Abstract
Aims
We examined the influence of inoculation with five species/strains of diazotrophic bacteria on the modulation of two enzymes involved in the assimilation of N and on the soluble N fractions in the sugarcane varieties RB867515 (adapted for low fertility soils) and IACSP95-5000 (adapted for medium to high fertility soils) under high- (3 mM) and low (0.3 mM)-N conditions in hydroponic cultivation for 59 days.
Methods
The sugarcane plants were produced in three steps to obtain the hydroponic cultivation: the supply of 3 mM N for 30 days (first harvest), N depletion for 72 h (second harvest), and cultivation in high- and low-N conditions over 26 days (final harvest). Inoculation was performed by immersion of the minisetts in a diluted solution of five diazotrophic bacteria. After the final harvest, plants were divided into roots and shoots to assess their dry weight and N, P, and K accumulation.
Results
The variety played an important role in the interaction with diazotrophs, each showing distinct behavior in the activity of their N-assimilation enzymes. The nitrate reductase activity (NRa—EC 1.7.1.1) was increased in var. RB867515 by 26% in the shoots and by 48% in the roots after 72 h under N depletion, while var. IACSP95-5000 showed a reduced enzymatic activity in the roots (by 62%) but not in the shoots. Under high-N conditions, the inoculated IACSP95-5000 plants showed 31% higher glutamine synthetase activity (GSa—EC 6.31.2) compared with 19% in RB867515. Under low-N conditions, the GSas were 21% and 16% higher in the inoculated RB867515 and IACSP95-5000 plants, respectively, compared with that of the control. The content of nitrogen in the form of nitrate (N-nitrate) confirmed these varietal differences, but the soluble sugar content did not.
Conclusions
The varieties utilized N sources differently, and inoculation modified the activity of two N-assimilation enzymes as well as the biomass accumulation, with the highest improvement seen in the low fertility-adapted variety RB867515; it showed a greater response to inoculation compared with that of the high fertility-adapted variety IACSP95-5000, with an increase in biomass and nutrient accumulation (N, P, K), especially when cultivated under low-N conditions. This suggests that the best response to inoculation with diazotrophs will be achieved using low fertility-adapted sugarcane varieties under low-N conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane is associated with a diverse community of bacteria and fungi that can contribute to plant growth and protection from environmental stress (Souza et al. 2016). Following the isolation and description of diazotrophs associated with sugarcane, it was observed that the contribution of biological nitrogen fixation can be up to 40 kg N/ha obtained by the natural process of N2 reduction (Urquiaga et al. 2012). Based on this observation, efforts have been made to improve N accumulation in sugarcane by biological nitrogen fixation processes using bacterial inoculation (Oliveira et al. 2006; Schultz et al. 2014).
Sugarcane propagation is based on cuttings from the stalks, and the size and number of buds can vary depending on the method of planting. By using a single bud (stem node), it is possible to reduce planting costs as a smaller number of stalks is needed to obtain the propagules. A method commonly applied in Brazil is the use of a single node containing one bud to produce plantlets grown in tubes, which are subsequently transplanted to the field (Landell et al. 2012). This procedure reduces the amount of millable stalks per area from 8 to 10 t to 3–4 t. The approach of using one bud as a propagule and inoculation with bacteria can be easily adopted by farmers for seedling production. Selecting diazotrophic bacteria adapted to the respective plant habitat and associated with other properties that promote plant growth can be a strategy to reduce N application, improve root development by the production of diverse phytohormones, and contribute to plant survival after transplanting to the field, which can reduce losses (Santos et al. 2017).
Although sugarcane plants can extract 100–300 kg of N from the soil to produce 100 Mg ha−1 of millable stalks per cycle (Fortes et al. 2013), this crop is well known for its low response to nitrogen addition during the first plant-cane cycle (Cantarella et al. 2007). Nitrogen fertilizer recovery by sugarcane is also low, and ranges from 20 to 40% of the applied fertilizer, depending on the N source used (Franco et al. 2010). Several mechanisms of N loss are involved in this low recovery, including leaching and ammonia volatilization (Thorburn et al. 2005). One of the possible mechanisms is related to the low-N necessity of the plant regarding inorganic sources present in the soil solution (ammonium and nitrate), and recent data suggest that organic forms of nitrogen can also contribute to N nutrition in plants (Vinall et al. 2012). The contribution of microbiota to sugarcane plant nutrition is normally associated with the abundance of bacteria on the plant, and the microbiome diversity is strongly correlated with the efficiency of the biological nitrogen fixation (BNF) process (Dong et al. 2018). Previous studies have described the successful association of bacteria with various plant parts responsible for N reduction, including the roots and shoots of sugarcane and sorghum inoculated with Herbaspirillum and Gluconacetobacter diazotrophicus (James et al. 1994; 1997; James and Olivares 1998); however, the respective mechanisms of these occurrences remain to be described comprehensively.
Diazotrophs can modify nitrogen uptake by improving the root architecture of plants and can also affect the enzymes responsible for N utilization (Santos et al. 2017). This improvement is one of the explanations for the promotion of growth after inoculation with different bacterial species or strains, as observed in several studies (Cassán et al. 2014; Costa et al. 2002). Moreover, growth improvement depends on the plant variety (Manter et al. 2010). Typically, the varieties selected for cultivation under suboptimal soil conditions also respond positively to inoculation with diazotrophic bacteria (Schultz et al. 2014). However, the nitrogen metabolism in relation to enzymatic activity or partitioning of N in specific plant tissues of inoculated plants is not yet comprehensively described. We hypothesized that inoculation using selected diazotrophs can alter N acquisition, assimilation, and distribution in sugarcane plants. In this context, a hydroponic sugarcane experiment was performed using two sugarcane varieties in order to assess the nitrate reductase (NRa) and glutamine synthetase activity (GSa), as well as the soluble fractions of N, in plant tissues after applying an inoculant composed of five diazotrophs during the initial growth under high- and low-N levels using hydroponics.
Material and methods
The experiment was conducted in a greenhouse with automated humidity and temperature controls. The greenhouse was located at Embrapa Agrobiology in the municipality of Seropédica, Rio de Janeiro (RJ) (22°44′38′′ S and 43°42′28′′ W, 26 m a.s.l.). The experiment was conducted in a randomized block design in a factorial scheme (2 × 2 × 2) with six replicates, using two sugarcane varieties (RB867515 and IACSP95-5000) in the presence or not of the inoculation treatment using a mixture of five diazotrophs, and two nitrogen doses (low, 0.3 mM and high, 3.0 mM).
Sugarcane varieties and planting procedure
The sugarcane varieties RB867515 and IACSP95-5000 were obtained from the experimental station of Embrapa Agrobiologia. Certain differences between the varieties are described as follows: RB867515 has excellent adaptability and production stability in soils with low fertility, and IACSP95-5000 grows well in soils of medium to high fertility. Fresh stems of each variety were cut into pieces containing one bud (minisetts), and after cutting, the minisetts were immersed in water at 52 °C for 30 min (short heat treatment), as described by Sanguino et al. (2006). This heat treatment was suggested to control ratoon stunting disease and reduces the bacterial biome in the minisetts while not eliminating the bacteria (Reis et al. 1994). After this, the minisetts were immersed in a fungicide solution containing methyl N-{2-[1-(4-chlorophenyl)-1H-pyrazol-3-yloxymethyl] phenyl} (N-methoxy) carbamate (Pyraclostrobin 250 g L−1) solution at 0.1% for 3 min. After the cleaning steps, the minisetts were maintained at 25 °C for 1 h to dry the excess water.
Inoculation
The inoculation treatment comprised the use of five diazotrophic bacteria strains preselected by Oliveira et al. (2006), all of which were isolated from sugarcane. The species/strains used were as follows: Gluconacetobacter diazotrophicus strain BR11281T (PAL-5T) described by Cavalcante and Döbereiner (1988); Herbaspirillum seropedicae BR11335 (HRC54) described by Baldani et al. (1986); Herbaspirillum rubrisubalbicans BR11504 (HCC103) described by Baldani et al. (1996); Paraburkholderia tropica BR11366T (PPe 8 T), formerly belonging to the genus Burkholderia, described by Reis et al. (2004) and recently reclassified by Oren and Garrity (2015); and Nitrospirillum amazonense BR11145 (CBAMc), formerly belonging to the genus Azospirillum, described by Magalhães et al. (1983) and reclassified by Lin et al. (2014). All strains were deposited in the diazotrophic bacteria collection of the Johanna Döbereiner Biological Resource Center (CRB-JD) (BR numbers).
To produce the inoculation medium, each bacterial strain was individually cultured in a DYGS culture medium (Baldani et al. 2014). After confirming purity, one colony was inoculated in 5 mL of DYGS medium and grown in a rotary shaker at 150 rpm and 30 °C for 48 h. After this, 1 mL of the culture was transferred to 75 mL DYGS medium for culturing under the same conditions for approximately 24 h until a population density of 108 cells mL−1 was reached (measured using the Neubauer counting method). This volume of each strain was added to 175 mL of neutralized sterile peat to produce 250 g inoculant per strain. A mixture of the inoculants was produced for inoculation as described by Santos et al. (2017). Each bag contained 108 bacterial cells g−1 peat before planting. This mixture (1250 g = five bags) containing the different immobilized bacteria was added to 50 L of distilled water to produce an equalized population of 107 cells mL−1 for the immersion procedure. For inoculation, the minisetts were immersed in the diluted inoculation mixture for 30 min. The inoculant counts and plant population after the tube phase were performed using the most probable number technique, as described by Baldani et al. (2014), with four semi-solid N-free media, LGI-P, JNFb, LGI, and JMV, suggested for G. diazotrophicus, Herbaspirillum sp., N. amazonense, and P. tropica, respectively.
Experimental setup
The substrate of plants in the sprouting stage was composed of a mixture of sand and vermiculite at a ratio of 2:1 (v/v). The substrate was sterilized by autoclaving twice (121 °C for 30 min) on alternate days. The substrate showed the following characteristics: pH (water), 4.7; exchangeable elements (cmolc dm−3): Al, 0.2; Ca + Mg, 0.9; individual Ca and Mg, not detected; available P (Mehlich-1), 3.0 mg dm−3; K, 10.0 mg dm−3; no traces of organic matter or N. At this initial budding stage, no fertilizer was applied as the propagation material contained sufficient nutrients for growth. The minisetts were distributed in randomized blocks with four replicates. Each replicate comprised 15 minisetts planted at a depth of 4 cm in a plastic container. After 16 days, the plants were transplanted to tubes (Table 1).
The plants were grown in 180-cm3 tubes (63-mm diameter, 135-mm height) containing the commercial substrate Multiplant™ (Buschle & Lepper SA, Joinville, Santa Catarina, Brazil). The substrate showed the following characteristics: pH (water), 5.5; exchangeable elements (in g kg−1): Ca, 20.2; Mg, 12.2; K, 2.12; available P (resin), 2.0; and N, 6.7. The plants were grown in the tubes for 30 days, which is the amount of time necessary for developing the secondary root system, depending on the environmental conditions and plant variety, among other factors. After this, the plants were transferred to a hydroponic system (Table 1). Before the transfer, the node was removed from the newly formed plant to avoid the confounding effects of nutrients present in the node.
In the hydroponic system, a modified Hoagland nutrient solution was used (Hoagland and Arnold 1950). Plants were grown in plastic pots of 7.5 L filled with 5.5 L of constantly aerated nutrient solution. The composition used contained macronutrients at 50% ionic strength and micronutrients at 100% ionic strength. The chemical composition of the nutrient solution with high N (3.0 mM) in g L−1 was as follows: N–NO3−, 0.035; N–NH4+, 0.007; P, 0.0155; K, 0.117; S–SO4−, 0.0954; Ca, 0.080, and Mg, 0.024. The chemical composition of the nutrient solution with low N (0.3 mM) in g L−1 was as follows: N–NO3−, 0.0035; N–NH4+, 0.0007; P, 0.0155; K, 0.117; S–SO4−, 0.124; Ca, 0.080; and Mg, 0.024. Then, 1 mL L−1 of micronutrient solution (in g L−1: Fe, 0.002; B, 0.0005; Cu, 0.00002; Mn, 0.0005; Zn, 0.00005; and Mo, 0.00001) was added to the two solutions. The salts, acids, or bases used in the solutions were as follows: Mg(NO3)2.6H2O, Ca(NO3)2.4H2O, (NH4)2SO4, K2HPO4, MgSO4, CaSO4.2H2O; C10H12N2NaFeO8.3H2O, H3BO3, CuSO4.5H2O, MnSO4.7H2O, ZnSO4.7H2O, and Na2MoO4.
The plants were cultivated with the high-N solution for 30 days, after which a new solution without nitrogen was applied for 72 h. After this starvation period, half of the plant pots were filled with the high-N solution (3.0 mM), and the other half were filled with the low-N solution (0.3 mM). Every 2 days, the pH was measured and adjusted to 6 using 0.05 N NaOH solution and/or 0.03 N hydrochloric acid (HCl). The nutrient solution was renewed each week. The plants were grown under these conditions from day 40 to day 59; it means, 26 days after hydroponics (DAH) (Table 1).
Three harvests were performed to examine enzymatic activity and soluble metabolites of nitrogen metabolism (Table 1). In the first harvest (after 30 DAH), 15 g of root and leaf tissue (the first fully expanded leaf from the leaf apex, without the central vein [leaf +1]) was collected after 72 h without a supply of N. From the final harvest (59 DAH and 105 days after inoculation; Table 1), the leaf and root tissues were collected for analyses. The soluble fractions were prepared in 80% alcohol, and 0.5 g was used to test the nitrate reductase activity (NRa), and 0.5 g frozen in liquid nitrogen and stored at − 70 °C was used to test the glutamine synthetase activity (GSa). The samples were stored at − 70 °C until GSa analysis, and samples for the soluble fraction analysis were stored in ethyl alcohol diluted to 80% (v/v) with deionized water. The dry mass and N, P, and K contents were measured after the final harvest.
Tested parameters
In the first and second harvest, only 2.0 g of the leaf +1 and 2.0 g of root tissue were collected and used for analyses of nitrate reductase activity (NRa), glutamine synthetase activity (GSa), and soluble fractions. After the third harvest, plants were separated into leaves, stem, and roots and were dried at 60 °C in a forced-air oven until constant weight. The dry material was ground for nutrient accumulation analyses. The NRa analysis was performed 24 h and 7 days after applying the nutrient solution containing 3.0 mM N.
Nitrate reductase activity (EC 1.7.1.1)
NRa (EC 1.7.1.1) analysis was performed using an in vivo method according to Jaworski (1971) and adapted for sugarcane by Santos et al. (2014). Samples were collected during the day and removed in order of blocks to avoid delays in the analysis. The sampling material comprised 0.5 g of ground root tissue and the leaf +1 cut approximately 1 cm from the stem insertion using liquid nitrogen. The ground tissue samples were placed in the dark in 10 mL of extractive solution (2.5 mL of phosphate buffer prepared with KH2PO4 at a concentration of 285 mmol dm−3 and pH 7.3, 2.5 mL of KNO3 at a concentration of 300 mmol dm−3, 1.0 mL of 0.6% (v/v) Tween 20, and 4.0 mL of deionized water). The samples were then immediately subjected to a vacuum treatment (600 mmHg) for 3 min, and then placed in a water bath at 32 °C for 90 min. After the incubation period, aliquots of 0.5 mL of the incubation solution of each sample were collected and transferred to test tubes to which 0.5 mL of 1% sulphanilamide and 0.5 mL of 0.02% N-naphthyl-ethylenediamine were added. The mixtures were incubated for 20 min, after which 4 mL of deionized water was added. The absorbance was measured at 540 nm using a spectrophotometer (2000 UV model; Bel Photonics Company-Brazil) to determine the NO2− concentrations according to a standard curve using NaNO2. Enzymatic activity was estimated based on the amount of NO2− released by the plant tissues in the incubation solution and was expressed in μmol of NO2− h−1 g−1 fresh weight. Nitrate assimilation by the roots is regulated by photosynthesis, and thus shows a marked circadian activity pattern; therefore, samples were collected during the expected peak of activity.
Glutamine synthetase activity (EC 6.31.2)
Samples of fresh leaf tissue and roots (0.5 g) were treated with liquid nitrogen to produce a fine powder to which 1.5 mL of extraction buffer (5 mL of 1 M Tris-HCl at pH 8.0, 0.2 mL of 0.5 M EDTA at pH 8.0, 1.5 g of polyvinylpolypyrrolidone, 0.154 g of dithiothreitol, 30 mL of glycerol, 0.5 mL of 200 mM phenylmethylsulphonyl fluoride, and 100 mL of deionized water) was added. The homogenate was centrifuged at 14,000 rpm in a refrigerated centrifuge (5415 R; Eppendorf) at − 4 °C for 30 min. The total protein content was determined by absorbance measurement using a spectrophotometer at 540 nm and with bovine serum albumin (Sigma) as a standard. Protein extracts containing 50 μg mL−1 of protein were used to quantify the GSa. For this purpose, an extraction solution was prepared from 2.5 mL of 1 M imidazole-HCl pH 7.5, 2.5 mL of 0.1 M hydroxylamine-Tris pH 7.5, 0.203 g of MgCl2·6H2O, 0.184 g of L-glutamate, 0.138 g of ATP, and 17.5 μL of β-mercaptoethanol and added to 100 mL of deionized water. A 0.45 mL aliquot of this buffer was added to 0.5 mL of the protein extract. This mixture was then incubated at 30 °C for 30 min, after which the reaction was terminated by the addition of 0.35 mL of a ferric chloride reagent added to trichloroacetic acid and dissolved in HCl 0.5 N. The absorbance was measured at 540 nm using an Anthos Zenyth 200 ST microplate reader (Biochrom) with standard γ-glutamyl monohydroxamate (Sigma). GSa was expressed in μmol of γ-glutamyl monohydroxamate min−1 μg- 1 protein.
Nitrogen soluble fractions and soluble sugars
One-gram samples of leaf and root tissue were added to 20 mL of ethyl alcohol diluted to 80% (v/v) with deionized water, ground for 3 min using a Turratec grinder, model TE-102 (12 mm propeller; Tecnal), homogenized, and filtered using four layers of gauze. The filtrate was collected and transferred to a separatory funnel using chloroform for separation of the polar and non-polar phases. The non-polar phase was discarded, and the polar phase was used for analyses. The levels of nitrate and nitrite were determined simultaneously according to Miranda et al. (2001), N-amino (free amino acids)-free content was measured using the ninhydrin method according to Yemm and Cocking (1955), ammonium content was determined using the colorimetric method described by Mitchell (1972), and soluble sugar content was measured according to Yemm and Willis (1954).
Total N, P, and K
The leaves, stems, and roots were dried in a forced-air oven at 65 °C to a constant weight, after which the samples were ground using a Wiley mill, and the total N, P, and K concentrations were analyzed. Sulfuric digestion and distillation were used to determine the N content, and nitro-perchloric digestion was used to determine the P and K content (Sarruge and Haag 1974). The nutrient concentrations (kg kg−1 dry weight) were multiplied by the corresponding dry mass of the plant and the respective amount was expressed for each nutrient in terms of mg plant−1.
Statistical analyses
The data were analyzed for normality (Lilliefors test) and homogeneity of variance (Cochran and Bartlett test) using the statistical software package SAEG 9.0 (Euclydes 2004). After this, an analysis of variance was performed using the SISVAR program 5.3 (Ferreira 2010). When significant results were obtained, Tukey’s test was performed. Statistical significance is reported at p < 0.05. Pearson’s correlation coefficients were calculated using Past 3.14 software (Harper and Ryan 2001) to test linear relations between the variables.
Results
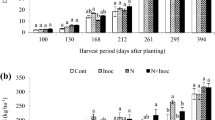
Thirty days after the beginning of the hydroponic cultivation, the first measurement of NRa was performed in the roots and leaves of the two sugarcane varieties to compare the effect of high-N conditions (3 mM N) with that of no N addition for 72 h (Fig. 1). Under high-N conditions, NRa remained stable in both tissues in var. RB867515 but was reduced in the roots of var. IACSP95-5000; however, the enzymatic activity in the roots was four times lower than that observed in the shoots (Fig. 1a, c). Var. RB867515, adapted to poor soils, showed a higher NRa in the roots and shoots when inoculated in the absence of N (Fig. 1b, d). The enzymatic activity in roots was reduced by 60% in the absence of N and even under low-N conditions; NRa activity in IACSP95-5000 was reduced by the inoculation treatment (Fig. 1b).
Nitrate reductase activity (μmol NO2− h−1 g−1 fresh weight) on leaves and roots of two sugarcane varieties inoculated or not with the bacterial mixture, grown for 30 days in a 3.0 mM dose of N modified Hoagland solution (a, c) and after 72 h of N depletion (b, d). Columns represent mean value and bars represent the standard error of 12 repetitions. Columns with the same lower case letter compare inside each cultivar are not significant different at p < 0.05
Glutamine synthetase activity (GSa) was measured in the shoots and roots, but in the roots the activity was very low and could not differentiate between the treatments applied. The colorimetric method was not sufficiently sensitive to measure the enzymatic activity in the roots (Fig. 2a, b). In the shoots, both varieties responded positively to inoculation in the presence or absence of N (Fig. 2c, d). The absence of N in the Hoagland’s solution for 72 h enhanced GSa by 8% in both varieties in response to N stress, as plants start to use accumulated cellular N reserves (Fig. 2d).
Glutamine synthetase activity μmol γ-glutamil monohydroxamate mg protein−1) in shoots and roots of two sugarcane varieties inoculated or not with the bacterial mixture, grown for 30 days in a 3.0 mM dose of N modified Hoagland solution (a, c) and after 72 h of N depletion (b, d). Columns represent mean value and bars represent the standard error of 12 repetitions. Columns with the same lower case letter compare inside each cultivar are not significant different at p < 0.05
The N-nitrate content in the shoots and roots of the two sugarcane varieties was higher after 30 days of cultivation with 3 mM N and decreased rapidly after 72 h without N addition to the Hoagland’s solution (Fig. 3). Inoculation did not produce an effect under high-N conditions, but after N depletion, the enzymatic activity in the shoots was enhanced in the inoculated plants of both varieties.
Nitrate content in the shoots and roots of two sugarcane varieties inoculated or not with the bacterial mixture, grown for 30 days in a 3.0 mM dose of N modified Hoagland solution (a, c) and after 72 h of N depletion (b, d). Columns represent mean value and bars represent the standard error of 12 repetitions. Columns with the same lower case letter compare inside each cultivar are not significant different at p < 0.05
The opposite pattern was observed regarding the N-amino fraction, which was higher after N depletion (Fig. 4). The N-amino content was lower in the roots during the initial growth phase, as this N form is typically transferred to the shoots. After 72 h without N, N-amino increased rapidly as a response to N deficiency.
N-amino contents in the shoots and roots of two sugarcane varieties inoculated or not with the bacterial mixture, grown for 30 days in a 3.0 mM dose of N modified Hoagland solution (a, c) and after 72 h of N depletion (b, d). Columns represent mean value and bars represent the standard error of 12 repetitions. Columns with the same lower case letter compare inside each cultivar are not significant different at p < 0.05
The soluble sugar content in the roots and shoots did not differ between varieties and inoculation treatments, in accordance with observed the N-amino content (Fig. 5). Higher values were observed in the shoots, where N is incorporated in carbon metabolites produced by photosynthesis. The contents of both N-amino and soluble sugars increased in the roots after N-depletion, and inoculation did not have an effect on this pattern.
Sugar contents in in the shoots and roots of two sugarcane varieties inoculated or not with the bacterial mixture, grown for 30 days in a 3.0 mM dose of N modified Hoagland solution (a, c) and after 72 h of N depletion (b, d). Columns represent mean value and bars represent the standard error of 12 repetitions. Columns with the same lower case letter compare inside each cultivar are not significant different at p < 0.05
Third harvest
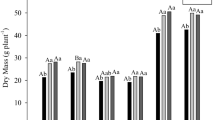
In response to the different amounts of N applied during plant development in the first 59 days, both varieties differed in biomass accumulation between the treatments, particularly var. RB867515 (Fig. 6). Under high-N conditions (3 mM N), inoculation did not affect the shoot dry weight in both varieties (Fig. 6a). Under low-N conditions, the shoot dry weight increased by 47% in var. RB867515, reaching similar values to those of plants grown under high-N conditions (Fig. 6b). The roots of var. RB867515 also responded to inoculation regarding both N levels, whereas no response to the inoculation treatment was observed in var. IACSP95-5000 (Fig. 6b, c). In var. RB867517, the inoculation treatment produced a similar total biomass accumulation in both low- and high-N-treated plants (Fig. 6e, f). Plants at this stage are shown in SM1 (Fig. 11; SM1).
Biomass accumulation of two sugarcane varieties grown in a modified Hoaglands solution and inoculated or not with the bacterial mixture at 59 DAH. Seedlings were submitted to high dose of N (3.0 mM) for 30 days, then the N of the solution was suppressed for 72 h and then resuspended with the dose 3.0 mM (a, c, and e) or 0.3 mM (b, d, and f) during 26 days. Columns represent mean value and bars represent the standard error of 6 repetitions. Columns with the same lower case letter compare inside each cultivar are not significant different at p < 0.05
The accumulated N differed between treatments and varieties at the end of the experiment (Fig. 7). Under high-N conditions (3 mM), var. RB867515 accumulated 50% more N in the shoots when inoculated compared with that in the control (Fig. 7a). The same pattern was observed under low-N conditions (Fig. 7b). In var. IACSP95-5000, inoculation had no effect (Fig. 6) on the N (Fig. 7b), P, and K (Fig. 7d, f) contents. Inoculation led to a higher dry mass production and P and K accumulation in var. RB867515 (Figs. 6 and 7c, e).
Nutrient accumulation (N, P, and K) in the shoots of two sugarcane varieties using hydroponic and inoculated or not with the bacterial mixture. Plants were submitted to high dose of N (3.0 mM) for 30 days, then the N of the solution was suppressed for 72 h and then resuspended with the dose 3.0 mM (a, c, and e) or 0.3 mM (b, d, and f) for 26 days. Columns represent mean value and bars represent the standard error of 6 repetitions. Columns with the same lower case letter compare inside each cultivar are not significant different at p < 0.05
In contrast, inoculation increased the total P and K under low-N conditions in var. RB867515, but did not affect the N concentration in the roots of both varieties (Fig. 8). The total K was also higher in the inoculated var. RB867515 under high-N conditions (Fig. 8e).
Nutrient accumulation (N, P, and K) in root of two sugarcane varieties using hydroponic and inoculated or not with the bacterial mixture. Plants were submitted to high dose of N (3.0 mM) for 30 days, then the N of the solution was suppressed for 72 h and then resuspended with the dose 3.0 mM (a, c, and e) or 0.3 mM (b, d, and f) for 26 days. Columns represent mean value and bars represent the standard error of 6 repetitions. Columns with the same lower case letter compare inside each cultivar are not significant different at p < 0.05
Enzyme activities and soluble fractions at the final harvest presented smaller effects of the inoculation treatment than those observed previously when plants were completely depleted of N for 72 h (Table 2). Maintenance of 0.3 mM N reduced the efficiency of inoculation observed by a comparison of high- and low-N conditions, and differed between varieties. The NRa was lower in both tissues of var. RB867515, compared with those in var. IACSP95-5000, especially in inoculated plants at 3 mM N. In the control plants, the NRa was higher in the shoots than in the roots (3.5-fold in RB867515 and 5-fold in IACSP95-5000). Inoculation reduced this difference (3- and 4.6-fold shoot/root ratio). Similar values of NRa were observed under low-N conditions (0.3 mM,) ranging from 0.134 to 0.272 μmol NO2− h−1 g−1 fresh weight.
The GSa increased in the shoots of the var. RB867515 upon inoculation and at this final harvest; in addition, the roots showed improved Gsa activities in both varieties under low-N conditions (Table 2). The N-amino and sugar contents were not affected by inoculation or N condition. The two varieties showed different N-amino contents in the inoculated roots and under low-N conditions; IACSP95-5000 presented a higher level than RB867515, independent of inoculation. The N–NO3− content in the shoots also increased in the inoculated plants of both varieties tested, independent of the N level. However, this was reduced in the inoculated roots, especially in var. IACSP95-5000. Under both N conditions, higher N–NO3− concentrations were observed in the roots than in the shoots (Table 2).
Discussion
N metabolism in plant tissue occurs in the roots and shoots, and nitrate and ammonium can be assimilated in different ways. Nitrate is typically reduced to ammonium followed by the assimilation of ammonium into amino acids (Masclaux-Daubresse et al. 2010). The results of the two sugarcane varieties RB867515 and IACSP95-5000 showed that the variety plays an important role in this process, and the interaction of variety and inoculation can also affect enzymatic activities (Figs. 1 and 2). The enzymatic activity at this initial growth stage differed between varieties tested, plant compartments, and N levels. For example, var. RB867515 enhanced the NRa in the leaves and roots of the inoculated plants after 72 h without N (26% and 48% respectively) compared with that in the control. The opposite occurred in the IACSP95-5000 roots (by 62%), whereas no effect of inoculation was observed in the shoots (Fig. 1).
Glutamine is the primary product of nitrogen assimilation from all inorganic nitrogen sources (nitrate, direct ammonium uptake) and is well studied in leguminous plants due to the importance of BNF in nodulated species. Glutamine synthetase is involved in ammonium assimilation and is released by several metabolic pathways (Lea and Miflin 2010). The GSa plays a role in nitrogen assimilation processes, such as photorespiration and amino acid catabolism, and is typically detected in chlorophyllous tissue (Bernard and Habash 2009; Verma et al. 1993); thus, low levels are expected in the roots, as observed in Fig. 2. Like NRa, the GSa was also modified by the N level as well as the inoculation, which improved its activity (31% in IACSP95-5000 and 19% in RB867515 compared with that in the control; Fig. 2c). This pattern was also observed under low-N conditions and was 21% and 16% higher in the inoculated RB867515 and IACSP95-5000 plants, respectively (Fig. 2d).
For instance, in the 20 combinations of treatments (hydroponics, shoots and roots, two varieties, N depletion, and low- and high-N conditions [0.3 and 3 mM and time of harvest]), the increment or reduction in each tested variety was used as a comparison for the inoculation treatment and variety tested (Fig. 9). Inoculation significantly increased the NRa and GSa in almost all evaluations performed with var. RB867515, except for under high-N conditions (3 mM). The opposite occurred in var. IACSP95-5000; a small increase in the NRa was seen in the roots without N, and a higher increase in GSa was observed in the roots under high-N conditions and in the shoots independent of N level. Based on these two enzyme activities, we can differentiate the control from the above inoculation treatments.
Increment or not, observed in hydroponics in two sugarcane varieties. Increment (%) = inoculated minus control. NR, nitrate reductase activity (μmol NO2− h−1 g−1 fresh weight); GS, glutamine synthetase activity (μmol γ-glutamil monohydroxamate μg protein−1 min−1); NC, nitrate content dry weight (g plant−1); NAC, N-amino content (μmol N-amino g−1 fresh weight); SC, sugar content (mg glucose g−1 fresh weight); R, root; S, shoot
Previous studies using other bacterial species found effects of inoculation on the NRa in cereals, such as maize and wheat (Reis Junior et al. 2008; Ribaldo et al. 2005; Aliasgharzad et al. 2014). In sugarcane, Santos et al. (2017) observed the highest NRa in var. RB966928 under N depletion, depending on the strain used. Nogueira et al. (2001) used inoculation with G. diazotrophicus and Herbaspirillum to study gene expression and observed that genes related to nitrate reduction and uptake were upregulated in the inoculated plants. In contrast, the GSa is involved in nitrogen sensing and the regulation of primary and secondary metabolism; it is tightly linked to energy consumption by the plant and by microorganisms, and to the nitrogen status of the cell in both organisms (Wagner et al. 2013). Purcino et al. (1996) compared the GSa and yield of six maize varieties inoculated with different strains of Azospirillum, and the GSa was not influenced by the N supply. Pereira-Defilippi et al. (2017) also compared maize genotypes and two expressed sequence tags coding for key enzymes of nitrogen metabolism in maize, which are nitrate reductase and glutamine synthetase in the presence of Azospirillum inoculation; in this case, the N level and genotype affected gene expression.
The N-nitrate content was decreased by inoculation in the shoots for both varieties under N depletion for 72 h; however, in the roots, the opposite occurred and the N-nitrate content was increased 100% by the inoculation treatment in var. RB867515 (Fig. 9). The N-amino content presented high levels compared with those of N-nitrate, but inoculation did not play a role in this fraction evaluated in both N levels (Fig. 4). This suggests that the increase of the N-amino pool is positively related to growth in both varieties (Fernandes and Rossiello 1995); however, in RB867515, this difference was affected by inoculation, which indicated that inoculation increased the N-amino pool and resulted in higher growth. The N-amino content had an opposite response, depending on the variety tested, as expected by the NRa activity. The soluble sugar content was a less sensitive parameter in almost all tested N levels and inoculations, decreasing in seven of eight instances. Donato et al. (2003) also observed a lower effect of N application on the sugar content in six sugarcane varieties at 45 days after planting under high-N conditions. Moreover, a low-N-concentration growth medium increased the sugar content in a sugarcane cell suspension, as observed by Veith and Komor (1993).
The biomass accumulation and nutrient content of the shoots and roots after 59 days of hydroponics were higher in var. RB867515. All evaluated parameters of biomass and N, P, and K accumulation were increased by inoculation in RB867515 but not in IACSP95-5000, which is selected for soils with high natural fertility (Fig. 10). It is important to note that the control of the two examined varieties is naturally associated with a microbiome, which can contribute to N supply and growth; however, in the responsive genotype RB867515, growth was stimulated by inoculation, especially under low-N conditions (90% increase in total N compared with that in the uninoculated control). It is known that sugarcane varieties differ in nitrogen accumulation and their contribution of natural BNF (Urquiaga et al. 2012). Moreover, inoculation and growth effects differ between varieties in sugarcane (Oliveira et al. 2006), maize (Alves et al. 2015), and rice (Vargas et al. 2012), among others.
Increment or not, observed in the last harvest, at 59 days in hydroponics in two sugarcane varieties. Increment (%) = inoculated minus control. DWS, dry weight shoot (g plant−1); DWR, dry weight roots (g plant−1); TDW, total dry weight (g plant−1); N, total N (mg plant−1); P, total P (mg plant−1); K, total K (mg plant−1); HN, 3 mM; LN, 0.3 mM
Comparing the results, the improvement of biomass was correlated with the NRa and N-nitrate content but not with the GSa or N-amino content (Table 3; SM2). These results can be partially explained by the importance of this nitrate N form for sugarcane growth. This can be expected, as NO3− is one of the most abundant sources of N in natural and agricultural systems (von Wiren et al. 2000). As the tested variety was selected for the tropical soils of Brazil, it can be assumed to be suitable for several regions and climates; thus, the response to NO3− could be described as a preference for a particular N source in this variety, in contrast to IACSP95-5000, which also uses this N-source, but in a different way. In addition, NO3− can act as a signaling molecule that modulates gene expression in a wide range of processes, including plant growth and root architecture (Alvarez et al. 2012).
Furthermore, the dry weight accumulation was positively correlated with the soluble sugar content and negatively correlated with the N-nitrate and N-amino contents (Table 3, SM2). Sugarcane, used for sugar production, has a sink–source system, which is not normally observed in other plants, including members of the Poaceae family; it accumulates sugars on the stem nodes as a final product, not grains. During initial growth, stems start to produce new nodes, and it is known that this plant accumulates sucrose both inside and outside of the cell, in the apoplast and in the symplast (Wang et al. 2013). Bacteria used in the inoculant mixture are considered endophytes and normally inhabit the apoplast space (James and Olivares 1998). The sugar content showed a reduced trend in both varieties; inoculation, however, did not seem to have an effect (Fig. 5).
Growth promotion associated with diazotrophic inoculation has been studied over several decades, and new insights of this association continue to emerge. The five bacterial species/strains tested in the present study produce different plant regulators belonging to the classes of auxins (Fuentes-Ramirez et al. 1993; Rodrigues et al. 2008), gibberellins (Bastian et al. 1998), and cytokinins (da Silva et al. 2015). These compounds were suggested to be responsible for root development and water and nutrient uptake (Cassán et al. 2014). P and Zn solubilization is also a mechanism described in Gluconacetobacter diazotrophicus (Crespo et al. 2011; Saravanan et al. 2007). One of the predicted effects of bacterial inoculation, which produces plant regulators, is that these hormones enhance the expression and activity of various enzymes involved in plant nitrogen metabolism, and the biomass increment observed in the responsive var. RB867515 supports this suggestion. However, var. IACSP95-5000 did not exhibit a positive interaction and can be considered as neutral by the lack of response in several of the examined parameters. Partida-Martínez and Heil (2011) classified the plant–bacteria interaction according to the observed consequence into negative, neutral, and positive effects, and correlated these classes with the abundance of bacteria. In sugarcane, the stem is colonized by a diverse microbiome which changes over time (Souza et al. 2016). The minisetts used in the experiment were naturally colonized by this microbiome and the inoculation treatment may have interacted with this differently in each variety (Table 4; SM3). The abundance of bacteria present in the plants at the time of planting or at the time of harvest might not differ between inoculated plants and controls, as all of the applied strains occur naturally in the nodes of sugarcane plants. The natural population counted before transplanting to the hydroponics produced values above 10,000 cells g−1 fresh weight (Table 4; SM3). Inoculation increased these numbers 10- to 100-fold, approximately. However, a single immersion of the stem pieces in the inoculant produced effects on different parameters, which were not correlated with the bacterial abundance in this case.
The respective mechanisms have not been comprehensively studied in sugarcane, particularly regarding the effect of inoculation. As the NRa and GSa were not correlated with the biomass at the final harvest (Table 3; SM2), it can be speculated that sugarcane plants can compensate for N stress by utilizing N sources that are not related to inorganic N, or that NO3− may act as a signaling molecule to improve root development (Fig. 6c, d), with a higher effect in responsive varieties.
Nitrogen accumulation and efficiency differ between sugarcane varieties (Robinson et al. 2007; Whan et al. 2010). RB867515 is one of the most commonly used varieties in Brazil; it is adapted to poor tropical soils and is also highly productive and resistant to diseases, besides other favorable characteristics (RIDESA 2015). Schultz et al. (2012, 2014) compared different varieties under field conditions using the same five bacterial strains tested in the present study, and RB867415 and RB72454 showed differences in the stalk yield depending on the location and sugarcane growth cycle. The tested bacterial strains also differed regarding their growth promotion effect (Suman et al. 2013). The optimal combination of bacteria and plant variety remains to be identified. Efforts must be made to understand the mechanisms involved in the plant–bacteria interaction and the efficiency of the N utilization of responsive varieties is a measure that can contribute to future breeding programs and the selection of adequate bacterial strains.
Nitrogen plays a crucial role in plant growth, and the selection of bacterial strains that can be applied as an inoculant for sugarcane production is difficult and cannot be compared with the seed inoculation of legumes or cereals, such as maize and wheat. The technology has several disadvantages, as the propagation of this culture uses pieces of the mother plant that are already colonized by a natural microbiome. Applying a single inoculant species or a mixture of strains using a single node is therefore a feasible approach.
Growth promotion and the direct contribution of nitrogen fixation are mechanisms that have been investigated in sugarcane, and the association of the rate of BNF with that plant variety has been reported by Urquiaga et al. (2012). However, potential markers for evaluating the responsiveness of a variety to inoculation have not yet been described. Since Oliveira et al. (2006), the above mixed inoculant is being used under field conditions in Brazil (Schultz et al. 2012, 2014). Sugarcane crop production, represented by tons of fresh stalks with a higher sugar accumulation, is the final objective of this research. Using selected varieties in association with selected diazotrophs is a feasible approach and can be used to reduce N losses and improve N acquisition by the plant. Elucidating how this plant–bacteria association is modulated by N is one of the initial steps to improving this technology.
References
Aliasgharzad N, Heydaryan Z, Sarikhani MR (2014) Azospirillum inoculation alters nitrate reductase activity and nitrate uptake in wheat plant under water deficit conditions. Int J Adv Sci Eng Infor Technol 4:94–98. https://doi.org/10.18517/ijaseit.4.4.422
Alvarez M, Vidal EA, Gutiérrez RA (2012) Integration of local and systemic signaling pathways for plant N responses. Curr Opin Plant Biol 15:185–191. https://doi.org/10.1016/j.pbi.2012.03.009
Alves GC, Videira SS, Urquiaga S, Reis VM (2015) Differential plant growth promotion and nitrogen fixation in two genotypes of maize by several Herbaspirillum inoculants. Plant Soil 387:307–321. https://doi.org/10.1007/s11104-014-2295-2
Baldani JI, Baldani VLD, Seldin L, Döbereiner J (1986) Characterization of Herbaspirillum seropedicae gen. nov. sp. nov. a root associated nitrogen fixing bacterium. Int J Syst Bacteriol 36:86–93. https://doi.org/10.1099/00207713-36-1-86
Baldani JI, Pot B, Kirchhof G, Falsen E, Baldani VLD, Olivares FL, Hoste B, Kersters K, Hartman A, Gillis M, Döbereiner J (1996) Emended description of Herbaspirillum; inclusion of [Pseudomonas] rubrisubalbicans, a mild plant pathogen, as Herbaspirillum rubrisubalbicans and classification of a group of clinical isolates (EF group 1) as Herbaspirillum species 3. Int J Syst Bacteriol 46:802–810
Baldani JI, Reis VM, Videira SS, Boddey LH, Baldani VLD (2014) The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384:413–431. https://doi.org/10.1007/s11104-014-2186-6
Bastian F, Cohen A, Piccoli P, Luna V, Baraldi R, Bottini R (1998) Production of indole-3-acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically defined culture media. Plant Growth Regul 24:7–11. https://doi.org/10.1023/A:100
Bernard SM, Habash DZ (2009) The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol 182:608–620. https://doi.org/10.1111/j.1469-8137.2009.02823.x
Cantarella H, Trivelin PCO, Vitti AC (2007) Nitrogênio e enxofre na cultura da cana-de-açúcar. In: Yamada T, Abdalla SRS, Vitti GC (eds) Nitrogênio e enxofre na agricultura brasileira. IPNI, Piracicaba, pp 355–412
Cassán F, Vanderleyden J, Spaepen S (2014) Physiological and agronomical aspects of phytohormone production by model plant-bacteria-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J Plant Growth Regul 33:440–459. https://doi.org/10.1007/s00344-013-9362-4
Cavalcante VA, Döbereiner J (1988) A new acid tolerant nitrogen- fixing bacterium associated with sugarcane. Plant Soil 108:23–31
Costa C, Dwyer LM, Zhou X, Dutilleul P, Hamel C, Reid LM, Smith D (2002) Root morphology by contrasting maize genotypes. Agron J 94:96–101
Crespo J, Boiardi J, Luna M (2011) Mineral phosphate solubilization activity of Gluconacetobacter diazotrophicus under P-limitation and plant root environment. Agricult Sci 2:16–22. https://doi.org/10.1590/S0100-204X2003001200003
da Silva JM, dos Santos TMC, de Albuquerque LS, Montaldo YC, de Oliveira JUL, da Silva SGM, Nascimento MS, Teixeira RRO (2015) Potential of the endophytic bacteria Herbaspirillum spp. and Bacillus spp. to promote sugarcane growth. AJCS 9:754–760 http://www.cropj.com/silva_9_8_2015_754_760.pdf
Donato VMTS, Andrade AG, Souza ES, França JGE (2003) Metabolismo de plantas de cana-de-açúcar cultivadas in vitro sob diferentes concentrações de nitrogênio. Pesq agrop bras 38:1373–1379 http://www.scielo.br/pdf/pab/v38n12/a03v38n12.pdf
Dong M, Yang Z, Cheng G, Peng L, Xu Q, Xu J (2018) Diversity of the bacterial microbiome in the roots of four Saccharum species: S. spontaneum, S. robustum, S. barberi, and S. officinarum. Front in Microbiol 9:267. https://doi.org/10.3389/fmicb.2018.00267
Euclydes R (2004) Sistema para análises estatísticas (SAEG 9.0). Funarbe, Viçosa
Fernandes MS, Rossiello OP (1995) Mineral nitrogen plant physiology and plant nutrition. Crit Rev Plant Sci 14:111–148
Ferreira D (2010) Sisvar: versão 5.3. Lavras: UFLA
Fortes C, Trivelin PCO, Vitti AC, Otto R, Franco HCJ, Forini CE (2013) Stalk and sucrose yield in response to nitrogen fertilization of sugarcane under reduced tillage. Pes agrop bras 48:88–96. https://doi.org/10.1590/S0100-204X2013000100012
Franco HCJ, Otto R, Faroni CE, Vitti AC, Oliveira ECA, Trivelin PCO (2010) Nitrogen in sugarcane derived from fertiliser under Brazilian field conditions. Field Crops Res 121:29–41. https://doi.org/10.1016/j.fcr.2010.11.011
Fuentes-Ramirez LE, Jiménez-Salgado T, Abarca-Ocampo IR, Caballero-Mellado J (1993) Acetobacter diazotrophicus, an indole acetic acid producing bacterium isolated from sugarcane cultivars of Mexico. Plant Soil 154:145–150. https://doi.org/10.1007/BF00
Harper D, Ryan P (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9 https://palaeo-electronica.org/2001_1/past/past.pdf
Hoagland DR, Arnold DI (1950) The water-culture method for growing plants without soil. Circular Number 347. California Agricultural Experiment Station
James EK, Reis VM, Olivares FL, Baldani JI, Döbereiner J (1994) Infection of sugar cane by the nitrogen-fixing bacterium Acetobacter diazotrophicus. J Exp Bot 45:757–766
James EK, Olivares FL, Baldani JI, Dobereiner J (1997) Herbaspirillum, an endophytic diazotroph colonizing vascular tissue in leaves of Sorghum bicolor L. Moench J Exp Bot 48:785–797
James EK, Olivares FL (1998) Infection and colonization of sugarcane and other graminaceous plants by endophytic diazotrophs. Crit Rev Plant Sci 17:77–119. https://doi.org/10.1080/07352689891304195
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Bioch Biophys Res Com 43:1274–1279. https://doi.org/10.1016/S0006-291X(71)80010-4
Landell MGA, Campana MP, Figueiredo P, Xavier MA, Anjos IA, Dinardo-Miranda LL, Scarpari MS, Garcia JC, Bidóia MA, Silva DN, Mendonça JR, Kanthack RAD, Campos MF, Brancalião SR, Petri RH, Miguel PEM (2012) Sistema de multiplicação de cana-de-açúcar com uso de mudas pré-brotadas (MPB), oriundas de gemas individualizadas, Campinas: Instituto Agronomico/ Fundação IAC (Boletim 109). 22p. http://www.udop.com.br/ebiblio/pagina/arquivos/2013_sistema_multiplicacao_cana_com_mudas_pre_brotadas.pdf
Lea PG, Miflin BJ (2010) Nitrogen assimilation and its relevance to crop improvement. Annu Plant Rev 42:1–40. https://doi.org/10.1002/9781444328608.ch1
Lin SY, Hameed A, Shen FT, Liu YC, Hsu YH, Shahina M, Lai WA, Young CC (2014) Description of Niveispirillum fermenti gen. nov., sp. nov., isolated from a fermentor in Taiwan, transfer of Azospirillum irakense 1989 as Niveispirillum irakense comb. nov., and reclassification of Azospirillum amazonense 1983 as Nitrospirillum amazonense gen. nov. Anton Leeuw 105:1149–1162. https://doi.org/10.1007/s10482-014-0176-6
Magalhães FM, Baldani JI, Souto SM, Kuykendall JR, Döbereiner J (1983) A new acid-tolerant Azospirillum species. Anais Acad Bras Ciên 55:417–430 https://springerlink.bibliotecabuap.elogim.com/content/pdf/10.1007%2FBF02370096.pdf
Manter DK, Delgado JA, Holm DG, Stong RA (2010) Pyrosequencing reveals a highly diverse and cultivar-specific bacterial endophyte community in potato roots. Microb Ecol 60:157–166. https://doi.org/10.1007/s00248-010-9658-x
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105:1141–1157. https://doi.org/10.1093/aob/mcq028
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:67–71. https://doi.org/10.1006/niox.2000.0319
Mitchell L (1972) Microdetermination of nitrogen in plant & tissues. J AOAC 1:1–3
Nogueira EM, Vinagre F, Masuda HP, Vargas C, Pádua VLM, Silva FR, Santos RV, Baldani JI, Ferreira PCG, Hemerly AS (2001) Expression of sugarcane genes induced by inoculation with Gluconacetobacter diazotrophicus and Herbaspirillum rubrisubalbicans. Gen Mol Biol 24:199–206. https://doi.org/10.1590/S1415-47572001000100027
Oliveira ALM, Canuto EL, Urquiaga S, Reis VM, Baldani JI (2006) Yield of micropropagated sugarcane varieties in different soil types following inoculation with diazotrophic bacteria. Plant Soil 284:23–32. https://doi.org/10.1007/s11104-006-0025-0
Oren A, Garrity GM (2015) List of new names and new combinations previously effective, but not validly, published. Int J Syst Evol Microbiol 65:2017–2025
Partida-Martínez LP, Heil M (2011) Themicrobe-freeplant:fact or artifact? Front Plant Sci 2:100. https://doi.org/10.3389/fpls.2011.00100
Pereira-Defilippi L, Pereira EM, Silva FM, Moro GV (2017) Expressed sequence tags related to nitrogen metabolism in maize inoculated with Azospirillum brasilense. Genet Mol Res 16(2):gmr16029682
Purcino AAC, Paiva E, Silva MR, de ASRM (1996) Influence of Azospirillum inoculation and nitrogen supply on grain yield, and carbon- and nitrogen-assimilating enzymes in maize. J Plant Nut 9:1045–1060
Reis Junior FB, Machado CTT, Machado AT, Sodek L (2008) Inoculação de Azospirillum amazonense em dois genótipos de milho sob diferentes regimes de nitrogênio. Rev Bras Ciênc Solo 32:1139–1146 http://www.scielo.br/pdf/rbcs/v32n3/a22v32n3.pdf
Reis VM, Olivares FL, Döbereiner J (1994) Improved methodology for isolation of Acetobacter diazotrophicus and confirmation of its endophytic habitat. World J Microbiol Biotechnol 10:401–405. https://doi.org/10.1007/BF00144460
Reis VM, Estrada-de los Santos P, Tenorio-Salgado S, Vogel J, Stoffels M, Guyon S, Mavingui P, Baldani VL, Schmid M, Baldani JI, Balandreau J, Hartmann A, Caballero-Mellado J (2004) Burkholderia tropica sp. nov., a novel nitrogen-fixing, plant-associated bacterium. Int J Syst Evol Microbiol 54:2155–2162. https://doi.org/10.1099/ijs.0.02879-0
Ribaldo CM, Rondanini DP, Trinchero GD, Curá JA (2005) Effect of Herbaspirillum seropedicae inoculation on maize nitrogen metabolism. Maydica 51:481–485
RIDESA (2015) Rede Interinstitucional de Desenvolvimento do Setor Sucroenergético Censo Varietal Brasil 2015. https://www.ridesa.com.br/variedades. Accessed 30 April 2019
Robinson N, Fletcher A, Whan A, Critchley C, Von Wirén N, Lakshmanan P, Schmidt S (2007) Sugarcane genotypes differ in internal nitrogen use efficiency. Funct Plant Biol 34:1122–1129. https://doi.org/10.1071/FPO7183
Rodrigues E, Rodrigues L, de Oliveira A, Baldani V, Teixeira K, Urquiaga S, Reis V (2008) Azospirillum amazonense inoculation: effects on growth, yield and N2 fixation of rice (Oryza sativa L.). Plant Soil 302:249–261. https://doi.org/10.1007/s11104-007-9476-1
Sanguino A, Moraes VA, Casagrande M (2006) Curso de formação e condução de viveiros de mudas de cana-de-açúcar, vol 43
Santos CLR, Cazetta JO, Saran LM, Sanches A (2014) Otimização da análise da atividade da redutase do nitrato e sua caracterização em folhas de cana-de-açúcar. Pesq Agropec Bras 49:384–394. https://doi.org/10.1590/S0100-204X20140005000008
Santos SG, Ribeiro FS, Fonseca CS, Pereia W, Santos LA, Reis VM (2017) Development and nitrate reductase activity of sugarcane inoculated with five diazotrophic strains. Arch Microbiol 199:863–873. https://doi.org/10.1007/s00203-017-1357-2
Saravanan VS, Madhaiyan M, Thangaraju M (2007) Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere 66:1794–1798. https://doi.org/10.1016/j.chemosphere.2006.07.067
Sarruge JR, Haag HP (1974) Análises químicas em plantas. ESALQ/USP, Piracicaba. 56p
Schultz N, Morais RF, Silva JA, Baptista RB, Oliveira RP, Leite JM, Pereira W, Carneiro JB Jr, Alves BJR, Baldani JI, Boddey RM, Urquiaga S, Reis VM (2012) Avaliação agronômica de variedades de cana-de-açúcar inoculadas com bactérias diazotróficas e adubadas com nitrogênio. Pesq Agropec Bras 47:261–268. https://doi.org/10.1590/S0100-204X2012000200015
Schultz N, Silva JAD, Sousa JS, Monteiro RC, Oliveira RP, Chaves VA, Pereira W, Silva MF, Reis VM, Urquiaga S (2014) Inoculation of sugarcane with diazotrophic bacteria. Rev Bras Ciênc Solo 38:407–414. https://doi.org/10.1590/S0100-06832014000200005
Souza RSC, Okura VK, Armanhi JSL, Jorrín B, Lozano N, Silva MJ, González-Guerreiro M, Araújo LM, Verza NC, Bagheri HC, Imperial J, Arruda P (2016) Unlocking the bacterial and fungal community’s assemblages of sugarcane microbiome. Sci Rep 6:28774. https://doi.org/10.1038/srep28774
Suman A, Singh P, Lal M (2013) Effects of diverse habitat biofertilizers on yield and nitrogen balance in plant–ratoon crop cycle of sugarcane in subtropics. Sugar Tech 15:36–43. https://doi.org/10.1007/s12355-012-0191-8
Thorburn PJ, Meier EA, Probert ME (2005) Modelling nitrogen dynamics in sugarcane systems: recent advances and applications. Field Crop Res 92:337–351. https://doi.org/10.1016/j.fcr.2005.01.016
Urquiaga S, Xavier R, Morais RF, Batista R, Schultz N, Leite JM, Resende A, Alves BJR, Boddey RM (2012) Evidence from field nitrogen balance and 15N natural abundance data of the contribution of biological N2 fixation to Brazilian sugarcane varieties. Plant Soil 356:5–21. https://doi.org/10.1007/s11104-011-1016-3
Vargas L, de Carvalho TLG, Ferreira PCG, Baldani VLD, Baldani JI, Hermerly A (2012) Early responses of rice (Oryza sativa L.) seedlings to inoculation with beneficial diazotrophic bacteria are dependent on plant and bacterial genotypes. Plant Soil 356:127–137. https://doi.org/10.1007/s11104-012-1274-8
Veith R, Komor E (1993) Regulation of growth, sucrose storage and ion content in sugarcane cells, measured with suspension cells in continuous culture grown under nitrogen, phosphorus or carbon limitation. J Plant Physiol 142:414–424. https://doi.org/10.1016/S0176-1617(11)81246-0
Verma DPS, Hirel B, Miao GH, Verma DPS (1993) Metabolic and developmental control of glutamine synthetase genes in legume and non-legume plants. In: Verma DPS (ed) Plant Gene Expression. CRC Press Inc., Boca Raton, FL, pp 443–458
Vinall K, Schmidt S, Brackin R, Lakshmanan P, Robinson N (2012) Amino acids are a nitrogen source for sugarcane. Funct Plant Biol 39:503–511. https://doi.org/10.1071/FP12042
von Wiren N, Gazzarrini S, Gojon A, Frommer WB (2000) The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol 3:254–261. https://doi.org/10.1016/S1369-5266(00)80074-6
Wagner D, Wiemann P, Huß K, Brandt U, Fleißner A, Tudzynski B (2013) A sensing role of the glutamine synthetase in the nitrogen regulation network in Fusarium fujikuroi. PLoS One 8(11):e80740. https://doi.org/10.1371/journal.pone.0080740
Wang J, Nayak S, Ming R (2013) Carbon portioning in sugarcane (Saccharum species). Front Plant Sci 4(201). https://doi.org/10.3389/fpls.2013.00201
Whan A, Robinson N, Lakshmanan P, Schmidt S, Aitken K (2010) A quantitative genetics approach to nitrogen use efficiency in sugarcane. Funct Plant Biol 37:448–454. https://doi.org/10.1071/FP09260
Yemm EW, Cocking EC (1955) The determination of aminoacid with ninhydrin. Analyst 80:209–213
Yemm EW, Willis AJ (1954) The estimation of carbohydrate in plants extracts by anthrone. Biochem J 57:508–514
Acknowledgments
The authors express their gratitude to the Coordination of Improvement of Higher Education Personnel - CAPES and the Foundation Carlos Chagas de Amparo à Pesquisa do Estado do Rio de Janeiro - FAPERJ for the fellowships and to the National Council of Scientific and Technological Development - CNPq [grant number INCT 456133/2014-2 and 470824/2013-1].
Funding
This work was supported by Newton Fund grant BB/N013476/1 “Understanding and Exploiting Biological Nitrogen Fixation for Improvement of Brazilian Agriculture”, co-funded by the Biotechnology and Biological Sciences Research Council (BBSRC) and the Brazilian National Council for State Funding Agencies (CONFAP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Euan K. James.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos, S.G., da Silva Ribeiro, F., Alves, G.C. et al. Inoculation with five diazotrophs alters nitrogen metabolism during the initial growth of sugarcane varieties with contrasting responses to added nitrogen. Plant Soil 451, 25–44 (2020). https://doi.org/10.1007/s11104-019-04101-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04101-1