Abstract
Aims
Moso bamboo (Phyllostachys edulis) invasions into adjacent forests are becoming increasingly common. Moso bamboo invasions affect litter quality, soil nutrients, and microbial community composition. Although these effects likely vary among invaded sites and forest types, this has not been investigated.
Methods
We investigated moso bamboo invasion effects on carbon (C) and other major nutrients of litter and soil, as well as soil microbial community composition determined by phospholipid fatty acids (PLFAs) in broadleaf or coniferous forests at three different sites in China.
Results
Ordinations indicated that the effects of invasions on soil nutrients, litter nutrients, and soil microbial composition each varied among forest types and sites. Invasions consistently decreased litter C. Invasions tended to have larger effects on soil nutrients in coniferous forests. Except for bacterial groups in one coniferous forest site, invasions had positive effects on every soil group.
Conclusions
Variations in direction and magnitude of invasion effects on litter properties, soil properties, and soil communities among community types and sites suggest that studies of effects of invasions on soils in a single invaded community may not be able to predict effects of an invasion at other locations, even when the original community is similar or occurs in the same site.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Moso bamboo (Phyllostachys edulis, Carrière J. Houz.; synonym: P. pubescens Mazel ex J. Houz), is native to warm temperate and subtropical regions of China, and has been introduced to other countries or regions including Japan, North Korea, and North America (Suzuki and Nakagoshi 2008; Wang et al. 2016c). The area of moso bamboo forest in China was reported to be 4.4 million ha, accounting for 73.7% of China’s bamboo forests (State Forestry Administration 2014). Moso bamboo has biological characteristics of fast growth and strong reproductive capacity, which allow it to invade adjacent natural forests (Song et al. 2016). Invasions by moso bamboo can alter forest succession and affect litter, soil physical and chemical properties, and microbial community diversity, which affect litter decomposition and alter nutrient release rates to the soil (Carrillo et al. 2012; Wu et al. 2013).
Litter decomposition is a process releasing and returning nutrients to soil, which is important for material cycling in terrestrial ecosystems (Aerts 1997) and plant-soil carbon (C), nitrogen (N) and phosphorus (P) cycling (Suthar and Gairola 2014; Li et al. 2015). Generally, plants eventually return nutrients back to soil via litter decomposition (Song et al. 2015), which is a major component of biogeochemical cycling (Wu et al. 2013). High litter N concentration (i.e., low C: N) can increase litter decomposition and hence N release rate, which is conducive to soil nutrient accumulation and microbial reproduction (Manzoni et al. 2010). Due to differences in litter quality between moso bamboo and the tree species they displace, moso bamboo invasions will potentially alter litter decomposition rate.
In general, coniferous forest litter has higher C concentration than that of broadleaf and moso bamboo forest (Chang and Chiu 2015; Song et al. 2016; Wang et al. 2016c). Therefore, moso bamboo invasions are thought to increase litter decomposition rate in coniferous forests they invade (Wang et al. 2016a). Because more materials will be input into soil ecosystems during litter decomposition, it has been suggested there will be increased C addition (Rubino et al. 2010) and hence more soluble organic C (Shiau and Chiu 2017) or even higher soil respiration rate (Song et al. 2014b) following moso bamboo invasions. Moreover, more soil organic matter may be decomposed by microorganisms due to priming effects (Kuzyakov et al. 2000; Pausch and Kuzyakov 2012; Shiau and Chiu 2017), reducing the amount of degradable nutrients. In addition, the lower N concentration in litter produced by moso bamboo relative to that in the invaded ecosystems will decrease both N release and N cycling rates between plant and soil. Soil quality and fertility (Shiau and Chiu 2017) as well as other factors impacting litter decomposition, such as major nutrients including soil N and P, and microbial communities, may gradually become negatively influenced by moso bamboo invasion (David et al. 1991; Wardle et al. 2006; Sayer et al. 2009).
Soil N and P are key factors limiting plant growth and forest productivity. Nitrogen not only promotes plant root growth, but can also stimulate the secretion of organic acids that increase soil P availability (Yang et al. 2011; Feng et al. 2018; Zhang et al. 2018a, b). Moso bamboo invasions have been shown to impact soil N cycling (Lovett et al. 2004) by increasing soil ammonification and decreasing N nitrification rates (Song et al. 2016; Bai et al. 2016). In another study, soil total P decreased and available P increased in a coniferous forest invaded by moso bamboo (Wu et al. 2018a). All the changes in soil physiochemical properties induced by moso bamboo directly affect the environment for soil microorganisms, which can alter soil microbial communities.
A previous study showed that soil arbuscular mycorrhizal fungal (AMF) communities and biomass in a broadleaf forest were substantially altered by moso bamboo invasion (Qin et al. 2017). Soil microbial communities are directly involved in soil ecological processes, including litter decomposition, humus formation, and nutrient recycling. Vegetation type is one of the main factors affecting the soil microbial community (Waid 1999). Moso bamboo invasion causes changes in forest species composition that can have direct impacts on soil microbial communities (Grayston and Prescott 2005; Lucas-Borja et al. 2012). Soil microorganisms in turn affect plant development, plant community composition and ecosystem function (Batten et al. 2008; Merilä et al. 2010). Studies on soil microbial community changes following moso bamboo invasion reported substantial changes in microbial community composition (Xu et al. 2015; Shiau and Chiu 2017). Particularly, soil bacterial and fungal phospholipid fatty acids (PLFAs) were increased significantly in a broadleaf forest (Wang et al. 2016c), but PLFAs of gram-positive bacteria (G+) and gram-negative bacteria (G-) were found to be decreased in a coniferous forest (Chang and Chiu 2015). Therefore, changes in soil microbial communities affected by moso bamboo invasions may be forest type dependent and may also vary among sites. However, such variations have not been investigated, limiting our understanding of the overall effects on litter, soil, and microbial communities induced by moso bamboo invasion. To understand the overall effects of moso bamboo invasion on litter, soil and microbial communities, three invaded sites and two common moso bamboo-invaded forest types (broadleaf and coniferous forests) were studied. We predicted that moso bamboo invasion effects on litter, and soil C, N, and P properties, as well as microbial community compositions will depend on original forest type across invaded sites.
Materials and methods
Site description
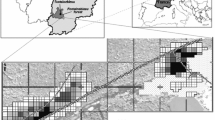
Three regions (Wugong Mountain (Shan) = WGS, Lu Mountain = LS, Yangjifeng Mountain = YJF) in Jiangxi province, China, that include two forest types (broadleaf and coniferous forests) with serious moso bamboo expansion were selected in 2016 (Table 1; Fig. 1). Moso bamboo invaded vegetation types at WGS include broadleaf (evergreen and deciduous) and coniferous forests. At LS, moso bamboo invades coniferous forests (Cryptomeria japonica) with broadleaf (evergreen and deciduous) forests not commonly invaded. Moso bamboo expansion at YJF is into broadleaf deciduous forests.
Locations of the study plots within each region (YJF = circles, LS = squares, WGS = triangles – three plots were located in a 10 m × 10 m area) and vegetation type (moso bamboo forest = black, broadleaf forest = light grey, coniferous forest = white, mixed forest with moso bamboo = dark grey). Numbers indicate elevation in meters
Within each region, sampling areas were established along the moso bamboo expansion gradient: broadleaf and/or coniferous forest, mixed forest (partially invaded by moso bamboo), moso bamboo forest. Along the gradient, six elevations were chosen with three squares (10 × 10 m) in each that were spaced at least 20 m apart. In each region there were 9 bamboo forest squares, 3 mixed forest squares, and 6 uninvaded forest squares (LS = 6 coniferous, YJF = 6 broadleaf, WG = 3 coniferous & 3 broadleaf). Within each square there were three sampling plots (54 plots per region = 6 × 3 × 3).
Litter and soil sampling and chemical analyses
Soil and litter samples were collected during the growing season (August) and non-growing season (January). Five soil samples of 0–20 cm depth were obtained following an S-shape in each sampling plot after the litter layer was removed. Plant roots and stones were removed and samples were kept cold for transport back to the lab. Subsamples were air dried for further determination of organic C, total N, total P, and total potassium (K). The newly produced litter was collected as litter samples, and brought back to the laboratory, air-dried, and ground to pass through a 0.149 mm sieve.
Litter and soil organic C contents were determined by the potassium dichromate (H2SO4-K2Cr2O7) oxidation-external heating method (Lu 2000). The H2SO4-H2O2 digestion method was used in litter and soil analyses of N, P and K with HClO4 added for soil samples. Litter and soil K were measured by the potassium dichromate oxidation-external heating method and then N and P were measured by an automatic discrete chemical analyzer (Smart Chem 200, Westco, Italy). Fresh samples were used for determination of soil available N (AN, including ammonium N and nitrate N), available P (AP), and microbial PLFAs. Soil AN and AP were extracted by 2 mol L−1 potassium chloride solution and 0.5 mol L−1 NaHCO3 solution, respectively, and also determined with the automatic discrete chemical analyzer.
Soil microbial PLFAs analysis
Soil microbial PLFAs were analyzed according to published methods (Bossio et al. 1998). Fresh soil samples equivalent to 8 g dry weight were extracted with a chloroform buffer: methanol: phosphoric acid (volume ratio was 1:2:0.8), shaken for 2 h and centrifuged for 10 min. The supernatant was discarded and the precipitate in the lower layer was extracted again with the buffer. The extracted solutions were mixed well and stored overnight. The next day, the chloroform layer was dried using high purity N2. Concentrated PLFAs were transferred to active silica gel columns using a chloroform solution, and eluted with chloroform, acetone and methanol in turn. The collected methanol eluent after reaction was dried using high purity N2 and then well mixed with a methanol: toluene mixture (volume ratio 1:1) and a 1 ml 0.2 mol L−1 KOH methanol solution. After a water bath treatment, 0.3 mol L−1 acetic acid, 2 ml n-hexane, and 2 ml of pure water were added and the upper solution was extracted after low-speed oscillation for 10 min. The remaining solution was extracted again with 2 ml n-hexane. The upper solutions from the two extractions were mixed and dried using high purity N2 to obtain methylated fatty acid samples. The sample was dissolved in 200 μ n-hexane and analyzed by a MIDI system (MIDI, Inc., Newark, DE) on a gas chromatograph (Hewlett-Packard 6890, Agilent, Santa Clara, CA, USA) with flame ionization detector, using 19:0 methyl ester as the internal standard to determine the content of each PLFA component. According to the fatty acid nomenclature, the results were classified into bacteria, actinobacteria, gram-positive bacteria (G+), gram-negative bacteria (G-), fungi, protozoa, and unknown.
Statistical analysis
We performed separate principal component analyses (PCA; 3 axes, simple correlations; proc. factor; SAS 9.4 for all analyses) to reduce the dimensionality of the soil variables (C, N, P, K, AP, NH4+, NO3−), litter variables (C, N, P, K), PLFA estimated taxa abundances (bacteria, actinobacteria, G-, G+, fungi, protozoa, unknown), and PLFA chemicals (38 PLFAs). Then we used analysis of variance (ANOVAs) to test the dependence of these PCA axes on region (YJF, LS, WGS), forest type (moso bamboo forest, mixed forest with moso bamboo, broadleaf forest, or coniferous forest), and their interaction. We used adjusted means partial difference tests to examine differences among means for significant effects.
We conducted multivariate ANOVAs (MANOVAs; proc. mixed) to test the dependence of soil, litter, PLFA chemical, and PLFA taxonomic variables on region, forest type and their interaction. We conducted follow-up ANOVAs to investigate the variables that were contributing to significant multivariate effects.
In order to identify soil factors (N, P, K, AP, NH4+, NO3−) that influenced litter properties (C, N, P, K), we performed a canonical correspondence analysis (CCA; proc. cancorr). We performed a second CCA to identify how these litter and soil factors influenced the taxonomic composition of the soil community (bacteria, gram negative bacteria, gram positive bacteria, fungi, protozoa, unknown). We used multivariate tests to determine if axes were significantly related, and used individual F-tests to determine the significance of specific axes.
We calculated Hedges g values to examine the effects of moso bamboo invasion on litter, soil, PLFA chemical properties, and PLFA estimated taxa abundances in YJF broadleaf forest, LS coniferous forest, WGS broadleaf forest, and WGS coniferous forest. For each of these four forest types we used the difference between the mean of the moso bamboo forest and the uninvaded forest (along with associated standard deviations) to calculate Hedges g, its associated standard error, and 95% confidence interval (Yang et al. 2015). We considered changes to be significant when the 95% CI did not overlap zero and we considered effects on forests to be different when their 95% CIs did not overlap.
Results
Ordinations
The first PCA axis for soil variables depended on region (YJF < LS < WGS), the second axis depended on region (YJF < WGS < LS) and forest types (coniferous<mixed~bamboo<broadleaf), and the third axis depended on the interaction of region and type (Table 2, Fig. 2a, c). Axis 1 had strongly positive loadings of C, N, P, AP and NO3− and a strongly negative loading of K (Table S1). Axis 2 had a strongly positive NH4+ loading (Table S1). Axis 3 had strongly positive loadings of AP and NO3− and strongly negative C and NH4+ loadings (Table S1).
The dependence of PCA axes for soil properties (C, N, P, K, AP, NH4+, NO3−)(a, c) and litter properties (C, N, P, K)(b, d) on region (YJF = circles, LS = squares, WGS = triangles) and vegetation type (moso bamboo forest = black, broadleaf forest = light grey, coniferous forest = white, mixed forest with moso bamboo = dark grey)
The first and second PCA axes for litter variables depended on region (PCA1: YJF < LS < WGS; PCA2: YJF < WGS~LS) and type (PCA1: coniferous<broadleaf~bamboo~mixed; PCA2: bamboo<mixed~broadleaf<coniferous) and the third axis depended on the interaction of region and type (Table 2, Fig. 2b, d). Axis 1 had strongly positive N, P and K loadings, axis 2 had strongly positive C and P loadings, and axis 3 had strongly positive C and K loadings and strongly negative N and P loadings (Table S2).
The first PCA axis for PLFA taxa depended on the interaction of region and type, the second axis depended on region (LS~WGS < YJF), and the third axis did not depend on any predictors (Table 2, Fig. 3a, c). Axis 1 had positive loadings of all taxa (but loadings were weak for protozoa and unknown taxa), axis 2 had a strongly positive unknown taxa loading, and axis 3 had a strongly positive protozoa loading (Table S3).
The first PCA axis for PLFA chemicals depended on the interaction of region and type (Table 2, Fig. 3b, d), the second axis depended on region (LS < WGS~YJF) and type (coniferous<broadleaf<. mixed~bamboo), and the third axis depended on region (YJF < WGS~LS). All the chemicals had positive loadings on the first axis (especially large for 18:3 ω6c (6,9,12) and i15:1 ω9c), axis 2 has a mix of positive (especially large for 18:1 ω5c and 20:4 ω6,9,12,15c) and negative loadings (especially large for 20:00 and 18:0 2OH), and axis 3 also had a mix of positive (especially large for 16:1 2OH and 18:1 2OH) and negative loadings (especially large for 10Me 19:0, 11Me 18:1 ω7c, and 16:1 ω11c; Table S4).
Multivariate analyses
Soil, litter, PLFA chemical, and PLFA taxonomic variables each depended on region, type and their interaction in their MANOVAs (Table 3). Every soil variable depended on region×type in follow-up ANOVAs except for P which only varied with region. All litter variables depended on region×type except for C which only depended on type. For PLFA types, i:a varied with region and type, cy:pre only depended on region and sat:mono depended on region×type. PLFA taxonomic groups depended on region×type (bacteria, actinobateria, G+), only region (G-, fungi, unknown) or no predictors (protozoa) in the follow-up ANOVAs.
Canonical correspondence analysis
In the litter properties CCA, three axes explained a significant amount of variance (multivariate: P < 0.0001; axis 1: P < 0.0001, 27.94% of variance explained; axis 2: P < 0.0001, 24.83% explained; axis 3: P = 0.0006, 29.45% explained). The first axis, which had positive contributions from soil C (r = +0.87), N (r = +0.79), and P (r = +0.83) and a negative contribution from soil K (r = −0.70), separated YJF and WGS with LS intermediate (Fig. 4a). The second axis, which had negative contributions from soil K (r = −0.63), C (r = −0.39) and N (r = −0.24) and a positive contribution from soil AP (r = +0.17) separated WGS and LS with YJF intermediate (Fig. 4a). The third axis, which had positive loadings from soil AP (r = +0.79) and P (r = +0.38), separated forest types (bamboo>mixed>broadleaf >coniferous; Fig. 4a).
Canonical correspondence analysis (CCA). a. The influence of soil factors on litter properties. b. The influence of soil and litter properties on the soil community. Shape indicates region (YJF = circles, LS = squares, WGS = triangles) and color indicates vegetation type (moso bamboo forest = black, broadleaf forest = light grey, coniferous forest = white, mixed forest with moso bamboo = dark grey). The vectors in B show the contributions of different soil and litter variables. Dotted lines indicate zero
In the PLFA taxa CCA, two axes explained a significant amount of variance (multivariate: P < 0.0001; axis 1: P < 0.0001, 19.49% of variance explained; axis 2: P = 0.0020, 3.90%). The contributions of litter N, litter K and soil AP were opposite to those of soil K and litter C with those of soil C, soil N, soil P, soil NO3− and litter P in an orthogonal direction to these other two groups (Fig. 4b). The first axis separated YJF from LS and WGS and the second axis separated WGS and LS with YJF intermediate (Fig. 4b). Forest types did not appear to differ though some combinations of site and forest type (such as WGS bamboo and LS conifer) were widely separated on axis 2 (Fig. 4b).
Hedges g
Moso bamboo invasion reduced litter C for all forests but reductions were larger for coniferous forests than broadleaf forests (Fig. 5). Moso bamboo invasion reduced litter nitrogen in YJF broadleaf forest but increased it in other forests, especially LS coniferous forest. Moso bamboo invasion increased litter P in both WGS forests but reduced it in YJF broadleaf and LS coniferous forest with the LS effect being larger. Moso bamboo invasion decreased litter K in WGS forests (especially broadleaf) and increased it in YJF broadleaf and LS coniferous forests, with the latter being a larger increase.
The dependence of Hedge’s g (mean and 95% CI) for litter variables, soil variables, PLFA chemical properties, and PLFA estimated taxa abundances on moso bamboo invasion in different forest types (YJF broadleaf forest = grey circles, LS coniferous forest = white squares, WGS broadleaf forest = grey triangles, WGS coniferous forest = white triangles). See Table S5 for PFLA treatment means
Moso bamboo invasions decreased soil C in YJF broadleaf and LS coniferous forests and soil N in LS coniferous forests. Soil P was insensitive to moso bamboo invasion. Soil K was lower with moso bamboo invasion in YJF broadleaf forests but higher for other forests, especially LS coniferous forests. AP was higher with moso bamboo invasion in coniferous forests. NH4+ was decreased by moso bamboo invasion in WGS broadleaf forests but increased in LS coniferous forests. NO3− was decreased by moso bamboo invasion in WGS coniferous forests and increased in LS coniferous forests. Moso bamboo invasion decreased soil AN in WGS forests but increased it in LS coniferous forests. I: a was decreased by moso bamboo invasion in LS coniferous and YJF broadleaf forests. Cy: pre was increased in all but LS coniferous forests. Sat: mono was increased in WGS broadleaf forest and decreased in YJF broadleaf and LS coniferous forests with the latter decrease being larger. Bacteria were more abundant in all forest types except LS coniferous forests in which moso bamboo invasion decreased their abundance. Moso bamboo invasion increased actinobacterial abundance in broadleaf forests. Both G+ and G- bacteria were increased by moso bamboo invasion in all but LS coniferous forests. Moso bamboo invasion increased fungi in WGS forests and protozoa in WGS coniferous forests.
Discussion
In general, soil, litter, PLFA chemical, and PLFA taxonomic variables each depended on region, forest type and their interaction with moso bamboo invasion profoundly affecting litter C, N and P, soil K and AP, and most of the PLFA taxonomic variables (Table 3, Fig. 5). Differences in litter properties among different forest types reflected strong effects of soil AP and P but variation among regions was also related to soil K (Fig. 4). Soil P, litter C and litter N contributed the most to the differentiation of PLFA taxa across different regions but not forest types (Fig. 4b).
Moso bamboo invasion increased litter N in coniferous forests and litter P in WGS (Fig. 5), which may in turn facilitate N and P cycling as has been reported for other invasive species (Zhang et al. 2014a, b, 2016; Wang et al. 2016b). Moso bamboo invasion also reduced litter C in both invaded forest types (Fig. 5), which was consistent with previous studies on moso bamboo (Song et al. 2016) and other invasive species (Zhang et al. 2014a, b, 2016). In combination, the increase and decrease in litter C and N after moso bamboo invasions into coniferous forests (Fig. 5), respectively, indicated that moso bamboo invasions have profoundly altered litter stoichiometric characteristics (Song et al. 2016), such as C:N ratio. Litter C:N ratio has been widely used as an index of litter quality controlling litter decomposition rate (Zhang et al. 2014b, 2016, 2017). The decreased C:N ratio following moso bamboo invasions we found here indicated potentially increased litter decomposition rate and hence litter N and P release in the invaded coniferous forests. Moreover, increased leaf N and P content will generally increase total primary productivity (Tang et al. 2018), especially species that are invading divergent ecosystems (Eppinga et al. 2011; Zhang et al. 2016; Wu et al. 2018b). Therefore, the increased litter N and P following moso bamboo invasions in WGS suggest potentially increased primary productivity, which would increase soil organic matter input (Song et al. 2014a).
Moso bamboo invasion effects on litter P and K depended more on site than invaded forest type (Table 3, Fig. 5). However, litter P and K showed opposite responses to moso bamboo invasion across sites and forest types (Table 3, Fig. 5), indicating moso bamboo invasion interacted with sites or forest types impacting litter characteristics. The increased litter P in the WGS area, which could be related to the higher P mineralization ability of phosphate-solubilizing fungi inhabiting moso bamboo rhizosphere soil (Zhang et al. 2018a, b), would positively impact P cycling rates in forests invaded by moso bamboo. In addition, the larger increase in litter P of coniferous forests was consistent with the corresponding increase in soil AP (Fig. 5), which was also in accordance with the larger loading by soil AP in its effects on litter properties across forest types (Fig. 4a). As a feedback, litter P will be returned to soil with accelerated litter decomposition, potentially providing more readily available P for newly sprouted moso bamboo. On the other hand, K plays an important role in regulating plant photosynthesis and metabolism (Oosterhuis et al. 2014). The decreased litter K in WGS invaded forests suggested substantial alteration in plant physiological traits between original species and moso bamboo (Fig. 5). Similarly, the decrease in litter N and P content and the increase in litter K at YJF (Fig. 5) might be caused by species differences or the ecological variations in protein synthesis rate (Mathers et al. 1993; Güsewell 2004). Moreover, the increased litter K at LS and YJF (Fig. 5) could stimulate the release of root exudates and soil enzyme activity (Ali et al. 2018) or even soil microbial activities across region or forest types (Table 3, Fig. 4b).
Changes in litter C and other nutrients caused by moso bamboo invasion would directly affect soil physiochemical properties. Soil organic C and N are highly dependent on the physical and chemical properties and composition of forest litter. In general, moso bamboo litter contains more O-alkyl-C (Li et al. 2017) but less lignin, which is easier to decompose than litter produced in coniferous forests, resulting in relatively faster C cycling and hence SOC reduction in moso bamboo forests (Wang et al. 2016a). The reduction of SOC content caused by moso bamboo invasion is one of its adverse effects on invaded ecosystems (Bai et al. 2016), as observed at LS and YJF as well as in previous studies (Table 4, Fig. 5).
As the main source of soil organic matter, litter input impacts N transformation (Guan et al. 2015; Song et al. 2016). Both ammonium N and nitrate N were increased by bamboo invasions into LS coniferous forest, while those at WGS decreased (Fig. 5). Moso bamboo invasions into broadleaf forest inhibit N mineralization and alter total soil N transformations (ammonification and nitrification)(Song et al. 2016), but complete moso bamboo invasion leads to increased soil AN (all studies in Table 4), which is consistent with the results found at LS, indicating substantial contribution of litter input to soil N cycling in these invasions. Because N transformations were not included in this study, it is not clear whether ammonification or nitrification has been altered by moso bamboo invasions. However, the inconsistent response of AN to moso bamboo invasions found here indicated the effects were both site and forest type dependent. More studies that include these variables would be necessary to understand how N transformations are affected by moso bamboo invasions.
Soil AP in coniferous forest was consistently increased following moso bamboo invasions (Table 3, Fig. 5), and contributed substantially to soil effects on litter properties across different forest types (Fig. 4a). Because decomposition of organic matter provides substantial quantities of P in soil (Peng et al. 2018), the higher AP might have been induced by easily decomposed litter. Indeed, moso bamboo invasion consistently decreased and increased litter C and N in coniferous forests (Tables 3 and 4, Fig. 5), respectively, which would increase litter decomposition rate (Wall et al. 2008). Other possible reasons including increased phosphatase activity (Wu et al. 2018a) and hosting of phosphate-solubilizing fungi (Zhang et al. 2018b) by moso bamboo, could also accelerate the rate of P mineralization, resulting in an increase in soil AP. The increased soil K in invaded forests at WGS and LS would impose positive effects on moso bamboo invasion. As one of the most important elements associated with plant photosynthesis (Oosterhuis et al. 2014), K can increase plant uptake of N and P, activate enzyme activities, promote organic acid metabolism, and enhance plant resistance (Oosterhuis et al. 2014; Ali et al. 2018).
Changes in SOC and other soil nutrients would further affect soil PLFA compositions (Grayston and Prescott 2005; Frey et al. 2008). Three fatty acids ratios, including cyclopropane fatty acid to its precursor fatty acid, isomeric PLFAs to anteiso PLFAs, and saturated fatty acids to monounsaturated fat have been used as indicators of environmental stress (Bossio et al. 1998; Hedlund 2002; McKinley et al. 2005). The higher cy:pre in invaded WGS and YJF forests suggests increased environmental stress with moso bamboo invasion (Table 3, Fig. 5), which may adversely impact the persistence of original plants. However, both i:a and sat:mono were lower in LS coniferous and YJF broadleaf forests (Fig. 5), indicating decreased environmental stress levels in these two invaded regions. In addition, PLFA taxonomic composition also showed substantial region variations (Table 3), which could further be explained by the first two axes of the CCA examining effects of both soil and litter properties on soil microbial communities (Fig. 4b). Hence, invading region could be an important factor regulating ecological effects of moso bamboo invasions (Meiners and Cadenasso 2001; Vila et al. 2006)(Table 4), which should be considered in management of moso bamboo and other invasive species (Lin et al. 2014; Chang and Chiu 2015; Wang et al. 2016a; Shiau and Chiu 2017).
Moreover, changes in overall bacterial and fungal PLFAs following moso bamboo invasion also showed substantial variation with sites and forest types (Figs. 4b and 5). Chang and Chiu (2015) reported that moso bamboo invasion negatively impacted bacterial activity in a coniferous forest, leading to a decrease in soil bacteria, which is consistent with changes in bacterial PLFAs in LS. Interestingly, while moso bamboo invaded coniferous forest at WGS slightly increased bacterial PLFAs, invaded forests at LS and those studied by Chang and Chiu (2015) were both dominated by the same coniferous tree species, Cryptomeria japonica, indicating the consistent results could be associated with original tree species in the invaded forests and not indicative of coniferous forests in general. Similarly, studies on Solidago canadensis invasions into Phragmites australis and Citrus madurensis reported different effects on soil N cycling rate, suggesting ecological effects by invasive species could be influenced by original dominant species of the invaded ecosystems (Zhang et al. 2009, 2014b), which could be another factor influencing ecological effects of plant invasions.
Soil fungal communities are vital in soil organic matter decomposition and plant nutrient uptake, and their diversity increases with the ratio of degradable C to SOC (Qin et al. 2017; Li et al. 2017). The increase in soil fungal PLFAs of WGS forests is consistent with the positive effects of moso bamboo (Qin et al. 2017) and spotted knapweed (Centaurea stoebe)(Harner et al. 2010) invasions on AMF biomass, which would promote soil aggregate formation and positively affect soil C pools and might have been induced by the relatively higher SOC at WGS compared with that at LS and YJF (Fig. 5). Increased soil fungi could enhance soil fertility and P availability (Zhang et al. 2018b), and hence further invasion by moso bamboo. Therefore, in areas with serious moso bamboo invasions, alterations in soil fungi may impact the management of invading bamboo.
The consistent increase in PLFAs of G+, G-, and actinobacteria in broadleaf forests invaded by moso bamboo (Fig. 5) suggested similar changes within broadleaf forests experiencing moso bamboo invasions. Indeed, while Chang and Chiu (2015) proposed that changes in G+ and G- content related to SOC, which is in accordance with the decrease in G+ in LS, actinobacteria have been shown to decompose complex organic matter, and improve soil quality by stimulating the formation of soil aggregates (Frey et al. 2008; Okoro et al. 2009). Therefore, more substrate preferred by actinobacteria could be expected in moso bamboo invaded broadleaf forests, and the soil quality would be much more improved by moso bamboo invasions, which might also enhance further invasion of moso bamboo.
Conclusions
Moso bamboo invasions substantially altered litter, soil, and microbial characteristics, but the responses of most variables depended on site and forest type. Moso bamboo invasion consistently decreased litter C, but increased litter N and soil AP in coniferous forests, and PLFAs of total bacteria, actinobacteria, G+, and G- in broadleaf forests. While soil AP and P contributed the most to variations in litter properties across forest types, soil and litter property effects on soil microbial communities did not show obvious variation in forest type combined with site. Moso bamboo invasion effects on litter, soil, and microbial communities are highly dependent on original forest type. Future studies determining the overall effects of invasions on ecological variables or element cycling process are still needed to understand the variability among invaded communities and the characteristics that underlie those variable effects.
References
Administration SF (2014) Report for Chinese forest resource—the 8th national forest inventory. China Forestry Publishing House
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Ali S, Hafeez A, Ma X, Tung SA, Liu A, Shah AN, Chattha MS, Zhang Z, Yang G (2018) Potassium relative ratio to nitrogen considerably favors carbon metabolism in late-planted cotton at high planting density. Field Crop Res 223:48–56
Bai S, Conant RT, Zhou G, Wang Y, Wang N, Li Y, Zhang K (2016) Effects of moso bamboo encroachment into native, broad-leaved forests on soil carbon and nitrogen pools. Sci Rep 6:31480
Batten KM, Scow KM, Espeland EK (2008) Soil microbial community associated with an invasive grass differentially impacts native plant performance. Microb Ecol 55:220–228
Bossio DA, Scow KM, Gunapala N, Graham KJ (1998) Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microb Ecol 36:1–12
Carrillo Y, Ball BA, Strickland MS, Bradford MA (2012) Legacies of plant litter on carbon and nitrogen dynamics and the role of the soil community. Pedobiologia 55:185–192
Chang E, Chiu C (2015) Changes in soil microbial community structure and activity in a cedar plantation invaded by moso bamboo. Appl Soil Ecol 91:1–7
David J, Ponge J, Arpin P, Vannier G, David J, Ponge J (1991) Reactions of the macrofauna of a forest mull to experimental perturbations of litter supply. Oikos 61:316–326
Eppinga MB, Kaproth MA, Collins AR, Molofsky J (2011) Litter feedbacks, evolutionary change and exotic plant invasion. J Ecol 99:503–514
Feng J, Wu J, Zhang Q, Zhang D, Li Q, Long C, Yang F, Chen Q, Cheng X (2018) Stimulation of nitrogen-hydrolyzing enzymes in soil aggregates mitigates nitrogen constraint for carbon sequestration following afforestation in subtropical China. Soil Biol Biochem 123:136–144
Frey SD, Drijber R, Smith H, Melillo J (2008) Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol Biochem 40:2904–2907
Grayston SJ, Prescott CE (2005) Microbial communities in forest floors under four tree species in coastal British Columbia. Soil Biol Biochem 37:1157–1167
Guan F, Tang X, Fan S, Zhao J, Peng C (2015) Changes in soil carbon and nitrogen stocks followed the conversion from secondary forest to Chinese fir and Moso bamboo plantations. Catena 133:455–460
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Harner MJ, Mummey DL, Stanford JA, Rillig MC (2010) Arbuscular mycorrhizal fungi enhance spotted knapweed growth across a riparian chronosequence. Biol Invasions 12:1481–1490
Hedlund K (2002) Soil microbial community structure in relation to vegetation management on former agricultural land. Soil Biol Biochem 34:1299–1307
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Li X, Yin X, Wang Z, Fan W (2015) Litter mass loss and nutrient release influenced by soil fauna of Betula ermanii forest floor of the Changbai Mountains, China. Appl Soil Ecol 95:15–22
Li Y, Liang X, Li Y, Wang Q, Chen J, Xu Q (2016) Effects of Phyllostachys edulis invasion of native broadleaf forest on soil fungal community. Chinese J Appl Ecol 02:585–592
Li Y, Li Y, Chang SX, Xu Q, Guo Z, Gao Q, Qin Z, Yang Y, Chen J, Liang X (2017) Bamboo invasion of broadleaf forests altered soil fungal community closely linked to changes in soil organic C chemical composition and mineral N production. Plant Soil 418:507–521
Li Y, Liang X, Tang C, Li Y, Chen Z, Chang SX, Guo Z, Shen Y, Xu Q (2018) Moso bamboo invasion into broadleaf forests is associated with greater abundance and activity of soil autotrophic bacteria. Plant Soil 428:163–177
Lin Y, Tang S, Pai C, Whitman WB, Coleman DC, Chiu C (2014) Changes in the soil bacterial communities in a cedar plantation invaded by Moso bamboo. Microb Ecol 67:421–429
Lovett GM, Weathers KC, Arthur MA, Schultz JC (2004) Nitrogen cycling in a northern hardwood forest: do species matter? Biogeochemistry 67:289–308
Lu R (2000) Methods of soil and agro-chemical analysis. China Agricultural Science &Technology Press, Beijing (in Chinese)
Lucas-Borja ME, Candel D, Jindo K, Moreno JL, Andrés M, Bastida F (2012) Soil microbial community structure and activity in monospecific and mixed forest stands, under Mediterranean humid conditions. Plant Soil 354:359–370
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80:89–106
Mathers EM, Houlihan DF, McCarthy ID, Burren LJ (1993) Rates of growth and protein synthesis correlated with nucleic acid content in fry of rainbow trout, Oncorhynchus mykiss: effects of age and temperature. J Fish Biol 43:245–263
McKinley VL, Peacock AD, White DC (2005) Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils. Soil Biol Biochem 37:1946–1958
Meiners SJ, Cadenasso ML (2001) Effects of plant invasions on the species richness of abandoned agricultural land. Ecography 24:633–644
Merilä P, Malmivaara-Lämsä M, Spetz P, Stark S, Vierikko K, Derome J, Fritze H (2010) Soil organic matter quality as a link between microbial community structure and vegetation composition along a successional gradient in a boreal forest. Appl Soil Ecol 46:259–267
Okoro CK, Brown R, Jones AL, Andrews BA, Asenjo JA, Goodfellow M, Bull AT (2009) Diversity of culturable actinomycetes in hyper-arid soils of the Atacama Desert, Chile. Antonie Van Leeuwenhoek 95:121–133
Oosterhuis DM, Loka DA, Kawakami EM, Pettigrew WT (2014) The physiology of potassium in crop production. Adv Agron 126:203–233
Pausch J, Kuzyakov Y (2012) Soil organic carbon decomposition from recently added and older sources estimated by δ13C values of CO2 and organic matter. Soil Biol Biochem 55:40–47
Peng Y, Yang W, Yue K, Tan B, Huang C, Xu Z, Ni X, Zhang L, Wu F (2018) Temporal dynamics of phosphorus during aquatic and terrestrial litter decomposition in an alpine forest. Sci Total Environ 642:832–841
Qin H, Niu L, Wu Q, Chen J, Li Y, Liang C, Xu QF, Fuhrmann JJ, Shen Y (2017) Bamboo forest expansion increases soil organic carbon through its effect on soil arbuscular mycorrhizal fungal community and abundance. Plant Soil 420:407–421
Rubino M, Dungait JAJ, Evershed RP, Bertolini T, De Angelis P, D Onofrio A, Lagomarsino A, Lubritto C et al (2010) Carbon input belowground is the major C flux contributing to leaf litter mass loss: evidences from a 13C labelled-leaf litter experiment. Soil Biol Biochem 42:1009–1016
Sayer EJ, Sutcliffe LME, Ross RIC, Tanner AEVJ (2009) Arthropod abundance and diversity in a lowland tropical forest floor in Panama: the role of habitat space vs. nutrient concentrations. Biotropica 2:194–200
Shiau Y, Chiu C (2017) Changes in soil biochemical properties in a cedar plantation invaded by moso bamboo. Forests 8:222
Song XZ, Zhang H, Jiang H, Peng C (2014a) Combination of nitrogen deposition and ultraviolet-B radiation decreased litter decomposition in subtropical China. Plant Soil 380:349–359
Song XZ, Peng C, Zhao Z, Zhang Z, Guo B, Wang W, Jiang H, Zhu Q (2014b) Quantification of soil respiration in forest ecosystems across China. Atmos Environ 94:546–551
Song XZ, Zhou G, Gu H, Qi L (2015) Management practices amplify the effects of N deposition on leaf litter decomposition of the Moso bamboo forest. Plant Soil 395:391–400
Song Q, Yang Q, Liu J, Yu D, Fang K, Xu P, He Y (2013) Effects of Phyllostachys edulis expansion on soil nitrogen mineralization and its availability in evergreen broadleaf forest. Chinese J Appl Ecol 02:338–344
Song Q, Ouyang M, Yang Q, Lu H, Yang G, Chen F, Shi J (2016) Degradation of litter quality and decline of soil nitrogen mineralization after moso bamboo (Phyllostachys pubscens) expansion to neighboring broadleaved forest in subtropical China. Plant Soil 404:113–124
Suthar S, Gairola S (2014) Nutrient recovery from urban forest leaf litter waste solids using Eisenia fetida. Ecol Eng 71:660–666
Suzuki S, Nakagoshi N (2008) Expansion of bamboo forests caused by reduced bamboo-shoot harvest under different natural and artificial conditions. Ecol Res 23:641–647
Tang Z, Xu W, Zhou G, Bai Y, Li J, Tang X, Chen D, Liu Q, Ma W, Xiong G, He H, He N, Guo Y, Guo Q, Zhu J, Han W, Hu H, Fang J, Xie Z (2018) Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China’s terrestrial ecosystems. Proc Natl Acad Sci U S A 115:4033–4038
Vila M, Tessier M, Suehs CM, Brundu G, Carta L, Galanidis A, Lambdon P, Manca M, Medail F, Moragues E, Traveset A, Troumbis AY, Hulme PE (2006) Local and regional assessments of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. J Biogeogr 33:853–861
Waid J Saville (1999) Does soil biodiversity depend upon metabiotic activity and influences? Appl Soil Ecol 13:151–158
Wall DH, Bradford MA, St. John MG, Trofymow JA, Behan-Pelletier V, Bignell DE, Dangerfield JM, Parton WJ et al (2008) Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob Chang Biol:2661–2677
Wang Q, Xu Q, Jiang P, Qin H (2009) DGGE analysis of PCR of 16S rDNA V3 fragments of soil bacteria community in soil under natural broadleaf forest invaded by Phyllostachy pubescens in Tianmu mountain nature reserve. Acta Pedologica Sinica 04:662–669
Wang H, Tian G, Chiu C (2016a) Invasion of moso bamboo into a Japanese cedar plantation affects the chemical composition and humification of soil organic matter. Sci Rep 6:32211
Wang W, Sardans J, Zeng C, Tong C, Wang C, Peñuelas J (2016b) Impact of plant invasion and increasing floods on total soil phosphorus and its fractions in the Minjiang river estuarine wetlands, China. Wetlands 36:21–36
Wang X, Sasaki A, Toda M, Nakatsubo T (2016c) Changes in soil microbial community and activity in warm temperate forests invaded by moso bamboo (Phyllostachys pubescens). J For Res 21:235–243
Wardle DA, Yeates GW, Barker GM, Bonner KI (2006) The influence of plant litter diversity on decomposer abundance and diversity. Soil Biol Biochem 38:1052–1062
Wu J, Jiang P, Wang Z (2008) The effects of Phyllostachys pubescens expansion on soil fertility in national nature reserve of mount Tianmu. Acta Agriculturae Universitatis Jiangxiensis 04:689–692
Wu D, Li T, Wan S (2013) Time and litter species composition affect litter-mixing effects on decomposition rates. Plant Soil 371:355–366
Wu C, Mo Q, Wang H, Zhang Z, Huang G, Ye Q, Zou Q, Kong F et al (2018a) Moso bamboo (Phyllostachys edulis (Carriere) J. Houzeau) invasion affects soil phosphorus dynamics in adjacent coniferous forests in subtropical China. Ann Forest Sci 75:24
Wu A, Liu J, He F, Wang Y, Zhang X, Duan X, Liu Y, Qian Z (2018b) Negative relationship between diversity and productivity under plant invasion. Ecol Res 33:949–957
Xu Q, Jiang P, Wu J, Zhou G, Shen R, Fuhrmann JJ (2015) Bamboo invasion of native broadleaf forest modified soil microbial communities and diversity. Biol Invasions 17:433–444
Yang Y, Luo Y, Finzi AC (2011) Carbon and nitrogen dynamics during forest stand development: a global synthesis. New Phytol 190:977–989
Yang H, Zhang Q, Dai Y, Liu Q, Tang J, Bian X, Chen X (2015) Effects of arbuscular mycorrhizal fungi on plant growth depend on root system: a meta-analysis. Plant Soil 389:361–374
Zhang CB, Wang J, Qian BY, Li WH (2009) Effects of the invader Solidago canadensis on soil properties. Appl Soil Ecol 43:163–169
Zhang L, Wang H, Zou J, Rogers WE, Siemann E (2014a) Non-native plant litter enhances soil carbon dioxide emissions in an invaded annual grassland. PLoS One 9:e92301
Zhang L, Zhang Y, Zou J, Siemann E (2014b) Decomposition of Phragmites australis litter retarded by invasive Solidago canadensis in mixtures: an antagonistic non-additive effect. Sci Rep 4:5488
Zhang L, Ma X, Wang H, Liu S, Siemann E, Zou J (2016) Soil respiration and litter decomposition increased following perennial forb invasion into an annual grassland. Pedosphere 26:567–576
Zhang L, Zou J, Siemann E (2017) Interactive effects of elevated CO2 and nitrogen deposition accelerate litter decomposition cycles of invasive tree (Triadica sebifera). Forest Ecol Manag 385:189–197
Zhang Y, Chen F, Wu X, Luan F, Zhang L, Fang X, Wan S, Hu X, Ye J (2018a) Isolation and characterization of two phosphate-solubilizing fungi from rhizosphere soil of moso bamboo and their functional capacities when exposed to different phosphorus sources and pH environments. PLoS One 13(7):e0199625
Zhang Q, Xiong G, Li J, Lu Z, Li Y, Xu W, Wang Y, Zhao C, Tang Z, Xie Z (2018b) Nitrogen and phosphorus concentrations and allocation strategies among shrub organs: the effects of plant growth forms and nitrogen-fixation types. Plant Soil 427:305–319
Zhao Y, Fan S, Luo J (2017) The influence of Phyllostachys edulis expanding into evergreen broadleaf forest on soil property and its related analysis. Forest Research 02:354–359
Acknowledgements
This project was funded by the National Natural Science Foundation of China (31560203, 31560204, 41501317), and China Postdoctoral Science Foundation (2017 M612153). We acknowledge assistance by Hanrui Ji, Meiling Feng, Miao Dou and Bangliang Deng in the field and laboratory work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Cindy Prescott.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Liu, X., Siemann, E., Cui, C. et al. Moso bamboo (Phyllostachys edulis) invasion effects on litter, soil and microbial PLFA characteristics depend on sites and invaded forests. Plant Soil 438, 85–99 (2019). https://doi.org/10.1007/s11104-019-04010-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04010-3