Abstract
Awareness of global warming has stimulated research on environmental controls of soil methane (CH4) consumption and the effects of increasing atmospheric carbon dioxide (CO2) on the terrestrial CH4 sink. In this study, factors impacting soil CH4 consumption were investigated using laboratory incubations of soils collected at the Free Air Carbon Transfer and Storage I site in the Duke Forest, NC, where plots have been exposed to ambient (370 μL L−1) or elevated (ambient + 200 μL L−1) CO2 since August 1996. Over 1 year, nearly 90% of the 360 incubations showed net CH4 consumption, confirming that CH4-oxidizing (methanotrophic) bacteria were active. Soil moisture was significantly (p < 0.01) higher in the 25–30 cm layer of elevated CO2 soils over the length of the study, but soil moisture was equal between CO2 treatments in shallower soils. The increased soil moisture corresponded to decreased net CH4 oxidation, as elevated CO2 soils also oxidized 70% less CH4 at the 25–30 cm depth compared to ambient CO2 soils, while CH4 consumption was equal between treatments in shallower soils. Soil moisture content predicted (p < 0.05) CH4 consumption in upper layers of ambient CO2 soils, but this relationship was not significant in elevated CO2 soils at any depth, suggesting that environmental factors in addition to moisture were influencing net CH4 oxidation under elevated CO2. More than 6% of the activity assays showed net CH4 production, and of these, 80% contained soils from elevated CO2 plots. In addition, more than 50% of the CH4-producing flasks from elevated CO2 sites contained deeper (25–30 cm) soils. These results indicate that subsurface (25 cm+) CH4 production contributes to decreased net CH4 consumption under elevated CO2 in otherwise aerobic soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil methane (CH4) consumption by CH4-oxidizing (methanotrophic) organisms is of crucial importance to the global CH4 budget. It is the largest terrestrial CH4 sink, oxidizing nearly half of all soil-produced CH4, and it consumes 40–60 Tg per year of CH4 directly from the atmosphere (Watson et al. 1990; Reeburgh et al. 1993). CH4 oxidation is strongly influenced by soil moisture, organic matter content, texture, and nutrient status, but quantifying their effects on net CH4 consumption in soils has been a challenge for microbial ecologists, in part because of the relatively recent finding that CH4 production, a process dependent on an absence of O2, can occur in macroscopically oxic soils (Yavitt et al. 1995; Horz et al. 2002). As a result, modeling efforts can be stymied by the existence of soil CH4 sources and sinks in close proximity to one another, even within the same soil particle (Nedwell 1996; von Fischer and Hedin 2002).
Moisture is clearly one of the most important environmental factors influencing atmospheric CH4 consumption in unsaturated soils (Born et al. 1990; Koschorreck and Conrad 1993). CH4 diffusion is 10,000-fold slower in water than in air, and thus, increased soil moisture reduces diffusive transport of atmospheric CH4 to the subsurface methanotrophs, limiting rates of soil CH4 consumption (Koschorreck and Conrad 1993; Castro et al. 1994). Increased soil moisture can also promote soil anoxia and subsequent methanogenesis that may contribute to diminished net CH4 consumption (Sexstone and Mains 1990; Yavitt et al. 1995).
Recent research efforts into environmental controls of soil CH4 consumption have focused on the effects of increasing atmospheric carbon dioxide (CO2) on the terrestrial CH4 sink, studies stimulated by an increased awareness of global warming and the roles that these two greenhouse gases will play in mediating future climate change. At some sites, soil CH4 consumption has been shown to decrease by 23 to 67% under conditions of elevated atmospheric CO2 (Ineson et al. 1998; Ambus and Robertson 1999; Phillips et al. 2001b), but the mechanisms controlling these reductions are largely unclear. Plant-mediated soil moisture increases have corresponded to decreased net methanotroph activity at some elevated CO2 sites (Ambus and Robertson 1999; McLain et al. 2002). However, decreases in CH4 oxidation under elevated CO2 with no concomitant increases in soil moisture have also been reported (Phillips et al. 2001b), raising the possibility that other changes to the soil environment contribute to reduced CH4 oxidation.
Over 2 years (March 1999 to February 2001), we monitored the effects of elevated atmospheric CO2 on CH4 cycling at the Free Air Carbon Transfer and Storage (FACTS)-I facility in the Duke University forest, a loblolly pine (Pinus taeda L.) ecosystem exposed to Free Air Carbon Dioxide Enrichment (FACE) technology. These soils embody a substantial CH4 sink, consuming more than 200 mg CH4 m−2 year−1 (McLain et al. 2002), but from 1999 to 2001, atmospheric CO2 enrichment decreased net CH4 consumption by 26%, an inhibition that corresponded to 20% higher volumetric soil moisture in the elevated CO2 plots (McLain et al. 2002). While soil moisture accounted for a significant proportion of the variability in the surface CH4 fluxes, statistical analyses showed that the moisture differences mediated only half of the measured decrease in CH4 consumption in elevated CO2 plots (McLain et al. 2002). Laboratory incubations using sieved soils collected at 0–15-cm depths on three sampling dates (May to October 1999) confirmed the decreased CH4 consumption but revealed no causative factors such as moisture increases or CH4 production (Phillips et al. 2001a).
This work extends the findings of Phillips et al. (2001a) by reporting the results of laboratory activity assays conducted using soils collected at three sampling depths (0–25 cm) on ten sampling dates over 11 months (February to December 2000). By obtaining laboratory observations of CH4 cycling activity from soil samples collected at individual depths over four seasons and by incubating these unsieved soils under atmospheric CH4 concentrations closely resembling those found in situ, we hoped to more closely identify the changes to the soil ecology that were contributing to the observed year-round decreases in surface CH4 flux in FACTS-I-elevated CO2 soils. In addition, by incubating soils under a range of depths from 0 to 30 cm, we hoped to identify the soil layer in which methanotroph activities were most strongly inhibited. Results of this intensive study will aid in the understanding of potential changes to CH4 cycling that may occur in future climates.
Materials and methods
Site description
The FACTS-I site consists of three control and three treatment plots, each 30 m in diameter, in a 25-year-old loblolly pine plantation in the Duke Forest, Orange County, NC (35°58′N, 70°05′W). Since fumigation began in August 1996, CO2 has been delivered to maintain a concentration of ∼200 μL L−1 above ambient or 570 ± 30 μL L−1 in treatment plots (Hendrey et al. 1999), a level projected to occur within the next 50 years if current rates of atmospheric CO2 increase are sustained (Schlesinger 1997). Control plots are fumigated with ambient air to replicate micrometeorological effects of the FACE technology.
The FACTS-I forest is dominated by a loblolly pine plantation. Soils at the site are of the Enon Series, Ultic Alfisols, with low nitrogen and phosphorus availability typical of many upland areas in the Southeast. The soils are derived from igneous rock, yielding an acidic (pH ≈ 5.75), well-developed profile, consisting of a thick organic layer at the soil surface (0–5 cm) underlain by mixed clay mineralogy (25+ cm) (USDA-SCS 1977).
Soil sampling and physical analyses
Each FACTS-I plot is divided radially into four equal-sized sampling sectors. On each sampling date, soil cores (1.9 × ∼35 cm) were removed from two sectors of each ring at random locations, but avoiding sites used for previous cores. Samples were obtained with an unslotted stainless steel soil recovery probe (AMS, American Falls, ID) fitted with a butyrate plastic liner (Forestry Suppliers, Jackson, MS) and a slide-hammer attachment (AMS). After the probe was withdrawn, the liner was removed, clipped to remove unfilled portions, sealed with polyethylene caps (Forestry Suppliers), and transported to the laboratory on ice.
In the laboratory, soil cores were placed in an anoxic glovebox (Coy Laboratory Products, Grass Lake, MI) under a N2/H2 ratio of 98:2 to prevent introduction of O2. Subsamples at 0–5-, 15–20-, and 25–30-cm depths were analyzed for gravimetric water content (mL H2O g−1 dry soil) by oven drying samples at 105°C for 48 h. Soil water-holding capacities (WHCs; mL H2O g−1 dry soil) were measured three times during the study period using a pressure plate (Soilmoisture Equipment, Santa Barbara, CA) and the method of Klute (1965). Briefly, a saturated soil sample was placed on a porous ceramic plate inside a pressure cylinder, whereupon N2 was forced into the apparatus to attain a net pressure, forcing water from the soil. WHC was calculated as the amount of water held by a soil sample between pressures of 0.1 (field capacity) and 15 bars (permanent wilting point). The gravimetric water content of each soil subsample was converted to %WHC by dividing the water content by the WHC (mL H2O g−1 dry soil) and multiplying by 100. Soil pH was measured using the method of Van Lierop (1990), in which 2.5 g of sieved, air-dried soil and 5 mL of ultrapure H2O were combined and vortexed vigorously for ∼30 s. Solids were allowed to settle, and the pH of the supernatant was determined with an Orion 420A meter and a Ross model 8005 electrode (Thermo Fisher Scientific, Waltham, MA). Soil C and N contents were determined by a dry combustion analyzer interfaced with a Europa Hydra 20/20 IRMS (Europa Scientific, Crewe, UK).
To minimize destructive sampling inside the FACTS-I rings, bulk densities (BD) at the 0–5-, 15–20-, and 25–30-cm soil depths were measured directly outside of the fumigated plots using the metal ring sampler technique (Foth et al. 1982). Briefly, a 30-cm-deep trench was dug, and a metal cylinder with an interior volume of 161.1 cm3 was inserted into the side of the trench at the given depth to remove a known volume of soil. Soil samples were oven dried at 105°C for 48 h, weighed, and the BD (g cm−3 dry soil) was calculated.
Methane consumption activity assays
During construction of the FACTS-I site, soil gas wells were installed at 15- and 30-cm depths in each sector of each ring, allowing for the collection of porespace gases from individual depths corresponding to the soil core subsample depths. The gas wells consist of a 5 cm diameter × 10 cm height pipe (internal volume ∼200 cm3) situated vertically in the soil such that its open bottom rests at the depth of interest, while its top is closed with a two-holed rubber stopper. Plastic tubes extend from the buried cylinder to the soil surface where they are sealed by Kynar® caps attached with stainless steel Swagelok connectors. On each sampling date, the plastic tubes were evacuated by pulling 45 cm3 of gas from the buried cylinder, whereupon soil gas samples were withdrawn from the cylinder using a stainless steel vacuum manifold designed according to Andrews et al. (1999) and 75 cm3 Whitey stainless steel cylinders that had been evacuated to 10–5 Pa before sampling. Gas samples were then transported to the laboratory to be used as headspace gas in the activity assays. Gas sampling from these cylinders was performed at a minimum of 2-week intervals to allow for equilibration between the cylinders and the porespace air. Additional information on the cylinder design and use can be found in Andrews et al. (1999).
Net CH4-cycling activity was measured ten times in vitro from February through December 2000 using subsamples of the soil cores described above. A total of 360 incubations (ten sampling dates × six FACE rings × two soil samples per ring × three depths) were performed in which individual subsamples (3.0 g fresh weight) from three depths (0–5, 15–20, and 25–30 cm) were transferred from the soil cores into sterile 30-mL serum bottles and sealed with teflon-lined butyl rubber stoppers with crimp seals. Each serum bottle was purged with N2 for 1 min, evacuated, and refilled with soil gases from the corresponding depth to reproduce atmospheric conditions in situ as closely as possible. Bottles were pressurized to 1.1 atm to prevent vacuum development during subsequent sampling. Replicate bottles without soil controlled for potential gas emission by rubber stoppers, and sterile controls were obtained by autoclaving core samples at 121°C for 1 h on each of 3 consecutive days.
Bottles were incubated at their respective field temperatures (4–20°C) in the dark, and 0.5-mL gas samples were removed from each bottle at 0, 4, 8, 12, 24, and 48 h and analyzed for CH4 by gas chromatography/flame ionization detector (Varian 3400, GS-Q divinylbenzene homopolymer-fused silica capillary column [J&W Scientific, Folsom, CA], 30 m × 0.53 mm inner diameter, at 150°C with 250°C detector and 30 mL min−1 He carrier gas). Certified CH4 standards were used for calibration (Scott Specialty Gases, Plumsteadville, PA). Data revealed first-order decreases or increases in CH4 with time, and net CH4 cycling rates were calculated, by dividing the slope from the linear portion of the exponential decrease or increase (Koschorreck and Conrad 1993) of CH4 within the incubation by the molar volume of the gas held in each incubation flask, to achieve a CH4-cycling rate in moles. After completion of the assay, incubated soils were oven dried at 105°C for 48 h to determine gravimetric water content, and each CH4 cycling rate was corrected for the moisture content of the soil sample.
Statistical analyses
Differences in CH4 cycling between treatments were assessed using repeated-measures analysis of variance, with Tukey’s post-hoc test to locate differences between the means. Fixed-effects linear regression models were used to investigate the effects of soil moisture on CH4 cycling. All statistical estimation procedures and null hypothesis tests for regression coefficients within each model were performed using the software package Splus2000 (Professional release 1; copyright 1998–1999 MathSoft). All models were fit using Splus functions lm, lme, and aov.
Results and discussion
Soil physical and chemical analyses
Analysis of cores collected within each ring showed that elevated CO2 soils had significantly (p = 0.03) greater surface (0–5 cm) organic C contents (Table 1), in agreement with other studies performed at the FACTS-I site (Schlesinger and Lichter 2001). No differences in C or N between the ambient and elevated CO2 soils were detected in any other depth. Soil pH values were similar at all depths (Table 1), and no differences in pH were found between ambient and elevated CO2 soils.
Many studies of microbial production and consumption of gases express activity in absolute terms (e.g., CO2 production g−1 dry soil h−1). However, it has been reported that the expression of methanotroph activity on a gravimetric basis in soils of differing BD can lead to biases in data analysis (Bradford et al. 2001). FACTS-I soils showed increased (p < 0.01) BD with depth (Table 1), with no differences in BD found (p = 0.46) between ambient and elevated CO2 soils. Thus, data resulting from soil incubations was standardized using the BD of the corresponding soil depth to express CH4-cycling activity on a volumetric soil basis.
Methane oxidation activity: depth dependence
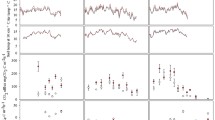
Throughout the study, a total of 360 activity measurements, representing two replicates of each sample from each depth (0–5, 15–20, and 25–30 cm), were performed. Initial headspace concentrations of CH4 within the incubation flasks decreased with depth, but these concentrations were equal between CO2 treatments (Table 2). Activity assays confirmed that CH4-cycling microbial communities were active throughout the 0–30-cm layer of FACTS-I soils, as 87.0% (313 flasks) showed net CH4 consumption during the 48-h assay, while 6.1% (22 flasks) had no net CH4 consumption or production, and 6.9% (25 flasks) exhibited net CH4 production. As reported in a variety of temperate ecosystems (Koschorreck and Conrad 1993; Castro et al. 1994; Roslev et al. 1997), the strongest CH4 sink was located below the surface (0–5 cm) layer. The 15–20-cm soils consumed the most (p < 0.01) CH4 by volume, averaging −3.7 ± 1.3 ng CH4 cm−3 soil day−1 over the study period (Fig. 1), while consumption rates in surface soils (0–5 cm; −2.2 ± 0.8 ng CH4 cm−3 soil day−1) were equal (p = 0.52) to those in the 25–30-cm layer (−2.0 ± 1.3 ng CH4 cm−3 soil day−1).
Net CH4 consumption in soil subsamples collected at 0–5- (a), 15–20- (b), and 25–30-cm (c) depths in FACTS-I sites over the 11-month sampling period. Symbols represent the average of two duplicate incubations performed at each depth under each CO2 treatment (elevated and ambient), ±1 standard deviation. Dotted line represents division between CH4 production (above) and consumption (below)
Results of the in vitro activity assays agreed with in situ surface flux measurements (Phillips et al. 2001b; McLain et al. 2002) in that net CH4 consumption was inhibited in elevated CO2 soils (Fig. 1). At 0–5 cm, CH4 oxidation in ambient CO2 rings (−2.5 ± 0.8 ng CH4 cm−3 soil day−1) was 26% higher than that of elevated CO2 rings (−1.9 ± 0.8 ng CH4 cm−3 soil day−1), but this inhibition was only marginally significant (p = 0.07). No significant differences in CH4 consumption were found between treatments in the 15–20-cm depth, where ambient CO2 soils consumed −4.1 ± 1.1 ng CH4 cm−3 soil day−1, and elevated CO2 soils consumed −3.3 ± 1.5 ng CH4 cm−3 soil day−1, a difference of 21%. Significant (p < 0.01) inhibition in CH4 oxidation was measured in the elevated CO2 treatment soils collected from the 25–30-cm depth, which consumed 70% less CH4 (−0.9 ± 1.3 ng CH4 cm−3 soil day−1) than the same depth of ambient CO2 soils (−3.0 ± 1.0 ng CH4 cm−3 soil day−1).
The CH4 oxidation rates in the 0–15-cm soil layer were lower than those reported by Phillips et al. (2001a) in FACTS-I soil laboratory incubations (−3.0 to −6.2 ng CH4 cm−3 day−1). However, Phillips et al. (2001a) performed incubations using sieved (5 mm) soils, while measurements in the current study were performed using intact soil core subsamples. It is highly likely that sieving the FACTS-I soils increased soil diffusivity (Bradford et al. 2001), thereby increasing the rates of CH4 oxidation found by Phillips et al. (2001a).
Soil moisture: depth dependence
WHCs of FACTS-I soil samples showed some variation with depth (Table 3). The organic 0–5-cm soils had higher (p < 0.01) WHC than the deeper mineral layers, while WHC in the 15–20- and 25–30-cm depths were equal (p = 0.65). There were no significant differences in WHC between the six FACTS-I rings, and the average (0–30 cm) WHC of elevated CO2 soils was equal to that of the ambient CO2 soils (Table 3). These significant differences in WHC precluded the use of absolute expressions of water content (g H2O g−1 dry soil) in this study for comparison of moisture effects on microbial activities (Gulledge and Schimel 1998). Instead, percent WHC, a parameter that parallels percent water-filled porespace (Linn and Doran 1984) and can thus provide an index of diffusivity across a range of physically different soils (Aulakh et al. 1991; Gulledge and Schimel 1998), was utilized to compare moisture effects on microbial activities between the three measured depths.
Moisture content measurements of the soil subsamples revealed seasonal patterns in the upper (0–5 and 15–20 cm) soil layers, where soils were wettest in mid-winter and drier in the summer and early fall (Fig. 2). One exception to this occurred on the August sampling date, after a rainfall event on August 1–5 during which more than 75 mm of precipitation fell at the site (State Climate Office of North Carolina 2004). Moisture contents measured in soil cores were statistically equal in the ambient and elevated CO2 rings at the 0–5- (p = 0.83) and 15–20-cm (p = 0.30) depths. At the 25–30-cm soil depth, elevated CO2 soil moisture contents were significantly (17.2%; p = 0.01) higher than ambient. In situ measurements using time domain reflectometry probes confirmed that from February to December 2000, volumetric soil moisture averaged 17.0% higher in the 0–30-cm layer of elevated CO2 soils compared to ambient (Duke FACE Facility 2007). Similar increases in soil moisture under CO2 fumigation have also been reported in grasslands (Owensby et al. 1997; Niklaus et al. 1998) and in hardwood stands (Ambus and Robertson 1999).
Moisture contents (percent water-holding capacity) of soil subsamples collected at 0–5 (a), 15–20 (b), and 25–30-cm (c) depths in FACTS-I sites over the 11-month monitoring period. Symbols represent the average of two duplicate incubations performed at each depth under each CO2 treatment (elevated and ambient), ±1 standard deviation
The causes of the increased soil moisture in elevated CO2 plots of FACTS-I soils have been the subject of debate. The moisture differences increased over time from a difference of 0.03 ± 0.01 mL cm−3 during the first year of CO2 fumigation (1997) to 0.08 ± 0.05 mL cm−3 in 1999 and to 0.10 ± 0.05 mL cm−3 in 2000 (Schäfer et al. 2002). The moisture increases in elevated CO2 plots may reflect a reduction in water loss through evaporation from the soil surface. Increased plant biomass accumulation resulted in 29% more litter deposited in elevated CO2 plots relative to ambient in 1999 (Schlesinger and Lichter 2001), and this thicker litter layer may inhibit surface evaporation, possibly increasing soil moisture retention under elevated CO2 in FACTS-I soils (Schäfer et al. 2002).
Methane oxidation activity measurements: moisture dependence
Soil moisture is a key regulator of diffusive transport of atmospheric CH4 into soils (Born et al. 1990; Dörr et al. 1993). Researchers have often reported negative correlations between soil moisture and CH4 consumption in field soils (Koschorreck and Conrad 1993; Castro et al. 1995), and in situ surface CH4 flux in FACTS-I soils was significantly (p < 0.01) negatively correlated with volumetric moisture from March 1999 to February 2001 (McLain et al. 2002). Linear regression confirmed that the average %WHC from 0 to 30 cm accounted for significant variability in CH4 consumption (also averaged 0–30 cm) in the soil incubations from both the ambient (p = 0.05) and elevated (p = 0.02) CO2 rings (Fig. 3), with decreasing CH4 oxidation correlated with increasing soil moisture. A significant relationship between net soil CH4 oxidation and WHC has also been reported for European forest soils (Koschorreck and Conrad 1993).
Although a significant relationship was found between CH4 consumption and %WHC when averaged over 0–30 cm (Fig. 3), %WHC did not account for significant variability in CH4 oxidation in the 0–5 cm FACTS-I soils from the elevated CO2 rings (p = 0.37), while the relationship in the ambient CO2 rings was near significant (p = 0.07). At the 15–20-cm depth, %WHC was not correlated with CH4-cycling activity in the elevated CO2 rings (p = 0.61) but was significantly so in the ambient CO2 rings (p = 0.04). Thus, while methanotroph activity results show strong moisture/CH4 oxidation correlations in the upper soil layers under ambient CO2, results from the elevated CO2 rings suggest the presence of additional factors impacting net CH4 oxidation. Soil moisture was not strongly correlated with CH4 oxidation in the 25–30-cm layer (p = 0.18 and 0.81 in elevated and ambient CO2 rings, respectively). The higher clay content of the deeper soils most probably accounted for this observation, as soil gas diffusion is more strongly controlled by texture than by moisture content in finer-textured soils (Dörr et al. 1993).
Net CH4 production
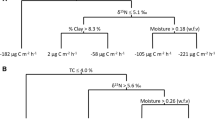
Of the 360 activity assays performed during this study, a total of 25 flasks showed net CH4 production at ambient temperature and moisture (Fig. 4). Thus, although FACTS-I soils are macrosopically oxic from 0 to 30 cm (McLain et al. 2002), localized anaerobic conditions that stimulate the activity of methanogens may increase in response to environmental stimuli (e.g., root respiration or moisture increases). The consistent dominance of methanotroph activity, evidenced by net surface CH4 consumption at all sites during a 2-year period (McLain et al. 2002) and the dominance of net CH4 oxidation in activity flasks, indicates that only very low levels of CH4 production may be occurring. Yet, methanogen activity has been documented in the upper layers of macroscopically oxic soils in other ecosystems (Yavitt et al. 1995; Castro et al. 2000; Horz et al. 2002), as O2-deficient microniches exist in generally oxic soils (Sexstone et al. 1985), and several studies have reported enhancements in soil CH4 production in response to environmental disturbance (Steudler et al. 1996; Castro et al. 2000).
The CH4-producing flasks were equally distributed among soil depths, but 80% (20 of 25) of the CH4-producing samples were collected from elevated CO2 soils (Fig. 4). Moreover, more than half of the CH4-producing flasks from elevated CO2 soils (11 of 20) contained soils from 25 to 30 cm. Increased rates of respiration in elevated CO2 soils (Andrews et al. 1999; McLain et al. 2002) may have depleted porespace O2 in these deeper, finer-textured soils, producing microsites where CH4 could be generated (Verchot et al. 2000). Although McLain et al. (2002) reported that porespace O2 concentrations were indistinguishable between CO2 treatments in the top 30 cm of FACTS-I soils, detection of small differences in O2 may have been hampered by a relatively high detection limit (0.1%) of the instrument used. In deeper soils (70–200 cm), O2 concentrations were lower at under elevated CO2, with differences averaging ∼6% across sampling dates (McLain et al. 2002).
These findings of in vitro CH4 production differ from those of Phillips et al. (2001a), who reported no CH4 production after applying a methanotroph inhibitor to laboratory incubations of sieved FACTS-I soils. However, this lack of agreement may be due to differing sampling and incubation protocols between the two studies. The sieving of soils by Phillips et al. (2001a) may have destroyed aggregates where microsites supporting the activity of methanogens would form in an otherwise aerobic soil (Smith 1980; Sexstone et al. 1985). In addition, while the headspace of incubation flasks used in the Phillips et al. (2001a) study contained ambient air, this study utilized porespace air, exposing the soils to slightly lower O2 concentrations and possibly encouraging stronger methanogen activity.
The presence of net methanogenesis under macroscopically aerobic conditions suggests that net CH4 fluxes in FACTS-I soils reflect a balance between soil production and consumption. Furthermore, increased methanogenesis in soils exposed to elevated CO2 indicate that the fundamental changes occurring in these soils, including porespace moisture and CO2 increases, are inducing CH4 production. While the incubations utilized in the current study revealed interesting patterns in the net consumption and production of CH4, it is hoped that these findings will stimulate further work utilizing isotope pool dilution experiments (Andersen et al. 1998; von Fischer and Hedin 2002) to separate and quantify gross CH4 cycling in elevated CO2 soils.
Conclusions
Efforts to understand increases in tropospheric CH4 have focused primarily on increased emissions of CH4 from biogenic sources to the atmosphere. However, recent research has indicated that a portion of the increase may also result from reduced strength of the CH4 sink. Our work confirms that soil CH4 consumption is reduced under elevated CO2 in temperate forest soils and that increased moisture levels and anoxia in deep (25+ cm) soils are critical factors contributing to this inhibition. Our results further suggest that CH4 production must also be considered as a contributor to decreased net CH4 consumption under elevated CO2 in some otherwise aerobic soils. By using a suite of approaches, including activity measurements, in situ net oxidation measurements, and statistical analyses, important insights into the CH4 dynamics of a soil were obtained, allowing for more confident predictions of the response of CH4 flux to future environmental change.
References
Ambus P, Robertson GP (1999) Fluxes of CH4 and N2O in aspen stands grown under ambient and twice-ambient CO2. Plant Soil 209:1–8
Andersen BL, Bidoglio G, Leip A, Rembges D (1998) A new method to study simultaneous methane oxidation and methane production in soils. Glob Biogeochem Cycles 12:587–594
Andrews JA, Harrison KG, Matamala R, Schlesinger WH (1999) Separation of root respiration from total soil respiration using carbon-13 labeling during Free-Air Carbon Dioxide Enrichment (FACE). Soil Sci Soc Am J 63:1429–1435
Aulakh MS, Doran JW, Walters DT, Power JF (1991) Legume residue and soil water effects on denitrification in soils of different textures. Soil Biol Biochem 23:1161.S–1167.S
Born M, Dörr H, Levin I (1990) Methane consumption in aerated soils of the temperate zone. Tellus 42B:2–8
Bradford MA, Ineson P, Wookey PA, Lappin-Scott HM (2001) Role of CH4 oxidation, production and transport in forest soil CH4 flux. Soil Biol Biochem 33:1625–1631
Castro MS, Melillo JM, Steudler P, Chapman JW (1994) Soil moisture as a predictor of methane uptake by temperate forest soils. Can J Forest Res 24:1805–1810
Castro MS, Steudler PA, Melillo JM, Aber JD, Bowden RD (1995) Factors controlling atmospheric methane consumption by temperate forest soils. Glob Biogeochem Cycles 9:1–10
Castro MS, Gholz HL, Clark KL, Steudler PA (2000) Effects of forest harvesting on soil methane fluxes in Florida slash pine plantations. Can J Forest Res 30:1534–1542
Dörr H, Katruff I, Levin I (1993) Soil texture parameterization of the methane uptake in aerated soils. Chemosphere 26:697–713
Duke FACE Facility (2007) FACTS-I data archive. Avaliable at: http://face.env.duke.edu/main.cfm
Foth HD, Withee LV, Jacobs HS, Thien SJ (1982) Laboratory manual for introductory soil science, 6th edn. William C. Brown, Dubuque, IA
Gulledge J, Schimel JP (1998) Moisture control over atmospheric CH4 consumption and CO2 production in diverse Alaskan soils. Soil Biol Biochem 30:1127–1132
Hendrey GR, Ellsworth DS, Lewin KF, Nagy J (1999) A free-air enrichment system for exposing tall forest vegetation to elevated atmospheric CO2. Glob Change Biol 5:293–309
Horz H-P, Raghubanshi AS, Heyer J, Kammann C, Conrad R, Dunfield PF (2002) Activity and community structure of methane-oxidising bacteria in a wet meadow soil. FEMS Microbiol Ecol 41:247–257
Ineson P, Coward PA, Hartwig UA (1998) Soil gas fluxes of N2O, CH4 and CO2 beneath Lolium perenne under elevated CO2: the Swiss free air carbon dioxide enrichment experiment. Plant Soil 198:89–95
Klute A (1965) Water capacity. In: Black CA (ed) Methods of soil analysis, part I. American Society of Agronomy, Madison, WI, pp 274–278
Koschorreck M, Conrad R (1993) Oxidation of atmospheric methane in soil: measurements in the field, in soil cores, and in soil samples. Glob Biogeochem Cycles 7:109–121
Linn DM, Doran JW (1984) Aerobic and anaerobic microbial populations in no-till and plowed soils. Soil Sci Soc Am J 48:794–799
McLain JET, Kepler TB, Ahmann D (2002) Below-ground factors mediating changes in methane consumption in a forest soil under elevated CO2. Global Biogeochem Cy 16:1050
Nedwell DB (1996) Methane production and oxidation in soils and sediments. In: Microbiology of atmospheric trace gases. NATO ASI Ser I, pp 33–49
Niklaus PA, Spinnler D, Körner C (1998) Soil moisture dynamics of calcareous grassland under elevated CO2. Oecologia 117:201–208
Owensby CE, Ham JM, Knapp AK, Bremer D, Auen LM (1997) Water vapour fluxes and their impact under elevated CO2 in a C4-tallgrass prairie. Glob Change Biol 3:189–195
Phillips RL, Whalen SC, Schlesinger WH (2001a) Response of soil methanotrophic activity to carbon dioxide enrichment in a North Carolina coniferous forest. Soil Biol Biochem 33:793–800
Phillips RL, Whalen SC, Schlesinger WH (2001b) Influence of atmospheric CO2 enrichment on methane consumption in a temperate forest soil. Glob Change Biol 7:557–563
Reeburgh WS, Whalen SC, Alperin MJ (1993) The role of methylotrophy in the global methane budget. In: Murrell JC, Kelly DP (eds) Microbial growth on C1 compounds. Intercept, Andover, UK, pp 1–14
Roslev P, Iversen N, Henriksen K (1997) Oxidation and assimilation of atmospheric methane by soil methane oxidizers. Appl Environ Microb 63:874–880
Schäfer KVR, Oren R, Lai C-T, Katul GG (2002) Hydrologic balance in an intact temperate forest ecosystem under ambient and elevated atmospheric CO2 concentration. Glob Change Biol 8:895–911
Schlesinger WH (1997) Biogeochemistry: an analysis of global change, 2nd edn. Academic, San Diego, CA
Schlesinger WH, Lichter J (2001) Limited carbon storage in soil and litter of experimental forest plots under increased atmospheric CO2. Nature 411:466–469
Sexstone AJ, Mains CN (1990) Production of CH4 and ethylene in organic horizons of spruce forest soils. Soil Biol Biochem 22:135–139
Sexstone AJ, Revsbech NP, Parkin TB, Tiedje JM (1985) Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci Soc Am J 49:645–651
Smith KA (1980) A model of the extent of anaerobic zones in aggregated soils and its potential application to estimates of denitrification. J Soil Sci 31:263–277
State Climate Office of North Carolina (2004) Raleigh–Durham NC climate summary. North Carolina State University, Raleigh, NC. Avaliable at: http://www.nc-climate.ncsu.edu/
Steudler PA, Melillo JM, Feigl BJ, Neill C, Piccolo MC, Cerri CC (1996) Consequence of forest-to-pasture conversion on CH4 fluxes in the Brazilian Amazon Basin. Global Biogeochem Cy 101:18547–18554
USDA-SCS (1977) Soil survey of Orange County, North Carolina. US Government Printing Office, Washington, DC
Van Lierop W (1990) Soil pH and lime requirement determination. In: Westerman RL (ed) Soil testing and plant analysis, 3rd edn. Soil Science Society of America, Madison, WI, pp 73–126
Verchot LV, Davidson EA, Cattânio JH, Ackerman IL (2000) Land-use change and biogeochemical controls of methane fluxes in soils of Eastern Amazonia. Ecosystems 3:41–56
von Fischer JC, Hedin LO (2002) Separating methane production and consumption with a field-based isotope pool dilution technique. Glob Biogeochem Cycles 16:1034
Watson RT, Rodhe H, Oeschger H, Siegenthaler U (1990) Greenhouse gases and aerosols. In: Houghton JT, Jenkins GJ, Ephraums JJ (eds) Climate change: the IPCC scientific assessment. Cambridge University Press, Cambridge, UK, pp 1–40
Yavitt JB, Fahey TJ, Simmons JA (1995) Methane and carbon dioxide dynamics in a Northern hardwood ecosystem. Soil Sci Soc Am J 59:796–804
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McLain, J.E.T., Ahmann, D.M. Increased moisture and methanogenesis contribute to reduced methane oxidation in elevated CO2 soils. Biol Fertil Soils 44, 623–631 (2008). https://doi.org/10.1007/s00374-007-0246-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-007-0246-2