Abstract
Aims
Microbial turnover processes are typically restricted by low substrate availability in the subsoil. We hypothesized that SOM decomposition increases with plant density and decreases with N fertilization: We expected a greater rate of C allocation to the rhizosphere in the topsoil than in the subsoil treatments.
Methods
In order to simulate different degrees of rhizodeposition, wheat was planted in pots at four different densities. The plants were continuously labeled with 13C-depleted CO2. Soil CO2 efflux was partitioned for root- and SOM-derived CO2. Moreover, we determined the enzyme kinetics by measuring catalytic efficiency and enzyme stoichiometry in both topsoil and subsoil.
Results
Shoot biomass and the shoot to root ratio were significantly higher for plants grown in the topsoil compared with the subsoil, which demonstrated higher relative C allocation to root biomass in the subsoil treatment. Despite the similar size of the rhizosphere, root-derived CO2 was always higher in the topsoil compared with the subsoil treatment, indicating enhanced root exudation. Effect sizes of all enzyme activities showed stronger magnitudes for the subsoil treatments. This was in line with a two-times increase of the effect size of SOM decomposition in the subsoil relative to topsoil.
Conclusions
Overall, the plants in the subsoil treatments allocated more C to root biomass, less C to shoot biomass, and substantially less C to root exudates. However, the effect sizes of both SOM decomposition and enzyme activities were higher in the subsoil than in the topsoil, reflecting a stronger sensitivity to C inputs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventionally managed agricultural soils are characterized by low soil organic carbon (SOC) stocks and high mineral nitrogen (N) contents due to the addition of inorganic fertilizers. Crops take up about 30–50% of N applied in inorganic form (Tilman et al. 2002). In arable systems with external input of mineral fertilizers, the topsoil is more relevant for nutrient acquisition of crops than the subsoil (Hirte et al. 2018; Kautz et al. 2013) because the majority of root biomass is located in the topsoil, especially in agricultural lands. Therefore, research has been focused on topsoil rather than subsoil processes, and topsoil SOC dynamics are better studied than subsoil SOC dynamics (Salomé et al. 2010). However, subsoil C represents a large reservoir of organic C. SOC in subsoils is primarily stabilized due to selective preservation from microbial decomposition (Rumpel et al. 2004; Wordell-Dietrich et al. 2017) and is controlled by the input and availability of fresh substrates (Fontaine et al. 2007; Guenet et al. 2012; Wordell-Dietrich et al. 2017). Assimilated C is quickly transferred to belowground pools through rhizosphere processes (Cheng and Gershenson 2007; Kuzyakov et al. 2001; Ostle et al. 2000). The C input from deep rooting plants (Lorenz and Lal 2005; Schenk and Jackson 2002) and leached dissolved organic C provide a notable resources for microorganisms in subsoils (Kalbitz et al. 2000). The acquisition of subsoil SOC may support sustainable agricultural practices especially for nutrient and water acquisition when topsoils run dry and diffusion gradients are small (Lorenz and Lal 2005; Lynch and Wojciechowski 2015; Schenk and Jackson 2002).
Plants allocate photoassimilated C to above- and belowground organs to provide C and energy for metabolism, growth, and storage pools (Kuzyakov and Cheng 2001; Larcher 2003). Plant leaves produce photosynthates and plant stems distribute these carbohydrates throughout the plant (Smith and Dukes 2013). In general, the assimilation rate of C limits root growth. By contrast, the growth of leaves is limited by root-supplied water and nutrients. The belowground C pool is regulated by root-associated C fluxes (Cheng and Gershenson 2007; Schimel 1995).
In addition to root litter and dissolved organic C, the main source of C input into subsoils is rhizodesposits of deep-rooting plants. Rhizodeposition is to a large extent driven by photosynthesis (Gavrichkova et al. 2010; Kuzyakov and Gavrichkova 2010), which fosters microbial growth (Loeppmann et al. 2018; Panikov 1995; Blagodatskaya et al. 2009), enzyme production (Loeppmann et al. 2016a), and decomposition of organic compounds (Paterson 2003). Therefore, rhizodeposition may be more important in the subsoil where SOC is limited. Root exudation is known to increase with planting density, because more C is assimilated and allocated belowground. Photosynthesis also affects root and rhizomicrobial respiration, which may depend more on internal circadian cycles in regulating exudation rather than on sunlight directly (Bahn et al. 2013; Gavrichkova and Kuzyakov 2017). Belowground CO2 efflux can be partitioned into two distinct processes: (1) rhizosphere respiration or root-derived CO2, including root respiration and microbial respiration resulting from the mineralization of living root material, and (2) microbial decomposition of soil organic compounds, or soil-derived CO2. If rhizosphere processes are closely connected to plant photosynthesis and rhizodeposition (Hoberg et al. 2001; Kuzyakov and Cheng 2001), this synchronicity may be beneficial to plants. Here, we address the ways in which plant-soil interactions may affect C allocation to rhizodeposits and root growth in topsoil and subsoil.
The quantity of enzymes produced in the rhizosphere correlates to the activity level of rhizomicrobial populations (Burns 1982). The production of extracellular enzymes by plants, microbes, and soil fauna in both topsoil and subsoil is regulated by nutrient availability (Jones et al. 2018; Liang et al. 2019) and energy demand (Heuck et al. 2015; Sinsabaugh et al. 2009). Therefore, enzymatic activity is an excellent indicator of SOM decomposition and is closely interrelated with soil quality (Bending et al. 2004; Paudel et al. 2011). Tillage and fertilization increase soil aeration and N availability which enhances SOM decomposition, organic N mineralization, and nitrification. However, Zang et al. (2017) recently found that N fertilization increased the assimilation of rhizosphere C into microbial biomass, increased rhizomicrobial turnover rates, and reduced the rate of SOM decomposition. The effects of N fertilization on rhizomicrobial enzymatic catalytic efficiency and stoichiometry under wheat production are largely unknown in both the topsoil and the subsoil.
In a pot experiment, we planted spring wheat in different densities either in topsoil or in subsoil and conducted a continuous 13C-CO2 labeling in order to partition soil CO2 efflux. With increasing planting density, we expected higher root and shoot biomass and greater rhizodeposition. We anticipated that this would enhance microbial biomass and SOM decomposition for both the topsoil and subsoil treatments. A second set of treatments with ten plants per pot received nitrogen fertilizer since SOM decomposition and microbial activity strongly depend on the availability of mineral N in the soil. We hypothesized that SOM decomposition increases with plant density and decreases with N fertilization. We expected a higher amount of aboveground biomass, a greater rate of photosynthesis, and a greater rate of C allocation to the rhizosphere (respiration and exudation) in the topsoil treatments.
We integrated the activities of enzymes involved in the same process as indicators of organic matter degradation and nutrient transformation. It is assumed that the sum of major C-acquiring enzyme activities is a better indicator of total C acquisition than e.g. BG solely (Bell et al. 2014; Loeppmann et al. 2016b; Nannipieri et al. 2012). Thus, enzyme activity ratios were used as a tool for examining relative allocation to energy versus nutrient acquisition (Hill et al. 2014; Moorhead et al. 2013). Extracellular hydrolytic enzyme stoichiometry and the catalytic efficiency were used to explain differences in the exploitation of resources between topsoil and subsoil (Gianfreda et al. 1995; Koshland 2002; Nannipieri et al. 2012). Microbial biomass C was determined, since it is the crucial pool facilitating the decomposition of organic material. This study addresses some of the underlying mechanisms driving plant-soil interactions, especially nutrient acquisition and competition in topsoil and subsoil systems.
Material and methods
Plants and soil
To manipulate root C input, we planted spring wheat (Triticum aestivum L.) with the following planting densities Table 1): very low (2 plants pot−1; T2), low (5 plants pot−1; T5), common for agricultural systems (10 plants pot−1; T10), and high (20 plants pot−1; T20). In addition, unplanted controls for each the topsoil and subsoil treatments were prepared. A second set of the T10 treatment received N fertilizer (Table 1). Plants were grown in PVC pots (15 cm diameter, 40 cm height, equipped with an inlet tube at the bottom for aeration and soil CO2 trapping). A nylon bag filled with 1500 g sand was placed at the bottom of each pot to improve air circulation. Soil from two contrasting horizons was used in this experiment: (1) topsoil (0–20 cm, Ap horizon), (2) subsoil (70–90 cm, B horizon). The soil was taken from an organic farm at the University of California, Santa Cruz. The soil is classified as a sandy loam Mollisol. The Ap horizon had an average C to N ratio of 9.8, a δ13C value of − 23.3‰, and a pH value of 5.8. The B horizon showed an average C to N ratio of 7.8, a δ13C value of − 17.2‰, and a pH value of 6.1. In order to achieve a high degree of soil homogeneity and to reduce variability among treatments and replicates, the soil was air-dried then sieved (< 4 mm). All pots were filled with 6 kg dry weight soil equivalent and wetted with deionized water to 80% of the water holding capacity, estimated separately for filled topsoil and subsoil pots.
To minimize the influence of a high CO2 efflux due to soil disturbance by sieving, we pre-incubated the soils after water addition in the greenhouse for 2 weeks before the start of the experiment. The CO2 concentration during pre-incubation was monitored by an infrared gas analyzer (Li-820 CO2 gas analyzer Biosciences serial O/I). After 2 weeks of pre-incubation, the water lost by evapotranspiration was refilled based on the weight of the filled pots. Ammonium nitrate was dissolved in water and added at a rate of 15 g N m−2 (0.3 g N pot−1) with the first water amendment after pre-incubation to half of the unplanted pots and to additional pots in the T10 treatment. For planting, the wheat seeds were presoaked overnight. Twice as many seeds were sown for each treatment, and the seedlings were thinned to the respective number after emergence. The soil moisture content was measured gravimetrically and adjusted daily to 80% of the water holding capacity. The location of the pots in the greenhouse was changed twice a week by mixing them randomly to guarantee similar growing conditions for the plants. Artificial lighting (1100-W lights, P.L. Light Systems, Beamsville, ON) was used to ensure an adequate energy flux during the experiment. The light intensity was kept above 900 W m−2 and the photoperiod was set from 4:30 a.m. to 4:30 p.m. The relative air humidity was kept constant at 45% by a dehumidifier (Kenmore Elite 70 pint, Sears, Chicago, IL, USA). The inlet tube at the bottom of each pot was connected to an aquarium pump to aerate the pots. This was done two times during the dark period to avoid contamination of the growth chamber δ13C signal with that of soil-derived CO2 during the assimilation period.
13CO2 continuous labeling
The experiment was conducted in a greenhouse equipped with supplies for continuous labeling with 13C-depleted CO2 (Cheng and Dijkstra 2007; Zhu and Cheng 2012; Pausch et al. 2013, 2016). Plants were continuously, labeled from the emergence of the first leaf until harvest, exposed to 13C-depleted CO2 (− 18.0 ± 0.6‰). Soil CO2 efflux was measured 5 weeks after planting by means of a closed-circulation CO2 trapping system (Cheng et al. 2003; Pausch et al. 2013). Soil CO2 was trapped in 400 mL of 1 M NaOH for 24 h. Afterwards, an aliquot of each NaOH solution was analyzed for total inorganic C using a Shimadzu TOC-5050A Total Organic Carbon Analyzer. Another aliquot was precipitated as SrCO3 (Harris et al. 1997) and analyzed for δ13C by means of a Carlo Elba 1108 elemental analyzer interfaced to a continuous flow isotopic ratio mass spectrometer Thermo Finnigan Delta Plus XP isotope ratio mass spectrometer at the Isotope Facility of University of California, Santa Cruz.
After CO2 trapping, the pots were destructively harvested. The shoots were cut at the base. The soil of each pot was pulled out and the roots were separated by hand-picking. The soil adhering to the roots was collected by slightly shaking the roots and was defined as rhizosphere. Roots were rinsed under deionized water. Shoots and roots were dried at 60 °C for 3 days, weighed, ground in a ball mill, and measured for δ13C.
Calculation of root- and SOM-derived C
The contribution of CO2 derived from SOM decomposition (CSOM-derived, mg C day−1 kg−1 soil) to total soil respiration was calculated using linear two-source isotopic mixing model:
where CTOTAL is the total CO2 efflux of the planted treatment (mg C day−1 kg−1 soil) and δ13CTOTAL the corresponding δ13C value of CO2 from SOM decomposition measured in the unplanted pots (‰). CROOT-DERIVED is the root-derived CO2 in the planted pot (mg C day−1 kg−1 soil) with δ13CROOT-DERIVED as the corresponding δ13C value (‰). The isotopic composition of root-derived CO2 was calculated by the δ13C value of the roots plus a fractionation factor of 0.87 (Zhu and Cheng 2011). This fractionation factor was determined for wheat with a similar experimental setup as compared to the present study.
Determination of soil microbial biomass and mineral nitrogen
Soil microbial biomass C (MBC) was determined on all remaining soil samples by the chloroform fumigation extraction method described by Vance et al. (1987) with the modification that fumigated and non-fumigated soil samples (30 g fresh soil) were extracted for 2 h with 60 mL of 0.5 M K2SO4 solution. The samples were filtered and the extracts were analyzed for total organic carbon by means of a Shimadzu TOC-5050A Total Organic Carbon Analyzer. The difference between the extracts of fumigated and non-fumigated samples corrected for a kEC factor of 0.45 (Wu et al. 1990) gives the total amount of microbial biomass C. To determine the water content, 30 g fresh soil was dried at 105 °C for 3 days and weighed thereafter.
Soil mineral N (Nmin; NO3− + NH4+) was extracted from 30 g fresh soil with 60 ml of 2 M KCl solution. Samples were shaken for 2 h and filtered and the extracts were analyzed for NO3− and NH4+ by a flow injection analyzer (Lachat QuikChem 8000, Milwaukee, WI).
Enzyme kinetics
We determined the enzyme activities of β-cellobiohydrolase (exo-1,4-β-glucanase, EC 3.2.1.91), β-glucosidase (EC 3.2.1.21), acid phosphatase (EC 3.1.3.2), β-xylosidase (EC 3.2.2.27), leucine-aminopeptidase (EC 3.4.11.1), and N-acetyl-β--glucosaminidase (EC.3.2.1.14) respectively. β-Glucosidase (BG), β-cellobiohydrolase (CE), and β-xylosidase (BX) represented enzymes in the C cycle, whereas leucine-aminopeptidases (LE) represented N cycling enzymes. Acid phosphatase (AP) is responsible for substrate utilization in the P cycle. N-Acetyl-β-d-glucosaminidase (Chitinase, NAG) catalyzes the hydrolytic cleavage of the β-1 → 4-glycoside bond present in biopolymers of N-acetylglucosamine, primarily in chitin (C and N cycles). Indeed, chitinases are widely distributed in living organisms and are found in fungi, bacteria, parasites, plants, and animals.
We added half a gram of soil to 50 ml sterile water in autoclaved jars and dispersed by an ultrasonic disaggregation (50 J s−1 for 120 s; De Cesare et al. 2000). Aliquots of 50 ml were withdrawn while stirring the suspension and dispensed into 96-well microplates (Brand pureGrade, black). Fifty microliter of buffer was added (0.1 M MES buffer, pH 6.1 for carbohydrases and phosphatase, 0.05 M TRIZMA buffer, pH 7.8 for leucine-aminopeptidase) (Loeppmann et al. 2016b; Marx et al. 2005). Finally, 100 ml of substrate solution was added at a series of concentrations (20, 40, 60, 80, 100, 200, 400 μmol substrate g−1 soil). Plates were kept at 22 °C, agitated and measured fluorometrically (excitation 360 nm; emission 450 nm) after 1-h, 2-h, and 3-h incubation using an automated fluorometric plate reader (Wallac 1420, PerkinElmer, Turku, Finland). Fluorescence was converted into an amount of 4-methylumbelliferone or 7-amino-4-methylcoumarin by reference to the fluorescence of standard solutions, which had been prepared in sub-samples of the various soil suspensions. Each replicate was measured as an analytical triplicate. We determined the catalytic efficiency for all measured enzymes (Gianfreda et al. 1995; Koshland 2002; Moscatelli et al. 2012). It reflects the total enzyme catalytic process combining enzyme-substrate complex dissociation and the rate of enzyme-substrate complex formation (Cornish-Bowden 1995; Koshland 2002).
Statistics
The values presented in the figures and tables are given as means ± standard errors of the means (±SEM). A Shapiro-Wilk test was applied to test for Gaussian distribution. We used the Pearson correlation coefficients to interpret the degree of linear relationships. Significant differences of the measured data between top- and subsoil and between the plant density treatments were obtained by analysis of variance (ANOVA) in combination with a post hoc unequal N HSD test. Parameter optimization of enzyme properties was restricted to the applied model equation (Michaelis-Menten) as indicated by maximum values of statistic criteria: r2, the fraction of total variation explained by the model defined as ratio of model weighted sum of squares to total weighted sum of squares. Identification of outliers was performed by the ROUT method, based on the false discovery rate (FDR) (Motulsky and Brown 2006). The standardized data (z-scoring) was analyzed by principal component analysis (PCA), using the software PAST 3.14 (Hammer et al. 2001).
Results
Shoot and root biomass, soil respiration, and microbial biomass
The shoot biomass and the shoot to root ratio were higher for plants grown in the topsoil compared with the subsoil (Table 1), which demonstrated higher relative C allocation to roots in subsoil possibly for nutrient acquisition. The shoot to root ratios for the topsoil treatment ranged from 4 to 7.5, whereas those for the subsoil treatments ranged from 0.9 to 1.2. The shoot biomass ranged from 0.8 to 4.3 g DW pot−1 in topsoil and from 0.2 to 1.6 g DW pot−1 in the subsoil.
Nutrient limitation in the subsoil and better plant growth in topsoil were also indicated by a 3-times steeper slope of the positive linear relationship between root biomass and shoot biomass in the topsoil treatments compared with the subsoil treatments (Fig. 1).
The ratio of rhizosphere soil to total soil increased with plant density but did not differ significantly between top- and subsoil (Fig. 2). Root-derived CO2 was always higher in the topsoil treatments (Fig. 3A) and had a significant positive correlation with shoot biomass (Fig. 4a). This is also supported by a steeper slope of the linear regression between root biomass and root-derived CO2 in the topsoil treatments compared with the subsoil treatments (Fig. 4b). The topsoil treatments produced greater amounts of photosynthetically active biomass, allocated more assimilates belowground, and had higher microbial biomass. Overall, the topsoil treatments produced much more CO2 than the subsoil treatments (Table 1). The most striking result was that wheat plants used much more C for rhizodeposition and respiration (indicated by higher root-derived CO2) when grown in topsoil compared with subsoil, despite a lower root biomass of plants grown in topsoil.
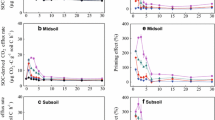
Contribution of rhizosphere to total soil in topsoil (top) and subsoil (bottom) with increasing plant density. The common plant density in agroecosystems was established with and without addition of mineral N fertilizer (10 plants pot−1). Significant treatment effects were assessed by ANOVA (P < 0.05) and indicated by different lowercase letters. The asterisk denotes significant effects between topsoil and subsoil
Root-derived CO2 (A) and SOM-derived CO2 (B) for the topsoil and the subsoil treatments; The horizontal dashed line indicates the rhizosphere priming effect. The common plant density in agroecosystem was established with and without addition of mineral N fertilizer (10 plants pot−1). Significant treatment effects were assessed by ANOVA (P < 0.05) and indicated by different lowercase letters. The asterisk denotes significant effects between topsoil and subsoil
All planted treatments showed consistently positive priming effects; however, these effects were not statistically significant from zero (Fig. 3B). Although the total SOM-derived CO2 efflux was significantly higher in topsoil than in subsoil (Fig. 3B), the relative difference in SOM-derived CO2 between the planted treatment and the respective unplanted control was higher in sub- than in topsoil, reflecting intensified decomposition of organic compounds. However, two-times greater effect size of SOM-derived CO2 was observed in the subsoil than in the topsoil (Fig. 6).
Overall, microbial biomass was higher in the topsoil compared with the subsoil across all planting densities (Table 1). PCA for planted topsoil revealed strong magnitudes of SOM-derived CO2, root-derived CO2, total CO2 efflux, and shoot biomass, which are explained by PC1, whereas MBC was best explained by PC2 (not shown).
Enzyme activity and catalytic efficiency
The effect of increasing C input via exudation on extracellular enzyme activities (Vmax) was evident in both soils. Especially, in the T20 treatment, Vmax significantly increased compared with densities < 20 plants per pot (Supplementary Fig. 3). All enzyme activities were higher in the topsoil compared with the subsoil, except for AP (Supplementary Fig. 3). However, the effect sizes of enzyme activities were higher in all plant densities (Fig. 6). In the subsoil, the AP activity significantly increased for both 10 and 20 plants per pot compared with all other treatments (Supplementary Fig. 3). In the topsoil, the AP activity was exclusively high for T20 (P < 0.05). Most enzyme activities were positively correlated to root biomass (Fig. 5). Regression analyses revealed steeper enzyme activity slopes in planted topsoil than in subsoil, except for AP (Fig. 5). The effect sizes of all enzyme activities were higher in the subsoil than in the topsoil, indicating enhanced sensitivity to C input (Fig. 6).
Linear regression between enzyme activity (Vmax) of (a) cellobiohydrolase, (b) chitinase, (c) acid phosphatase, (d) β-xylosidase, (e) β-glucosidase, and (f) leucine-aminopeptidase in rhizosphere soil (RS) and the root biomass. The asterisk denotes the significant effects (****P ≤ 0.0001; ***P ≤ 0.001; **P ≤ 0.01; *P ≤ 0.05)
Effect size of enzyme activities (left) over all plant densities for (a) topsoil and (b) subsoil are shown in the boxplot. Whiskers refer to 5 to 95% percentiles. The median is denoted by the line in the box. Effect size of SOM-derived CO2 relative to the bulk soil–derived CO2 (right) over all plant densities for topsoil and subsoil treatments. All data was log-transformed before analysis
The proportional enzyme activities of C and P acquiring enzymes varied significantly between the top- and subsoil. Across all plant densities, the C to P ratio was higher in the topsoil (up to 4-fold in T10) compared with subsoils, reflecting stronger P limitation in the subsoil (Supplementary Fig. 4). The proportional enzyme activities of C versus N acquiring enzyme did not show any effect between topsoil and subsoil (Supplementary Fig. 4).
Planting density had the most pronounced effect on the catalytic efficiency (Ka). Ka was strongly depleted exclusively for T20 in the topsoil whereas in the subsoil the opposite was the case (Supplementary Fig. 1). Overall, Ka of AP, CE, and BG was lower in subsoil, whereas the Ka of BX, LE, and partly NAG showed almost equal efficiencies in top- and subsoil (Supplementary Fig. 1).
Effect of N addition
Mineral N addition significantly reduced the root C to N in all treatments (Table 1). Nmin was always higher in the subsoil treatments and increased up to 2-fold with N addition in S10 (data not shown), reflecting stronger plant uptake in topsoil at all plant densities. This is in line with a strong non-linear negative relationship between Nmin and root biomass, whereas a linear decrease was observed for subsoil (Fig. 7). The catalytic efficiency of acid phosphatase was 5-fold lower in the subsoil compared with the topsoil in the T10 Nmin treatments (Supplementary Fig. 1).
In summary, wheat altered the allocation of carbon differently in the top- and subsoil treatments in response to the addition of Nmin. Wheat allocated more C to root biomass, less to shoot biomass, and, more importantly, substantially less to root-derived CO2 (respiration of roots and decomposition of exudates) when grown in the subsoil, resulting in lower microbial activity and SOM decomposition (Fig. 8).
Discussion
Topsoil versus subsoil
Higher enzyme activities corresponded to higher microbial biomass in the topsoil, inducing a strong reduction of mineral N (Table 1, Supplementary Fig. 3, Fig. 7). This enhanced plant shoot production and resulted in a higher shoot to root ratio as compared with plants grown in the subsoil (Table 1, Fig. 1). Moreover, the tendency for greater root biomass production in the subsoil (yet not significant for all of the plant densities) reflected the increased plant need for investments into the roots to increase nutrient acquisition from subsoil (Table 1). Lynch (2007) reported lower shoot growth than root growth when nutrients are scarce. This might be attributed to the functional equilibrium between C allocation and resource capture rates of shoots and roots (Gregory 2006; Pinton et al. 2007). Both shoots and roots obtain resources, which are commonly thought of as a relatively stable ratio. Therefore, root growth is contingent not only on C from the shoot but also on its own acquisition of N (Farrar and Jones 2000; Thornley 1977).
About 50% of photosynthates are translocated belowground in wheat plants at early tillering. Of this, approximately one-half is respired and one-quarter each is recovered in roots and soil (Keith et al. 1986). Above- and belowground processes were found to be closely coupled irrespective of soil horizon. This is demonstrated by the relationship between shoot biomass and root-derived CO2 for both the top- and subsoil treatments (Fig. 3). These results are consistent with previous findings which suggest that respiration is largely driven by C assimilation via photosynthesis and that photosynthesis modulates the belowground C allocation of plants (El-Sharkawy et al. 1990; Kuzyakov and Gavrichkova 2010). Despite a similar ratio of rhizosphere soil to bulk soil in each treatment (Fig. 2), root-derived CO2 was consistently and significantly higher in the topsoil compared with the subsoil (Fig. 3). This may indicate that the amount of photosynthetically active biomass is a more influential factor than the amount of root biomass in predicting belowground C allocation. Assuming an equal amount of root biomass in top- and subsoil, the greater amount of root-derived CO2 produced in the topsoil, resulted from higher allocation of assimilates to the roots due to greater shoot biomass and therefore higher photosynthesis. A shortage of resources in the rhizosphere is known to cause a shift of C assimilates in favor of the root system relative the shoot (Brouwer 1983). Farrer and Jones (2000) proposed several C allocation mechanisms such as push, pull, and shared control hypothesis. They concluded that there is qualitative and quantitative evidence supporting the hypothesis that control of C flux into roots is shared between the many processes which contribute to whole plant C flux, including those determining the loss of C from the root to soil and its organisms (Farrer and Jones 2000). Top-down, the C allocation to the roots is strongly dependent on the photomorphogenesis and the storage of assimilates, whereas the bottom-up pathway is strongly controlled by the soil nutrient availability and abiotic factors (Farrer and Jones 2000). The shared control hypothesis (Farrer and Jones 2000) may describe the C allocation best in our study.
We obtained a positive linear relationship between root-derived CO2 and root biomass (Fig. 4b). Field experiments demonstrated that root growth is strongly correlated to rhizodeposition and root-derived CO2, which supports our findings (Prikryl and Vancura 1980; Remus and Augustin 2016). Crops and grasses have been found to allocate a greater amount of C to roots than to net rhizodeposits. In crop species, net rhizodeposition accounts for 3% of assimilated C and root-derived CO2 accounts for 8% of assimilated C (Pausch and Kuzyakov 2017). Net rhizodeposition may remain steady in a system despite increases in gross rhizodeposition if microbial decomposition of rhizodeposition and SOM increases simultaneously via rhizosphere priming (Huo et al. 2017; Pausch et al. 2016; Shahbaz et al. 2018). For this reason, gross rhizodeposition may provide a better measure of changes in C allocation than net rhizodeposition (Pausch et al. 2016). Taking these factors into account, there was no significant change in the rate of SOM decomposition in any of the planted treatments compared with the unplanted control treatments. Nevertheless, we found a trend of increasing decomposition of organic compounds as plant density increased which suggests that the direction and magnitude of the rhizosphere priming effect may be impacted by the availability of labile root-derived C in the C limited subsoil. Here, higher microbial biomass and higher enzyme production induced intensive decomposition of organic compounds, which resulted in higher SOM-derived CO2 in the topsoil relative to the subsoil (Fig. 4a) reflecting lower rhizodeposition in subsoil treatments. The turnover rate of recent C pools is higher compared with older ones in deeper soil horizon, and decreases with time after the C entered the soil (Lützow et al. 2006; Pausch and Kuzyakov 2012). Subsoil SOM is often enriched in microbial-derived C compounds, as it is highly processed with time (Banfield et al. 2017; Tückmantel et al. 2017). In contrast, topsoil SOM primarily comprised of energy-rich plant material (Rumpel and Kögel-Knabner 2011). The subsoil plays a pronounced role in nutrient mobilization and re-allocation across the whole profile (Kautz et al. 2013), and it provides an additional nutrient reservoir for future sustainable plant production (Eilers et al. 2012). Deep-growing roots supply extra access to N and P (Tarkalson et al. 1998) and C-limited subsoil organisms may get access to labile root-derived C compounds affecting SOM decomposition (Zhang et al. 2017). During drought or periods of nutritional deficiency in the topsoil, plants may increase their access to resources by subsoil rhizosphere priming, inferring considerable contribution to plant nutrition. Here, we demonstrated that subsoil properties encouraged shifts in the C allocation pattern and soil C dynamics in wheat.
Enzyme properties in topsoil and subsoil
The stronger correlation between most enzyme activities and root biomass in the topsoil than in the subsoil (Fig. 2) indicated intensified production of extracellular enzymes by plants to acquire soil nutrients, likely induced by higher C supply due to increased photosynthesis of plants grown in topsoil. This results in strong competition or facilitation between individuals sharing resources under limited nutrient availability (Kuzyakov and Xu 2013). The change in Ka of acid phosphatases indicated lower P availability in the subsoil (T20) (Supplementary Fig. 1), since Vmax was enhanced in both planted top- and subsoil (Fig. 5). As the enzyme affinity to the substrate shifted, catalytic efficiency of acid phosphatases was lower in planted topsoil. This shift was also demonstrated by the proportional enzyme activities of C- versus P-acquiring enzymes (Supplementary Fig. 4). The contrasting Ka of CE and BG between top- and subsoil (Supplementary Fig. 1), caused by the lower microbial biomass in the subsoil (Table 1), reflected the varying investments in extracellular enzyme hydrolysis of insoluble cellulose requiring endoglucanases, exoglucanases (cellobiohydrolases), and β-glucosidases working in synergy (Van Dyk and Pletschke 2012).
Plant and enzymatic responses on mineral N addition
The response of cereals to applications of N fertilizer has been shown to significantly increase the shoot to root ratio (Barraclough 1984). Here, this response was unique to the topsoil (Table 1). Poeplau et al. (2017) did not find any effect of N fertilization on the ratio, whereas Hirte et al. (2018) demonstrated that shoot to root ratios are regulated by fertilization intensity than by site. A strong non-linear negative relationship between Nmin and root biomass was found in the topsoil, whereas a linear decrease was observed for the subsoil (Fig. 7), which may be explained by much higher shoot biomass production in topsoil and more complete depletion of nutrients. A recent study, mimicking root exudation by glucose addition, showed that N limitation largely controlled C turnover in the subsoil (Liang et al. 2019). Nitrogen addition did not have any effect on respiration (SOM-derived, root-derived CO2 efflux) in the presence of plants (Fig. 3), contradicting several other studies (e.g., Ramirez et al. 2010). However, organic N cycling may decline due to a reduction in the activity of enzymes that target nitrogenous compounds (Allison et al. 2010), which was indicated by the slightly lower leucine-aminopeptidase activity when N was added irrespective of top- or subsoil (Supplementary Fig. 3).
Conclusion
The subsoil treatments had lower rhizosphere activity, greater root biomass, and lower shoot to root ratios than the topsoil treatments. This indicates a reduced allocation of C from shoot to rhizodeposition in the subsoil treatments. The presence of plants increased the rate of SOM decomposition in both the topsoil and subsoil treatments relative to the unplanted control. Root-derived CO2 increased with increasing plant density, suggesting a close relationship with photosynthetic activity. Overall, plants grown in topsoil used higher amounts of C to supply rhizodeposition and respiration processes (indicated by root-derived CO2) than plants grown in subsoil, thereby maintaining higher microbial activity in the rhizosphere. SOM decomposition tended to increase with plant density in both the topsoil and subsoil, which may indicate that the quantity of root exudates determines the impact on SOM decomposition. Enhanced subsoil sensitivity to C input demonstrated the importance of the rhizosphere inputs on microbial activity and SOM decomposition in deeper soil layers. Based on these results, we conclude that rhizosphere activity is mainly driven by aboveground C assimilation and allocation to rhizosphere processes.
References
Allison, S. D., Weintraub, M. N., Gartner, T. B., & Waldrop, M. P. (2010). Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In Soil enzymology (pp. 229-243). Springer, Berlin, Heidelberg.
Bahn M, Lattanzi FA, Hasibeder R, Wild B, Koranda M, Danese V, Brüggemann N, Schmitt M, Siegwolf R, Richter A (2013) Responses of belowground carbon allocation dynamics to extended shading in mountain grassland. New Phytol 198(1):116–126
Banfield CC, Dippold MA, Pausch J, Hoang DT, Kuzyakov Y (2017) Biopore history determines the microbial community composition in subsoil hotspots. Biol Fertil Soils 53(5):573–588
Barraclough PB (1984) The growth and activity of winter wheat roots in the field: root growth of high-yielding crops in relation to shoot growth. J Agric Sci 103(2):439–442
Bell C, Carrillo Y, Boot CM, Rocca JD, Pendall E, Wallenstein MD (2014) Rhizosphere stoichiometry: are C:N:P ratios of plants, soils, and enzymes conserved at the plant species level? New Phytol 201:505–517
Bending GD, Turner MK, Rayns F, Marx MC, Wood M (2004) Microbial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimes. Soil Biol Biochem 36(11):1785–1792
Blagodatskaya EV, Blagodatsky SA, Anderson TH, Kuzyakov Y (2009) Contrasting effects of glucose, living roots and maize straw on microbial growth kinetics and substrate availability in soil. Eur J Soil Sci 60(2):186–197
Brouwer R (1983) Functional equilibrium: sense or nonsense? Neitherlands J Agric Sci 31:335–348
Burns RG (1982) Enzyme activity in soil: location and a possible role in microbial ecology. Soil Biol Biochem 14(5):423–427
Cheng W, Dijkstra FA (2007) Theoretical proof and empirical confirmation of a continuous labeling method using naturally 13C-depleted carbon dioxide. J Integr Plant Biol 49(3):401–407
Cheng W, Gershenson A (2007) Carbon fluxes in the rhizosphere. Rhizosphere:31–56
Cheng W, Johnson DW, Fu S (2003) Rhizosphere effects on decomposition. Soil Sci Soc Am J 67(5):1418–1427
Cornish-Bowden A (1995) Introduction to the enzyme kinetics. In: Fundamentals of enzyme kinetics. Portland Press Limited, Portland, pp 243–252
De Cesare, F., Garzillo, A. M. V., Buonocore, V., & Badalucco, L. (2000). Use of sonication for measuring acid phosphatase activity in soil. Soil Biology and Biochemistry, 32(6), 825-832.
Eilers KG, Debenport S, Anderson S, Fierer N (2012) Digging deeper to find unique microbial communities: the strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol Biochem 50:58–65
El-Sharkawy MA, Cock JH, Lynam JK, del Pilar Hernàndez A, Cadavid LFZ (1990) Relationships between biomass, root-yield and single-leaf photosynthesis in field-grown cassava. Field Crop Res 25(3–4):183–201
Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450(7167):277–280
Farrar, J. F., & Jones, D. L. (2000). The control of carbon acquisition by roots. The New Phytologist, 147(1), 43-53.
Gavrichkova O, Kuzyakov Y (2017) The above-belowground coupling of the C cycle: fast and slow mechanisms of C transfer for root and rhizomicrobial respiration. Plant Soil 410(1–2):73–85
Gavrichkova O, Moscatelli MC, Kuzyakov Y, Grego S, Valentini R (2010) Influence of defoliation on CO2 efflux from soil and microbial activity in a Mediterranean grassland. Agric Ecosyst Environ 136(1–2):87–96
Gianfreda LA, De Cristofaro A, Rao MA, Violante A (1995) Kinetic behavior of synthetic organo- and organo-mineral-urease complexes. J Soil Sci Soc Am 59:811–815
Gregory PJ (2006) Roots, rhizosphere and soil: the route to a better understanding of soil science? Eur J Soil Sci 57(1):2–12
Guenet B, Juarez S, Bardoux G, Abbadie L, Chenu C (2012) Evidence that stable C is as vulnerable to priming effect as is more labile C in soil. Soil Biol Biochem 52:43–48
Hammer Ø, Harper DAT, Ryan PD (2001) PAST-palaeontological statistics, ver. 1.89. Palaeontol Electron 4(1):1–9
Harris D, Porter LK, Paul EA (1997) Continuous flow isotope ratio mass spectrometry of carbon dioxide trapped as strontium carbonate. Commun Soil Sci Plant Anal 28(9–10):747–757
Heuck C, Weig A, Spohn M (2015) Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol Biochem 85:119–129
Hill BH, Elonen CM, Jica TM, Kolka RK, Lehto LLP, Sebestyen SD, Siefert-Monson LR (2014) Ecoenzymatic stoichiometry and microbial processing of organic matter in northern bogs and fens reveals a common P-limitation between peatland types. Biogeochemistry 120:203e224
Hirte J, Leifeld J, Abiven S, Oberholzer HR, Mayer J (2018) Below ground carbon inputs to soil via root biomass and rhizodeposition of field-grown maize and wheat at harvest are independent of net primary productivity. Agric Ecosyst Environ 265:556–566
Hoberg E, Marschner P, Lieberei R (2001) Pflanze-Bakterien-Interaktionen bei Phosphatmangel in Sterilkultur. In: Physiologie und Funktion von Pflanzenwurzeln. Vieweg+ Teubner Verlag, pp 64–69
Huo C, Luo Y, Cheng W (2017) Rhizosphere priming effect: a meta-analysis. Soil Biol Biochem 111:78–84
Jones DL, Magthab EA, Gleeson DB, Hill PW, Sánchez-Rodríguez AR, Roberts P, Ge T, Murphy DV (2018) Microbial competition for nitrogen and carbon is as intense in the subsoil as in the topsoil. Soil Biol Biochem 117:72–82
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165(4):277–304
Kautz T, Amelung W, Ewert F, Gaiser T, Horn R, Jahn R et al (2013) Nutrient acquisition from arable subsoils in temperate climates: a review. Soil Biol Biochem 57:1003–1022
Keith H, Oades JM, Martin JK (1986) Input of carbon to soil from wheat plants. Soil Biol Biochem 18(4):445–449
Koshland DE (2002) The application and usefulness of the ratio kcat/Km. Bioorg Chem 30:211–213
Kuzyakov Y, Cheng W (2001) Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol Biochem 33(14):1915–1925
Kuzyakov Y, Gavrichkova O (2010) Time lag between photosynthesis and carbon dioxide efflux from soil: a review of mechanisms and controls. Glob Chang Biol 16(12):3386–3406
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198(3):656–669
Larcher W (2003) Physiological plant ecology, 4th edn. Springer, Berlin
Liang Z, Olesen JE, Jensen JL, Elsgaard L (2019) Nutrient availability affects carbon turnover and microbial physiology differently in topsoil and subsoil under a temperate grassland. Geoderma 336:22–30
Loeppmann S, Semenov M, Blagodatskaya E, Kuzyakov Y (2016a) Substrate quality affects microbial-and enzyme activities in rooted soil. J Plant Nutr Soil Sci 179(1):39–47
Loeppmann S, Blagodatskaya E, Pausch J, Kuzyakov Y (2016b) Enzyme properties down the soil profile-a matter of substrate quality in rhizosphere and detritusphere. Soil Biol Biochem 103:274–283
Loeppmann S, Semenov M, Kuzyakov Y, Blagodatskaya E (2018) Shift from dormancy to microbial growth revealed by RNA:DNA ratio. Ecol Indic 85:603–612
Lorenz K, Lal R (2005) The depth distribution of soil organic carbon in relation to land use and management and the potential of carbon sequestration in subsoil horizons. Adv Agron 88:35–66
Lützow MV, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions–a review. Eur J Soil Sci 57(4):426–445
Lynch JP (2007) Rhizoeconomics: the roots of shoot growth limitations. HortScience 42(5):1107–1109
Lynch JP, Wojciechowski T (2015) Opportunities and challenges in the subsoil: pathways to deeper rooted crops. J Exp Bot 66(8):2199–2210
Marx MC, Kandeler E, Wood M, Wermbter N, Jarvis SC (2005) Exploring the enzymatic landscape: distribution and kinetics of hydrolytic enzymes in soil particle-size fractions. Soil Biol Biochem 37(1):35–48
Moorhead DL, Rinkes ZL, Sinsabaugh RL, Weintraub MN (2013) Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: informing enzyme based decomposition models. Front Terrestrial Microbiol 4(223):1–12
Moscatelli MC, Lagomarsino A, Garzillo AMV, Pignataro A, Grego S (2012) b-Glucosidase kinetic parameters as indicators of soil quality under conventional and organic cropping systems applying two analytical approaches. Ecol Indic 13(1):322–327
Motulsky HM, Brown RE (2006) Detecting outliers when fitting data with nonlinear regression e a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 7:123
Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B, Masciandaro G, Fornasier F, Moscatelli MC, Marinari S (2012) Soil enzymology: classical and molecular approaches. Biol Fertil Soils 48(7):743–762
Ostle N, Ineson P, Benham D, Sleep D (2000) Carbon assimilation and turnover in grassland vegetation using an in situ 13CO2 pulse labelling system. Rapid Commun Mass Spectrom 14(15):1345–1350
Panikov NS (1995) Microbial growth kinetics. Springer Science & Business Media
Paterson E (2003) Importance of rhizodeposition in the coupling of plant and microbial productivity. Eur J Soil Sci 54:741–750
Paudel BR, Udawatta RP, Anderson SH (2011) Agroforestry and grass buffer effects on soil quality parameters for grazed pasture and row-crop systems. Appl Soil Ecol 48(2):125–132
Pausch J, Kuzyakov Y (2012) Soil organic carbon decomposition from recently added and older sources estimated by δ13C values of CO2 and organic matter. Soil Biol Biochem 55:40–47
Pausch, J., Kuzyakov, Y., 2017. Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Global change biology
Pausch J, Tian J, Riederer M, Kuzyakov Y (2013) Estimation of rhizodeposition at field scale: upscaling of a 14 C labeling study. Plant Soil 364(1–2):273–285
Pausch J, Loeppmann S, Kühnel A, Forbush K, Kuzyakov Y, Cheng W (2016) Rhizosphere priming of barley with and without root hairs. Soil Biol Biochem 100:74–82
Pinton R, Varanini Z, Nannipieri P (2007) The rhizosphere: biochemistry and organic substances at the soil-plant interface. CRC press
Poeplau C, Kätterer T, Leblans NI, Sigurdsson BD (2017) Sensitivity of soil carbon fractions and their specific stabilization mechanisms to extreme soil warming in a subarctic grassland. Glob Chang Biol 23(3):1316–1327
Prikryl Z, Vancura V (1980) Root exudates of plants VI. Wheat root exudation as dependant on growth, concentration gradient of exudates and the presence of bacteria. Plant Soil:57–69
Ramirez KS, Craine JM, Fierer N (2010) Nitrogen fertilization inhibits soil microbial respiration regardless of the form of nitrogen applied. Soil Biol Biochem 42(12):2336–2338
Remus R, Augustin J (2016) Dynamic linking of 14C partitioning with shoot growth allows a precise determination of plant-derived C input to soil. Plant Soil 408(1–2):493–513
Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter—a key but poorly understood component of terrestrial C cycle. Plant Soil 338(1–2):143–158
Rumpel C, Eusterhues K, Kögel-Knabner I (2004) Location and chemical composition of stabilized organic carbon in topsoil and subsoil horizons of two acid forest soils. Soil Biol Biochem 36(1):177–190
Schenk HJ, Jackson RB (2002) Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J Ecol 90(3):480–494
Salomé, C., Nunan, N., Pouteau, V., Lerch, T. Z., & Chenu, C. (2010). Carbon dynamics in topsoil and in subsoil may be controlled by different regulatory mechanisms. Global Change Biology, 16(1), 416-426.
Schimel DS (1995) Terrestrial ecosystems and the carbon cycle. Glob Chang Biol 1:77–91
Shahbaz M, Kumar A, Kuzyakov Y, Börjesson G, Blagodatskaya E (2018) Priming effects induced by glucose and decaying plant residues on SOM decomposition: a three-source 13 C/14 C partitioning study. Soil Biol Biochem 121:138–146
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462(7274):795–798
Smith NG, Dukes JS (2013) Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Glob Chang Biol 19(1):45–63
Tarkalson DD, Jolley VD, Robbins CW, Terry RE (1998) Mycorrhizal colonization and nutrition of wheat and sweet corn grown in manure-treated and untreated topsoil and subsoil. J Plant Nutr 21(9):1985–1999
Thornley JHM (1977) Growth, maintenance and respiration: a re-interpretation. Ann Bot 41(6):1191–1203
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418(6898):671–677
Tückmantel T, Leuschner C, Preusser S, Kandeler E, Angst G, Mueller CW, Meier IC (2017) Root exudation patterns in a beech forest: dependence on soil depth, root morphology, and environment. Soil Biol Biochem 107:188–197
Van Dyk J, Pletschke B (2012) A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—factors affecting enzymes, conversion and synergy. Biotechnol Adv 30(6):1458–1480
Vance, E. D., Brookes, P. C., & Jenkinson, D. S. (1987). An extraction method for measuring soil microbial biomass C. Soil biology and Biochemistry, 19(6), 703-707.
Wordell-Dietrich P, Don A, Helfrich M (2017) Controlling factors for the stability of subsoil carbon in a Dystric Cambisol. Geoderma 304:40–48
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass-C by fumigation-extraction-an automated procedure. Soil Biol Biochem 22:167–169
Zang H, Blagodatskaya E, Wang J, Xu X, Kuzyakov Y (2017) Nitrogen fertilization increases rhizodeposits incorporation into microbial biomass and reduces soil organic matter losses. Biol Fertil Soils 53:419–429
Zhang X, Han X, Yu W, Wang P, Cheng W (2017) Priming effects on labile and stable soil organic carbon decomposition: pulse dynamics over two years. PLoS One 12(9):e0184978
Zhu B, Cheng W (2011) 13 C isotope fractionation during rhizosphere respiration of C 3 and C 4 plants. Plant Soil 342(1–2):277–287
Zhu, B., & Cheng, W. (2012). Nodulated soybean enhances rhizosphere priming effects on soil organic matter decomposition more than non-nodulated soybean. Soil Biology and Biochemistry, 51, 56-65.
Acknowledgments
We thank Susann Enzmann for the helping hand in the lab.
Funding
This study received funding from the Deutscher Akademischer Austauschdienst (DAAD) 57051794. Furthermore, this study also received funding from the H.W. Schaumann Stiftung.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Simon Jeffery.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1142 kb)
Rights and permissions
About this article
Cite this article
Loeppmann, S., Forbush, K., Cheng, W. et al. Subsoil biogeochemical properties induce shifts in carbon allocation pattern and soil C dynamics in wheat. Plant Soil 442, 369–383 (2019). https://doi.org/10.1007/s11104-019-04204-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04204-9