Abstract
Background and aims
Elevated CO2 (eCO2) alters plant rhizosphere processes and soil microbial properties which in turn change the decomposition of native soil organic carbon (SOC), the process termed rhizosphere priming effect (RPE). This study examined the effect of eCO2 on RPEs of plant species contrasting in root system and exudation.
Methods
Two C3 species, wheat (Triticum aestivum L. cv. Yitpi) and white lupin (Lupinus albus L. cv. Kiev), were grown under ambient CO2 (400 ± 15 μmol mol−1) and eCO2 (800 ± 30 μmol mol−1) for 34 and 62 days in a C4 soil. The amounts of CO2 derived from SOC and plant roots were quantified.
Results
Elevated CO2 increased the RPEs of white lupin by 78% and 47% at days 34 and 62, respectively. It increased microbial respiration (63%) and biomass carbon (43%) in the rhizosphere soil of white lupin at Day 62. In contrast, eCO2 decreased wheat RPE by 22% and did not affect rhizosphere soil respiration and microbial biomass carbon at Day 62. Moreover, eCO2 increased the concentration of soluble organic carbon in the rhizosphere of white lupin but not wheat.
Conclusions
The enhanced RPE of white lupin but not wheat under eCO2 had resulted from an increase in root exudation of white lupin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The CO2 concentration in the atmosphere is expected to reach 600–800 μmol mol−1 by the end of this century (Meinshausen et al. 2011). High atmospheric CO2 concentration stimulates plant photosynthesis and enhances photosynthetic inputs into below-ground via rhizodeposition (Pendall et al. 2004; Paterson et al. 2008; De Graaff et al. 2009; Phillips et al. 2011), which provides labile substrates to relative C-limited soil microorganisms to degrade indigenous soil organic carbon (SOC) (Van Groenigen et al. 2014). This rhizodeposition-induced change in the decomposition of SOC is termed rhizosphere priming effect (RPE) (Cheng et al. 2014).
The contribution of root exudates accounts for 58–95% of the observed RPEs (Shahzad et al. 2015). Therefore, any changes in root exudation by elevated CO2 (eCO2) would yield differences in RPEs. Greater RPEs are anticipated under eCO2 by comparison to aCO2 due to an increased input of easily decomposable C. From another perspective, eCO2 changes the chemical composition of plants to more production of carbon-based secondary compounds (e.g. phenolics) (Peñuelas et al. 1996, 1997). The increases of such substances may facilitate soil C sequestration because they are biologically resistant to microbial decomposition. If eCO2 alters the chemical composition of root exudates, RPEs are expected to change under eCO2. Furthermore, eCO2 enhances biological N2 fixation in legumes, which in turn provides extra N for these plants (Hartwig and Sadowsky 2006; Lam et al. 2012; Butterly et al. 2016; Tobita et al. 2016). As a result, eCO2 may affect the N status of legumes and cereals differently, which in turn affects the chemical composition of their root exudates and hence RPEs.
Except for the changes in the amount and quality of root exudates, eCO2 could also alter RPEs via its influences on soil variables such as soil N availability and pH. For example, eCO2 has been reported to decrease soil N availability, mainly in the form of nitrate (NO3 −) (Hovenden et al. 2008) possibly by increasing microbial immobilisation and reducing the rate of gross N mineralisation (Berntson and Bazzaz 1997; Hungate et al. 1999). Stimulated labile C inputs and lower soil N availability under eCO2 can increase SOC mineralisation because soil microorganisms utilize labile substrates as energy to acquire N from more stable soil organic matter (the so-called ‘microbial N mining’ theory) (Craine et al. 2007). Moreover, pH is a dominant factor affecting soil microbial activities, community structures and functions, such as SOC decomposition (Blagodatskaya and Kuzyakov 2008). Elevated CO2 could change rhizosphere soil pH by affecting cation-anion uptake of plant roots (Guo et al. 2012) and through this way the RPEs would also be changed by eCO2.
Plant species differ naturally in their releasing patterns of root exudates. For example, some legume plants (e.g. white lupin) exude large amounts of low-molecular-weight organic anions (e.g. carboxylates) into their rhizospheres, especially when soil P is limited (Veneklaas et al. 2003). In contrast, cereal plants (e.g. wheat) release extremely low amounts of root exudates (Weisskopf et al. 2008). The release of root exudates is also controlled by plant developmental stages. For instance, white lupin secretes small amounts of organic acids (mainly malate) at early stages but exudes larger amounts of citrate at the mature stage (Sugiyama and Yazaki 2012). The changes in the composition and amounts of root exudates could drive distinct microbial growth and function as well as their decomposition of SOC. Besides, root architecture could be another plant trait that affects RPE (Bardgett et al. 2014). Cereal species such as wheat have longer and finer roots which can explore larger volumes of soil than legumes (e.g. white lupin). This possibly means more SOC in wheat rhizosphere are subjected to microbial decomposition than in white lupin rhizosphere (Weisskopf et al. 2008). However, it is unknown whether eCO2 alters RPE via its effect on root growth traits or exudation.
The objectives of this study were 1) to examine the effect of eCO2 on RPE and 2) to compare the effects of cereal and legume species on RPEs under eCO2. Wheat and white lupin were chosen as test plants. They represent common cereal and leguminous crop species, respectively, and differ substantially in root exudation and root morphology. We hypothesized that 1) eCO2 would stimulate the RPEs due to an increase in root exudation; 2) white lupin would have greater RPE stimulation than wheat under eCO2 due to its higher quantity and quality of root exudates.
Materials and methods
Surface soil (0–10 cm) was collected from a native C4 kangaroo grassland at Merotherie, New South Wales, Australia (32°11′ S, 149°33′ E). The site had been dominated by C4 kangaroo grasses (Themeda triandra) for more than 150 years. After collection, the soil was air-dried and sieved to pass a 2-mm mesh. The basic properties of the soil were: SOC 28 mg g−1, total N 1.6 mg g−1, pH (0.01 M CaCl2) 5.0, clay 130 mg g−1, and δ13C −19.3‰.
A column experiment was carried out in four growth cabinets (Fitotron SGC 120, Loughborough, Leicestershire, UK). It consisted of two CO2 concentration levels, two plant species and three replicates for the first harvest and four replicates for the second harvest. Two growth cabinets were supplied with ambient CO2 concentration (aCO2, 400 ± 15 μmol mol−1) and another two with elevated CO2 concentration (eCO2, 800 ± 30 μmol mol−1). Pure CO2 (Coregas, Yennora, New South Wales, Australia) was pumped into the growth cabinets and mixed with the air inside to get the desired CO2 concentrations. The concentrations of CO2 inside the growth cabinets were also monitored throughout the experiment using portable carbon-dioxide analysers (Extech SD800, Nashua, New Hampshire, USA). To eliminate possible effects of below-ground CO2 on CO2 partitioning, the growth cabinets were fluxed with fresh air and re-supplied with pure CO2 daily. Temperatures were controlled at 22 °C day and 18 °C night with a day length of 14 h. Relative humidity was maintained at 70%. The photosynthetic active photon flux density at the top of the canopy was approximately 400 μmol m−2 s−1. The plant species were wheat (Triticum aestivum L. cv. Yitpi) and white lupin (Lupinus albus L. cv. Kiev).
Growing system
Plants were grown in polyvinyl chloride (PVC) columns (diameter 7.5 cm, height 40 cm). Each column was bottom-capped and had an air-inlet and an air-outlet. To prevent anaerobic conditions and to facilitate CO2 trapping, a pouch of 300 g plastic beads was packed at the bottom of each column before packing into 1.24 kg of air-dried soil. The soil was mixed with basal nutrients at the following rates (mg kg−1): CO(NH2)2, 64.3; KH2PO4, 180; CaCl2·2H2O, 180; K2SO4, 120; MgSO4·7H2O, 50; MnSO4·H2O, 15; ZnSO4·7H2O, 9; CuSO4·5H2O, 6; Na2MoO4·2H2O, 0.4; FeEDTA, 5.5.
Prior to planting, plant seeds were pre-soaked in Milli-Q water and germinated at 25 °C for 48 h. Eight pre-germinated seeds of wheat and four of white lupin were sown in a row into each column and seedlings were thinned to four for wheat and two for white lupin plants one week after emergence. Urea was applied at the rate of 30 mg N kg−1 soil at a one-week interval from the fourth week. An additional set of unplanted columns was used as controls without urea application. The planted and control columns were transferred into growth cabinets. Soil water content was maintained at 80% field capacity by adding reverse-osmosis water daily based on weight loss. The soil columns were randomly reallocated between two replicated growth cabinets weekly to ensure homogenous growing conditions. Plants were harvested at 34 (3 replicates) and 62 (4 replicates) days after planting, representing the early vegetative and booting stages for wheat and the early vegetative and flowering stages for white lupin, respectively.
Below-ground CO2 trapping
CO2 trapping was conducted to quantify below-ground CO2 released from soil columns before each harvest but 3–4 days after urea application. To form an air-tight entirety, the top of each column was sealed with two PVC plates around plant stems, using Blu-Tack (Bostik, Thomastown, Victoria, Australia). The integrity of the seal was tested by pumping CO2-free air through the column and observing the bubbles produced in NaOH solution. Before each trapping, CO2-free air was pumped through the columns for 30 min to remove the initial CO2. Total below-ground CO2 was trapped for two days in 150 ml of 0.3 M NaOH solution for 30 min between 9:00 and 23:00 and three times per day (6-h intervals). To accelerate gas movement, a vacuum was attached at the end of the trapping apparatus. More details can be found in Wang et al. (2016). Total CO2 trapped was determined by titrating the excessive NaOH solution with 0.25 M HCl using the phenolphthalein indicator after precipitation of the carbonate with 0.6 M BaCl2. Another subsample of the trapping solution was mixed with excessive SrCl2 (0.6 M) solution to get SrCO3 precipitates (Cheng et al. 1998). To prevent the formation of Sr(OH)2 and to minimize the contamination of atmospheric CO2, 0.25 M HCl was added drop by drop to neutralize the pH of the suspension. The precipitates were washed three times with Milli-Q water, dried in an oven at 60 °C and analysed for δ13C using an isotope mass spectrometer (Sercon 20–22, Gateway, Crewe, UK).
The following equations were used to separate total below-ground CO2 efflux (Ctotal) into SOC-derived CO2 (Csoil) and root-derived CO2 (Croot) (Cheng 1996):
where δ13Croot is the δ13C value of roots. δ13Ctotal is the δ13C value of total below-ground CO2 efflux (Ctotal) from planted columns. δ13Csoil is the mean δ13C value of CO2 derived from SOC in unplanted treatments (Ccontrol) which was −14.91‰. f is the contribution of SOC decomposition to total below-ground CO2 efflux. In this study, the 13C abundance of roots other than the δ13C value of root-derived CO2 was used to calculate RPE. This might lead to bias in absolute rhizosphere priming effect as isotopic fractionation might occur during microbial utilisation of root-derived labile C (Werth and Kuzyakov 2010), but this should not affect the treatment difference.
Plant and soil analysis
Plants were destructively harvested after each CO2 trapping. Shoots were cut at the soil surface and roots were collected by hand-picking using a 2-mm sieve. Roots were washed and scanned with an EPSON EU-35 scanner (Seiko Epson Corp., Suwa, Japan). The root images were then analysed using WinRHIZO Pro 2003b (Regent Instruments, Quebec City, Canada). Shoot and root materials were oven-dried at 70 °C for 48 h and weighed.
The soil attached to the root was collected and defined as rhizosphere soil. The soil samples were sieved to less than 2 mm and divided into two parts, with one part being stored at −4 °C when necessary for the analysis of microbial biomass C (MBC) and rhizosphere extractable inorganic N, and the other being air-dried for chemical measurements.
Rhizosphere soil respiration was measured as cumulative microbial respiration from a laboratory incubation (Wang et al. 2016). Briefly, 8 g of rhizosphere soil was incubated in a 1-L Mason jar at 25 °C for 14 h. The amount of CO2 released during this period was measured using an infrared gas analyser (Servomex 4210, Crowborough, East Sussex, UK).
The chloroform-fumigation extraction method was adopted to measure MBC. Briefly, a subsample of 8 g fresh rhizosphere soil was extracted with 40 ml of 0.5 M K2SO4 solution. Another subsample of soil was fumigated with chloroform for 24 h in dark, and subsequently extracted with 0.5 M K2SO4 solution. Fumigated and non-fumigated extracts were digested and analysed for total organic C using a Varian Cary 50 Bio UV-Visible Spectrophotometer (Agilent Technologies, Santa Clara, California, USA). The MBC was calculated as the differences in total organic C between the fumigated and non-fumigated extracts with a conversion factor of 0.45 (Vance et al. 1987). Soil extracts from non-fumigated samples were also analysed for NH4 + and NOx − (NO2 − and NO3 −) using a Lachat’s QuikChem 8500 Series 2 Flow Injection Analysis System (Lachat Instruments, Loveland, Colorado, USA).
Oven-dried shoot and root samples were ball-milled and analysed by a Sercon 20–22 Isotope Ratio Mass Spectrometer (Sercon, Gateway, Crewe, UK) for δ13C abundance. A CHNS/O analyser (PerkinElmer EA2400, Shelton, Connecticut, USA) was used to determine total C and N contents in all soil and plant samples.
Statistical analysis
The effects of CO2 levels, species and their interaction were assessed at each harvest using a two-way ANOVA with a block design. Differences between means were tested using Duncan’s multiple range test at P = 0.05. The ANOVA was conducted using Genstat (v17; VSN International, Hemel Hempstead, UK).
Results
Plant growth
While wheat had greater biomass than white lupin, eCO2 tended to increase the shoot and root biomass of both species at the first harvest (Table 1). At the second harvest, the two plant species differed in their responses to CO2 treatment. Elevated CO2 increased the shoot and root biomass of white lupin by 36% and 80%, respectively, but it had no effect on the biomass of wheat, leading to a significant CO2 × species interaction. Elevated CO2 did not change the root length of either species throughout the experiment although the total root length of wheat was 3 and 9 folds greater than those of white lupin at the first and second harvest, respectively.
Plant C and N
Elevated CO2 decreased the N concentrations in both shoot and root of wheat at the first harvest, and decreased the root N concentration (by 14%) at the second harvest (Table 1). It decreased N concentration in the shoot by 16% but not in the root of white lupin at the second harvest. On average, white lupin had 16–107% higher N concentrations than wheat. At the second harvest, wheat was N-limited as shown by an apparent N-deficiency symptom (pale leaves).
Elevated CO2 had no effect on C:N ratio in either shoot or root of white lupin throughout the experiment except root C:N at the first harvest. However, it increased the shoot and root C:N ratios of wheat by 41% and 32% at the first harvest, and the root C:N ratio by18% at the second harvest. At both harvests, white lupin had lower C:N ratios than wheat (Table 1).
A significant CO2 × species interaction on root δ13C occurred at the first harvest with eCO2 decreasing the δ13C values of wheat only. However, eCO2 did not affect the root 13C composition of either species at the second harvest (Table 1).
Total below-ground respiration and its 13C signature
The magnitude of eCO2 effect on below-ground respiration depended on plant species and growth stage (Fig. 1, Table 2). Elevated CO2 had no significant effect on below-ground CO2 evolved from wheat columns at both growth stages (Fig. 1). However, it increased total below-ground respirations under white lupin by 39% and 19% for the first and second harvest, respectively (Fig. 1). The total below-ground CO2 efflux did not differ between two species at the first harvest, but was 2.2 times higher for white lupin than for wheat at the second harvest (Fig. 1, Table 2). Moreover, the below-ground respiration was 2.4 times greater at the second than the first harvest for white lupin (Fig. 1).
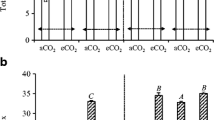
Total below-ground CO2 efflux (soil-derived CO2-C and root-derived CO2-C) from soil columns with wheat and white lupin grown for 34 (A) and 62 (B) days under aCO2 (400 μmol mol−1) and eCO2 (800 μmol mol−1). Error bars represent standard errors of means of four replicates. Means with a common upper-case letter (root-derived CO2-C) or lower-case letter (soil-derived CO2-C) within the same harvest are not significantly different at P = 0.05 using the Duncan’s new multiple range test
Elevated CO2 increased the root-derived CO2 from white lupin-soil system only, leading to a significant CO2 × species interaction (Fig. 1, Table 2). The increases were 44% and 15% at the first and second harvest, respectively (Fig. 1). At the second harvest, the root-derived CO2 from white lupin was 2.9 times greater than that from wheat.
The 13C abundance of the CO2 released via below-ground respiration of planted columns ranged from −26.5 to −25.2‰ at Day 34 and from −25.2 to −22.2‰ at Day 62 (Table 1). Elevated CO2 decreased the 13C abundance at Day 34, and tended to decrease it though not significant at Day 62. The CO2 evolved from white lupin columns showed invariably lower δ13C abundances when compared to those from wheat columns (Table 1).
Rhizosphere priming effect
Elevated CO2 tended to increase the soil-derived CO2 under wheat at the first harvest, but decreased it by 16% at the second harvest (Fig. 1). Elevated CO2 increased the soil-derived CO2 under white lupin and the increases were 21% and 30% at the first and second harvest, respectively (Fig. 1). On average, the soil-derived CO2 was 18% higher under white lupin than under wheat at the first harvest, but was 9% lower at the second harvest (Fig. 1, Table 2).
Rhizosphere priming effects were determined by taking the basal soil respiration rates out of the soil-derived CO2. The basal soil respiration rates in the control amounted at 7.11 and 5.51 mg CO2-C kg−1 soil d−1 at the first and second harvest, respectively. At the first harvest, eCO2 showed no impact on the RPE of wheat but it increased white lupin RPE by 78% (Fig. 2A). On average, the RPE was 1.8 folds higher under white lupin than under wheat (Fig. 2, Table 2). At the second harvest, eCO2 decreased the wheat RPE by 22%, but it increased the white lupin RPE by 47% (Fig. 2B, Table 2). When averaged two CO2 treatments, the RPEs were 6.6 and 3.5 folds higher at the second harvest than at the first harvest for wheat and white lupin, respectively (Fig. 2).
Rhizosphere priming effects (Primed soil C) of wheat and white lupin grown for 34 (A) and 62 (B) days under either aCO2 (400 μmol mol−1) or eCO2 (800 μmol mol−1). Error bars represent standard errors of means of four replicates. Means with a common letter within the same harvest are not significantly different at P = 0.05 using the Duncan’s new multiple range test
Rhizosphere soil respiration and microbial biomass C
Elevated CO2 had no significant effect on the rhizosphere soil respirations of either species at the first harvest and that of wheat at the second harvest (Fig. 3A, B, and Table 2). However, it increased the rhizosphere soil respiration of white lupin by 63% at the second harvest (Fig. 3B). On average, the rhizosphere soil respiration of white lupin was 2.0 and 3.8 times greater than those of wheat at the first and second harvest, respectively (Fig. 3A, B, Table 2).
Rhizosphere soil respiration (A, B) and microbial biomass C (C, D) in soil with wheat and white lupin grown for 34 (A, C) and 62 (B, D) days under either aCO2 (400 μmol mol−1) or eCO2 (800 μmol mol−1). The arrow-ended short lines represent the values obtained from unplanted control soils. Error bars represent standard errors of means of three replicates for Day 34 and four replicates for Day 62. Means with a common letter within the same harvest are not significantly different at P = 0.05 using the Duncan’s new multiple range test
The significant CO2 effect on soil MBC was only observed in the rhizosphere of white lupin at the second harvest (Fig. 3C, D). Elevated CO2 induced a 43% increase in MBC in white lupin rhizosphere when compared to aCO2. Although there was no difference in MBC between wheat and white lupin at the first harvest, growing white lupin resulted in a 149% increase in MBC at the second harvest by comparison with wheat (Fig. 3C, D, and Table 2). Additionally, MBC in the rhizosphere of white lupin was 115% higher at the second than the first harvest (Fig. 3C, D).
Rhizosphere K2SO4-extractable C
Elevated CO2 had no significant effect on K2SO4-extractable C (EOC) in the rhizosphere of wheat at the first harvest (Fig. 4A). However, it increased the EOC in white lupin rhizosphere by 53% and 22% at the first and second harvest, respectively (Fig. 4). On average, the concentrations of EOC in the rhizosphere of white lupin were 1.8 and 5.5 times greater than those of wheat at the first and second harvest, respectively (Fig. 4, Table 2). Furthermore, rhizosphere EOC of white lupin increased 3 folds at the second harvest compared to the first harvest (Fig. 4).
Concentrations of K2SO4-extractable organic C in the rhizospheres of wheat and white lupin grown for 34 (A) and 62 (B) days under either aCO2 (400 μmol mol−1) or eCO2 (800 μmol mol−1). The arrow-ended short lines represent the values obtained from unplanted control soils. Error bars represent standard errors of means of three replicates for Day 34 and four replicates for Day 62. Means with a common letter within the same harvest are not significantly different at P = 0.05 using the Duncan’s new multiple range test
Rhizosphere K2SO4-extractable N
The concentrations of K2SO4-extractable inorganic N (NH4 +-N + NOx −-N) in the rhizosphere of wheat and white lupin were lower under eCO2 than aCO2. For example, at the first harvest, the extractable inorganic N were 59% and 45% lower under eCO2 than aCO2 for wheat and white lupin, respectively (Fig. 5A). At the second harvest, the concentration of extractable inorganic N in white lupin rhizosphere was 23% lower under eCO2 (Fig. 5B). The concentrations of extractable inorganic N in the rhizosphere were higher under white lupin than under wheat (Fig. 5, Table 2). The decreases in the concentrations of extractable inorganic N under eCO2 was mainly attributed to the decline in NOx −-N concentrations (Fig. 5).
Concentrations of K2SO4-extractable inorganic N (NH4 +-N and NOx −-N) in the rhizosphere of wheat and white lupin grown for 34 (A) and 62 (B) days under either aCO2 (400 μmol mol−1) or eCO2 (800 μmol mol−1). Error bars represent standard errors of means of three replicates for Day 34 and four replicates for Day 62. Means with a common upper-case letter (NOx −-N) or lower-case letter (NH4 +-N) within the same harvest are not significantly different at P = 0.05 using the Duncan’s new multiple range test
Discussion
This study demonstrated that the CO2 effect on the rhizosphere priming of SOC decomposition differed between plant species and between developmental stages. Elevated CO2 increased the RPEs of white lupin at both growth stages but decreased the RPE of wheat at the later growth stage, partly supporting our hypothesis. This species variation could be mainly attributed to possible variations in the quantity and quality of root exudates between the two species.
In contrast to our hypothesis, eCO2 had no effect on RPE of wheat plants at the vegetative stage (Day 34), but decreased the amount of primed soil C under wheat by 22% at the booting stage (Day 62). At the early vegetative growth stage, eCO2-induced labile C input from wheat roots might be too small to induce a measurable change in microbial growth (Fig. 3A, C) and hence rhizosphere primed C. This is evidenced by no increases in root-derived CO2 or extractable organic C in rhizosphere soil under eCO2 relative to aCO2. The decreased RPE under eCO2 at the booting stage might be attributed to the poor quality of root-derived substrates as indicated by the increased root C:N ratio in this and previous studies (e.g. Jin et al. 2015) or increased phenolic and non-structural carbohydrates (Goufo et al. 2014). Poor substrate quality might restrict microbial decomposition of SOC (Cotrufo and Ineson 1996; Viswanath et al. 2010).
Kuikman et al. (1990) also reported that eCO2 decreased SOC decomposition under wheat (Triticum aestivum L. cv. Ralle) at a late growth stage (Day 49) but not at an early stage (Day 22). They suggested that the decrease could be caused by microbial preferential utilisation of root-released labile C that was stimulated by eCO2. Soil microbial community shifted their substrate preference to readily decomposable root-derived C at eCO2, decreasing their dependence on SOC and the decomposition of existing SOC. However, this explanation is not applicable to this study because eCO2 did not increase the quantities of root exudates as indicated by root-derived CO2 and extractable organic C in the rhizosphere (Figs. 1B, 4B). Our results were inconsistent with previous findings that plants often exhibit stimulated growth and higher root-to-shoot ratio, greater rhizodeposition and higher rhizosphere respiration under eCO2 (Kuzyakov 2010; Cheng et al. 2014; Nie et al. 2015). For example, Cheng and Johnson (1998) found that eCO2 (700 μmol mol−1) increased the total mass of wheat by 49% and the concentration of rhizosphere soluble C by 60%. The larger input of rhizodeposits under eCO2 was considered as an important source of substrates for soil organisms to decompose SOC (Cheng and Gershenson 2007). This difference in CO2 effect on plant growth between this study and others is likely due to a difference in N availability (Billings and Ziegler 2008).
In contrast to wheat, eCO2 increased the RPE of white lupin by 78% and 47% at the vegetative stage (Day 34) and the flowering stage (Day 62), respectively. This observation was consistent with the increases in rhizosphere soil respiration and microbial biomass C under eCO2 (Fig. 3). In this study, eCO2 stimulated the input of labile C, as indicated by the increases in root-derived CO2 and extractable organic C (Figs. 1 and 4), and hence the ability of soil microorganisms to decompose SOC (Health et al. 2005; Nie et al. 2013; Van Groenigen et al. 2017). The prominent capacity of white lupin in releasing organic acids/anions such as citrate has been documented (Watt and Evans 1999; Wasaki et al. 2005). Previous hydroponic culture experiments showed that eCO2 increased the release of malate or citrate from white lupin roots via enhanced biomass production or specific release rate per unit of root biomass (Watt and Evans 1999; Campbell and Sage 2002). Furthermore, eCO2 is also expected to alter the composition of the root exudates of legumes with relatively higher N-rich substances due to the increased N2 fixation at eCO2 (De Graaff et al. 2006; Jin et al. 2012; Lam et al. 2012). These changes in the quality alongside the quantity of root-derived substrates under eCO2 stimulated microbial growth and activity which increased the decomposition of SOC by co-metabolism and/or enhanced extracellular enzyme production (Kuzyakov et al. 2000; Carney et al. 2007).
Except for the quantity and quality of root exudates, soil N availability might also affect the rhizosphere priming effect, as reported by other studies (Berntson and Bazzaz 1997; Craine et al. 2007; Zang et al. 2016). Elevated CO2 decreased the concentrations of K2SO4-extractable N in the rhizosphere of both species, mainly in the form of nitrate (Fig. 5) which concurred with those reported previously (Hovenden et al. 2008; Nie and Pendall 2016). This was mainly attributed to the extra N demand and uptake by plants to favour the enhanced plant growth under eCO2. The reduction of soil N availability under eCO2 could also be caused by increased microbial N immobilisation (Billings and Ziegler 2005). The decrease in soil N availability under eCO2 was thought to increase the intensity and duration of RPE (Cheng and Kuzyakov 2005) due to microbial N mining from soil organic matter (Chen et al. 2014). However, in this study, eCO2 decreased soil N availability, which might not have constrained soil microbes with white lupin because more N-rich root exudates under eCO2 due to enhanced biological N2 fixation might have met the microbial N requirement. In the wheat-soil system, the negative effect of eCO2 on N availability was stronger. This might have limited the activity of microorganisms to decompose SOC, which is in line with many other studies (Berntson and Bazzaz 1997; Bengtson et al. 2012). Moreover, lower N availability under eCO2 could also shift the composition of soil microbial community, as a result, changing the decomposition of SOC. For example, Carney et al. (2007) found that the increased priming effect at eCO2 was pertinent to the increase in fungal abundance which is possibly caused by the lower N availability under eCO2 as fungi demand less N than bacteria (Billings and Ziegler 2005).
The RPE of white lupin was higher than that of wheat at the first harvest. Conversely, there was more RPE under wheat than under white lupin when averaged across two CO2 levels at the second harvest. When both species had small root systems at the first harvest, white lupin might have secreted more easily decomposable C compounds than wheat as indicated by the higher root-derived CO2 and extractable organic C in the rhizosphere soil (Figs. 1A, 4A) because its specific release rate of root exudates per unit of root biomass is higher than wheat (Nuruzzaman et al. 2006; Pearse et al. 2006; Weisskopf et al. 2008). Besides, white lupin relying on N2 fixation had higher root N concentrations than wheat, indicating the rhizodeposits of white lupin were also more N-enriched than wheat. Nitrogen-rich labile compounds are thought to yield higher priming effects than sole C substrates (Dalenberg and Jager 1989; Cheng 2009). For instance, Knorr et al. (2005) found that the addition of N-rich substrates increased the decomposition of plant litters. The increased input of labile C compounds with higher N content from white lupin roots could have activated soil microbial growth and activity (Carney et al. 2007; Drake et al. 2013), leading to higher RPE under white lupin than under wheat. The higher RPE of wheat than white lupin at the second harvest might be due to its longer roots than white lupin in the small soil columns, that is, wheat roots explore more volume of soil than white lupin (Weisskopf et al. 2008). Moreover, soil microbial communities can be distinct between plant species due to species selective influences (due to the differences in quantity and quality of root exudates) on different microbial communities (Marschner et al. 2001; Hartmann et al. 2009). Future work is needed to explore plant traits and root exudation, and their impacts on soil microbial function and community structure to better understand the species variation in eliciting RPE.
The RPEs were consistently lower at the early than the late growth stage. The results are consistent with the findings by Cheng and Kuzyakov (2005) and Cheng (2009) reporting that the priming effects are lower and even negative at early growing stages, increase to the highest at the flowering stage, and decline thereafter. According to them, plant growth stage regulates the release of substrates in the rhizosphere and hence the pattern of RPE. This could account for the higher RPE of white lupin at Day 62 than Day 34 in our present study as rhizosphere extractable C, root-derived CO2, rhizosphere soil respiration and microbial biomass C were all greater at Day 62. However, there was less root-derived CO2 (P < 0.05), similar MBC and EOC in wheat rhizosphere at Day 62 when compared to Day 34. As stated above, the increase of wheat RPE at the later growth stage was caused by more rhizosphere soil when wheat roots expanded and explored more soil volumes than the first harvest (Table 1). More studies are needed to cover the entire growth stages to better understand the effect and mechanisms of plant phenology on RPE.
Conclusions
Elevated CO2 increased the RPEs of white lupin at both growth stages but decreased the RPE of wheat at the later growth stage. Changes in root exudate quality and quantity at eCO2 might account for the species-specific responses in RPEs to eCO2. This study suggests that future high atmospheric CO2 concentration may favour the decomposition of native SOC under legumes. The selection of proper crop species which release small amounts of root exudates could be conducive to SOC sequestration. Future studies on mechanistic understandings of the RPE should focus on the changes in the quantity and quality of root exudates at eCO2, and how microbes respond to these changes under various plant species at different growth stages.
Abbreviations
- aCO2 :
-
ambient atmospheric CO2 concentration
- eCO2 :
-
elevated atmospheric CO2 concentration
- EOC:
-
rhizosphere K2SO4-extractable C
- MBC:
-
microbial biomass C
- RPE:
-
rhizosphere priming effect
- SOC:
-
soil organic carbon
References
Bardgett RD, Mommer L, Vries FTD (2014) Going underground: root traits as drivers of ecosystem processes. Trends Ecol Evol 29:692–699
Bengtson P, Barker J, Grayston SJ (2012) Evidence of a strong coupling between root exudation, C and N availability, and stimulated SOM decomposition caused by rhizosphere priming effects. Ecol Evol 2:1843–1852
Berntson GM, Bazzaz FA (1997) Nitrogen cycling in microcosms of yellow birch exposed to elevated CO2: simultaneous positive and negative below-ground feedbacks. Glob Chang Biol 3:247–258
Billings SA, Ziegler SE (2005) Linking microbial activity and soil organic matter transformations in forest soils under elevated CO2. Glob Chang Biol 11:203–212
Billings SA, Ziegler SE (2008) Altered patterns of soil carbon substrate usage and heterotrophic respiration in a pine forest with elevated CO2 and N fertilization. Glob Chang Biol 14:1025–1036
Blagodatskaya Е, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131
Butterly CR, Armstrong R, Chen D, Tang C (2016) Free-air CO2 enrichment (FACE) reduces the inhibitory effect of soil nitrate on N2 fixation of Pisum sativum. Ann Bot 117:177–185
Campbell CD, Sage RF (2002) Interactions between atmospheric CO2 concentration and phosphorus nutrition on the formation of proteoid roots in white lupin (Lupinus albus L.) Plant Cell Environ 25:1051–1059
Carney KM, Hungate BA, Drake BG, Megonigal JP (2007) Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc Natl Acad Sci 104:4990–4995
Chen R, Senbayram M, Blagodatsky S et al (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Chang Biol 20:2356–2367
Cheng W (1996) Measurement of rhizosphere respiration and organic matter decomposition using natural 13C. Plant Soil 183:263–268
Cheng W (2009) Rhizosphere priming effect: its functional relationships with microbial turnover, evapotranspiration, and C-N budgets. Soil Biol Biochem 41:1795–1801
Cheng W, Gershenson A (2007) Carbon fluxes in the rhizosphere. In: Cardon ZG, Whitbeck JL (eds) The rhizosphere: an ecological perspective. Elsevier Academic Press, London, pp 31–56
Cheng W, Johnson DW (1998) Elevated CO2, rhizosphere processes, and soil organic matter decomposition. Plant Soil 202:167–174
Cheng W, Kuzyakov Y (2005) Root effects on soil organic matter decomposition. Agronomy monograph No. 48. In: Wright S, Zobel R (eds) Roots and soil management: interactions between roots and the soil. ASA-SSSA, Madison, pp 119–143
Cheng S-H, Moore BD, Seemann JR (1998) Effects of short-and long-term elevated CO2 on the expression of ribulose-1, 5-bisphosphate carboxylase/oxygenase genes and carbohydrate accumulation in leaves of Arabidopsis thaliana (L.) Heynh. Plant Physiol 116:715–723
Cheng W, Parton WJ, Gonzalez-Meler MA et al (2014) Synthesis and modeling perspectives of rhizosphere priming. New Phytol 201:31–44
Cotrufo MF, Ineson P (1996) Elevated CO2 reduces field decomposition rates of Betula pendula (Roth.) leaf litter. Oecologia 106:525–530
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113
Dalenberg JW, Jager G (1989) Priming effect of some organic additions to 14C-labelled soil. Soil Biol Biochem 21:443–448
De Graaff MA, Van Groenigen KJ, Six J et al (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Chang Biol 12:2077–2091
De Graaff MA, Van Kessel C, Six J (2009) Rhizodeposition-induced decomposition increases N availability to wild and cultivated wheat genotypes under elevated CO2. Soil Biol Biochem 41:1094–1103
Drake JE, Darby BA, Giasson M-A et al (2013) Stoichiometry constrains microbial response to root exudation- insights from a model and a field experiment in a temperate forest. Biogeosciences 10:821–838
Goufo P, Pereira J, Moutinho-Pereira J et al (2014) Rice (Oryza sativa L.) phenolic compounds under elevated carbon dioxide (CO2) concentration. Environ Exp Bot 99:28–37
Guo J, Zhang W, Zhang M et al (2012) Will elevated CO2 enhance mineral bioavailability in wetland ecosystems? Evidence from a rice ecosystem. Plant Soil 355:251–263
Hartmann A, Schmid M, van Tuinen D, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321:235–257
Hartwig UA, Sadowsky MJ (2006) Biological nitrogen fixation: a key process for the response of grassland ecosystems to elevated atmospheric [CO2]. In: Nösberger J, Long SP, Norby RJ et al (eds) Managed ecosystems and CO2. Springer, Heidelberg, pp 325–336
Health J, Ayres E, Possell M et al (2005) Rising atmospheric CO2 reduces sequestration of root-derived soil carbon. Science 309:1711–1713
Hovenden MJ, Newton P, Carran R et al (2008) Warming prevents the elevated CO2-induced reduction in available soil nitrogen in a temperate, perennial grassland. Glob Chang Biol 14:1018–1024
Hungate BA, Dijkstra P, Johnson D et al (1999) Elevated CO2 increases nitrogen fixation and decreases soil nitrogen mineralization in Florida scrub oak. Glob Chang Biol 5:781–789
Jin J, Tang C, Armstrong R, Sale P (2012) Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus-deficient Vertisol. Plant Soil 358:91–104
Jin J, Tang C, Sale P (2015) The impact of elevated carbon dioxide on the phosphorus nutrition of plants: a review. Ann Bot 116:987–999
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Kuikman PJ, Lekkerkerk LJA, Van Veen JA (1990) Carbon dynamics of a soil planted with wheat under an elevated atmospheric CO2 concentration. In: Wilson WS, Gray Timothy RG (eds) Advances in soil organic matter research: the impact on agriculture and the environment. The Royal Society of Chemistry, Special Publication, Cambridge, pp 267–274
Kuzyakov Y (2010) Priming effects: Interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Lam SK, Chen D, Norton R, Armstrong R (2012) Does phosphorus stimulate the effect of elevated [CO2] on growth and symbiotic nitrogen fixation of grain and pasture legumes? Crop Pasture Sci 63:53–62
Marschner P, Yang C, Lieberei R, Crowley DE (2001) Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol and Biochem 33:1437–1445
Meinshausen M, Smith SJ, Calvin K et al (2011) The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim Chang 109:213–241
Nie M, Pendall E (2016) Do rhizosphere priming effects enhance plant nitrogen uptake under elevated CO2? Agric Ecosyst Environ 224:50–55
Nie M, Pendall E, Bell C, Gasch CK, Raut S, Tamang S, Wallenstein MD (2013) Positive climate feedbacks of soil microbial communities in a semiarid grassland. Ecol Lett 16:234–241
Nie M, Bell C, Wallenstein MD, Pendall E (2015) Increased plant productivity and decreased microbial respiratory C loss by plant growth-promoting rhizobacteria under elevated CO2. Sci Report 5:1–6
Nuruzzaman M, Lambers H, Bolland MD, Veneklaas EJ (2006) Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 281:109–120
Paterson E, Thornton B, Midwood AJ et al (2008) Atmospheric CO2 enrichment and nutrient additions to planted soil increase mineralisation of soil organic matter, but do not alter microbial utilisation of plant- and soil C-sources. Soil Biol Biochem 40:2434–2440
Pearse SJ, Veneklaas EJ, Cawthray GR et al (2006) Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant Soil 288:127–139
Pendall E, Mosier AR, Morgan JA (2004) Rhizodeposition stimulated by elevated CO2 in a semiarid grassland. New Phytol 162:447–458
Peñuelas J, Estiarte M, Kimball BA et al (1996) Variety of responses of plant phenolic concentration to CO2 enrichment. J Exp Bot 47:1463
Peñuelas J, Estiarte M, Llusia J (1997) Carbon-based secondary compounds at elevated CO2. Photosynthetica 33:313–319
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14:187–194
Shahzad T, Chenu C, Genet P et al (2015) Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol Biochem 80:146–155
Sugiyama A, Yazaki K (2012) Root exudates of legume plants and their involvement in interactions with soil microbes. In: Vivanco JM, Baluška F (eds) Secretions and exudates biological systems, signaling and communication in plants. Springer, Heidelberg, pp 27–48
Tobita H, Yazaki K, Harayama H, Kitao M (2016) Responses of symbiotic N2 fixation in Alnus species to the projected elevated CO2 environment. Trees 30:523–537
Van Groenigen KJ, Qi X, Osenberg CW et al (2014) Faster decomposition under increased atmospheric CO2 limits soil carbon storage. Science 344:508–509
Van Groenigen KJ, Osenberg CW, Terrer C et al (2017) Faster turnover of new soil carbon inputs under increased atmospheric CO2. Glob Chang Biol 00:1–10. https://doi.org/10.1111/gcb.13752
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Veneklaas EJ, Stevens J, Cawthray GR et al (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248:187–197
Viswanath T, Pal D, Purakayastha TJ (2010) Elevated CO2 reduces rate of decomposition of rice and wheat residues in soil. Agric Ecosyst Environ 139:557–564
Wang X, Tang C, Severi J et al (2016) Rhizosphere priming effect on soil organic carbon decomposition under plant species differing in soil acidification and root exudation. New Phytol 211:864–873
Wasaki J, Rothe A, Kania A et al (2005) Root exudation, phosphorus acquisition, and microbial diversity in the rhizosphere of white lupine as affected by phosphorus supply and atmospheric carbon dioxide concentration. J Environ Qual 34:2157–2166
Watt M, Evans JR (1999) Linking development and determinacy with organic acid efflux from proteoid roots of white lupin grown with low phosphorus and ambient or elevated atmospheric CO2 concentration. Plant Physiol 120:705–716
Weisskopf L, Le Bayon R-C, Kohler F et al (2008) Spatio-temporal dynamics of bacterial communities associated with two plant species differing in organic acid secretion: A one-year microcosm study on lupin and wheat. Soil Biol Biochem 40:1772–1780
Werth M, Kuzyakov Y (2010) C fractionation at the root–microorganisms–soil interface: A review and outlook for partitioning studies. Soil Biol Biochem 42:1372–1384
Zang H, Wang J, Kuzyakov Y (2016) N fertilization decreases soil organic matter decomposition in the rhizosphere. Appl Soil Ecol 108:47–53
Acknowledgements
This research was supported under Australian Research Council’s Discovery Projects funding scheme (project DP120104100). We thanks anonymous reviewers for their constructive comments, Colin Seis for providing the C4 soil, Mark Richards for providing the white lupin seeds, and Jinlong Dong, Dominic Lauricella, Anan Wang and Eric Zhang for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric Paterson
Rights and permissions
About this article
Cite this article
Xu, Q., Wang, X. & Tang, C. Wheat and white lupin differ in rhizosphere priming of soil organic carbon under elevated CO2 . Plant Soil 421, 43–55 (2017). https://doi.org/10.1007/s11104-017-3431-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3431-6