Abstract
Background and aims
Neptunia amplexicaulis, endemic to Central Queensland (Australia), is one of the strongest selenium (Se) hyperaccumulators known globally, capable of accumulating up to 13 600 µg Se g− 1 in its leaves. This work aimed to elucidate root foraging in response to Se in N. amplexicaulis applied in two different chemical forms and concentrations compared to the sympatric non-accumulator N. gracilis.
Methods
Neptunia amplexicaulis and N. gracilis seeds were germinated and transplanted into rhizotrons filled with half control and half Se-dosed soils with low (5 µg Se g− 1) or high (30 µg Se g− 1) levels of Se in soluble (Na2SeO4) or insoluble (CaSeO3) form. After 3 weeks, the root density in the two areas of the rhizotrons was measured and plants were removed from the soil to determine biomass and for chemical analysis of Se and other elements.
Results
Major changes were observed in the low Se dosed side in Na2SeO4 form, and in the high Se dosed side in CaSeO3 form in N. amplexicaulis roots: a higher density, Se concentration, Se:S ratio, and a tendency to increase the biomass. In contrast, a reduction in the root density with 30 µg Se g− 1 in respose to the CaSeO3 form was observed in N. gracilis.
Conclusions
Neptunia amplexicaulis preferentially foraged in Se soluble enriched soil, which may be beneficial for the plant given the increase in the root biomass at low Se dosed soil. In contrast, a reduction in the root density in N. gracilis indicated avoidance of soils enriched with high insoluble form of Se.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hyperaccumulators are plants that have the ability to accumulate particular metal(loid) elements in extremely high concentrations in their aerial tissues without experiencing toxicity (Jaffré et al. 1976; van der Ent et al. 2013). Hyperaccumulation is rare globally (Baker and Brooks 1989), and the hyperaccumulation of the metal(loid) selenium (Se) is even rarer, recorded in only 45 taxa (Cappa and Pilon-Smits 2014; White 2016). Selenium hyperaccumulators are plants that concentrate > 1000 µg Se g− 1 in their shoots, however plants can also be classified as secondary Se accumulators (100–1000 µg Se g− 1 in shoots) (Anderson 1993; Brown and Shrift 1982). In contrast, most plants cannot tolerate more than 10–100 µg Se g− 1 in their tissues and show signs of Se toxicity when prevailing foliar Se is greater (Hartikainen et al. 2001), as such, plants with < 100 µg Se g− 1 are classified as non-accumulators (White et al. 2004). Families that contain Se hyperaccumulating species are variable, and while Se hyperaccumulation may occur in several species in the same genus, such as Astragalus which contains ∼25 Se hyperaccumulator species, Xylorhiza and Symphyotrichum (Asteraceae) contain only three Se hyperaccumulator species each, in many other cases Se hyperaccumulation may only occur in one or two species in a genus (Stanleya pinnata and S. bipinnata of the Brassicaceae family) (El Mehdawi et al. 2014; Rosenfeld and Beath 1964; White 2016). Currently, the strongest Se hyperaccumulators are species within the genus Astragalus which are able to accumulate upwards of 10 000 µg Se g− 1, while the soil on which they grow contains 2–10 µg Se g− 1 (Schiavon and Pilon-Smits 2016). Another of the strongest Se hyperaccumulator plants known globally is Neptunia amplexicaulis (Fabaceae), an herbaceous legume from Central Queensland, Australia (AVH 2019; Knott and McCray 1959). In Se-dosed glasshouse conditions, this speciesis capable of accumulating up to 13 600 µg Se g− 1 in young leaves (Harvey et al. 2020). In nature, when found growing on the most seleniferous area within its endemic habitat near Richmond, it was recorded accumulating on average 3028 µg Se g− 1 and up to 4334 µg Se g− 1in leaves, when growing on soils and rocky outcrops with 10 to 69 µg Se g− 1 (Knott and McCray 1959; McCray and Hurwood 1963). The larger Richmond area has largely variable Se soil content ranging from non-seleniferous to 32 µg Se g− 1 originating from highly seleniferous limestone outcrops (McCray and Hurwood 1963). The area also has several other species of Neptunia, whose Se concentrations range from near negligible to > 200 µg Se g− 1, notably the Se sensitive non-accumulator Neptunia gracilis which grows semi-sympatrically with N. amplexicaulis (AVH 2019; McCray and Hurwood 1963).
In well drained soils, such as those found at Richmond, highly bioavailable forms of Se such as selenate (SeO42−) are taken up through root sulphate transporters, before being metabolised through sulphate assimilation mechanisms in the shoot or root (Terry et al. 2000; White et al. 2004). Due to the strong molecular similarity between selenium and sulphur (S), and the enhanced ability of Se hyperaccumulators to discriminate between the two, Se hyperaccumulators typically have a substantially elevated Se:S ratio when compared to non-accumulators (White et al. 2007). As inorganic forms of Se are thought to cause more oxidative stress in plants, hyperaccumulators facilitate the conversion of inorganic to organic Se, which in part explains the Se hyper-tolerance of hyperaccumulators (Van Hoewyk 2013). Conversely, non-accumulators tend to accumulate more inorganic Se (Brown and Shrift 1982; Freeman et al. 2006; Pilon-Smits et al. 1999). Organic forms of Se can cause toxicity when Se amino acids are non-specifically incorporated into proteins (Brown and Shrift 1982; Stadtman 1990). Hyperaccumulators use the plastidic enzyme selenocysteine methyltransferase (SMT) to convert selenocysteine (SeCys) to methyl-SeCys, thus avoiding this type of toxicity (Brown and Shrift 1982; Neuhierl and Böck 1996; Sors et al. 2009).
Previously, N. amplexicaulis has been reported to contain several C-Se-C compounds including selenocystathionine, methyl-SeCys and seleno-methionine (Harvey et al. 2020; Peterson and Butler 1967). Selenium was found to accumulate primarily in the young leaves, flowers, pods and taproot, with lower Se concentrations in the fine roots and stem, while the old leaves contained the lowest Se concentrations overall (Harvey et al. 2020).
Neptunia amplexicaulis and N. gracilis use a taproot to access deep water stores when growing in arid environments, with multiple lateral-growing fine roots. Plants proliferate lateral roots preferentially in nutrient-rich zones to access essential nutrients in diverse soil microenvironments (Guan et al. 2014). Although this response is mainly associated with macronutrients, such as N, P and K, it has also been reported for hyperaccumulator plants in the presence of certain trace metals such as Zn, Cd and Ni, suggesting that hyperaccumulator plants might have a higher requirement for specific metals (Dechamps et al. 2008; Haines 2002; Liu et al. 2010; Schwartz et al. 1999; Whiting et al. 2000). Localised root proliferation, or ‘root foraging’, is one of the mechanisms for highly efficient metal uptake in the well-studied Ni-Cd-Pb hyperaccumulator Noccaea caerulescens (Assunção et al. 2003a, b; Gonneau et al. 2017; Schwartz et al. 1999). This species has been subjected to several investigations aimed at elucidating root foraging in response to Zn (Haines 2002; Whiting et al. 2000), Cd (Liu et al. 2010; Schwartz et al. 1999) and Ni (Dechamps et al. 2008; Tognacchini et al. 2020). Root foraging for Se has been observed in the hyperaccumulator S. pinnata, though to a relatively weak degree (Goodson et al. 2003).
Selenium is not commonly considered essential to plant metabolism, although there has been evidence of a beneficial growth effect in both hyperaccumulators and non-accumulators (Pilon-Smits et al. 2009). Adding Se to a variety of secondary accumulating and non accumulating crop plants including Indian mustard, lettuce and sorrel, has been noted to provide growth stimulation through antioxidant effects at low concentrations (Kong et al. 2005; Singh et al. 1980; Xue et al. 2001). Hyperaccumulator seedlings (Astragalus racemosus) grown without Se developed slower and produced significantly less biomass than their Se-dosed counterparts, and thus Se was suggested to match the criterion of a micronutrient for Se accumulators (Shrift 1969; Trelease and Trelease 1938). It has also been suggested that growth stimulation in Astragalus may be due to the role of Se in supressing sorption of toxic levels of P (Broyer et al. 1972). Additionally, higher Se levels in hyperaccumulators have also been shown to reduce rates of predation from both insects and mammals (Galeas et al. 2008; Quinn et al. 2008, 2010). As a result, it is plausible that Se hyperaccumulators, such as N. amplexicaulis, actively seek out higher Se concentrations in soil in order to maximise Se uptake.
The aim of this study was to address the following key questions: (i) does the hyperaccumulator N. amplexicaulis preferentially forage in Se-enriched zones? (ii) does a positive root response to Se enhance accumulation in N. amplexicaulis? (iii) How does this compare to the non-accumulator N. gracilis? To address these questions, we investigated the root responses of N. amplexicaulis and N. gracilis grown in rhizotrons with localised Se enrichment in order to observe active Se foraging vs. avoidance strategies.
Materials and methods
Biological material and growth conditions
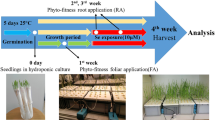
Neptunia amplexicaulis and N. gracilis seeds were collected in June 2018 from Richmond, Central Queensland (-20.648359, 143.098375). Germination was carried out by a pretreatment in which the seed coat was punctured with a scalpel, placed in petri dishes and submerged in distilled water for 24 hrs to promote germination. The seeds were then placed on moistened paper and kept at 25 °C for 24 hrs until the radicle emerged.
Natural soil from the UQ St Lucia Campus was used because it is relatively infertile, has a near neutral pH and has a dark colour (which assists in the imaging analyses of root distribution based on colour contrast of roots versus background). The soil was oven dried at 60 °C for 48 hrs and then sieved at < 2 mm and divided in equal parts of 1.2 kg. Two aliquots of soil were enriched with Se, each with a specific Se chemical form and the remaining soil was kept as a control. Two Se chemical forms with different solubility were chosen for the soil enrichment: (i) Na2SeO4 (water soluble) and (ii) CaSeO3 (water insoluble). The soil was spiked with three selenium concentrations (0, 5 or 30 µg Se g− 1) added in the form of Na2SeO4 or CaSeO3. Soil from each treatment was then watered up to field capacity.
Rhizotron experiment
In order to observe root growth responses of the tested plant species in the presence of Se, a rhizotron experiment was conducted. Rhizotrons consist of narrow transparent boxes filled with soil, which allow for non-destructive observations of roots on a transparent surface. The self-made rhizotrons were constructed from polycarbonate square Petri dishes (12 × 12 cm). Openings for seedling transplantation and watering were created on the upper part of the Petri dishes. The left half of the rhizotron was used as a control (filled with soil Se 0 µg g− 1 Se) and the right half was filled with soil with different Se concentrations and forms. A plastic foil was used to create a vertical separation while filling with soil, but was subsequently removed so that no physical barrier existed between the control soil and the Se enriched soil. The soil surface was then compacted to avoid inhomogeneities to appear during the plant growth as well as to allow for observations of the roots. After seedlings were transplanted with roots aligned with midline between soils, the rhizotrons were closed with the Petri dish cover plates, wrapped with aluminium foil to protect the roots from light and set up at an inclination of 45° with the rooted surface facing down. The two Se forms (Na2SeO4 and CaSeO3) were tested for in eight different treatments and three replicates from each condition. The experiment was conducted for three weeks in a growth cabinet with a 12 hrs per day of light, a temperature of 20–25 °C (night–day), 75 % humidity and light intensity of 350 µmol m2 sec− 1 photosynthetically active radiation (PAR) supplied using LED lights (Valoya B200). The rhizotrons were watered daily to field capacity.
Growth analyses
After 3 weeks, the plants were completely removed from the soil and washed several times with distilled water until all soil was removed. Shoot and root were then dried at 45 °C for 48 hrs and weighed to analyse changes in the growth. Soil and plants samples were used to determine prevailing Se and other elemental concentrations.
Chemical analysis of plant tissues
Dried samples were weighed (100 mg) in 10 mL polypropylene tubes, then pre-digested using 2 mL HNO3 (70 %) for 68 hrs, and then digested using a hot block (Thermo Scientific Digital Dry Bath) for 3 hrs at 125 °C. Samples were brought to volume (10 mL) with ultrapure water (Millipore 18.2 MΩ·cm at 25 °C) before analysis with Inductively coupled plasma atomic emission spectroscopy (ICP-AES) with a Thermo Scientific iCAP 7400 instrument for macro-elements (Na, Mg, Al, P, S, K, Ca), trace-elements (Cr, Mn, Fe, Co, Ni, Cu, Zn, Se) in radial and axial modes depending on the element and expected analyte concentration. In-line internal addition standardization using yttrium was used to compensate for matrix-based interferences.
Chemical analysis of soil
Weakly exchangeable elemental concentrations in the soil were determined using a Sr(NO3)2 extraction (0.01 M) based on a method adapted from Kukier and Chaney (2001) with solid/liquid ratio (m:v) of 1:4 and shaking for 2 hrs on an end-over-end shaker. Pseudo-total elemental concentrations in soils were determined by weighing ~ 100 mg soil sub-samples into quartz tubes and digesting them using reverse aqua regia (3:1 HNO3:HCL) for 16 min at 50 % power using a ColdBlock system (CB15S 15 channel system, ColdBlock Technologies Inc) with high-intensity infrared irradiation (Wang et al. 2014). The digest solutions were diluted to 30 mL with ultrapure deionised water (Millipore), filtered (0.45 µm syringe filters, Milipore) and analysed by ICP-AES. Soil pH and electrical conductivity (EC) was obtained in a 1:2 soil:water mixture after 2-hrs equilibrium time on an end-over-end shaker and 1-hr settling time.
Statistical analyses
At the end of the growing period (3 weeks) and before the harvesting of shoots, high resolution images of all rhizotrons were taken with a Canon 5D MkII (22.1-megapixel full-frame) camera with 50 mm prime lens. The images were then processed with the imaging software Image-J (Schneider et al. 2012) and converted to binary, where only black (“0”) and white (“255”) pixels were displayed. The colours of the pictures were then inverted and in the binary images, “0” (black/roots) and “255” (white/bulk soil) pixels were counted in each half of the rhizotrons with the Image-J program function “pixel count”. The root density in the two areas of the rhizotrons was than measured as the percentage (%) of black pixels (roots) in each half calculated from the total black pixels of the full surface. In addition to the pixel counts, roots were harvested from each half of the rhizotrons, thoroughly rinsed to remove soil particles and oven dried at 45 °C for 48 hrs. Dry weight was recorded and the root density in each side was measured as a percentage of the total root density for each rhizotron.
Differences in root density, root biomass and Se and other elemental concentrations in roots were assessed through two-way ANOVA and Fisher LSD post-hoc test considering treatment (control or enriched), and species as factors. Differences in the Se concentration in shoots was assessed through two-way ANOVA and Fisher LSD post-hoc test considering Se concentration in soil and species as factors. All statistical tests were performed with the software Statistical 7.0 considering a significance level of p < 0.05.
Results
Elemental concentrations in the experimental soils
Total elemental concentrations and exchangeable S and Se in soils were determined by two different methods as described above. The concentrations of Se detected with the exchangeable method were under the limit of detection (LOD) in the treatments with 5 µg Se g− 1 in soluble and insoluble forms of Se (Table 1). In the 30 µg g− 1 treatment, the CaSeO3 dosed side contained 1.44 µg Se g− 1, while the control side had a concentration < LOD. The high Se treatment in Na2SeO4 form had 4.34 and 9.85 µg Se g− 1 in the control and dosed side respectively.
Root density in the rhizotrons
The Se hyperaccumulator N. amplexicaulis and the Se sensitive N. gracilis (Fig. 1) were used to elucidate the root response under different concentrations and chemical forms of Se dosed in the soils of the rhizotrons. The rhizotron experiment was conducted for three weeks (Fig. 2). After that time root preference for Se was observed and then measured as root density (%) using the pixel count method (Fig. 3). Major changes were observed in N. amplexicaulis, which had a higher root density (90.3 ± 3.30 % of total roots) within 5 µg Se g− 1 soil enriched with Na2SeO4 compared to the control side (Fig. 2a). CaSeO3 induced a reduction in root density in N. gracilis, with only 20.6 ± 15.1 % of the roots on the 30 µg Se g− 1 dosed side compared to the control side (Fig. 2d). No significant changes in root density were observed for N. gracilis exposed to Na2SeO4, nor in N. amplexicaulis exposed to CaSeO3 (Fig. 2b, c).
Root density % in the two areas of the rhizotrons (Se enriched and control sides) calculated from imaging pixel counts for Neptunia amplexicaulis and Neptunia gracilis. Values are mean ± SE (n = 3). Different letters show statistical differences using two-way ANOVA considering soil condition (control or Se enriched sides) and species as factors (Fisher LSD test; p < 0.05)
Selenium concentrations in roots
Roots collected from enriched and control sides were processed and Se concentrations were measured using ICP-AES (Fig. 4). Neptunia amplexicaulis had higher Se concentration in the roots from soil enriched with 5 µg Se g− 1 in form of Na2SeO4 (177 ± 44.7 µg Se g− 1), and a higher Se concentration in the roots from soil enriched with 30 µg Se g− 1 in form of CaSeO3 (264 ± 64.6 µg Se g− 1) compared to roots from the control sides (Fig. 4a, d). In contrast, N. gracilis had no difference in Se concentration in roots from soil enriched with Na2SeO4, nor CaSeO3 at the 5 µg Se g− 1 concentration. The Se concentration in N. gracilis roots from the 30 µg Se g− 1 enriched side with the CaSeO3 form was 40.0 ± 20.8 µg Se g− 1; all other N. gracilis root values were < LOD in CaSeO3 treatments and respective controls (LOD = 8.66 µg Se g− 1). Between the two species, N. amplexicaulis had a significantly higher Se concentration in the roots from the high Se treatment with Na2SeO4 (177 ± 44.7 µg Se g− 1) compared to N. gracilis (47.2 ± 13.3 µg Se g− 1) grown under the same conditions (p < 0.05; Fig. 4a).
Selenium concentrations in the roots of the two areas of the rhizotrons (Se enriched and control side) for Neptunia amplexicaulis and Neptunia gracilis. Values are mean ± SE (n = 3). Different letters show statistical differences using two-way ANOVA considering soil condition (control or Se enriched side) and species as factors (Fisher LSD test; p < 0.05)
Root biomass
In order to determine the root growth response under Se enrichment, root biomass was measured. CaSeO3 induced changes in the root biomass in N. amplexicaulis grown at both low and high Se concentrations in CaSeO3 form (Fig. 5a, d); a higher biomass was present in roots from the enriched side at 5 µg Se g− 1 (18.1 ± 1.30 mg) compared to the control side (7.0 ± 2.02 mg), and a similar response was observed in roots from the enriched side at 30 µg Se g− 1 which had a higher biomass (12.3 ± 1.93 mg) compared to the control side (5.55 ± 2.0 mg). While there was no significant difference in root biomass between the enriched and control soils in N. gracilis grown in CaSeO3, Na2SeO4 induced a higher biomass in this species in roots from the enriched side (1.7 ± 0.35 mg) compared to the control side (0.9 ± 0.260 mg) at 30 µg Se g− 1 (Fig. 5c). Between the species, N. amplexicaulis had a significant higher biomass (4.4 ± 0.173 mg) compared to N. gracilis (1.7 ± 0.351 mg) in roots from the side dosed with 30 µg Se g− 1 in Na2SeO4 form (Fig. 5c).
Root weights of the two areas of the rhizotrons (Se enriched and control side) for Neptunia amplexicaulis and Neptunia gracilis. Values are mean ± SE (n = 3). Different letters show statistical differences using two-way ANOVA considering soil condition (control or Se enriched side) and species as factors (Fisher LSD test; p < 0.05)
Root Se:S ratios
Se:S ratio was calculated from the Se and S concentration measured from the roots (Fig. 6). Neptunia amplexicaulis had a significantly higher Se:S in the enriched side with 5 µg Se g− 1 in Na2SeO4 form compared to the control side (Fig. 6a). A similar result was observed in roots from soil enriched with 30 µg Se g− 1 in form of CaSeO3 (Fig. 4d). Most ratios could not be calculated for N. gracilis as most Se levels in the roots were below the LOD, except for the roots of the 30 µg Se g− 1 CaSeO3 treated roots, which exhibited Se:S ratios statiscally similar to the control roots of N. amplexicaulis from the same treatment. When comparing the two species, N. amplexicaulis had a significantly higher Se:S ratio in the roots from the high Se treatment with Na2SeO4 compared to N. gracilis grown under the same conditions (p < 0.05; Fig. 6a).
Se:S ratios in roots of the two areas of the rhizotrons (Se enriched and control side) for Neptunia amplexicaulis and Neptunia gracilis. Values are mean ± SE (n = 3). Different letters show statistical differences using two-way ANOVA considering soil condition (control or Se enriched side) and species as factors (Fisher LSD test; p < 0.05)
Selenium concentrations in shoots
Shoots collected from plants grown at the 5 and 30 µg Se g− 1 dose levels were processed and Se concentrations were measure using ICP-AES (Table 2). Both species developed higher Se concentrations in their shoots when exposed to the soils enriched with 30 µg Se g− 1 compared to the treatment with 5 µg Se g− 1 in Na2SeO4 form. A similar response was observed in the shoots from the CaSeO3 treatment in N. amplexicaulis (p < 0.05). When comparing the species, N. amplexicaulis had higher Se in the shoot than N. gracilis, but only in the CaSeO3 at 30 µg Se g− 1 treatment.
Sulphur concentrations in roots
Roots collected from Se-enriched and control sides were processed and S concentrations were measured using ICP-AES (Table 3). The S concentration in N. amplexicaulis roots decreased with the CaSeO3 treatment at 5 µg Se g− 1(p < 0.05). In contrast, N. gracilis had no statistical differences under the same conditions. Na2SeO4 did not affect the S concentration in roots, neither did the CaSeO3 treatment at 30 µg Se g− 1, where no differences between control and enriched side with either species were found. However, differences were found when comparing the two species under control conditions: N. amplexicaulis roots had higher concentration of S compared to N. gracilis, except in the rhizobox spiked with CaSeO3 30 µg Se g− 1, where no differences were observed between the species.
Macro and micro elements in roots
Concentrations of macro and micro elements are shown in Tables 4 and 5. Major differences were observed in the 30 µg Se g− 1 with Na2SeO4 form treatment where N. gracilis had a lower Ca, Mg, and Zn concentrations in the roots compared to the control side (p < 0.05). Additionally, in this treatment N. gracilis had a higher K concentrations in the control conditions, and higher P concentrations in the control and treatment conditions, compared to N. amplexicaulis. On the other hand, N. amplexicaulis had higher concentrations of K and Mg compared to the control side in the 5 µg Se g− 1 with CaSeO3 form treatment.
Discussion
Neptunia amplexicaulis and N. gracilis are two species belonging to the same genus of the Fabaceae family and naturally grow near Richmond, Queensland on seleniferous soils. Even though these species are taxonomically and ecologically similar, their relationship with Se differentiates them; Neptunia amplexicaulis is a well-known Se hyperaccumulator, whereas N. gracilis is Se sensitive. The characteristics of these two species provide ideal experimental subjects for understanding the mechanisms of Se hyperaccumulation in N. amplexicaulis. We studied the changes occurring in root proliferation and root and shoot biomass under different chemical forms and concentrations of Se dosed in the soil during the first three weeks of plant development.
Both insoluble (CaSeO3) and soluble (Na2SeO4) Se at low (5 µg Se g− 1) and high levels (30 µg Se g− 1) had differing effects on root behaviour and overall Se levels in the roots. For the hyperaccumulator N. amplexicaulis, root foraging as a percentage density was observed in the soluble Se dosed specimens at low Se concentration in the soil (Fig. 3a), and is related to an increase in the Se concentration in roots (Fig. 4a). Moreover, a tendency to increase the root biomass (although not statistically significant), was observed in N. amplexicaulis growing at low concentrations in the soluble form of Se (Fig. 5a). Insoluble forms of Se were also beneficial for N. amplexicaulis as it increased the root biomass at both low and high treatments. As the presence of low concentration or less available Se either increased root density and/or root biomass, these conditions may have a positive effect on growth and Se seeking behaviour for the hyperaccumulator.
The root preference for Se has also been described in the Se hyperaccumulator Symphyotrichum ericoides, where populations from seleniferous soil had directional growth towards selenate, as judged from more root biomass, longer individual roots, and larger total root length on the + Se side compared to the -Se side (Mehdawi et al. 2015). The Se hyperaccumulator S. pinnata was also reported to be foraging for Se under rhizotron conditions, although there was also considerable root proliferation in the non-Se dosed soils (Goodson et al. 2003). Additionally, Rao et al. (2020) recently reported that a 0.25 mg L− 1 Na2SeO4 treatment stimulated growth in the Se hyperaccumulator Cardamine violifolia. In studies on other trace element hyperaccumulator plants, root proliferation and plant biomass in response to Zn enriched soil patches have been observed in Noccaea caerulescens revealing that this species is actively foraging for Zn in the soil (Haines 2002; Whiting et al. 2000). Similar responses have been reported in this species in response to Cd-enriched soil patches (Schwartz et al. 1999; Whiting et al. 2000). While root foraging towards Ni was reported by Dechamps et al. (2008) in some accessions of N. caerulescens, Tognacchini et al. (2020) report minor avoidance in response to Ni in this species.
In contrast to the hyperaccumulator, N. gracilis did not show preference or avoidance in response to the soluble form of Se, however, a reduction in the root density was observed at high concentrations of Se in the insoluble form which suggests an avoidance response to high levels of Se (Fig. 3). However, N. gracilis had a significant, but minor, increase in the root biomass in the highly dosed soluble Se soil, where the root density and root Se concentrations were statistically indistinguishable, though root biomass in this treatment was far smaller than other treatments, even compared to N. amplexicaulis (Fig. 5c). This species may have some degree of tolerance to Se, given that it is found in seleniferous areas alongside N. amplexicaulis, but these specimens only grew for three weeks and may have experienced toxicity with prolonged exposure. The taproot of the secondary Se accumulator Brassica juncea under the same conditions grew down the division between Se and non-Se soils with indiscriminate lateral root proliferation, similar to the mostly indiscriminate root density results from N. gracilis (Goodson et al. 2003). Conversely, non-accumulators (Astragalus canadensis, Lacuta sativa, Lolium perenne) avoided lateral root proliferation even on low Se-dosed soils, a behaviour only observed in N. gracilis under high insoluble Se conditions (Hartikainen et al. 2001).
The highest Se concentration in roots was observed in N. amplexicaulis growing under the high Se dose level (with 3600 µg Se− 1; Fig. 4c). This concentration was 20.3-fold higher than the root Se concentration in the same species growing in the low Se dose level. This is a characteristic of the hyperaccumulators, in which the Se uptake depends on the concentration in the soil and on its availability (Brown and Shrift 1982). Despite this, no changes in the Se concentration in the roots were observed in the high Se dose level when the control and enriched sides were compared. Micro-analytical investigations have shown that Se in Neptunia is present almost exclusively in the phloem bundles in the plant, which is suggestive of intensive recycling of Se from roots to shoots back to roots via the phloem (Harvey et al. 2020). As such, the mature taproot of these species serves as the main store for organic Se with rapid translocation to young emerging leaves. As the plants in this study were young and had not yet developed a lignified taproot, these internal cycling processes may have led to significant Se concentrations in the entire root system. These internal Se cycling processes may be partially responsible for the levels of Se found in the control soil of the high level soluble Se rhizotrons at harvest, coupled with Se leaching from the dosed side. The previous rhizotron experiment with S. pinnata observed a weak but noticeable root foraging response, and the authors noted that higher Se levels and use of selenate may encourage a stronger response (Goodson et al. 2003). The authors did not use selenate due to the potential for Se leaching, but as observed here, N. amplexicaulis did exhibit root responses even when leaching may have occured.
Within soluble Se-dosed specimens of N. amplexicaulis, the roots on the low Se-dosed side had significantly more Se than control side roots, however insoluble Se-dosed specimens only had a significantly higher Se concentration in highly dosed roots compared to the control. Selenium uptake from insoluble Se forms would be a much slower process, reliant on rhizosphere alteration and geochemical weathering, which the three-week-old plants in a laboratory setting may have been unable to achieve. Even when the insoluble form becomes more soluble, selenite (SeO3) is taken up in hyperaccumulators through phosphate pathways, so higher levels of phosphate in the soil could have competed with available selenite, lowering their accumulation rates (Hopper and Parker 1999). In contrast, N. gracilis had no changes in the Se concentration in roots and exhibited a lower Se uptake compared to N. amplexicaulis (Fig. 4a). The insoluble Se dosed specimens exhibited little shoot Se uptake, and significant but relatively small root Se levels, indicating the highly soluble form was up taken effectively and rapidly when compared to CaSeO3, which first needs to be weathered to become soluble before uptake can take place. It should be noted, however, that the young plants did not produce much biomass, meaning that this may not reflect the accumulation capacity of larger, more mature specimens, nor the effects of toxicity due to ongoing exposure to Se.
A higher Se:S ratio in the shoots is a characteristic shared by Se hyperaccumulator plants (White et al. 2007). Higher Se:S ratios have also been shown in roots of hyperaccumulating populations of Sympotrichum ericoides exposed to increasing Se levels, compared with non-accumualting populations of the same species (El Mehdawi et al. 2014). Neptunia amplexicaulis had a significantly higher Se:S ratio in the low dosed side with the soluble form of Se compared to the control side and also compared to N. gracilis (Fig. 6a). Conversely, Se in soil did not affect the Se:S ratio in N. gracilis. As Se is chemically similar to S, it competes with S and is transported inside the plant through sulphate transporters present in the root plasma membrane (Sors et al. 2005; Li et al. 2008). We observed that under control conditions, the S concentration in roots in N. amplexicaulis is higher than N. gracilis. However, within the Se treatment, there is a slight non-significant reduction in S concentration in N. amplexicaulis, resulting in a statistically similar S level to N. gracilis. It is possible that a mechanism switches the uptake preference from S to Se in the hyperaccumulator in these conditions (Schiavon et al. 2015). The role of the high-affinity sulfate transporters (HASTs) has been attributed to the selectivity between selenate and sulphate in different species that have contrasting shoot Se:S ratios when grown under the same conditions (Rosenfeld and Beath 1964; Bell et al. 1992; Galeas et al. 2007). Two well-known examples are the hyperaccumulators A. bisulcatus and S. pinnata, that have always shown shoot Se:S ratios greater than those in the rhizosphere solution (Bell et al. 1992; Feist and Parker 2001; Ellis and Salt 2003; Galeas et al. 2007). It is, therefore, hypothesised that the dominant HASTs of Se-accumulator plants are selective for selenate, whereas those in other angiosperm species are selective for sulphate (White et al. 2004; Sors et al. 2005; Broadley et al. 2006).
Neptunia amplexicaulis preferentially foraged for Se in the Se-soluble enriched soil, which may be beneficial for the plant given the resultant increase in the root biomass in the low Se dosed soil. High levels of Se, but in the insoluble form, are also beneficial for this species. This may represent an ‘ideal’ concentration of Se in soils for hyperaccumulators, where lower levels induce foraging behaviour and higher concentrations allow non-foraging behaviour to still result in beneficial levels of Se uptake, especially considering the intensive cycling of Se within the root system.
References
Anderson JW (1993) Selenium interactions in sulfur metabolism. In: Sulfur nutrition and assimilation in higher plants: Regulatory, agricultural and environmental aspects. SPB Academic Publishing, The Hague, pp 49–60
Assunção AGL, Bookum WM, Nelissen HJM, Vooijs R, Schat H, Ernst WHO (2003a) Differential metal-specific tolerance and accumulation patterns among Thlaspi caerulescens populations originating from different soil types. New Phytol 159:411–419
Assunção AGL, Schat H, Aarts MGM (2003b) Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants, vol 159. Blackwell Publishing Ltd., Oxford
AVH (2019) The Australasian Virtual Herbarium. Council Heads of Australasian Herbaria. https://avh.chah.org.au/. Accessed 6/09 2019
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyper accumulate metallic elements. Biorecovery 1:81–126
Bell PF, Parker DR, Page AL (1992) Contrasting selenate sulfate interactions in selenium-accumulating and nonaccumulating plant species. Soil Sci Soc Am J 56:1818–1824
Broadley MR, White PJ, Bryson RJ, Meacham MC, Bowen HC, Johnson SE, Hawkesford MJ, McGrath SP, Zhao FJ, Breward N, Harriman M, Tucker M (2006) Biofortification of UK food crops with selenium. Proc Nutr Soc 65:169–181
Brown T, Shrift A (1982) Selenium: Toxicity and tolerance in higher plants. Biol Rev Camb Philos Soc 57:59–84
Broyer T, Johnson C, Huston R (1972) Selenium and nutrition of Astragalus. Plant Soil 36:635–649
Cappa J, Pilon-Smits E (2014) Evolutionary aspects of elemental hyperaccumulation. Planta 239:267–275
Dechamps C, Noret N, Mozek R, Draye X, Meerts P (2008) Root allocation in metal-rich patch by Thlaspi caerulescens from normal and metalliferous soil—new insights into the rhizobox approach. Plant Soil 310:211–224
El Mehdawi AF, Reynolds RJB, Prins CN, Lindblom SD, Cappa JJ, Fakra SC, Pilon-Smits EAH (2014) Analysis of selenium accumulation, speciation and tolerance of potential selenium hyperaccumulator Symphyotrichum ericoides. Physiol Plant 152:70–83
Ellis DR, Salt DE (2003) Plants, selenium and human health. Curr Opin Plant Biol 6:273–279
Feist LJ, Parker DR (2001) Ecotypic variation in selenium accumulation among populations of Stanleya pinnata. New Phytol 149:61–69
Freeman JL, Zhang LH, Marcus MA, Fakra S, McGrath SP, Pilon-Smits EAH (2006) Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiol 142:124–134
Galeas ML, Zhang LH, Freeman JL, Wegner M, Pilon-Smits EAH (2007) Seasonal fluctuations of selenium and sulfur accumulation in selenium hyperaccumulators and related nonaccumulators. New Phytol 173:517–525
Galeas ML, Klamper EM, Bennett LE, Freeman JL, Kondratieff BC, Quinn CF, Pilon-Smits EAH (2008) Selenium hyperaccumulation reduces plant arthropod loads in the field. New Phytol 177:715–724
Gonneau C, Noret N, Godé C, Frérot H, Sirguey C, Sterckeman T, Pauwels M (2017) Demographic history of the trace metal hyperaccumulator Noccaea caerulescens (J. Presl and C. Presl) F. K. Mey. in Western Europe. Mol Ecol 26:904–922
Goodson CC, Parker DR, Amrhein C, Zhang Y (2003) Soil selenium uptake and root system development in plant taxa differing in Se-accumulating capability. New Phytol 159:391–401
Guan P et al (2014) Nitrate foraging by Arabidopsis roots is mediated by the transcription factor TCP20 through the systemic signaling pathway. PNAS 111:15267–15272
Haines BJ (2002) Zincophilic root foraging in Thlaspi caerulescens. New Phytol 155:363–372
Hartikainen H, Pietola L, Simojoki A (2001) Quantification of fine root responses to selenium toxicity. Agric Food Sci 10:53–58
Harvey M-A et al (2020) Distribution and chemical form of selenium in Neptunia amplexicaulis from Central Queensland, Australia. Metallomics 12:514–527
Hopper JL, Parker DR (1999) Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant Soil 210:199–207
Jaffré T, Brooks RR, Lee J, Reeves RD (1976) Sebertia acuminata: A hyperaccumulator of nickel from New Caledonia. Science 193:579–580
Knott SG, McCray CWR (1959) Two naturally occurring outbreaks of selenosis in Queensland. Aust Vet J 35:332–334
Kong L, Wang M, Bi D (2005) Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul 45:155–163
Kukier U, Chaney RL (2001) Amelioration of nickel phytotoxicity in muck and mineral soils. J Environ Qual 30:1949–1960
Li HF, McGrath SP, Zhao FJ (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102
Liu J-Q, Allan DL, Vance CP (2010) Systemic signaling and local sensing of phosphate in common bean: Cross-talk between photosynthate and microrna399. Mol Plant 3:428–437
McCray CWR, Hurwood IS (1963) Selenosis in north west Queensland associated with marine cretaceous formation. Qld J Agric Sci 20:475–498
Mehdawi E, Paschke AF, Paschke MW, Pilon-Smits EAH (2015) Symphyotrichum ericoides populations from seleniferous and nonseleniferous soil display striking variation in selenium accumulation. New Phytol 206:231–242
Neuhierl B, Böck A (1996) On the mechanism of selenium tolerance in selenium-accumulating plants: purification and characterization of a specific selenocysteine methyltransferase from cultured cells of Astragalus bisulcatus.Eur J Biochem 239:235–238
Peterson PJ, Butler GW (1967) Significance of selenocystathionine in an Australian selenium-accumulating plant, Neptunia amplexicaulis. Nature 213:599–600
Pilon-Smits EAH et al (1999) Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction and tolerance. Plant Physiol 119:23–132
Pilon-Smits EA, Quinn CF, Tapken W, Malagoli M, Schiavon M (2009) Physiological functions of beneficial elements. Curr Opin Plant Biol 12:267–274
Quinn CF, Freeman J, Galeas ML, Klamper EM, Pilon-Smits EAH (2008) The role of selenium in protecting plants against prairie dog herbivory: implications for the evolution of selenium hyperaccumulation. Oecologia 155:267–275
Quinn CF, Freeman JL, Reynolds RJB, Cappa JJ, Fakra SC, Marcus MA, Lindblom SD, Quinn EK, Bennet LE, Pilot-Smits EAH (2010) Selenium hyperaccumulation offers protection from cell disruptor herbivores. BMC Ecol 10:19
Rao S, Yu T, Cong X, Xu F, Lai X, Zhang W, Liao Y, Cheng S (2020) Integration analysis of PacBio SMRT- and Illumina RNA-seq reveals candidate genes and pathway involved in selenium metabolism in hyperaccumulator Cardamine violifolia. BMC Plant Biol 20:492
Rosenfeld I, Beath OA (1964) Selenium: Geobotany, biochemistry, toxicity and nutrition. Academic Press, New York
Schiavon M, Pilon-Smits EA (2016) The fascinating facets of plant selenium accumulation – biochemistry, physiology, evolution and ecology. New Phytol 213:1582–1596
Schiavon M, Pilon M, Malagoli M, Pilon-Smits EA (2015) Exploring the importance of sulfate transporters and ATP sulphurylases for selenium hyperaccumulation-a comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Front Plant Sci 6:2
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schwartz C, Morel JL, Saumier S, Whiting SN, Baker AJM (1999) Root development of the Zinc-hyperaccumulator plant Thlaspi caerulescens as affected by metal origin, content and localization in soil. Plant Soil 208:103–115
Shrift A (1969) Aspects of selenium metabolism in higher plants. Annu Rev Plant Physiol 20:475–494
Singh KM, Singh KN, Bhandari KD (1980) Interaction of selenium and sulfur on the growth and chemical composition of Raya. Soil Sci 129:238–244
Sors TG, Ellis DR, Na GN, Lahner B, Lee S, Leustek T, Pickering IJ, Salt DE (2005) Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant J 42:785–797
Sors TG, Martin CP, Salt DE (2009) Characterization of selenocysteine methyltransferases from Astragalus species with contrasting selenium accumulation capacity Plant J 59:110–122
Stadtman TC (1990) Selenium biochemistry. Annu Rev Biochem 59:111–127
Terry N, Zayed AM, de Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol 51:401–432
Tognacchini A, Salinitro M, Puschenreiter M, van der Ent A (2020) Root foraging and avoidance in hyperaccumulator and excluder plants: a rhizotron experiment. Plant Soil 450:287–302
Trelease SF, Trelease HM (1938) Selenium as a stimulating and possibly essential element for indicator plants. Am J Bot 25:372–380
van der Ent A, Baker A, Reeves RD, Pollard A, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 362:319–334
Van Hoewyk D (2013) A tale of two toxicities: malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Annals Bot-London 112:965–972
Wang Y, Kanipayor R, Brindle ID (2014) Rapid high-performance sample digestion for ICP determination by ColdBlock™ digestion: part 1 environmental samples. J Anal At Spectom 29:162–168
White PJ (2016) Selenium accumulation by plants. Ann Bot 117:217–235
White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, Smith BM, Thomas B, Broadley MR (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937
White PJ, Bowen HC, Marshall B, Broadley MR (2007) Extraordinarily high leaf selenium to sulfur ratios define ‘Se-accumulator’ plants. Ann Bot 100:111–118
Whiting SN, Leake JR, McGrath SP, Baker AJM (2000) Positive responses to Zn and Cd by roots of the Zn and Cd hyperaccumulator Thlaspi caerulescens. New Phytol 145:199–210
Xue T, Hartikainen H, Piironen V (2001) Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 237:55–61
Acknowledgements
K. Pinto Irish and M-A. Harvey are the recipients of Australian Government Research Training Program (RTP) Scholarships at The University of Queensland and their research is supported by this funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Fangjie Zhao.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 316 KB)
Rights and permissions
About this article
Cite this article
Pinto Irish, K., Harvey, MA., Erskine, P.D. et al. Root foraging and selenium uptake in the Australian hyperaccumulator Neptunia amplexicaulis and non‐accumulator Neptunia gracilis. Plant Soil 462, 219–233 (2021). https://doi.org/10.1007/s11104-021-04843-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-04843-x