Abstract
Aims
In water-limited areas, shrubs influence biological soil crust (biocrust) composition and diversity via soil microenvironment alterations and through modifying biotic interactions amongst biocrust taxa. However, the relative contributions of shrubs to biocrust succession and assembly via the biotic and abiotic influences are poorly known.
Methods
The community composition of biocrusts and soil properties in the interspace and beneath the dominant shrub (Artemisia ordosica) along a biocrust succession sequence were investigated. Biocrust interspecific interactions at a small scale (within shrub) were evaluated based on the co-occurrence pattern using null models. A hypothetical multigroup structural equation model (SEM) was proposed to evaluate the influence of multiple variables on the biocrust richness and to investigate the path variance between successional stages.
Results
Along the biocrust succession, shrubs significantly increased the size of bare soil gap by 489%, decreased lichens by 43% and increased soil organic matter by 13%. In years 18, 31and 37, the paths in SEM explained 59% of the variation in richness, only the effect of abiotic amelioration was significant (0.62). In years 54 and 62, the shrubs had direct (0.37) and indirect effect (0.10) via species interaction to biocrust richness.
Conclusions
Shrubs directly and indirectly affected the community assembly of biocrusts. Biocrust species interactions are an important driver of biocrust diversity and primarily affect late succession. The increasing influences of shrubs suggests a close relationship between shrub and biocrust components in arid or semiarid ecosystems, with shrubs playing an important role in regulating biocrust assembly and maintaining biocrust richness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biocrusts are assemblages of cyanobacteria, eukaryotic algae, lichens, bryophytes and other microorganisms integrated with topsoil particles, and they are widespread in arid and semiarid ecosystems (Aguiar and Sala 1999; Li et al. 2017). It is widely recognized that the diversity and composition of biocrusts are closely related to the ecosystem services of biocrusts in drylands, such as soil stabilization (Zhang et al. 2008), hydrological ecosystem services (Belnap 2006), nutrient cycling (Turetsky 2003), supplying microhabitat for other organisms (Liu et al. 2013), and interactions with vascular plants (Breen and Lévesque 2011). In most water-limited ecosystems, the landscape features a mosaic of vegetative patches and interspersed biocrusts (Belnap 2003; Boeken and Shachak 1994; Cortina et al. 2010). Biocrusts are nested under a patchy mosaic of vegetation and bare soil cover, and their composition and diversity may be influenced by vegetation, especially perennial shrubs (Eldridge et al. 2011). However, the role of shrubs in the succession and community assembly of biocrusts has received limited scientific attention.
The canopy shade, litterfall, and the root activity of shrubs can alter the soil microenvironment, including the light intensity, soil moisture, stability and fertility, thus influencing biocrust assembly, development and distribution (Belnap et al. 2014; Csotonyi and Addicott 2004; Weber et al. 2016). Shade under canopies, for example, prolongs the wetting time, and reduced water stress causes species turnover in biocrusts (Kidron and Benenson 2014; Li et al. 2017). Litter input accelerates soil formation and increases the topsoil thickness, and decomposition of organic material also exerts a strong amelioration of soil fertility, enhancing the biocrust cover and richness (Bowker et al. 2005; Li et al. 2003). While litter cover also occupies the spatial niche and decreases photosynthetic activity, it is negatively correlated with biocrust cover (Serpe et al. 2013; Zhang et al. 2013). Plants affect soil stability by decreasing wind erosion (Hao et al. 2016) or altering the behavior of animal digging and trampling (Li et al. 2014). In general, vascular plants buffer the fluctuating environment for biocrust succession and assembly (Bowker et al. 2005; Soliveres and Eldridge 2014, 2020).
At a small scale, both abiotic environmental constraints and biotic species interactions are major factors driving community assembly (Drake 1990; Gotelli et al. 2010; Ulrich et al. 2016). Despite the extensive research about the influences of shrubs on microenvironments, how shrubs influence interspecific interactions of biocrusts has received limited scientific attention (Bowker et al. 2014; Soliveres and Eldridge 2020). Some researchers have found that the dominant interspecific competition within biocrust communities regulates their species diversities (Bowker et al. 2010; Maestre et al. 2008). Therefore, the shrubs could indirectly affect biocrust community assembly by altering the interspecific interactions of biocrusts. In research on vascular plant communities, the observed species interactions usually depend on the environmental gradients or life-history strategies of the species involved (Cahill 2007; Michalet et al. 2006). Therefore, varying responses of the community are expected to occur along with succession because of dramatic changes in the abiotic environment and community structure that are also frequently reported in the vascular plant community (Cahill 2007). Some evidence has also been reported for biocrusts. For example, dominant species at different successional stages have divergent abiotic requirements, e.g., mosses are more sensitive to aridity than other species, while lichens are more sensitive to the stability of topsoil (Li et al. 2017; Reynolds et al. 2001). Environmental stress has been ameliorated by biotic activity during succession, thus the intensity and frequency of species interactions usually change (Bowker et al. 2010; Michalet et al. 2006). Therefore, quantifying the abiotic and biotic effects of shrubs on succession of biocrusts is very important to obtain a better understanding of biocrust community assembly in arid ecosystems.
In this study, we used space-for-time substitution to study the succession and process of the community assembly of biocrusts. Specifically, the physical-chemical properties of soil, interspecific interactions and community compositions of biocrusts were investigated. Our first objective was to compare abiotic and biotic features with/without the influence of the dominant shrubs Artemisia ordosica along successional sequences. Our second objective was to investigate how shrubs affect the diversity of biocrust communities, either directly or indirectly, through abiotic or biotic means. The structural equation model (SEM) is ideal for partitioning direct and indirect effects of factors on responses. Since the influence of shrubs changed between successional stages, the multigroup SEM technique was used to evaluate the variability in pathways from early to late succession (Grace et al. 2010; Ulrich et al. 2016; Vandenberg and Lance 2000). In general, we hypothesized the following: (1) Shrubs will influence the soil condition and interspecific interactions of biocrusts, which may affect the biocrust composition; (2) shrubs will influence the diversity of biocrust community through abiotic and biotic constraints, and these influences will be expected to vary along a succession because of the developments of the biocrust community and edaphic conditions.
Materials and methods
Study region
The study site is located in the Shapotou region of the Ningxia Hui Autonomous Region, China, and in the southeastern fringe of the Tengger Desert (37°32′-37°26′ N, 105°02′-104°30′ E, 1300–1350 m above mean sea level (AMSL)). This region is typical of ecotones between sandy desert and desertified steppe, and it has an annual mean temperature of 9.6◦C, an annual mean precipitation of 186 mm, and an annual potential evaporation of 2900 mm (Li et al. 2007). The annual temperatures range is large. January’s lowest mean temperature is 6.9◦C, and July’s highest mean temperature is 24.3◦C. The temporal distribution of precipitation is uneven, and 80% of the annual precipitation falls between May and September. To protect the Baotou-Lanzhou railway from burial by sand, five 500 m wide sand-binding vegetation belts were built in chronosequence next to the railway (Fig. 1a) (Li et al. 2003). Initially, the establishment of Caragana korshinskii Kom. and Artemisia ordosica Krasch. shrubs and straw checkerboards were established directly on sand dunes in 1956, 1964, 1981, 1987, and 1990 (Li et al. 2007). The straw checkerboards have an area of 1 m2 and a height of 0.15–0.2 m above the ground, and they increase the roughness of the sand surface and reduce the wind velocity by 20–40% at 0.5 m above the surface; thus, these structures help planted xerophytic shrubs to persist in environments with wind erosion (Zou et al. 1981; Li et al. 2007). Biocrusts began to colonize on the stabilized sand surface, following a typical successional sequence in Tengger Desert, from algae and cyanobacteria crust to lichen crust to moss crust (Fig. 1b-d) (Li et al. 2007). To maintain successional sequences, a sixth sand-binding belt was built on the sand dunes using the identical method in 2000, located 1.3 km away at the Soil Water Balance Experimental Field of the Shapotou Desert Experimental Research Station, Chinese Academy of Science. The segments of the sand-binding vegetation belts we chose are surrounded by the Shapotou tourist area (Fig. 1a), which reduces the disturbance of sand and wind erosion from the desert that could otherwise form gradient disturbance for each belt. Moreover, samples were not collected in the outermost belt built in 1990 because it is closest to the tourist area and is affected by tourism.
The study areas along the Baotou–Lanzhou railway at the Shapotou region of the Tengger Desert, China. a The aerial map and the location of study areas. The green marks the segments of sand binding vegetation belts where the data are obtained. b-d Three typical landscapes in chronosequences, where communities of biocrusts are dominated by algae and cyanobacteria, lichens, and mosses, respectively
Field sampling
Within each sand-binding belt, twenty 1.5-m line intercept transects were sampled to evaluate the community properties. Among them, 10 line transects were placed on flat ground, and the other 10 line transects were placed under the north canopy of Artemisia ordosica, which is the dominant regenerative shrub in the Shapotou region. For sampling representativeness, 20 line transects were randomly placed on the flat terrain along the belts at 5–15 m intervals and at least 2 m from the nearest mound (Choler et al. 2001). Because many shrub canopies were not sufficient to cover the 1.5 m line transects, we folded them into an “L” shape with 0.75 m on each side and with the vertex pointing to the north. Along the line transect, we sprayed deionized water to increase the visibility of the biocrusts and then recorded the presence of mosses, lichens, cyanobacteria, algae, plants and bare soil at a 1 mm resolution. Since a field survey could not be used to identify cyanobacteria and algae at the species level and the richness of cyanobacteria and algae was not compatible with that of mosses and lichens (the richness was much higher than that of mosses and lichens) (Li et al. 2003), we combined cyanobacteria and algae crust as one “species” group in our study. Therefore, the line transects can be regarded as alternating sections of different species patches. For each line transect, we count the proportion of length for mosses, lichens, algae and cyanobacteria and open gaps (plants and bare soil) to indicate the composition of the biocrust community. The moss species included Bryum argenteum Hedw., Didymodon vinealis (Brid.) Zander, Syntrichia caninervis Mitt., and Tortula bidentata Bai Xue Liang. The lichen species included Collema coccophorum Tuck., Endocarpon pusillum Hedw., Fulgensia bracteata (Hoffm.) Rasanen, Xanthoparmelia camtschadalis (Ach.) Hale, Endocarpon simplicatum (Nyl.) Nyl., Fulgensia desertorum (Tomin) Poelt, and Psora decipiens (Hedw.) Hoffm..
Biotic indicator
In addition to the community composition and species richness, the community-level interspecific interactions were also evaluated by null model analyses based on the co-occurrence patterns. (Gotelli 2000; Gotelli and McCabe 2002). According to Gotelli and McCabe (2002), under a homogeneous resource background and without dispersal limitations, if the species co-occur less frequently than expected by chance, then the community is more likely to be structured by competition, and this relationship has been used to indicate the role of both competitive and facilitative interactions (Bowker et al. 2010; Maestre et al. 2008; Rooney 2008). In our study, limited dispersal and resource heterogeneity can only marginally affect the species co-occurrence and it is suitable to link the co-occurrence patterns to species interactions, because we intentionally set the short line transect in a homogenous environment (flat ground) and the dispersal characteristics of biocrusts make them quite unlikely to be dispersal limited at such small scale. To obtain a raw presence-absence matrix for the co-occurrence pattern analysis, we divided each 1.5 m line transect into 50 of 3 cm segments and counted the occurrences within each segment. Each row of the presence-absence matrix represented one single species, and each column represented a segment. We chose the C-score proposed by Stone and Roberts (1990) to quantify the co-occurrence pattern because it is more robust to noise in the data than other metrics when calculating the average segregation between all possible species pairs (Gotelli 2000). The C-score is denoted by the averaged \(({R}_{i}-S)\cdot ({R}_{j}-S)\) for all possible species pairs in the matrix, where \({R}_{i}\) and \({R}_{j}\) indicate the total numbers of rows containing species \(i\) and \(j\), respectively, and \(S\) is the total number of rows containing both species.
Because the number of species pairs affects the C-score, we used a null model based on the C-score as a baseline for how a community unstructured by species interactions would behave to facilitate comparisons (Connor and Simberloff 1979; Gotelli and McCabe 2002). The null model randomized the observed presence-absence matrix 10,000 times in our simulation to obtain a null matrix. Next, we reevaluated the C-score on the simulated null matrix to calculate the standardized effect size (SES) as \(\left({I}_{obs}-{I}_{sim}\right)/{S}_{sim}\), where \({I}_{obs}\) is the observed C-score, and \({I}_{sim}\) and \({S}_{sim}\) indicate the mean and standard deviation of the simulated C-score, respectively (Gotelli 2000). In the null model, we employed two swap algorithms to obtain the SES because the latent variable in SEM requires more than one manifest variable to reduce bias. The “fixed rows–equiprobable columns” algorithm (SESfe) retains the species frequencies and allows any number of species in each segment; The “fixed rows-fixed columns” algorithm (SESff) retains both the species frequencies and the number of species in each segment (for details, see Gotelli 2000). The two algorithms fix the rows; thus, the observed species occurrence frequencies can be maintained, and each species can occupy any column randomly. The performance of both indices has been extensively tested, and they show low type I error and good power for the detection of nonrandomness. A higher or lower SES according to the C-score indicates prevailing spatial segregation or aggregation, further suggesting the dominance of competitive or facilitative interactions.
Abiotic indicators
In this study, under an identical climatic regime, the abiotic variations were primarily explained by soil properties (Li et al. 2017). The soil samples were collected 0–3 cm under the biocrust layer, and four soil cores evenly along the 1.5-m line transect were mixed. Then, all 100 soil samples were air-dried, crushed and sieved (with a 2-mm mesh) for later parameter measurements, including the soil pH (pH), electrical conductivity (EC), soil soluble salt (SS), content of calcium carbonate (CaCO3), soil organic matter (SOM), total carbon (TC), total nitrogen (TN), total phosphorus (TP), and soil particle size. The pH and EC were measured by a portable multimeter (HQ30D, Hach Company, USA). SS was measured by the residue drying quality method, and CaCO3 accumulation was analyzed using ethylenediaminetetraacetic acid (EDTA) titration (Nanjing Institute of Soil Research 1980). SOM was determined with the dichromate oxidation method (Nelson et al. 1982). TC and TN were measured by elemental analysis (vario MACRO cube, Elementar Analysensysteme, Germany), and TP was measured by Mo-Sb colorimetry. The soil particle size was analyzed using the pipette method described by Smith and Mullins (2005).
Structural equation modeling
The influences of shrubs and succession through biotic and abiotic pathways on the community richness was evaluated by a multigroup SEM. In brief, the multigroup analysis has three main steps: Step 1 uses model specification; Step 2 estimates parameter estimation and model fit evaluation; and step 3 involves constraint-based modeling to analyze invariance or pathway changes (Iriondo et al. 2003; Lefcheck 2015).
-
Step 1) uses a priori background knowledge to construct a conceptual hypothesis and translates it into a series of equations in the form of a path diagram (Fig. 2). The working hypothesis in this study predicts that shrubs (Artemisia ordosica) affect the richness of the biocrust community during succession through both biotic and abiotic pathways, which is consistent with the assembly rule of Diamond (1975), which indicated that environmental constraints and internal dynamics in the community determine species filtering from the regional species pool to the local community. In the hypothetical structural model, “Succession” indicates the years from revegetation to the study period, and “Shrub” is a categorical variable indicating whether the biocrust community is in the interspace or beneath the shrub. The “Biotic effect” was a latent variable manifested by SES under two swap algorithms (SESfe and SESff), which reflect the interspecific interactions of each biocrust community. For “Abiotic effect”, 12 abiotic indicators are collinear and redundant; thus, we used an exploratory factor analysis to extract the main factor (MF1) from them (see Supplementary section S1). Then, we used MF1 to manifest the variable “Abiotic effect”. A preliminary statistical analysis found nonlinear relationships in the data (see Supplementary section S2); thus, we divided the complete dataset (100 samples) into two groups: an early successional stage (data of 18, 31, and 37 years after revegetation with 60 samples) and late successional stage (data of 54 and 62 years after revegetation with 40 samples).
Fig. 2 Hypothetical structural model showing the postulated effects from succession (years since revegetation) and shrubs (canopy vs. interspace) through biotic/abiotic effects on the biocrust richness. Circles indicate latent variables, where the biotic effect is manifested by standardized effect sizes based on both fixed rows–equiprobable columns algorithm (SESfe) and fixed rows-fixed columns algorithm (SESff). Rectangles indicate observed variables. Single headed arrows indicate an influence of one variable on another. The abiotic effect is manifested by a reduction to a single factor of measured soil indicators, including soil pH (pH), electrical conductivity (EC), soil soluble salt (SS), CaCO3 content (CaCO3), soil organic matter (SOM), total carbon (TC), total nitrogen (TN), total phosphorus (TP), and content of coarse clay (Clay1), fine clay (Clay2), silt (Silt) and dust (Dust). For invariance in the measurement structure and redundancy reduction, factor loadings for abiotic effect derived from preliminary exploratory factor analysis (EFA) are imposed for the structural equation model (SEM) (see Supplementary section S1), and factor loadings for the biotic effect are constrained to be equal for both groups (early/late successional stages)
In the multigroup SEM, invariance in the measurement structure is a prerequisite for the comparison of structural parameter estimates (i.e., equal factor loadings of latent variables are required (Vandenberg and Lance 2000)); thus, we fixed the factor loadings in the measurement structure for both groups. In exploratory factor analysis, the MF1 was extracted upon complete dataset. This dimension reduction technique can simplify the structure of the SEM and guarantee measurement equivalence in both groups.
-
Step 2) estimates the direct effects and correlations by fitting the observed variance-covariance matrix to the path diagram. Standardized path coefficients ranging from 0 to 1 that describe the effect size of each pathway in the model were estimated by the maximum likelihood technique. To evaluate the overall goodness-of-fit of the model for the dataset, we performed a \(\chi 2\) test and root mean square error of approximation (RMSEA) test and evaluated the comparative fit index (CFI) and Tucker-Lewis index (TLI) (Barrett 2007; Doncaster 2006).
-
Step 3) involves a comparison between a series of constrained models and a free model to analyze how pathways change between groups. The free model means that all path coefficients are free to vary between groups. The constrained model means the path coefficients between groups, are constrained to a single value determined by the entire dataset. If the constrained model and free model are not significantly different or if the former fits the data well, then we assume there is no variation in the path coefficients in the multigroup analysis. If they are significantly different, then we can determine which path is different between groups. In this study, we packed all the invariant paths into a hybrid model. The invariant paths were determined by a preliminary stepwise analysis (see Supplementary section S4). Then, we compared the hybrid model and the free model using a\(\chi 2\)difference test, which can help us guarantee path invariance from the perspective of overall performance.
Statistical analyses
The differences in soil attributes and community composition of the biocrusts during succession with/without the influence of shrubs were quantified by an analysis of covariance. The evaluation took the successional time (“Succession”) as a covariate and the binomial variable “Shrub” as an independent variable with an interaction term for the successional time. All the data analyses were run in the R environment by version 3.5.2 (R Development Core Team 2009), with the co-occurrence pattern and null model analyses performed with the “EcoSimR” package, version 0.1.0 (Gotelli et al. 2015), and SEM analysis performed with the “lavaan” package, version 0.65 (Rosseel 2012).
Results
Changes in biocrust community and soil properties
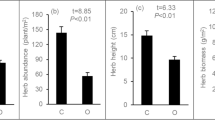
With increasing time after revegetation, all the soil properties significantly changed except for soil pH (Table 1). Artemisia ordosica did not have a significant influence on the soil condition throughout the whole succession. However, the shrubs increased the SOM by 13% among the 5 belts (P < 0.001), and the interactive effect between the shrubs and succession on SOM was significant. The community composition of the biocrusts also changed with succession, with mosses gradually increasing in proportion and lichens, algae and cyanobacteria and bare soil gaps decreasing (Fig. 3b). The shrubs significantly affected the proportion of lichens and bare soil gaps (P < 0.001), and the interactive effect between shrubs and succession was also significantly related to the proportion of gaps (P < 0.001). Table 1 shows that the interaction between shrubs and succession had a significant effect on the SESfe, SESff and biocrust richness (P = 0.0368, P = 0.0557, P = 0.0103) and the changes in SESfe, SESff and biocrust richness were nonlinear (Figs. 3a and 4). The richness gradually increased at the early successional stage and then declined at the late stage (Fig. 3a), and the species competition within biocrusts also decreased at the early stage and increased at the late stage (Fig. 4).
Changes in the community structure of biocrusts after revegetation among or beneath the shrub canopy (soil/shrub) Artemisia ordosica. a Biocrust richness. The error bars indicate the standard error for 10 samples. b Community composition of biocrusts in the functional group. The significance codes reflect the difference between two bars: *** indicates P < 0.001, ** indicates P < 0.01, * indicates P < 0.05, and . indicates P < 0.1
Changes in standardized effect sizes (SESs) from null models of cooccurrence patterns. The null model for SESfe is based on the “fixed rowsequiprobable columns” algorithm; the null model for SESff is based on the “fixed rows-fixed columns” algorithm. The significance codes reflect the difference between two bars: *** indicates P < 0.001, ** indicates P < 0.01, * indicates P < 0.05, and . indicates P < 0.1
Analysis of path invariance
Table 2 shows that the free model including both early and late successional stages fitted the hypothetical SEM very well according to the \(\chi 2\) test (\(\chi 2\) = 9.917, df = 10, P = 0.448), and the RMSEA, TLI and CFI were 0.000, 1.001 and 1.000, respectively. All the standardized path coefficients in the hybrid model were basically equal to those of free model (Fig. 5), although the goodness-of-fit of the hybrid model was slightly poorer (\(\chi 2\) = 16.670, P = 0.274, RMSEA = 0.062, TLI = 0.974, CFI = 0.987). The hybrid model was still not significantly different from the free model (\(\varDelta \chi 2\) = 6.752, P = 0.150), which further supports the path invariance for “Shrub → Abiotic effect”, “Succession → Abiotic effect”, “Abiotic effect → Richness”, and “Succession → Richness” between groups.
Results of multigroup structural equation modeling between the early and late successional stages. a, b indicate the results from the free model, where all path coefficients are free to vary between groups. c, d indicate the results from the hybrid model, where some paths are constrained to be equal between groups and are denoted by black lines. The breath of the arrows is proportional to the standardized path coefficient (SPC) labeled on the lines, where blue and red mark positive and negative values, respectively. Solid lines indicate significant paths with significance codes: *** P < 0.001, ** P < 0.01, * P < 0.05
Multigroup structural equation model
From the fitted standardized path coefficients from both the free and hybrid model (Fig. 5), the successional time significantly influenced the biotic effect (free model: -0.63, hybrid model: -0.63) and abiotic effect (free model: 0.81, hybrid model: 0.79), and indirectly influenced the biocrust richness (free model: 0.50, hybrid model: 0.51) at the early successional stage (Fig. 5a, c) while shrubs only influenced the biotic effect (free model: 0.24, hybrid model: 0.24). At the late successional stage, 64% (free model) or 62% (hybrid model) of the variations in richness were explained by the paths. Shrubs influenced the biotic effect (free model: -0.35, hybrid model: -0.34) and had direct influences (free model: 0.37, hybrid model: 0.43) and indirect influences (free model: 0.10, hybrid model: 0.05) on biocrust richness (Fig. 5a, c). Succession still had a strong influence on abiotic effect (free model: 0.51, hybrid model: 0.62).
Discussion
Shrubs influence the biocrust assembly along the succession gradient
Along the biocrust succession gradient, the composition of biocrusts underwent substantial change, which is consistent with the well-recognized standard (conceptual) model of primary and secondary succession of biocrusts with dominance changing from algae and cyanobacteria to lichens and mosses (Weber et al. 2016). Meanwhile, biocrusts apparently improved the soil condition over time, including soil fertility, stability and hydrologic characteristics (Duan et al. 2004). Over the background of successional variation, the shrubs had marginal influences on the community composition of biocrusts and soil properties. Shrub effects included decreased proportion of lichens, and increased soil gaps and SOM. The litter from shrubs likely caused the gap and decreased the lichens because the formation of lichens requires a stable topsoil, and lichens are more sensitive to burial than mosses (Dettweiler-Robinson et al. 2013; Kidron et al. 2010). In addition, Litter is the primary input of SOM, but also frequently buried the biocrust and restrict the development of biocrusts or even kills them (Maestre et al. 2010). In general, our results support previous research indicating that biocrusts in the interspace and beneath the shrubs show divergent community assembly processes in drylands (Soliveres and Eldridge 2020).
Changes of biocrusts are different between succession stages
Plant succession theory predicts different responses of the plant community to external stimuli at different successional stages. This is because the early successional community, consisting of the initial colonizing species, have a random spatial structure, and it usually shifts towards a more spatially and competitively structured assemblage during late succession (Connell and Slatyer 1977; Meiners et al. 2015). However, this theory has rarely been tested in biocrust communities. In our study, such a phenomenon was also found in the responses of richness and species interactions. The convex variation of biocrust richness during succession, whether in the interspace or beneath the shrubs, is consistent with many plant succession predictions in which peak richness is reached at mid-succession (Horn 1974; Howard and Lee 2003; Michalet et al. 2006). In addition, the neutral to positive interactions or random spatial distributions that promotes species coexistence are also evident at mid-succession. This well-established pattern is expected to arise from the trade-offs among dispersal, interspecific interactions, and resource allocation in vascular plants (Howard and Lee 2003; Tilman 1985). Species with different life-history strategies, such as competitors, stress tolerators and ruderals (Grime 1977), adapted to either terminus of succession are able to coexist during mid-succession. Analogously, Li et al. (2017) proposed a diagrammatic triangle that considers disturbance and water availability as two key factors for determining the relative composition of algae and cyanobacteria, lichens and mosses. As explained in this triangle, a mixture of lichens and mosses can be evident at mid-succession because the capture of aeolian fine particles by plants and biocrusts prolongs the wetting time and improves the topsoil structural stability, which are critical for mosses and lichens, respectively (Li et al. 2017; Reynolds et al. 2001).
Role of successional time decreases at the late succession
In our study, successional time was the dominant driving force that determined the soil properties, biocrust species interactions and biocrust richness, however, its role changed with different successional stages. In the early stage, successional time played the most important role in determining the community assembly and species interactions by mainly increasing richness through soil amelioration and decreasing competitive interactions in the biocrust community. The most significant soil properties affecting biocrust richness are soil texture, pH, and soil calcareousness, which usually determine the regional species pools (Bowker and Belnap 2009). At smaller local, intrasite, or microscales, because of the narrow range of soil physical and chemical properties, soil fertility, temperature or microtopography can ultimately determine the fine-scale distribution patterns (Bowker et al. 2006). In our study, the overall change in the soil was dramatic and coupled the facilitation effect from early species to late species, which explains the increase in the biocrust richness. The decreased competition may be associated with the strong competitive exclusion among early-colonizer algae, cyanobacteria and lichen, which improved the harsh initial succession conditions, thereby facilitating their replacement by mosses (Lan et al. 2015). By contrast, the change rate of the soil condition was basically constant during succession. Among the four invariant paths, only the path “Succession → Abiotic effect” (see Supplementary section S4) was significant at both stages, which means that the influences of succession on abiotic effects was basically equal and constant at two stages. This finding is consistent with the earlier observations and indicates that soil recovery is a slow process that occurs over decades to centuries in an extremely arid desert environment (Li et al. 2007).
Shrubs indirectly affect biocrust diversity by altering biotic interactions
Despite the dominant influences of succession time, shrubs slightly increased the competition within biocrust communities at the early successional stage. The presence of shrubs may increase the competitive hierarchy because the fragile algae and cyanobacteria crusts and lichen crusts are more sensitive to disturbances from shrub litter fall while moss favor shady and moist conditions (Li et al. 2017). The role of shrubs became more obvious during late succession, with shrubs directly and indirectly increasing the species richness. In addition to edaphic amelioration, previous research has indicated that shaded light and temperature, prolonged moisture time, accumulated litter and even root activity improved the microenvironment and increased species diversity (Hao et al. 2016; Kidron and Benenson 2014; Serpe et al. 2013), which may explain the strong direct influence of shrubs on richness. The indirect effects were caused by the decreased competition within biocrust community (Maestre et al. 2008). First, shrubs reduced the competitive interactions because of either a decreased frequency of interactions caused by increased gaps or a decreased competitive hierarchy among mosses and lichens (Bowker et al. 2010; Langhans et al. 2010). Second, decreased competition favored species coexistence because competition has been reported as a driving force to constrain diversity (Maestre et al. 2010). This finding broadly supports the work of Soliveres and Eldridge (2020), which reveal the fundamental role of biotic interactions of biocrusts in their assembly when growing beneath shrubs. In our study, biotic interactions are included in the background of succession, and successional stage is identified as an application condition.
Why does the role of shrubs change?
In conclusion, the role of shrubs in biocrust assembly is evident during succession and is mediated by species interactions in biocrusts. As reported in vascular plant research, these staged responses may be associated with different environmental conditions and community structures (Boeken and Orenstein 2001; Weber et al. 2016). One explanation is related to the different community compositions during succession, with moss usually dominating in late succession and lichens, algae and cyanobacteria dominating in early succession. Under the canopy of shrubs, moss is more favored due to the moderately decreased light intensity and prolonged wetting time (Kidron and Benenson 2014); however, the disturbance from shrub litter has a greater influence on lichens, algae and cyanobacteria (Serpe et al. 2013). The influence of shrubs differed at different successional stages. Another explanation is related to the competitive hierarchy that varies with environmental stress (Bowker et al. 2010; Soliveres et al. 2015); therefore, the pattern of interactions will differ in the interspace or beneath the shrubs because the weakening environmental stress during succession is altered by shrubs.
Conclusions
Arid or semiarid ecosystems are typically characterized by a patchy mosaic landscape consisting of shrubs and biocrusts, with the two important components interacting and working together to maintain the ecosystem functions and sustainability. Referring to working hypothesis 1, shrubs significantly affected the species interactions and compositions of the biocrust community. The soil properties were not affected except for the SOM, which suggests that the disturbance from litterfall may play a role. In accordance with the prediction of hypothesis 2, the richness of biocrust was also positively influenced by shrubs, although only during late succession, which indicates the dominant role of shrubs on maintaining of biocrust diversity. The decreased competition under shrubs caused the indirect influence from shrubs to biocrust richness (free model: 0.10, hybrid model: 0.05). The direct influence form shrubs to biocrust richness is stronger (free model: 0.10, hybrid model: 0.05), and the underlying forces deserve deeper investigation in the future. In summary, our study provides deeper insights into the community assembly of biocrusts and relationship between shrubs and biocrusts, which are important for maintaining diversity and sustainability in arid ecosystems. The findings also highlight the importance of shrubs and provide practical guidance for revegetation and ecological restoration of biocrusts in drylands.
Abbreviations
- SEM:
-
Structural equation model
- SES:
-
Standardized effect size
- EC:
-
Electrical conductivity
- SS:
-
Soil soluble salt
- SOM:
-
Soil organic matter
- TC:
-
Total carbon
- TN:
-
Total nitrogen
- TP:
-
Total phosphorus
- EC:
-
Electrical conductivity
- RMSEA:
-
Mean square error of approximation
- CFI:
-
Comparative fit index
- TLI:
-
Tucker-Lewis index
References
Aguiar MR, Sala OE (1999) Patch structure, dynamics and implications for the functioning of arid ecosystems. Trends Ecol Evol 14(7):273–277
Barrett P (2007) Structural equation modelling: Adjudging model fit. Personal Individ Differ 42(5):815–824
Belnap J (2003) The world at your feet: Desert biological soil crusts. Front Ecol Environ 1:181. https://doi.org/10.2307/3868062
Belnap J (2006) The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrol Process 20(15):3159–3178. https://doi.org/10.1002/hyp.6325
Belnap J, Miller DM, Bedford DR, Phillips SL (2014) Pedological and geological relationships with soil lichen and moss distribution in the eastern Mojave Desert, ca, USA. J Arid Environ 106(7):45–57. https://doi.org/10.1016/j.jaridenv.2014.02.007
Boeken B, Orenstein D (2001) The effect of plant litter on ecosystem properties in a mediterranean semiarid shrubland. J Veg Sci 12(6):825–832. https://doi.org/10.2307/3236870
Boeken B, Shachak M (1994) Desert plant communities in human-made patches–implications for management. Ecol Appl. https://doi.org/10.2307/1942001
Bowker MA, Belnap J (2009) A simple but informative classification of biological soil crust habitat on the colorado plateau (USA). J Veg Sci 19:831–840. https://doi.org/10.3170/2008-8-18454
Bowker MA, Belnap J, Phillips DSL (2005) Evidence for micronutrient limitation of biological soil crusts: Importance to arid-lands restoration. Ecol Appl 15:1941–1951. https://doi.org/10.1890/041959
Bowker MA, Belnap J, Davidson DW, Goldstein H (2006) Correlates of biological soil crust abundance across a continuum of spatial scales: support for a hierarchical conceptual model. J Appl Ecol 43(1):152–163. https://doi.org/10.1111/j.13652664.2006.01122.x
Bowker MA, Soliveres S, Maestre FT (2010) Competition increases with abiotic stress and regulates the diversity of biological soil crusts. J Ecol 98(3):551–560. https://doi.org/10.1111/j.13652745.2010.01647.x
Bowker MA, Maestre FT, Eldridge D, Belnap J, Castillo-Monroy A, Escolar C, Soliveres S (2014) Biological soil crusts (biocrusts) as a model system in community, landscape and ecosystem ecology. Biodiversity Conservation 23(7):1619–1637. https://doi.org/10.1007/s105310140658x
Breen K, Lévesque E (2011) Proglacial succession of biological soil crusts and vascular plants: Biotic interactions in the high arctic. Can J Bot 84:1714–1731. https://doi.org/10.1139/b06131
Cahill JF (2007) Positive Interactions and Interdependence in Plant Communities. Springer Netherlands, Dordrecht. https://doi.org/10.1007/978-1-4020-6224-7$4
Choler P, Michalet R, Callaway RM (2001) Facilitation and competition on gradients in alpine plant communities. Ecology 82(12):3295–3308. https://doi.org/10.1890/00129658(2001)082[3295:FACOGI]2.0.CO;2
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111(982):1119–1144. https://doi.org/10.1086/283241
Connor EF, Simberloff D (1979) The assembly of species communities: Chance or competition? Ecology 60:1132–1140. https://doi.org/10.2307/1936961
Cortina J, Martín N, Maestre FT, Bautista S (2010) Disturbance of the biological soil crusts and performance of stipa tenacissima in a semiarid mediterranean steppe. Plant Soil 334(s12):311–322. https://doi.org/10.1007/s11104-010-0384-4
Csotonyi JT, Addicott JF (2004) Influence of trampling-induced microtopography on growth of the soil crust bryophyte ceratodon purpureus in jasper national park. Can J Bot 82:1382–1392. https://doi.org/10.1139/b04098
Dettweiler-Robinson E, Bakker JD, Grace JB (2013) Controls of biological soil crust cover and composition shift with succession in sagebrush shrub-steppe. J Arid Environ 94:96–104. https://doi.org/10.1016/j.jaridenv.2013.01.013
Diamond JM (1975) Assembly of species communities. In: Ecology and evolution of communities. Harvard University Press, Cambridge, pp 342–444
Doncaster CP (2006) Structural equation modeling and natural systems. Fish Fish 8(4):368–369. https://doi.org/10.1111/j.1467-2979.2007.00260.x
Drake JA (1990) The mechanics of community assembly rules. J Theor Biol 147(2):213–233. https://doi.org/10.1016/s0022-5193(05)80053-0
Duan ZH, Xiao HL, Li XR, Dong ZB, Wang G (2004) Evolution of soil properties on stabilized sands in the Tengger desert, China. Geomorphology 59:237–246. https://doi.org/10.1016/j.geomorph.2003.07.019
Eldridge DJ, Bowker MA, Maestre FT, Roger E, Reynolds J, Whitford WG (2011) Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecol Lett 14(7):709–722. https://doi.org/10.1111/j.1461-0248.2011.01630.x
Gotelli NJ (2000) Null model analysis of species co-occurrence patterns. Ecology 81(9):2606–2621. https://doi.org/10.2307/177478
Gotelli NJ, McCabe DJ (2002) Species cooccurrence: A meta-analysis of J. M. Diamond’s assembly rules model. Ecology 83:2091–2096. https://doi.org/10.2307/3072040
Gotelli NJ, Graves GR, Rahbek C (2010) Macroecological signals of species interactions in the danish avifauna. PNAS 107(11):5030–5035. https://doi.org/10.1073/pnas.0914089107
Gotelli NJ, Hart E, Ellison A (2015) EcoSimR: Null model analysis for ecological data. CRAN. http://CRAN.R-project.org/package=EcoSimR. Accessed 15 Jul 2020
Grace JB, Anderson TM, Olff H, Scheiner SM (2010) On the specification of structural equation models for ecological systems. Ecol Monogr 80:67–87. https://doi.org/10.2307/27806874
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111(982):1169–1194. https://doi.org/10.1086/283244
Hao HM, Lu R, Liu Y, Fang NF, Wu GL, Shi ZH (2016) Effects of shrub patch size succession on plant diversity and soil water content in the water-wind erosion crisscross region on the loess plateau. Catena 144(144):177–183. https://doi.org/10.1016/j.catena.2016.05.015
Horn HS (1974) The ecology of secondary succession. Annu Rev Ecol Syst 5:25–37. https://doi.org/10.1146/annurev.es.05.110174.000325
Howard LF, Lee TD (2003) Temporal patterns of vascular plant diversity in southeastern new hampshire forests. For Ecol Manag 185(1–2):0–20. https://doi.org/10.1016/s0378-1127(03)00243-3
Iriondo JM, Albert MJ, Escudero A (2003) Structural equation modelling: an alternative for assessing causal relationships in threatened plant populations. Biol Cons 113(3):367–377. https://doi.org/10.1016/S00063207(03)001290
Kidron GJ, Benenson I (2014) Biocrusts serve as biomarkers for the upper 30 cm soil water content. J Hydrol 509(2):398–405. https://doi.org/10.1016/j.jhydrol.2013.11.041
Kidron GJ, Vonshak A, Dor I, Barinova S, Abeliovich A (2010) Properties and spatial distribution of microbiotic crusts in the negev desert. Israel Catena 82(2):92–101. https://doi.org/10.1016/j.catena.2010.05.006
Lan SB, Wu L, Zhang DL, Hu CX (2015) Analysis of environmental factors determining development and succession in biological soil crusts. Sci Total Environ 538:492–499. https://doi.org/10.1016/j.scitotenv.2015.08.066
Langhans TM, Storm C, Schwabe A (2010) Regeneration processes of biological soil crusts, macro-cryptogams and vascular plant species after fine-scale disturbance in a temperate region: Recolonization or successional replacement? Flora 205(1):46–60. https://doi.org/10.1016/j.flora.2008.12.001
Lefcheck JS (2015) piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol Evol 7. https://doi.org/10.1111/2041-210X.12512
Li XR, Zhou HY, Wang XP, Zhu YG, O’Conner PJ (2003) The effects of sand stabilization and revegetation on cryptogam species diversity and soil fertility in the Tengger desert, northern China. Plant Soil 251(2):237–245. https://doi.org/10.1023/a:1023023702248
Li XR, He MZ, Duan ZH, Xiao HL, Jia XH (2007) Recovery of topsoil physicochemical properties in revegetated sites in the sand-burial ecosystems of the Tengger desert, northern China. Geomorphology 88(34):0–265. https://doi.org/10.1016/j.geomorph.2006.11.009
Li XR, Gao YH, Su JQ, Jia RL, Zhang ZS (2014) Ants mediate soil water in arid desert ecosystems: Mitigating rainfall interception induced by biological soil crusts? Appl Soil Ecol 78:57–64. https://doi.org/10.1016/j.apsoil.2014.02.009
Li XR, Song G, Hui R, Wang ZR (2017) Precipitation and topsoil attributes determine the species diversity and distribution patterns of crustal communities in desert ecosystems. Plant Soil 420(12):1–13
Liu YM, Li XR, Xing XZ, Sand Zhao, Pan YX (2013) Responses of soil microbial biomass and community com position to biological soil crusts in the revegetated areas of the Tengger desert. Appl Soil Ecol 65:52–59. https://doi.org/10.1016/j.apsoil.2013.01.005
Maestre FT, Escolar C, Martinez I, Escudero A (2008) Are soil lichen communities structured by biotic interactions? a null model analysis. J Veg Sci 19(2):261–266. https://doi.org/10.3170/2007-818366
Maestre F, Bowker MA, Escolar C, Puche M, Soliveres S, Maltez-Mouro S, García-Palacios P, CastilloMonroy AP, Martínez I, Escudero A (2010) Do biotic interactions modulate ecosystem functioning along stress gradients? insights from semiarid plant and biological soil crust communities. Philos Trans Biol Sci 365(1549):2057–2070. https://doi.org/10.1098/rstb.2010.0016
Meiners SJ, Cadotte MW, Fridley JD, Pickett STA, Walker LR (2015) Is successional research nearing its climax? new approaches for understanding dynamic communities. Funct Ecol 29(2):154–164. https://doi.org/10.1111/13652435.12391
Michalet R, Brooker RW, Cavieres LA, Kikvidze Z, Lortie CJ, Pugnaire FI, Valiente-Banuet A, Callaway RM (2006) Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol Lett 9(7):767–773. https://doi.org/10.1111/j.1461-0248.2006.00935.x
Nanjing Institute of Soil Research CAoS (1980) Analysis of Soil Physicochemical Features, (in Chinese) edn. Shanghai Science and Technology Press, Shanghai
Nelson DW, Sommers L, Page AL, Miller RH, Keeney DR (1982) Total carbon, organic carbon, and organic matter. Methods Soil Anal 9:539–552. https://doi.org/10.2136/sssabookser5.3.c34
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna. http://www.R-project.org. Accessed 08 Sep 2020
Reynolds R, Belnap J, Reheis M, Lamothe P, Luiszer F (2001) Aeolian dust in colorado plateau soils: nutrient inputs and recent change in source. Proc Natl Acad Sci 98(13):7123–7127. https://doi.org/10.1073/pnas.121094298
Rooney TP (2008) Comparison of co-occurrence structure of temperate forest herb-layer communities in 1949 and 2000. Acta Oecol 34(3):354–360. https://doi.org/10.1016/j.actao.2008.06.011
Rosseel Y (2012) lavaan: An R package for structural equation modeling. J Stat Softw 48. https://doi.org/10.18637/jss.v048.i02
Serpe MD, Roberts E, Eldridge DJ, Rosentreter R (2013) Bromus tectorum litter alters photosynthetic characteristics of biological soil crusts from a semiarid shrubland. Soil Biol Biochem 60(6):220–230
Smith KA, Mullins CE (2005) Soil and environmental analysis, physical methods, 2nd edn. Taylor & Francis eLibrary
Soliveres S, Eldridge DJ (2014) Do changes in grazing pressure and the degree of shrub encroachment alter the effects of individual shrubs on understory plant communities and soil function? Funct Ecol 28(2):530–537. https://doi.org/10.1111/13652435.12196
Soliveres S, Eldridge DJ (2020) Dual community assembly processes in dryland biocrust communities. Funct Ecol 34(4). https://doi.org/10.1111/13652435.13521
Soliveres S, Maestre F, Ulrich W, Manning P, Boch S, Bowker M, Prati D, Delgado-Baquerizo M, Quero J, Schöning I, Gallardo A, Weisser W, Müller J, Socher S, Gomez M, Ochoa V, Ernst Detlef S, Fischer M, Allan E (2015) Intransitive competition is widespread in plant communities and maintains their species richness. Ecol Lett 18. https://doi.org/10.1111/ele.12456
Stone L, Roberts A (1990) The checkerboard score and species distributions. Oecologia 85(1):74–79. https://doi.org/10.1007/bf00317345
Tilman D (1985) The resource-ratio hypothesis of plant succession. Am Nat 125(6):827–852. https://doi.org/10.1086/284382
Turetsky MR (2003) The role of bryophytes in carbon and nitrogen cycling. Bryologist 106(3):395–409. https://doi.org/10.1639/05
Ulrich W, Zaplata MK, Winter S, Schaaf W, Fischer A, Soliveres S, Gotelli NJ (2016) Species interactions and random dispersal rather than habitat filtering drive community assembly during early plant succession. Oikos 125(5):698–707. https://doi.org/10.1111/oik.02658
Vandenberg R, Lance C (2000) A review and synthesis of the measurement invariance literature: Suggestions, practices, and recommendations for organizational research. Organ Res Methods 5(1):139–158. https://doi.org/10.1177/109442810031002
Weber B, Büdel B, Belnap J (2016) Biological soil crusts: an organizing principle in drylands. Springer, Berlin. https://doi.org/10.1007/9783319302140$4
Zhang Z, Dong D, Zhao A, Yuan W, Han L (2008) The effect of restored microbiotic crusts on erosion of soil from a desert area in China. J Arid Environ 72:710–721. https://doi.org/10.1016/j.jaridenv.2007.09.001
Zhang J, Liu GB, Xu MX, Xu M, Yamanaka N (2013) Influence of vegetation factors on biological soil crust cover on rehabilitated grassland in the hilly loess plateau, China. Environ Earth Sci 68(4):1099–1105. https://doi.org/10.1007/s126650121811z
Zou BG, Cong ZL, Liu SJ (1981) A preliminary observation on the basic characteristics of sand-carrying currents and the effects of adopted prevention and control measurement at Shapotou. Chin J Desert Res 1:33–39 (in Chinese with English abstract)
Acknowledgements
The authors thank Rongliang Jia for assisting with the field works, and Guiping Fan and Xin Zhao for assisting with the laboratory work. This work was supported by the National Natural Science Foundation of China (grant nos. 41621001, 41530746). We are grateful to section editor Dr. Matthew A. Bowker and two anonymous reviewers for their constructive comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible Editor: Matthew A. Bowker.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 919 kb)
Rights and permissions
About this article
Cite this article
Sun, J., Li, X. Role of shrubs in the community assembly of biocrusts: the biotic and abiotic influences along a biocrust succession gradient. Plant Soil 460, 163–176 (2021). https://doi.org/10.1007/s11104-020-04789-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04789-6