Abstract
Aims
Changes in plant net primary production due to climate change can influence aboveground and belowground litter inputs to forest soils. We aim to examine the effects of such changes on soil carbon in subtropical forest ecosystems where these effects have not been thoroughly investigated.
Methods
We manipulated aboveground litter inputs and excluded roots in a factorial design, and measured the effects of each treatment and their interactions on soil carbon (C) and soil microbial community structure.
Results
After only 3 years of treatment, aboveground litter addition and root exclusion respectively caused 9% and 21% reductions in soil C concentration in the 0–10 cm soil, likely through different mechanisms. The reduction of soil C with aboveground litter addition was attributed to a priming effect, while reduced root-derived C inputs were likely the cause of the C reduction associated with root exclusion. PLFA analysis showed that both aboveground and belowground litter manipulations reduced Gram-positive bacteria biomass (by 30%–58%) compare to the control, but only root exclusion significantly reduced the actinobacteria biomarkers (by 46%–58%). Fungi, arbuscular mycorrhizal fungi, and ratios of Gram-negative to Gram-positive bacteria and bacteria to fungi did not differ among treatments.
Conclusion
Our results show that root-derived C inputs exert a stronger control on soil C concentrations and microbial community structures than aboveground litter does in subtropical natural forest soils. Our study also highlights that that both increases in aboveground litter and decreases in belowground C input to soil can lead to reduced soil C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, soils store more organic carbon (C) than vegetation and the atmosphere combined (Pan et al. 2011). Soil C storage is mediated by microbes that use net primary production (NPP) from above- and belowground litter and soil organic matter (SOM) as sources of C. Therefore, alterations in the quality and quantity of aboveground and belowground litter inputs to soils have profound effects on soil C dynamics (Lajtha et al. 2014a, 2018; Bowden et al. 2014). Global change has the potential to affect NPP and its allocation. For instance, increases in atmospheric CO2 concentration, nitrogen deposition and air temperature are likely to promote plant productivity and increase litter inputs to soil, via increases in canopy N content and longer growing seasons (Drake et al. 2011; Hickler et al. 2008; Keenan et al. 2014). In addition, CO2 addition is likely to increase belowground C allocation while nitrogen deposition might decrease it (Finzi et al. 2007; Drake et al. 2011). In contrast, in drought events generally decrease productivity since suppression of photosynthesis during drought (Gatti et al. 2014; Doughty et al. 2015) but increase the proportional allocation of C belowground (Hasibeder et al. 2015).
A large number of manipulative studies have examined how changes in litter input affect forest soil C dynamics (Leff et al. 2012; Xu et al. 2013; Lajtha et al. 2014a; Liu et al. 2017; Chen and Chen 2018). Although experimental additions or removals of aboveground litter have been widely used to explore its role in soil C dynamics, belowground litter manipulations are typically limited to removal. In general, litter removal, both above- and belowground, reduces soil C storage, while the effect of aboveground litter addition on soil carbon storage is variable (Xu et al. 2013; Bowden et al. 2014; Lajtha et al. 2014a; Pisani et al. 2016; Chen and Chen 2018), ranging from decreasing (Lajtha et al. 2014a; Pisani et al. 2016), no effects (Bowden et al. 2014) to increasing (Leff et al. 2012; Lajtha et al. 2014b). A meta-analysis showed that subtropical forest soils are more responsive to changes in litter production than other ecosystems (Xu et al. 2013).
Counter-intuitive net losses of soil C following increases in aboveground litter inputs have been attributed to positive priming effects (i.e., labile carbon in fresh litter provides energy to micro-organisms, which increase activity and in turn stimulate the decomposition of existing soil organic carbon). However, some studies have shown that priming occurs only when roots are present, possibly due to the important role of root exudates on soil microbe activities (Subke et al. 2004; Schaefer et al. 2009). Root exclusion is commonly applied in litter manipulation studies to separate autotrophic and heterotrophic respiration. However, few in-situ litter manipulation studies have directly investigated the effects of root exclusion on priming. In addition, few studies of litter manipulation have been conducted in tropical and subtropical plantation forests despite their important role in the global C cycle (Cusack et al. 2018; Liu et al. 2017; Wang et al. 2017). Due to differences in SOC storage, forest floor properties, litterfall and fine root dynamics between different regions and between natural and plantation forests (Yang et al. 2009), the response of soil carbon concentration to aboveground and belowground litter manipulation may be different between these forests.
Soil microbes play a key role in ecosystem processes through a large number of critical biochemical processes, including the mineralization of carbon and nutrients in litter (Paul 2014). Microbial community composition and function may respond rapidly to changes in litter inputs (Brant et al. 2006a; Yarwood et al. 2013). For example, an experiment in temperate forests in Oregon reported that root exclusion increased soil actinobacteria biomass and decreased fungal biomass (Brant et al. 2006a). In contrast, root exclusion increased the biomass of bacteria, fungi and actinobacteria, but decreased the Gram-negative to Gram-positive bacteria ratio and the bacteria to fungi ratio in a subtropical Chinese fir plantation (Wang et al. 2013). Our limited knowledge of the effects of belowground C inputs on microbial community structure hinders our understanding of how climate change may affect soil C cycling as microorganisms are known to mediate critical carbon transformations in soils (Nicolardot et al. 1994; Tveit et al. 2013).
Aboveground litter removal and addition could have opposite effects on microbial structures. For instance, increasing aboveground litter inputs decreased the abundance of putative oligotrophic soil bacteria (Acidobacteria) but increased putative copiotrophic soil bacteria (Alphaproteobacteria) in a lowland tropical rain forest (Nemergut et al. 2010). A laboratory experiment also reported increases in putative copiotrophic bacteria in response to C addition (Cleveland et al. 2007). In contrast, some studies showed that microbial community structure did not differ between the aboveground litter addition and the control plots in subtropical plantations and temperate deciduous forests (Nadelhoffer et al. 2006; Wang et al. 2013). In a nutrient-poor temperate pine plantation in China, litter removal had little impact on microbial biomass and community structure (Yarwood et al. 2013). The inconsistent results of litter manipulation experiments suggest that the effects of altering litter input on soil microbial structure are complex and might be site- or ecosystem-specific. A thorough understanding of the mechanisms (e.g. priming) underlying the responses of soil C stocks and microbial community structure to litter manipulation is required to accurately predict the effect of changes in litter inputs on soil microbial community structure.
Climate change is likely to affect both above- and belowground litter input through the alternations on primary productivity (Liu et al. 2004), fine root turnover (Bai et al. 2010), and carbon allocation (Davidson et al. 2002). The multi-site Detritus Input and Removal Treatment (DIRT) experiment was designed to assess how rates and sources of above- and belowground litter inputs affect the long-term stability, accumulation, microbial community and chemistry of SOM in forest ecosystems (Nadelhoffer et al. 2006; Brant et al. 2006a; Lajtha et al. 2014a, 2018 Pisani et al. 2016). Many DIRT experiments have examined the effects of modifying aboveground or belowground litter on SOM properties (Nadelhoffer et al. 2006; Fekete et al. 2014; Lajtha et al. 2014a). However, few studies to date have simultaneously manipulated aboveground and belowground litter inputs to investigate the relative importance of aboveground and belowground litter to soil processes and soil microbial communities. The dynamics of SOM are simultaneously affected by aboveground and belowground litter inputs, both of which may be affected by global change drivers. However, it is difficult to add belowground litter without severely disrupting the soil and current increases in atmospheric CO2 concentration are likely to alter aboveground litter input via affecting primary production. Therefore, we conducted a factorial experiment manipulating both aboveground litter input (doubling and excluding) and belowground litter input (excluding) to the soil in a natural forest dominated by Castanopsis carlesii (~200 years old) in subtropical China. The objective of the study is to examine how aboveground litter alternation and root exclusion affect soil C and soil microbial community structures independently and interactively.
Materials and methods
Study site
This study was conducted at the Forest Ecosystem and Global Change Research Station (FEGCRS) (26° 11′ N, 117° 228′E, 386 m a.s.l.) in Sanming, Fujian Province, China. The study area has a humid mid-subtropical monsoon climate. Mean annual precipitation was 1630 mm with 75% occurring from March to August and annual mean temperature was 19.5 °C (9.4 °C in January and 28.4 °C in July) between 1956 and 2006. The soils, which generally exceed 1 m in depth, developed from sandstone and are classified as red soil according to the Chinese classification system, equivalent to Oxisol in the USDA Soil Taxonomy (State Soil Survey Service of China 1998; Soil Survey Staff 2014). The soils have a sandy texture, with 67% sand, 18% silt and 15% clay. Due to rapid litter decay associated with high temperature and moisture, there is no organic horizon besides a thin litter layer (Cusack et al. 2018; Fang et al. 2009). The soils have a pH (1:2.5 fresh soil/distilled water) of 3.8; total nitrogen (TN) of 2.34 g kg−1, and soil phosphorus (TP) concentration of 0.48 mg kg−1 (Liu et al. 2017).
The experimental site is a natural evergreen broadleaved forest dominated by Castanopsis carlesii (Fagaceae) (82% of basal area) and has not been disturbed by human activities for nearly 200 years. Other overstory trees include C. kawakamii, Schima superba (Theaceae), Litsea subcoriacea (Lauraceae), and Elaeocarpus decipiens (Elaecarpaceae). The total biomass, stem density, mean tree height and mean diameter at breast height are 397.4 Mg ha−1, 1192 tree ha−1, 19.2 m and 22.13 cm, respectively (Lin et al. 2017).
Experimental design
We conducted a factorial aboveground and belowground litter manipulation in the Catanopsis natural forest. The experiment consists of six treatments (Table 1): control (CT), no aboveground litter (NL), double aboveground litter (DL), no roots (NR), no roots and double aboveground litter (NRDL), and no litter input (NI, both above- and belowground litter were excluded). Each treatment was randomly applied in three 1 m2 plots located under forest canopy. Aboveground litter was excluded from litter removal plots with 1-mm nylon mesh suspended one meter above the ground. Double aboveground litter input was achieved by adding litter taken from litter removal plots and distributed with gentle raking (to avoid disturbance) biweekly since January 2013. To exclude roots, a narrow trench was excavated along the perimeter of the plot to approximately 0.6 m depth. Before backfilling, nylon mesh (45-μm) sheets (Sefar, Switzerland) were inserted to exclude root ingrowth but allow water and microbes to move freely. All aboveground plants within the plots was removed by hand to prevent root growth.
Soil sampling and measures
In March 2016, nine soil cores (diameter = 3.5 cm) from each plot were collected from the top layer of soil (0–10 cm) after removing the litter layer, and mixed to form a composite soil sample. Because Xu et al. (2013) showed with a meta-analysis that DIRT treatments strongly influence carbon concentration in the 0–5 cm mineral soil but not at greater depths, we conservatively decided to sample the top 10 cm of soil. Soil samples were kept at 4 °C during transport to laboratory, and then sieved to 2-mm and processed for soil chemical analyses. Subsamples were oven-dried for 48 h at 105 °C to calculate gravimetric moisture concentration. Soil pH was measured with a pH meter in a 1:2.5 soil/water suspension. Total soil C and N was analyzed in subsamples using a high-temperature combustion total CN analyzer (Elementar Vario MAX, Germany). All soil carbon was assumed to be organic; carbonate minerals do not persist in acidic soils such as those at our study site. Dissolved organic carbon (DOC) in the soils was extracted with 0.5 M K2SO4 in a ratio of 1:5 by shaking at 200 rpm for 1 h, then filtering through 0.45-mm Millipore filter paper (Jones and Willett 2006). Concentrations of DOC were determined with a Shimadzu TOC-TN analyzer (Shimadzu Corp., Kyoto, Japan). Microbial biomass C and N was determined on fresh soil samples using the chloroform fumigation extraction method with a 0.5 M K2SO4 solution (Vance et al. 1987; Carter and Gregorich 2006). MBC and MBN were calculated as the difference in extractable C before and after fumigation using a conversion factor (kc) of 0.45 and 0.5 (Joergensen 1996), respectively.
PLFA analysis
Soil microbial community structure was determined using phospholipid fatty acids (PLFAs) as described by (Wan et al. 2015). The abundance of individual fatty acids was expressed as nanomole per gram of dry soil. Briefly, 8 g of soil was freeze-dried using a one phase solvent consisting of a 0.8:1:2 mixture of potassium phosphate buffers (pH 7.4), chloroform, and methanol. The lipids were split into neutral lipids, glycolipids and phospholipids by eluting with chloroform, acetone and methanol from a silica-filled solid phase extraction column. After mild alkaline methanolysis to form fatty acid methyl esters (FAMEs), individual FAMEs were analyzed by gas chromatography (Hewlett Packard 5890 GC, Agilent, USA). Peak areas and the resulting amount of PLFA were calculated relative to the internal standard PLFA 19:0. The peaks were identified on the basis of their retention times in comparison with a standard mixture using an identification software (MIDI Inc., Newark, DE). Gram-positive (GP) bacteria were identified by summing i14:0, i15:0, a15:0, i16:0, i17:0 and a17:0. Gram-negative (GN) bacteria were identified by summing 16:1ω7c, 16:1ω9c, cy17:0, 18:1ω7c, 18:1ω5c, and cy19:0 (Frostegård et al. 2011). The 18:1ω6 and 18:2ω6,9 PLFA was used as a marker for fungi (Frostegård et al. 2011). Actinobacteria were identified using the 10Me16:0, 10Me17:0, and 10Me18:0 (Frostegård et al. 2011). The biomarker used for arbuscular mycorrhizal fungi (AMF) was 16:1ω5 (McKinley et al. 2005). While the dominant tree species C. carlesii is ectomycorrhizal, arbuscular plant species such as Schima superba are also abundant at the study site. Total bacterial PLFAs were calculated as the sum of GN and GP. The ratio of fungal to total bacterial PLFAs (F:B) was used to estimate the ratio of fungal to bacterial biomass in soils.
Data analysis
Data were checked for normality and homogeneity of variance prior to statistical analysis, and logarithmically transformed when appropriate. The differences between treatments in soil chemical parameters, microbial biomass C, microbial biomass N, DOC, GN, GP, Fungal, AMF, total PLFAs and F:B were analyzed using one-way analysis of variance (ANOVA) followed by Duncan multiple comparison tests. Two-way ANOVA was used to assess the effects of aboveground litter, belowground litter manipulations and their interactions on soil properties, MBC MBN, community of the five functional groups (GP, GN, AFM, actinobacteria and fungi), GP:GN and F: B. The significance threshold α was established at 0.05. All statistical analyses were conducted using R version 3.2.2 (R core team 2014), with the ‘agricolae’ package for Duncan multiple comparison tests. Data in tables and figures are reported as means ±1 standard error (SE).
Differences in soil microbial community structure among treatments were examined using principle components analysis (PCA) on the relative mole abundances of PLFAs in samples. A redundancy analysis (RDA) was used to explore the relationship between the microbial community structure and soil characteristics. Soil properties were tested for significant contributions to the variation in the PLFA data using the Monte Carlo permutation test (P < 0.05). The PCA and RDA were processed using Canoco software 5.0 (ter Braak and Smilauer 2012).
Results
Effects of above and below-ground litter manipulation on soil property and microbial biomass C, N
There were no significant differences in soil moisture, total N concentration, C:N or pH of the 0-10 cm soil among treatments (Table 2). Total C concentration was significantly lower in DL, NR NRDL and NI treatment than in CT, but not different between NL and CT (Table 2); DOC concentration did not differ between the CT and NR treatments but was 15% higher in the DL treatment than the control, while it was significantly lower in NL, NRDL and NI treatments by 14%, 7% and 48%, respectively, relatively to the control (Table 2). MBC and MBN were significantly different among treatments (Table 2). Root manipulation had a stronger effect on MBC and MBN than did aboveground litter manipulation. MBC was lower by 17% in DL, 20% in NL, 37% in NR, 71% in NRDL and 55% in NI compared to the control treatment while MBN concentration was lower by 23.3%, 11.6%, 50%, 26.7% and 32.6% in DL, NL, NR, NRDL and NI treatment, respectively compared to the control (Table 2). The ratio of MBC to MBN did not differ between aboveground litter manipulation and control plots; compared to controls, this ratio increased in NR plots but decreased in NRDL and NI plots.
Microbial PLFAs
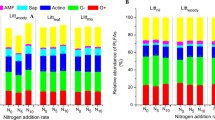
The aboveground litter manipulation and root exclusion treatments significantly reduced the PLFA concentrations (Table 3). Total PLFAs were lower in root-exclusion plots than in aboveground treatment plots, which in turn had lower total PLFAs than the control (Table 3). Compared to the control, the total PLFAs were 17% lower in DL, 31% in NL, 56% in NR, 53% in NRDL and 52% in NI. There were no significant differences in GP:GN ratio, F:B ratio, arbuscular mycorrhizal fungi and fungi were among treatments (Table 3). Biomass of GP was not different between CT and DL, but was significantly lower by 30%, 54%, 58% and 54% in NL, NR, NRDL and NI, respectively relative to the control. The GN showed similar patterns with GP. Actinobacteria were 56% lower in NR and 58% in NI relative to the control, but not significantly different among NL, DL, NRDL and CT (Table 3).
The controlling factors of soil microbial community structure
Approximately 98% of the variation of PLFA in soil samples can be explained by the first principal component (PC1 94.7%) and second principal component (PC2 3.5%) (Fig. 1). It was possible to distinguish two different groups in the PCA biplot, one group (upper left), was made up of samples from root exclusion root plots and the other group consisted of samples from plots with root presence. Aboveground treatments did not cluster cleanly in the biplot.
The RDA ordination biplot showed that soil properties explained 58.8% of the variance, with axis 1 explaining 58.2% of the variance and axis 2 explaining another 0.6% (Fig. 2). Soil microbial community structure was significantly related to soil the concentration s of MBC (Fig. 2, F = 16.4, P = 0.002).
Ordination biplot based on redundancy analysis (RDA) of PLFA in the soil samples. The dependent variables include biotic variable (solid arrow), gram-positive bacterial PLFAs (GP); gram negative bacterial PLFAs (GN); fungal PLFAs (Fungi); actinobacteria PLFAs (ACT); arbuscular mycorrhizal fungi PLFAs (AMF), total PLFAs (Total PLFAs) and abiotic variables (dashed arrow), microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN)
Discussion
Soil carbon concentration
The reduced soil C concentration in the double-litter input treatment relative to the control (Table 2) stands in contrast to the results of many DIRT experiments in temperate and tropical forests, which generally showed either no significant differences (Sulzman et al. 2005; Bowden et al. 2014; Lajtha et al. 2018) or increased soil C concentration (Leff et al. 2012; Fekete et al. 2014; Lajtha et al. 2014b) in response to aboveground litter addition. However, many other studies have shown that adding fresh litter may result in priming effects (Sulzman et al. 2005; Lajtha et al. 2014b, 2018), the mineralization of older soil organic matter (Kuzyakov et al. 2000), while the net effect on soil C concentration depends on the magnitude of the priming effects and the amount of aboveground litter added. It has also been suggested that greater inputs of new organic C may compensate for the release of older soil organic C by priming following litter addition (Xu et al. 2013). In our study site, increased fresh litter addition resulted in a strong priming effect as indicated by elevated enzyme activities and soil respiration (Liu et al. 2017). This priming is likely an important factor leading to reduced soil C in litter addition plots found in the current experiment.
In contrast to aboveground litter addition, we found that aboveground litter removal did not significantly affect soil C concentration (Table 2). Most studies in temperate and tropical forests showed that litter removal resulted in decreased soil total C due to the lack of the fresh C input (Leff et al. 2012; Xu et al. 2013; Fekete et al. 2014). Although our study found decreased soil DOC concentration and MBC concentration in response to aboveground litter removal (Table 1), DOC and MBC together contribute only approximately 1% of total soil C in forest soil (Whalen et al. 2000). Thus, decreases in soil DOC and MBC had very limited effects on soil total C concentration. A possible explanation for the lack of changes in soil C in response to aboveground litter removal could be the short experiment period (3 years), relative to the residence time of soil organic matter. Most studies have shown that aboveground litter removal reduced soil C concentration 8–20 years or longer after the treatment (Bowden et al. 2014; Lajtha et al. 2014b). However, Leff et al. (2012) found that aboveground litter removal from the forest floor drove a 26% reduction after only 2 years of treatment in the wet tropical forest in Costa Rica. The difference between our study and the study of Leff et al. (2012) may be partially explained by the warmer and wetter climate in the tropical forest in Costa Rica (~25 °C and 4430 mm) than at our study site (19.5 °C and 1630 mm). Temperature and rainfall are important factors affecting litter decomposition and the leaching of fresh DOC from litter into soils (Cleveland and Townsend 2006). The high temperature and rainfall in Costa Rica likely contributed to rapid decomposition and leaching of DOC. The removal of aboveground litter cut off the supply of C from litter to the soils and therefore reduced soil C concentration. The differences in soil C response to aboveground litter removal among forests with different climate and length of experimental treatments may illustrate the importance of climate and treatment time on the response of soil C to litter manipulation. In addition, our results suggest that soil C concentration is more sensitive to aboveground litter addition than to litter removal over short timescales (Table 2). However, it is important to note that changes in litter input due to climate change are not as drastic as is the manipulations in this and other DIRT experiments, some caution is needed when inferring the sensitivity of soil C to climate change from results of such studies.
Regardless of the treatment type (addition or removal) of aboveground litter treatment, soil C concentration was much lower in root removal treatments than in treatments with root presence (Table 2). Compared to the control, DOC was 7% lower in the NRDL treatment and 48% lower in the NI treatment. MBC was reduced by 37%, 71% and 55% in the NR, NRDL and NI plots, respectively. These results indicate that changes in belowground C supply plays a more important role on soil C than alteration in the quantity of aboveground litter. Several studies have suggested that roots may have greater contribution to the stable C pools than does aboveground litter (Brant et al. 2006a; Wang et al. 2013; Sokol and Bradford 2019). In contrast, the DIRT experiment at a Pennsylvania temperate deciduous forest found that aboveground litter was more important in maintaining soil C than was roots (Bowden et al. 2014). It appears that aboveground litter and belowground litter many exert different controls on soil C in different ecosystems.
Root exclusion is intended to prevent the flow of C fixed via photosynthesis from plant canopies to the soils, which occurs via multiple pathways: root turnover, allocation to mycorrhizae, and exudates (Epron et al. 2012; Gorka et al. 2019). The lack of difference in soil C concentration between NRDL and NR plots suggests that aboveground litter addition in the short term (i.e., three years) probably did not cause priming when live roots were excluded. That priming responses to aboveground litter manipulations are dependent on belowground C inputs is a key finding suggesting complex interactions between these functionally very different C inputs to the soil, which may be important to understand if the goal is to predict global change effects on soil C (Rasse et al. 2005). However, it is also important to note that short-term and long-term responses could be different. Given the lower C concentration in NRDL than NL, it is possible that continual DL inputs to the NR plots may induce observable decreases of soil C with additional priming.
Effects of C input manipulation on the microbial community
The PLFA analysis showed that plot-scale manipulation of the aboveground litter and belowground litter (roots) significantly changed the microbial community biomass and community structures (Table 3, Fig. 1), which is consistent with previous studies (Brant et al. 2006b; Nemergut et al. 2010; Wang et al. 2013). However, our finding that belowground C inputs exerted greater influence on the soil microbial community than aboveground C inputs has only been reported by DIRT experiments conducted by Brant et al. (2006a) in temperate forests, and by Wang et al. (2013) in a subtropical plantation. The greater effects of belowground than aboveground C input on soil microbial community is likely associated with greater reduction in soil labile organic C in the root exclusion treatments than in the aboveground litter treatments. The lower bacterial biomass (GP and GN) in the root exclusion and litter removal treatments than in the controls suggests that litter removal may has decreased labile C inputs to soil and thus decreased the GN bacteria biomass, because the growth of GN is highly dependent on labile C (Waldrop and Firestone 2004).
The smaller quantities of actinobacteria biomarkers in the root exclusion treatments (Table 3) was not consistent with studies in temperate forests and subtropical Chinese fir plantations that reported increased actinobacteria biomass in response to root exclusion (Brant et al. 2006a; Wang et al. 2013; Pisani et al. 2016). Actinobacteria are filamentous heterotrophic bacteria that degrade recalcitrant C compounds (McCarthy and Williams 1992; Khamna et al. 2009). Studies shows that root exclusion increases soil water concentration (due to lack of root uptake) which has a negative effect on actinobacteria biomass (Williams et al. 1972; Brant et al. 2006a) because actinobacteria has been reported to increase in relative abundance under low water availabilities (Griffiths et al. 1998; Šnajdr et al. 2008). However, the lack of difference among treatments in our study (Table 3) does not support the negative effects of root exclusion on actinobacteria biomass via reducing water stress (Table 2). The important role of root C input on actinobacteria is in agreement with the significant effect of root exclusion not litter manipulation on the biomass of actinobacteria (Table 3).
Conclusions
This study demonstrates that the soil C concentration is more sensitive to changes in belowground than aboveground litter input in a subtropical forest. Most notably, aboveground litter addition and root exclusion each significantly reduced soil C concentrations and microbial biomass in the 0–10 cm soil layer. These results suggest that root-derived C inputs exert a stronger control on soil C concentration and microbial community structures than aboveground litter in the studied subtropical natural forest. In light of this study, it is essential that plant-soil feedbacks should be taken into account in predictions of the C sequestration potential of subtropical natural forests under global change.
References
Bai W, Wan S, Niu S, Liu W, Chen Q, Wang Q, Zhang W, Han X, Li L (2010) Increased temperature and precipitation interact to affect root production, mortality, and turnover in a temperate steppe: implications for ecosystem C cycling. Glob Chang Biol 16:1306–1316
Bowden RD, Deem L, Plante AF, Peltre C, Nadelhoffer K, Lajtha K (2014) Litter input controls on soil carbon in a temperate deciduous Forest. Soil Sci Soc Am J 78:S66–S75
Brant JB, Myrold DD, Sulzman EW (2006a) Root controls on soil microbial community structure in forest soils. Oecologia 148:650–659
Brant JB, Sulzman EW, Myrold DD (2006b) Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol Biochem 38:2219–2232
Carter MR, Gregorich EG (2006) Soil sampling and methods of analysis. CRC Press, Taylor & Francis Group, Boca Raton
Chen X, Chen HY (2018) Global effects of plant litter alterations on soil CO2 to the atmosphere. Glob Chang Biol 24:3462–3471
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci U S A 103:10316–10321
Cleveland CC, Nemergut DR, Schmidt SK, Townsend AR (2007) Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition. Biogeochemistry 82:229–240
Cusack DF, Halterman SM, Tanner EVJ, Wright SJ, Hockaday W, Dietterich LH, Turner BL (2018) Decadal-scale litter manipulation alters the biochemical and physical character of tropical forest soil carbon. Soil Biol Biochem 124:199–209
Davidson EA, Savage K, Bolstad P, Clark DA, Curtis PS, Ellsworth DS, Hanson PJ, Law BE, Luo Y, Pregitzer KS (2002) Belowground carbon allocation in forests estimated from litterfall and IRGA-based soil respiration measurements. Agric For Meteorol 113:39–51
Doughty CE, Metcalfe D, Girardin C, Amézquita FF, Cabrera DG, Huasco WH, Silva-Espejo J, Araujo-Murakami A, da Costa M, Rocha W (2015) Drought impact on forest carbon dynamics and fluxes in Amazonia. Nature 519:78–82
Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML, Moore DJP, Oren R, Palmroth S, Phillips RP, Pippen JS, Pritchard SG, Treseder KK, Schlesinger WH, DeLucia EH, Finzi AC (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett 14:349–357
Epron D, Nouvellon Y, Ryan MG (2012) Introduction to the invited issue on carbon allocation of trees and forests. Tree Physiol 32:639–643
Fang HJ, Yu GR, Cheng SL, Mo JM, Yan JH, Li S (2009) 13C abundance, water-soluble and microbial biomass carbon as potential indicators of soil organic carbon dynamics in subtropical forests at different successional stages and subject to different nitrogen loads. Plant Soil 320:243–254
Fekete I, Kotroczó Z, Varga C, Nagy PT, Várbíró G, Bowden RD, Tóth JA, Lajtha K (2014) Alterations in forest detritus inputs influence soil carbon concentration and soil respiration in a central-European deciduous forest. Soil Biol Biochem 74:106–114
Finzi AC, Norby RJ, Calfapietra C, Gallet-Budynek A, Gielen B, Holmes WE, Hoosbeek MR, Iversen CM, Jackson RB, Kubiske ME (2007) Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc Natl Acad Sci U S A 104:14014–14019
Frostegård Å, Tunlid A, Bååth E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43:1621–1625
Gatti L, Gloor M, Miller J, Doughty C, Malhi Y, Domingues L, Basso L, Martinewski A, Correia C, Borges V (2014) Drought sensitivity of Amazonian carbon balance revealed by atmospheric measurements. Nature 506:76–80
Gorka S, Dietrich M, Mayerhofer W, Gabriel R, Wiesenbauer J, Martin V, Zheng Q, Imai B, Prommer J, Weidinger M, Schweiger P, Eichorst SA, Wagner M, Richter A, Schintlmeister A, Woebken D, Kaiser C (2019) Rapid transfer of plant photosynthates to soil bacteria via ectomycorrhizal hyphae and its interaction with nitrogen availability. Front Microbiol 10:168. https://doi.org/10.3389/fmicb.2019.00168
Griffiths B, Ritz K, Ebblewhite N, Dobson G (1998) Soil microbial community structure: effects of substrate loading rates. Soil Biol Biochem 31:145–153
Hasibeder R, Fuchslueger L, Richter A, Bahn M (2015) Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol 205:1117–1127
Hickler T, Smith B, Prentice IC, Mjöfors K, Miller P, Arneth A, Sykes MT (2008) CO2 fertilization in temperate FACE experiments not representative of boreal and tropical forests. Glob Chang Biol 14:1531–1542
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the k EC value. Soil Biol Biochem 28:25–31
Jones D, Willett V (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999
Keenan TF, Gray J, Friedl MA, Toomey M, Bohrer G, Hollinger DY, Munger JW, O’Keefe J, Schmid HP, Wing IS, Yang B, Richardson AD (2014) Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat Clim Chang 4:598–604
Khamna S, Yokota A, Lumyong S (2009) Actinomycetes isolated from medicinal plant rhizosphere soils: diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J Microbiol Biotechnol 25:649
Kuzyakov Y, Friedel J, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Lajtha K, Bowden RD, Nadelhoffer K (2014a) Litter and root manipulations provide insights into soil organic matter dynamics and stability. Soil Sci Soc Am J 78:S261
Lajtha K, Townsend KL, Kramer MG, Swanston C, Bowden RD, Nadelhoffer K (2014b) Changes to particulate versus mineral-associated soil carbon after 50 years of litter manipulation in forest and prairie experimental ecosystems. Biogeochemistry 119:341–360
Lajtha K, Bowden RD, Crow S, Fekete I, Kotroczó Z, Plante A, Simpson MJ, Nadelhoffer KJ (2018) The detrital input and removal treatment (DIRT) network: insights into soil carbon stabilization. Sci Total Environ 640:1112–1120
Leff JW, Wieder WR, Taylor PG, Townsend AR, Nemergut DR, Grandy S, Cleveland CC (2012) Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob Chang Biol 18:2969–2979
Lin KM, Lyu MK, Jiang MH, Chen YM, Li YQ, Chen GS, Xie JS, Yang YY (2017) Improved allometric equations for estimating biomass of the three Castanopsis carlesii H. forest types in subtropical China. New For 48:115–135
Liu C, Westman CJ, Berg B, Kutsch W, Wang GZ, Man R, Ilvesniemi H (2004) Variation in litterfall-climate relationships between coniferous and broadleaf forests in Eurasia. Glob Ecol Biogeogr 13:105–114
Liu XF, Lin TC, Yang ZJ, Vadeboncoeur MA, Lin CF, Xiong DC, Lin WS, Chen GS, Xie JS, Li YQ, Yang YY (2017) Increased litter in subtropical forests boosts soil respiration in natural forests but not plantations of Castanopsis carlesii. Plant Soil 418:141–151
McCarthy AJ, Williams ST (1992) Actinomycetes as agents of biodegradation in the environment-a review. Gene 115:189–192
McKinley V, Peacock A, White D (2005) Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils. Soil Biol Biochem 37:1946–1958
Nadelhoffer KJ, Boone RD, Bowden RD, Canary JD, Micks P, Ricca A, Aitkenhead JA, Lajtha K, McDowell WH (2006) The DIRT experiment. Forests in time: the environmental consequences of 1,000 years of change in New England, 300
Nemergut DR, Cleveland CC, Wieder WR, Washenberger CL, Townsend AR (2010) Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biol Biochem 42:2153–2160
Nicolardot B, Fauvet G, Cheneby D (1994) Carbon and nitrogen cycling through soil microbial biomass at various temperatures. Soil Biol Biochem 26:253–261
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993
Paul EA (2014) Soil microbiology and biochemistry, 2nd edn. Academic Press, San Diego
Pisani O, Lin LH, Lun OOY, Lajtha K, Nadelhoffer KJ, Simpson AJ, Simpson MJ (2016) Long-term doubling of litter inputs accelerates soil organic matter degradation and reduces soil carbon stocks. Biogeochemistry 127:1–14
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356
Schaefer DA, Feng W, Zou X (2009) Plant carbon inputs and environmental factors strongly affect soil respiration in a subtropical forest of southwestern China. Soil Biol Biochem 41:1000–1007
Šnajdr J, Valášková V, Merhautová VR, Herinková J, Cajthaml T, Baldrian P (2008) Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol Biochem 40:2068–2075
Soil Survey Staff (2014) Natural Resources Conservation Service 2014 National soil survey handbook. Title 430-VI. U.S. Government Printing Office, Washington D.C. Sec 602
Sokol NW, Bradford MA (2019) Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat Geosci 12:46–53
State Soil Survey Service of China (1998) China soil. China Agricultural Press, Beijing
Subke JA, Hahn V, Battipaglia G, Linder S, Buchmann N, Cotrufo MF (2004) Feedback interactions between needle litter decomposition and rhizosphere activity. Oecologia 139:551–559
Sulzman EW, Brant JB, Bowden RD, Lajtha K (2005) Contribution of aboveground litter, belowground litter, and rhizosphere respiration to total soil CO2 efflux in an old growth coniferous forest. Biogeochemistry 73:231–256
ter Braak CJF, Smilauer P (2012) Canoco reference manual and User's guide: software for ordination (version 5.0). Microcomputer Power, Ithaca
Tveit A, Schwacke R, Svenning MM, Urich T (2013) Organic carbon transformations in high-Arctic peat soils: key functions and microorganisms. ISME J 7:299–311
Vance E, Brookes P, Jenkinson D (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Waldrop MP, Firestone MK (2004) Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia 138:275–284
Wan XH, Huang ZQ, He ZM, Yu ZP, Wang MH, Davis MR, Yang YY (2015) Soil C: N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 387:103–116
Wang Q, He T, Wang S, Liu L (2013) Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agric For Meteorol 178:152–160
Wang Q, Yu Y, He T, Wang Y (2017) Aboveground and belowground litter have equal contributions to soil CO2 emission: an evidence from a 4-year measurement in a subtropical forest. Plant Soil 421:7–17
Whalen JK, Bottomley PJ, Myrold DD (2000) Carbon and nitrogen mineralization from light-and heavy-fraction additions to soil. Soil Biol Biochem 32:1345–1352
Williams ST, Shameemullah M, Watson ET, Mayfield C (1972) Studies on the ecology of actinomycetes in soil-VI. The influence of moisture tension on growth and survival. Soil Biol Biochem 4:215–225
Xu S, Liu L, Sayer E (2013) Variability of above-ground litter inputs alters soil physicochemical and biological processes: a meta-analysis of litterfall manipulation experiments. Biogeosciences 10:7423–7433
Yang Y, Guo J, Chen G, Yin Y, Gao R, Lin C (2009) Effects of forest conversion on soil labile organic carbon fractions and aggregate stability in subtropical China. Plant Soil 323:153–162
Yarwood S, Brewer E, Yarwood R, Lajtha K, Myrold D (2013) Soil microbe active community composition and capability of responding to litter addition after 12 years of no inputs. Appl Environ Microbiol 79:1385–1392
Acknowledgements
This research was jointly supported by Joint Fund for Promotion of Cross-strait Cooperation in Science and Technology (U1505233), National Natural Science Foundation of China (No. 31870601) and National Key Basic Research Program of China (2014CB954003). We are grateful to Cuicui Wei and Xianfeng Li for assistance in soil sampling and laboratory analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Lin, TC., Vadeboncoeur, M.A. et al. Root litter inputs exert greater influence over soil C than does aboveground litter in a subtropical natural forest. Plant Soil 444, 489–499 (2019). https://doi.org/10.1007/s11104-019-04294-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04294-5