker. Based on the results of the first experiment, we designed a second experiment testing the effect of the cumulative amount of rainfall during a season, on seed germination in the above two species plus one more species from the arid zone (I. petrana Dinsmore) and a species from the mesic coastal area (I. atropurpurea Dinsmore). The following questions were addressed: (1) How important are soil type and soil moisture for germination of Oncocyclus seeds and how do these factors affect the pattern of germination of Iris spp. over time, and (2) how should these environmental factors be manipulated to produce large numbers of seedlings from freshly collected Oncocyclus seeds? The latter question was important because most Iris species of the section Oncocyclus are rare, endemic and are a high priority for conservation (Avishai 1977; Shmida and Pollak 2007). Many populations of these species exhibit poor regeneration despite ample seed production (S. Volis, personal observations).
Methods
Two-species experiment
Fully matured seeds of two species, Iris mariae and I. atrofusca were used in an experiment testing the effects of soil type and amount of precipitation on seed germination in Oncocyclus irises. Seeds of Iris mariae and I. atrofusca used in this experiment were collected in April 2007 from a single population in the north-western Negev Desert (annual rainfall 100–200 mm) and northern Negev (annual rainfall 200–300 mm); population geographic coordinates 31°10′39.09″N, 34°19′33.88″E and 31°18′39.24″N, 34°47′23.20″E, respectively. For each species, seeds were collected from more than 30 individuals and the two seed pools were kept in paper bags in a roofed but open from the sides facility until the start of the experiment.
In fall 2007, 100 seed of each species were sown on each of two soil types: sand and loess, subjected to two precipitation conditions: natural precipitation and ample watering ("dry" and "wet" throughout the paper). In the latter, pots were watered during the growing season (see below) whenever the upper layer of soil became dry. The two soil types used in the experiment, sand and loess, that represent the soils in the natural habitat of I. mariae and I. atrofusca respectively differ in permeability and retention capacity. This experimental design was replicated five times with a total of 4000 seed sown (2 species × 2 treatments × 5 replicates × 100 seeds). For each treatment seeds were sown in 1 l pots and covered by 1 cm deep layer of the same soil in a net house of the Bergman Campus, Beer Sheva (annual precipitation around 200 mm of rainfall).
Pots were observed once a week during 11 consecutive growing seasons 2007–2018 (from the first rain event till approximately one month after the last rainy event). During these periods of time, emerging seedlings were counted and removed from the pots. During the dry season the pots were kept dry.
The effects of soil type and precipitation conditions, as well as their interactions on seed germination with a concomitant effect of species identity were analyzed separately for seasons when noticeable (over 20%) seedling emergence occurred in any treatment combination, and for the cumulative germination over 11 years. This was done using a Generalized linear model (GLZ) with binomial errors and probit link for the effects of three factors and their interactions on seed germination.
Four-species experiment
In the second experiment, only one environmental effect (amount of simulated rainfall) was tested, but, in addition to Iris mariae and I. atrofusca two more species represented by one population each were used (I. petrana, 31°1′57.09″N, 35°6′22.89″E; and I. atropurpurea, 32°17′17.45″N, 34°50′58.09″E). I. atropurpurea grows on the coastal plain, under a Mediterranean climate of 400–600 mm annual precipitation. I. petrana occurs in the Negev Desert under ca. 100 mm of rainfall. The seeds were collected in April 2009 from hand pollinated plants maintained at the Bergman Campus and kept in paper bags in a refrigerator until start of the experiment (November 2011). Germination of seeds of the four Oncocyclus species was tested under controlled conditions in a roofed but open from all sides growth facility at the Bergman Campus, Beer Sheva. For each species, one hundred seeds were buried at 1 cm depth in November 2011 in pots (bottom ∅ 12 cm, top ∅ 20 cm, height 12 cm) filled with a 50:50 mixture of sieved loess and sand and received one of five water treatment simulating precipitation of different quantities during the growing season. The amount of water applied was equivalent to 50, 100, 150, 200, and 250 mm of rainfall. Watering was done at regular intervals starting from November till March (the months when usually the first and the last event of natural precipitation occur). In August 2012 the seeds were dug out and classified as germinated (with a radicle protruded) or non-germinated. The non-germinated seeds were sown again in pots as above immediately after examination and stored outside. This procedure was repeated in the next 6 seasons. At the end of experiment, we counted the number of viable ungerminated seeds.
Effects of rainfall amount on germination of each species in the first four seasons and over seven seasons were tested by GLZ with binomial errors and probit link for the effects of two factors. In addition, an interactive effect of seed species identity and rainfall amount on germination in the first four seasons and cumulative germination over seven years was tested by the test of independence. All the statistical analyses were done in STATISTICA (StatSoft Inc. 2004).
Results
Two-species experiment
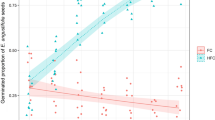
During the first two growing seasons (2007–2008 and 2008–2009) only 0.4% and 0.9% of sown seeds germinated, all in the “wet” treatment. Approximately equal number of I. atrofusca and I. mariae seeds germinated. In the third season, 22.6% of sown seeds germinated. In this season all main effects and interactions except for species × soil were significant (Table 1). Unlike in the previous two seasons, I. mariae had a higher germination percentage than I. atrofusca (17.2 vs. 5.4, respectively). In contrast to the first two seasons, germination in the third season occurred mostly in the “dry” treatments (93.1% of germinated seeds) (Fig. 1). In the fourth season, germination was low (3.4%), predominantly in the "wet" treatment (98.1% of germinated seeds) and of I. mariae (71.4%). In the fifth season, germination was negligible (0.6%), exclusively in the “wet” treatment. In the sixth season, 23.9% of seeds germinated, with a stronger pulse of germination in the "wet" treatment (76.4% of germinated seeds) (Fig. 1). In the seventh season, 1.3% of seeds germinated, exclusively in the "wet" treatment, In the eighth and ninth seasons no seeds germinated, while in the tenth and eleventh 0.8% of seeds germinated, exclusively in the "dry" treatment (Fig. 1).
In cumulative germination over 11 seasons, all main effects and interactions species × water and soil × species × water were significant (Table 1). From those seeds that germinated (52.6% of sown seeds) more seeds of I. mariae than I. atrofusca germinated (32.6 vs. 20.0%) and more in loess than in sand (29.5 vs. 23.1%).
Although soil had an effect, the interaction species × soil was not significant reflecting the fact that both species germinated better in loess than in sand (18.3 vs. 14.3% and 11.2 vs. 8.8%, I. mariae and I. atrofusca, respectively). The two species differed in their response to supplementary watering being positive for I. atrofusca (10.7 vs. 9.4%), but negative for I. mariae (14.8 vs. 17.8%).
Four-species experiment
GLZ revealed an effect of species identity and water amount on germination in the first two seasons, and cumulative germination over 7 years with I. atropurpurea having much greater germination than the other species in the first year. In the third season, no effect was significant, and in the fourth season only the water effect was significant (Table 2).
Analysis of pairwise species × water interactions revealed no interactive effect of seed origin and rainfall amount on germination percentage in the first year (except for the pair I. mariae—I. petrana), but there was a strong effect in the second year (except for the pair I. atrofusca—I. petrana). In the third season, the interaction was significant only for the pair I. atrofusca—I. atropurpurea. In the fourth season, there were no significant interactions for any species pair. In the following seasons the germination percentage was too low for testing an interaction between the two factors. For cumulative germination over seven years the interaction was significant only in comparisons involving I. atrofusca (Table 3).
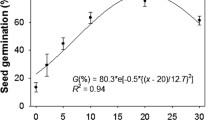
Germination percentages for each species under different amounts of rainfall in seven consecutive seasons are shown in Fig. 2. In the first season, there was no germination under 50 mm of rainfall for any species and close to zero germination percentage under all rainfall amounts in I. atrofusca and I. mariae but I. atropurpurea had much greater germination than the other species. In the second season, under all rainfall amounts germination percentage was much higher than in the first season in I. atrofusca, I. mariae, and I. petrana. However, as germination for I. atropurpurea was high in year 1 a lower proportion of seeds germinated in year 2 except for the 50 mm treatment where germination was over 60% and similar to I. petrana (Fig. 2). In the third season, noticeable germination was observed only for I. atrofusca and I. atropurpurea, in the former species in all water treatments but in the latter no seed germinated under 50 mm of rainfall. In the fourth year, germination was close to zero in all water treatments for I. atropurpurea, but relatively high under water treatments 150, 200, and 250 mm of rainfall in the other species. In the next 3 years germination was very low (Fig. 2). Despite this low germination, upon termination of the experiment, in all species except I. atropurpurea there was a substantial fraction of viable non-germinated seeds. This fraction across simulated rainfall amounts was 6–41% for I. mariae, 11–32% for I. atrofusca, and 11–18% for I. petrana. In contrast, in I. atropurpurea this fraction was very small (1–4%) but almost all seeds in the 100 and 150 mm treatments had germinated by year 3.
Discussion
Seed bank dynamics and a mechanism of dormancy
In species with deep dormancy and slow rate of germination over years, two factors can be responsible for this pattern: accumulation of germination-inhibiting substances in the seed coat during or shortly after seed maturation, and water impermeability of the seed coat. Blumenthal et al. (1986) reported that the pressure needed to pierce the seed coat of two species, I. lortetii and I. atropurpurea, the former species showing much stronger dormancy than the latter species, was 135 and 77 atmospheres, respectively. Extracts of the outer integument of the seed coat were toxic for the germinating embryo, but removal of this layer did not improve germination. Thus, Blumenthal et al. (1986) concluded that the main cause of seed dormancy in Oncocyclus is a high mechanical resistance of the seed coat at the micropylar area. The results of our experiments support this view. In the four-species experiment germination of seeds in the second year did not depend on amount of water supplied in the preceding year, although the latter varied considerably, from 50 to 250 mm of rainfall. In the two-species experiment, percentage of germinated seeds for both species was larger under ample watering only in certain years but in some years more seeds germinated under much lower natural precipitation. Significant germination occurred in the sixth season with 176 mm but was close to zero in the next three seasons when amount of rainfall was above 250 mm (Fig. 1). Thus, importance of leaching of germination-inhibiting substances in the seed coat for breaking dormancy in Oncocyclus is doubtful. Much more probable appears a hypothesis that a variation in germination timing is due to seed heteromorphism, i.e., that seeds in a capsule differ not only in size but also in seed coat thickness. The latter can be related to a position of a seed within a capsule. Produced in this way, the individual seeds can be programmed to come out of dormancy at a point of time determined by their seed coat thickness, with effect of soil moisture or temperature on the latter if present, being secondary.
Although in our study environmental effects appear to be secondary in importance, those related to soil moisture can advance or delay seedling emergence. The manipulations used in two experiments were related to soil moisture either directly (amount of water supplied) or indirectly (two soil types had different permeability and retention capacity). Generally, soil moisture is regarded as a dormancy breaking or alleviating agent, in contrast to germination stimulants like smoke or light (Baskin and Baskin 1998; Merritt et al. 2007; Turner et al. 2017; Elliott et al. 2019). However, effect of soil moisture on germination can not be directly inferred from amount of water supplied because soil moisture is regulated by an interaction between the rate of influx (i.e., precipitation) and the rate of removal (i.e., evapotranspiration) and both aspects should be considered in unison (Livingston 1910). Therefore, the cumulative effect of supplied water that triggered seed germination in I. atropurpurea should not be interpreted as a cumulative soil moisture effect because soil moisture and evapotranspiration in this experiment were not measured. For the other studied Oncocyclus species, water supply appears to have a positive effect on germination timing, but this effect cannot be described as a threshold, linear, or any other simple relationship.
Because we followed the seed cohort for many years under different environmental conditions, we can make some conclusions about Oncocyclus early stage population demography. The Israeli species from this section exhibited either cue non-responsive dormancy (sensu Meyer 2009) (I. atrofusca, I. petrana, and I. mariae) or cue-responsive dormancy (I. atropurpurea). In the latter species, high germination percentage was observed in the first year but only when the precipitation threshold (which is between 50 and 100 mm) was met, while in the former three species the dormancy break was not triggered by any particular environmental cue. In two such species for which we have data on germination over a decade period (I. atrofusca and I. mariae in the two-species experiment), distinct germination pulses were separated by years with no or very low germination, a pattern which contrasts with a linear trajectory of germination over years observed in other plant groups (e.g., hard-seeded legumes) (Meyer 2009). Germination in pulses was observed also in the four-species experiment spanning 7 years of observations for I. atrofusca, I. petrana, and I. mariae (Fig. 2).
Conservation implications
Most Iris species of the section Oncocyclus are threatened (Avishai 1977; Cohen and Avishai 2000; Shmida and Pollak 2007). In Israel, many natural populations of Oncocyclus species (e.g., I. atrofusca, I. atropurpurea, I mariae) are disappearing or declining at an alarming rate due to urbanization, agricultural and industrial development, and human population growth (Arafeh et al. 2002; Volis et al. 2016). Preservation in ex situ living collections turned out to be very problematic because rhizomes maintained in these collections tend to decay and die after a few years, probably due to viral infection (Shimshi 1967; Volis personal observations). However, the infection does not appear to be passed from the infected plants to their seeds (Shimshi 1967). Thus seeds are preferred to rhizomes (the latter either kept dormant under low temperature or as living plants), as the type of material for preservation ex situ. However, direct usage of Oncocyclus seeds in augmentation or translocation projects makes little sense because (i) the projects using seeds generally have a low probability of success (Guerrant and Kaye 2007; Menges 2008; Godefroid et al. 2011), and (ii) seedlings in Oncocyclus species are rarely observed, and those that do emerge have high mortality rate (S. Volis, personal observations and unpublished data). Thus, prior to introduction, the seeds must be germinated and seedlings grown to an appropriate size before transplantation. Currently a rhizome size that will ensure a high probability of survival in natural settings is not known for any Oncocyclus species. However, the rhizome size with a reasonable chance of flowering is known for I. atrofusca to be over 4 g (Volis et al. 2010).
Two methods have been proposed to germinate Oncocyclus seeds. Shimshi (1967) proposed an in vitro germination protocol that includes the use of very fresh seed, removal of its coat, and planting the embryo on a medium. Using this method combined with scarification, Dorman et al. (2009) successfully broke the innate dormancy and germinated seeds of three Oncocyclus species, I. mariae, I. atrofusca, and I. petrana (56%, 41%, and 46% germination, respectively). However this method demonstrated low success as, despite sterilization, fungal contamination reduced production of seedlings with true leave to 18% (I. mariae and I. petrana) and 20% (I. atrofusca).
Thus, the above method for obtaining large number of seedlings when financial and labor resources are scarce is of limited use. Fortunately, our results demonstrate that achieving mass germination is possible without any special seed treatment and facilities. For most Oncocyclus species sowing seeds in furrows ~ 3 cm deep in natural soil with or even without supplementary watering will result in mass germination not in the first year, but in the second or third year with very high probability. For I. atropurpurea and probably some other Oncocyclus species, it will happen in the first year.
Earlier, we recommended two procedures for artificial Oncocyclus seed germination for conservation purposes: (i) in vitro and (ii) in vivo with shading and watering ad libitum (Dorman et al. 2009). Recommendations from this study are that seeds should be sown about 3 cm deep in natural soil and ambient environmental conditions and monitored for three or more years. Supplementary watering (if feasible) will speed up the germination process but is not a requirement.
References
Arafeh RMH, Sapir Y, Shmida A, Iraki N, Fragman O, Comes HP (2002) Patterns of genetic and phenotypic variation in Iris haynei and I. atrofusca (Iris sect. Oncocyclus = the royal irises) along an ecogeographical gradient in Israel and the West Bank. Mol Ecol 11:39–53
Avishai M (1977) Species relationships and cytogenetic affinities in section Oncocyclus of the genus Iris. PhD Thesis, The Hebrew University of Jerusalem
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press, New York
Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Sci Res 14:1–16
Blumenthal A, Lerner HR, Werker E, Poljakoff-Mayber A (1986) Germination preventing mechanisms in Iris seeds. Ann Bot 58:551–561
Cohen D (1966) Optimizing reproduction in a randomly varying environment. J Theor Biol 12:119–129
Cohen O, Avishai M (2000) The Irises still exist: the conservation status of species Iris section Oncocyclus in Israel, a century after their description. Annali Di Botanica 58:145–160
Dorman M, Melnikov P, Sapir Y, Volis S (2009) Factors affecting dormancy of Oncocyclus iris seeds. Isr J Plant Sci 57:329–333
Elliott CP, Lewandrowski W, Miller BP, Barrett M, Turner SR (2019) Identifying germination opportunities for threatened plant species in episodic ecosystems by linking germination profiles with historic rainfall events. Aust J Bot 67:256–267
Fenner M, Thompson K (2005) The ecology of seeds. Cambridge University Press, Cambridge
Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171:501–523
Godefroid S, Piazza C, Rossi G, Buord S, Stevens A-D, Aguraiuja R, Cowell C, Weekley CW, Vogg G, Iriondo JM, Johnson I, Dixon B, Gordon DR, Magnanon S, Valentin B, Bjureke K, Koopman R, Vicens M, Virevaire M, Vanderborght T (2011) How successful are plant species reintroductions? Biol Conserv 144:672–682
Guerrant EOJ, Kaye TN (2007) Reintroduction of rare and endangered plants: common factors, questions and approaches. Aust J Bot 55:362–370
Livingston BE (1910) Relation of soil moisture to desert vegetation. Bot Gaz 50:241–256
Long RL, Gorecki MJ, Renton M, Scott JK, Colville L, Goggin DE, Commander LE, Westcott DA, Cherry H, Finch-Savage WE (2015) The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biol Rev 90:31–59
Menges ES (2008) Restoration demography and genetics of plants: when is a translocation successful? Aust J Bot 56:187–196
Merritt DJ, Turner SR, Clarke S, Dixon KW (2007) Seed dormancy and germination stimulation syndromes for Australian temperate species. Aust J Bot 55:336–344
Meyer SE (2009) Studying the seed bank dynamics of rare plants. Calochortiana 3:46–55
Shimshi D (1967) Raising irises from embryo culture. Teva Va’Arez 4:225–229 (In Hebrew)
Shmida A, Pollak G (2007) Red Data Book: Endangered Plants of Israel. Israel Nature and Parks Authority. Authority Press, Nature-Parks Jerusalem
StatSoft Inc (2004) STATISTICA (data analysis software system), version 7. www.statsoft.com
Turner SR, Lewandrowski W, Elliott CP, Merino-Martín L, Miller BP, Stevens JC, Erickson TE, Merritt DJ (2017) Seed ecology informs restoration approaches for threatened species in water-limited environments: a case study on the short-range Banded Ironstone endemic Ricinocarpos brevis (Euphorbiaceae). Aust J Bot 65:661–677
Vleeshouwers LM, Bouwmeester HJ, Karssen CM (1995) Redefining seed dormancy: an attempt to integrate physiology and ecology. J Ecol 83:1031–1037
Volis S (2019) Plant conservation: the role of habitat restoration. Cambridge University Press, Cambridge
Volis S, Blecher M, Sapir Y (2010) Application of complex conservation strategy to Iris atrofusca of the Northern Negev, Israel. Biodivers Conserv 19:3157–3169
Volis S, Zhang Y-H, Dorman M, Blecher M (2016) Iris atrofusca genetic and phenotypic variation, the role of habitat-specific selection in this variation structuring, and conservation implications using quasi in situ guidelines. Isr J Plant Sci 63:347–354
Acknowledgements
This project was supported by a Grant from the Israel Ministry of Sciences and a grant from Israel Nature and Parks Authority.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Philip Ladd.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Volis, S., Dorman, M. Effects of soil type, period of burial and moisture levels on the germination of Oncocyclus iris seeds. Plant Ecol 220, 1021–1028 (2019). https://doi.org/10.1007/s11258-019-00971-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-019-00971-8