Abstract
Background and aims
We aimed to investigate the effects of root carboxylate exudation in the interaction between Azospirillum brasilense and Zea mays. We hypothesized that root carboxylate exudation is a mechanism that increases colonization of the maize rhizosphere by A. brasilense and that carboxylate exudation would increase at a low soil phosphorus (P) availability.
Methods
We conducted a greenhouse experiment, using maize seeds inoculated and uninoculated with A. brasiliense. Seeds were planted in pots, supplied with nutrient solution, varying in P concentration. After 45 days we measured total plant biomass, root length and area, plant nutrient status, and the root carboxylate-exudation rate.
Results
Inoculation increased the root length and area, and this effect increased with increasing P supply. Inoculated plants also showed an increased root carboxylate-exudation rate. For inoculated treatments, the exudation rate was positively correlated with root architecture parameters; however, it was negatively correlated with leaf manganese concentration, a proxy for the amount of carboxylates in the rhizosphere.
Conclusion
Inoculation of A. brasilense stimulated root carboxylate exudation, which was positively correlated with root length and area. These positive correlations are probably mediated by the effect of carboxylates on the rhizosphere microbial community. This indicates a positive feedback in which A. brasilense inoculation stimulates root carboxylate exudation, influencing the rhizosphere microbial community. It results in positive effects on maize root architecture. The root length of inoculated plants was positively correlated with P supply, indicating that P supply positively affects the microbial community, modulating the interaction between A. brasilense and Z. mays.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Continuous development of innovative agricultural techniques is essential to improve the efficiency and sustainability of food production, and to increase food availability and accessibility. The current agricultural model is based on the application of large amounts of chemical fertilizers, resulting in an increased use of resources (World Bank 2014), causing serious environmental changes (Tilman 1999). In tropical regions, the use of phosphorus (P) is especially problematic; due to soils with low P availability, large amounts of exogenous P are needed to maintain high crop yields (Johnston et al. 2014; Roy et al. 2016). In this scenario, the use of free-living plant growth-promoting bacteria (PGPBs) has been proposed to enhance the efficiency of fertilizers, using less resources for the same crop yield which would positively impact food production (Cordell et al. 2009).
PGPBs are plant-associated microorganisms that inhabit the roots or rhizosphere, and positively affect their host (Bashan and Holguin 1997; Compant et al. 2010). These bacteria have multiple attributes that may benefit host plants: they may suppress populations of phytopathogenic soil microorganisms, fix atmospheric nitrogen (N), secrete phytohormones (such as auxins, cytokinins and gibberellins), or break down soil pollutants (Steenhoudt and Vanderleyden 2000; Bais et al. 2004; Ryu et al. 2004; Lugtenberg and Kamilova 2009; Thuita et al. 2012). A well-studied PGPB is Azospirillum brasilense, a diazotrophic bacterium that can interact with crop species like maize (Zea mays L.), sorghum (Sorgum spp.), and wheat (Triticum spp.; Dobbelaere et al. 2001). The mode of action of A. brasilense is commonly attributed to its ability to produce phytohormones, like indole-3-acetic acid (Spaepen et al. 2007; Fibach-Paldi et al. 2012) and gibberellins (Bottini et al. 2004), which modify root architecture and development (Kapulnik et al. 1985); its N-fixing capacity (Steenhoudt and Vanderleyden 2000); and its ability to release gluconic acid, which has been shown to solubilize sparingly-available calcium phosphates in vitro (Rodriguez et al. 2004). Generally, the interaction between A. brasilense and crop species results in an increased growth and yield of the plants; however, due to inconsistency of the results, the commercial application of this inoculant is not common (Dobbelaere et al. 2001).
We propose that the inconsistencies observed are related to unidentified factors that affect the interaction between A. brasilense and crop plants. Specifically, we investigated the role of soil P as a controlling factor. We focus on P, because this nutrient suppresses root carboxylate exudation (Neumann and Römheld 1999), whereas in soils with low P availability the exudation of carboxylates by roots is stimulated. These exudates may act as precursors for microbe-derived phytohormones (Bais et al. 2006), and as a source of carbon (C) and energy for microorganisms (Haichar et al. 2008), stimulating their proliferation in the rhizosphere (Bais et al. 2006). Therefore, we expect that in soils with a low P availability, the carboxylate-exudation rate of maize root will be faster, which would results in the proliferation of microorganisms including A. brasilense in the rhizosphere. This proliferation would account for observed differences in growth between non-inoculated and inoculated maize plants.
In this paper we used the known effects of A. brasilense (e.g., increased root length, increased biomass, increased concentration of N in shoots) as a proxy of the PGPB activity and rhizosphere colonization. In addition, the total shoot P content was measured in order to identify the effects of the PGPB on the acquisition of this nutrient, because of the reported potential of A. brasilense to solubilize P in vitro (Rodriguez et al. 2004). We measured the root carboxylate-exudation rate, and used the leaf manganese (Mn) concentration as a proxy for carboxylate concentrations in the rhizosphere (Lambers et al. 2015).
The specific aim of this study was to investigate the effects of root carboxylate exudation in the interactions between A. brasilense and Z. mays. Because carboxylates are precursors of phytohormones and also a source of C and energy for microorganisms, we hypothesized that faster root carboxylate-exudation rates result in more intense effects of A. brasilense in Z. mays (increase in total biomass, root length and area), either via stimulation of microbial activity or via stimulation of the proliferation of A. brasilense in the rhizosphere. Because root carboxylate-exudation rates tend to increase with decreasing soil P availability, we expected that increasing P supply would reduce the effect of A. brasilense on Z. mays.
Material and methods
Greenhouse experiment
We grew maize (cultivar BR 1060, Embrapa) plants in 15 L pots, in a low-nutrient soil (Table 1). Treatments were divided into non-inoculated (I-) and inoculated (I+). For I+, the seeds were inoculated with A. brasilense before sowing, (strains Ab-V4, Ab-V5), applying 4 mL of a commercial inoculant to 800 g of seeds, mixing both components in a plastic bag, which was kept for 10 min protected from sunlight. For both treatments, once a week we added 200 mL of a complete Hoagland solution per pot (Shipley and Keddy 1988), varying the concentration of P, which was supplied at four concentrations according to the treatment: 0, 50, 100, 200 mg P L−1, in the form of KH2PO4. Thus, there were eight treatments organized in a complete 2 × 4 factorial design: I + 0, I + 50, I + 100, I + 200, and I-0, I-50, I-100, and I-200, with five replicates for each treatment. The experiment was conducted in a greenhouse located at the University of Campinas, Campinas, SP, Brazil. Plants in the glasshouse were subjected to natural daily and seasonal cycles of solar radiation (with a daily peak of photosynthetically active radiation of 470–840 μmol photons m−2 s −1), natural temperature (8–39 °C) and relative humidity (10–100 %).
Soil collection and analysis
We collected soil at a depth of 0 to 30 cm in an area near the University of Campinas, SP, Brazil. We mixed it with sand in a 3:1 proportion of soil: sand. We sent a subsample (ca. 500 g) for chemical analysis to a private soil analysis laboratory (IBRA – Instituto Brasileiro de Análises), in Sumaré, SP, Brazil. Phosphorus, potassium, calcium, and magnesium were extracted with ion exchange resins (van Raij et al. 1986); copper, iron, zinc, and Mn were extracted in a chelating solution (DTPA) (Lindsay and Norvell 1978), and boron was extracted in barium chloride (BaCl2). Soil pH was determined in calcium chloride (CaCl2, Thomas 1996), and aluminum was extracted in potassium chloride (KCl) and determined by acid-base titration curves (Bertsch and Bloom 1996).
Plant growth and carboxylate analysis
After 45 days, plants were harvested and separated into roots and shoots. The root system was gently shaken to remove most of the soil, and what remained on the roots was considered rhizosphere soil (Veneklaas et al. 2003). To determine rhizosphere carboxylate exudation rate, the 10 cm apex of a selected axis of the root was submerged in 4 mL of deionized water for 10 s. These samples were frozen and lyophilized, and then re-suspended in 0.5 mL of deionized water. Ultra-High Performance Liquid Chromatography-Mass Spectrometry (UHPLC-MS) was used for the analysis of citric and malic acid (Abrahão et al. 2014). The exudation rates (μg cm−1 s−1) were calculated as the mass of each organic acid (μg) released from the root system, divided by the length of root used for collection of root exudates (10 cm), and divided by time (10 s).
To determine root length, we measured root length in aliquots and then extrapolated for the entire root system, based on the dry mass of the root samples. We collected and weighed a part of each root and then stained the roots with methylene blue (Bouma et al. 2000), followed by digitization of the roots in a flatbed scanner, with 600 dpi of resolution. The RootEdge software (Kaspar and Ewing 1997) was used for the analysis of root length.
Plants were oven dried for 48 h at 70 °C, and root and shoot parts were weighed using a semi-analytical balance. Shoot parts (leaves and stem) were ground and sent for N, P and Mn analyses to the “Luiz de Queiroz” college of Agriculture, University of São Paulo, Piracicaba-SP, Brazil. The following methods were used for nutrient determinations: N was determined by Kjeldahl distillation, after sulfuric acid digestion (Miyazawa et al. 2009); P was determined by spectrophotometry after nitric and perchloric acid digestion (Motomizu and Oshima 1987); Mn was determined by acid digestion with hydrochloric acid followed by atomic absorption (Miyazawa et al. 2009).
Data analysis
To test if there were significant effects (p < 0.05) of bacteria inoculation and the amount of P supplied on maize dry mass, root architecture, nutrient status and carboxylate concentration in the rhizosphere (dependent variables), and if the effects of the bacteria and the P supplied were dependent of each other, we performed a factorial analysis of variance (ANOVA). When necessary, the data were log-transformed in order to normalize and homogenize the variance (Zar 2010). For the analysis and graphs we used the software R v3.2.1 (R Development Core Team 2013).
To assess the effect of the carboxylate-exudation rates on plant dry mass, height, root length and aboveground P, N and Mn content, we performed a simple linear regression for both treatments (I-, I+), considering total carboxylates in the rhizosphere as the independent variable and plant traits as dependent variables.
Results
In general, plant dry mass and N, P and Mn content were not related to the inoculation (Table 2). However, plants root length and area were positively affected by inoculation (Table 2). Root length of the plants of I + 200 and I + 100 was greater than that of I-50. Root area of the plants of I + 200 differed from that of I-50 and I + 0, while plants of I-200 and I + 100 had a larger root area than that of I-50. The effects of P supply and inoculation on plants dry mass, root length and nutrient status are shown in supplementary material.
We expected a greater effect of the bacteria in plants growing without P fertilization. We did not find any effect of the bacteria on the nutrient status and the biomass of plants, and the effects of the bacteria in root architecture parameters were greater in treatments with more P supplied, while we expected the opposite.
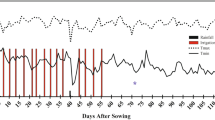
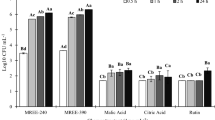
Interestingly, the carboxylate-exudation rate for both malic and citric acid was faster in inoculated treatments (Table 2, Fig. 1 a, b), but, surprisingly, it was not affected by the amount of P supplied (Table 2). The I + 200 plants showed a faster exudation rate of malic acid than the I-50 plants did, and a faster exudation rate of citric acid than I + 0 plants did.
Zea mays root carboxylate-exudation rates according to the inoculation and phosphorus (P) supply. The points represent the median and the segments indicate the maximum and minimum values obtained. Non-inoculated individuals (I-) (○). Inoculated individuals (I+) (●). Different letters indicate statistical differences between treatments using Tukey’s HSD test (p < 0.05)
For non-inoculated plants, the root architecture parameters were not correlated with root carboxylate-exudation rate (Fig. 2 a, b). However, for inoculated plants, root length and area were correlated with the exudation rate of citric acid. Also, the shoot Mn concentration was negatively correlated with the carboxylate-exudation rate for inoculated plants, whereas there was no correlation for uninoculated plants (Fig. 3).
Discussion
The inoculation of maize with A. brasilense resulted in greater root length and area, as reported in other studies (Kapulnik et al. 1985; Molina-Favero et al. 2008). Those effects are accounted for by phytohormones produced by the bacteria (Mantelin and Touraine 2004; Bashan and de-Bashan 2010) and are expected to increase the roots’ contact surface with soil which results in more water and nutrient uptake by the plant (Kapulnik et al. 1985; Bashan and Holguin 1997; Dobbelaere et al. 2001; Bashan et al. 2004). However, the shoot, root and total dry mass, and the nutrient contents of the plants were not affected by inoculation with A. brasilense. This suggests that the effects of A. brasilense in maize roots are not necessarily related to increase in plant dry mass and nutrient contents, and this is possibly the source of inconsistencies observed (Dobbelaere et al. 2001). Thus, it may be important to evaluate under which conditions the A. brasilense modifications of root architecture result in increased plant dry mass, yields and nutrient contents.
The root architecture parameters analyzed, used as a proxy of the A. brasilense inoculation effects in Z. mays, were positively correlated with the root exudation rates, corroborating our initial hypothesis. Interestingly, this correlation was found only for the inoculated treatment; furthermore, the inoculated treatment showed faster exudation rates. These results suggest that A. brasilense stimulated carboxylate exudation by maize roots; microorganisms are able to modify the nature and amounts of root exudates (Barber and Lynch 1977; Přikryl and Vančura 1980; Kraffczyk et al. 1984). This stimulation of carboxylate exudation may have major effects on the plant-microorganism interaction, because the effects of inoculation on plant parameters were enhanced with increased carboxylate-exudation rate. We provide evidence supporting a causal effect, in which faster carboxylate-exudation rates indirectly affect the root length and area. Organic acids are an important source of C and energy for rhizosphere microorganisms (Haichar et al. 2008; Lambers et al. 2009), stimulating their activity (Phillips et al. 2011), and also influencing bacterial flagellar mobility, adhesion and chemotaxis (Burdman et al. 2001; de Weert et al. 2002; Somers et al. 2004), and thus promoting rhizosphere colonization by microorganisms. Even small amounts of those compounds may activate beneficial soil microorganisms (‘priming effect’; Kuzyakov et al. 2000; Fontaine et al. 2003). Since we found a positive correlation between root parameters and carboxylate-exudation rate only for inoculated plants, the increased carboxylate-exudation rate likely affected the A. brasilense population, probably intensifying their production of phytohormones, which resulted in increased root length and area. This hypothesis is corroborated by the fact that leaf Mn concentration, a proxy for carboxylate concentrations in the rhizosphere (Lambers et al. 2015), was negatively correlated with root exudation rate of inoculated plants, suggesting that the exuded organic acids were consumed by the rhizobacteria, rather than accumulated. Therefore, our results suggest a positive feedback, in which the exudation of carboxylates is stimulated by A. brasilense; the carboxylates, in turn, are consumed by the rhizosphere microbial community, resulting in positive effects in the plant.
The present effect of A. brasilense on root architecture was positively related to P supply, and corroborates our initial hypothesis that P affects the interaction between the bacteria and plants. However, the mechanism of action differs from the one we proposed. We expected a negative correlation between P supply and the bacterial effects on plants, since these effects would be related to root exudation of carboxylates, a parameter that is usually negatively related to P availability in soil (Neumann and Römheld 1999). Instead, our results show that P fertilization affected the microbial activity. Fertilization has been reported to stimulate the soil microbial activity and biomass (Marschner et al. 2003; Chu et al. 2007), and this might be the case in our experiment. It is possible that the P addition stimulated A. brasilense, which, consequently, intensifies its effects in the plant. Furthermore, the root carboxylate-exudation rates were not related to the P supply and, thus, the effect of P in the studied interaction was not mediated by the carboxylate exudation in the manner we hypothesized, requiring further investigation.
In conclusion, our results suggest a positive feedback between A. brasilense and Z. mays, in which inoculation with A. brasilense stimulated the root carboxylate exudation, which affected the rhizosphere microbial community. The increased carboxylates exudation is, then, related to a more intense effect of the bacteria on the plant, such as the observed increase in root length and area. Phosphorus supply also positively affected the interaction between bacteria and plants, and our data suggest that this is a consequence of a direct effect of P supply on the activity of A. brasilense, intensifying its effects on the plant.
References
Abrahão A, Lambers H, Sawaya ACHF, Mazzafera P, Oliveira RS (2014) Convergence of a specialized root trait in plants from nutrient-impoverished soils: phosphorus-acquisition strategy in a nonmycorrhizal cactus. Oecologia 176:345–355. doi:10.1007/s00442-014-3033-4
Bais HP, Fall R, Vivanco JM (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319. doi:10.1104/pp.103.028712
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. doi:10.1146/annurev.arplant.57.032905.105159
Barber DA, Lynch JM (1977) Microbial growth in the rhizosphere. Soil Biol Biochem 9:305–308. doi:10.1016/0038-0717(77)90001-3
Bashan Y, De-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. Adv Agron 108:77–136. doi:10.1016/S0065-2113(10)08002-8
Bashan Y, Holguin G (1997) Azospirillum – plant relationships: environmental and physiological advances (1990–1996. Can J Microbiol 43:103–121. doi:10.1139/m97-015
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum - plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003). Can J Microbiol 50:521–577. doi:10.1139/w04-035
Bertsch PM, Bloom PR (1996) Aluminum. In: DL S, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis. Part 3 - chemical methods. Soil Science Society of America Inc., USA, pp. 517–550
Bottini R, Cassán F, Piccoli P (2004) Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl Microbiol Biotechnol 65:497–503. doi:10.1007/s00253-004-1696-1
Bouma TJ, Nielsen KL, Koutstaal B (2000) Sample preparation and scanning protocol for computerised analysis of root length and diameter. Plant Soil 218:185–196. doi:10.1023/A:1014905104017
Burdman S, Dulguerova G, Okon Y, Jurkevitch E (2001) Purification of the major outer membrane protein of Azospirillum brasilense, its affinity to plant roots, and its involvement in cell aggregation. Mol Plant-Microbe Interact MPMI 14:555–561. doi:10.1094/MPMI.2001.14.4.555
Chu H, Lin X, Fujii T, Morimoto S, Yagi K, Hu J, Zhang J (2007) Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biol Biochem 39:2971–2976. doi:10.1016/j.soilbio.2007.05.031
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678. doi:10.1016/j.soilbio.2009.11.024
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Chang 19:292–305. doi:10.1016/j.gloenvcha.2008.10.009
de Weert S, Vermeiren H, Mulders IH, Kuiper I, Hendrickx N, Bloemberg GV, Vanderleyden J, De Mot R, Lugtenberg BJ (2002) Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol Plant-Microbe Interact MPMI 15:1173–1180. doi:10.1094/MPMI.2002.15.11.1173
Dobbelaere S, Croonenborghs A, Thys A, Ptacek D, Vanderleyden J, Dutto P, Labandera-Gonzalez C, Caballero-Mellado J, Aguirre JF, Kapulnik Y, Brener S, Burdman S, Kadouri D, Sarig S, Okon Y (2001) Responses of agronomically important crops to inoculation with Azospirillum. Funct Plant Biol 28:871–879. doi:10.1071/PP01074
Fibach-Paldi S, Burdman S, Okon Y (2012) Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiol Lett 326:99–108. doi:10.1111/j.1574-6968.2011.02407.x
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843. doi:10.1016/S0038-0717(03)00123-8
Haichar FZ, Marol C, Berge O, Rangel-Castro JI, Prosser JI, Balesdent J, Heulin T, Achouak W (2008) Plant host habitat and root exudates shape soil bacterial community structure. ISME J 2:1221–1230. doi:10.1038/ismej.2008.80
Johnston AE, Poulton PR, Fixen PE, Curtin D (2014) Phosphorus: its efficient use in agriculture. Adv Agron 123:177–228
Kapulnik Y, Okon Y, Henis Y (1985) Changes in root morphology of wheat caused by Azospirillum inoculation. Can J Microbiol 31:881–887. doi:10.1139/m85-165
Kaspar TC, Ewing RP (1997) Rootedge: a software for measuring root length from desktop scanner images. Agron J 89:932–940. doi:10.2134/agronj1997.00021962008900060014x
Kraffczyk I, Trolldenier G, Beringer H (1984) Soluble root exudates of maize: influence of potassium supply and rhizosphere microorganisms. Soil Biol Biochem 16:315–322. doi:10.1016/0038-0717(84)90025-7
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498. doi:10.1016/S0038-0717(00)00084-5
Lambers H, Mougel C, Jaillard B, Hinsinger P (2009) Plant-microbe-soil interactions in the rhizosphere: an evolutionary perspective. Plant Soil 321:83–115. doi:10.1007/s11104-009-0042-x
Lambers H, Hayes PE, Laliberté E, Oliveira RS, Turner BL (2015) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90. doi:10.1016/j.tplants.2014.10.007
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428. doi:10.2136/sssaj1978.03615995004200030009x
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556. doi:10.1146/annurev.micro.62.081307.162918
Mantelin S, Touraine B (2004) Plant growth promoting bacteria and nitrate availability: impacts on root development and nitrate uptake. J Exp Bot 55:27–34. doi:10.1093/jxb/erh010
Marschner P, Kandeler E, Marschner B (2003) Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol Biochem 35:453–461. doi:10.1016/S0038-0717(02)00297-3
Miyazawa M, Pavan MA, Muraoka T, Carmo CAFS, Melo WJ (2009) Análise química de tecido vegetal. In: Silva FC (ed) Manual de análises químicas de solos, plantas e fertilizantes. Embrapa Informação Tecnológica, Brazil, pp. 191–234
Molina-Favero C, Creus CM, Simontacchi M, Puntarulo S, Lamattina L (2008) Aerobic nitric oxide production by Azospirillum brasilense sp245 and its influence on root architecture in tomato. Mol Plant-Microbe Interact 21:1001–1009. doi:10.1094/MPMI-21-7-1001
Motomizu S, Oshima M (1987) Spectrophotometric determination of phosphorus as orthophosphate based on solvent extraction of the ion associate of molybdophosphate with malachite green using flow injection. Analyst 112. doi:10.1039/AN9871200295
Neumann G, Römheld V (1999) Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 211:121–130. doi:10.1023/A:1004380832118
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14:187–194. doi:10.1111/j.1461-0248.2010.01570.x
Přikryl Z, Vančura V (1980) Root exudates of plants. Plant Soil 57:69–83. doi:10.1007/BF02139643
R Development Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna ISBN 3-900051-07-0, URL http://www.R-project.org
Rodriguez H, Gonzalez T, Goire I, Bashan Y (2004) Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften 91:552–555. doi:10.1007/s00114-004-0566-0
Roy ED, Richards PD, Martinelli LA, Coletta LD, Lins SRM, Vazquez FF, Willig E, Spera SA, VanWey LK, Porder S (2016) The phosphorus cost of agricultural intensification in the tropics. Nat Plants 16043. doi:10.1038/nplants.2016.43
Ryu CM, Farag MA, CH H, Reddy MS, Kloepper JW, Paré PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026. doi:10.1104/pp.900104
Shipley B, Keddy PA (1988) The relationship between relative growth rate and sensitivity to nutrient stress in twenty-eight species of emergent macrophytes. J Ecol 76:1101–1110. doi:10.2307/2260637
Somers E, Vanderleyden J, Srinivasan M (2004) Rhizosphere bacterial signalling: a love parade beneath our feet. Crit Rev Microbiol 30:205–240. doi:10.1080/10408410490468786
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448. doi:10.1111/j.1574-6976.2007.00072.x
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506. doi:10.1111/j.1574-6976.2000.tb00552.x
Thomas GW (1996) Soil pH and soil acidity. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis Part 3 - chemical methods. Soil Science Society of America Inc, USA, pp. 475–490
Thuita M, Pypers P, Herrmann L, Okalebo RJ, Othieno C, Muema E, Lesueur D (2012) Commercial rhizobial inoculants significantly enhance growth and nitrogen fixation of a promiscuous soybean variety in Kenyan soils. Biol Fertil Soils 48:87–96. doi:10.1007/s00374-011-0611-z
Tilman D (1999) Global environmental impacts of agricultural expansion: the need for sustainable and efficient practices. Proc Natl Acad Sci U S A 96:5995–6000. doi:10.1073/pnas.96.11.5995
van Raij B, Quaggio JA, Silva NM (1986) Extraction of phosphorus, potassium, calcium, and magnesium from soils by an ion-exchange resin procedure. Commun Soil Sci Plant Anal 17:547–566. doi:10.1080/00103628609367733
Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248:187–197. doi:10.1023/A:1022367312851
World Bank (2014). Fertilizer consumption (kilograms per hectare of arable land) http://data.worldbank.org/indicator/AG.CON.FERT.ZS/countries?display=graph. Accessed 9 Dec. 2014
Zar JH (2010) Data transformations. In: Zar JH (ed) Bioestatistical analysis, 5th edn. Pearson Education, USA, pp. 286–295
Acknowledgments
The study was financially supported by the Programa Institucional de Bolsa de Iniciação Científica (PIBIC), of the National Council of Technological and Scientific Development (CNPq), by São Paulo Research Foundation (FAPESP Grant no. 2010/17204-0, FAPESP/Microsoft Grant no. 2011/52072-0), and by the Universidade Estadual de Campinas (UNICAMP). RSO received research productivity scholarship from CNPq. The fellowship between UNICAMP and University of Western Australia (UWA) was granted by the Ciências sem Fronteiras (CsF) program (CAPES: 88887.108541/2015-00). We are thankful for four anonymous reviewers for their very insightful and constructive comments. We thank I. Marriel, of Embrapa- Milho e Sorgo (CNPMS), for the donation of maize seeds used in this experiment, and F.B. Sei, of Total Biotecnologia, for the donation of the inoculant of Azospirillum brasilense used in this experiment. We are also thankful to A. Abrahão for critical reading of this manuscript; and A.L. Mansur for the assistance with the installation of the experiment; and P. Mazzafera for the use of the equipment (FAPESP 2008/58035-6).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stéphane Compant.
Electronic supplementary material
ESM 1
(DOCX 82 kb)
Rights and permissions
About this article
Cite this article
D’Angioli, A.M., Viani, R.A.G., Lambers, H. et al. Inoculation with Azospirillum brasilense (Ab-V4, Ab-V5) increases Zea mays root carboxylate-exudation rates, dependent on soil phosphorus supply. Plant Soil 410, 499–507 (2017). https://doi.org/10.1007/s11104-016-3044-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3044-5