Abstract
Low effectiveness of native strains remains a limitation to soybean productivity in sub-Saharan Africa; while in other countries commercial inoculants are produced that provide effective strains that stimulate N fixation and growth. An experiment was set up to evaluate the response of a dual purpose promiscuous soybean variety (TGx1740-2F) and a non-promiscuous variety (Nyala) to commercial rhizobium inoculants in soils from central and coastal Kenya. Highest nodulation was observed in some of the treatments with commercial inoculants applied with nodule weights of 4.5 and 1.0 g plant−1 for TGx1740-2F and Nyala, respectively. Average biomass yields of TGx1740-2F (16 g plant−1) were twice as large as of Nyala (7.5 g plant−1) at the podding stage. Nitrogen fixation was higher in TGx1740-2F than in Nyala, and positively affected by a number of commercial inoculants with more than 50% N derived from the atmosphere. Nodule occupancy was 100% on both soybean varieties, indicating that the commercial strains were extremely infective in both of the tested soils. These results showed that commercial strains can be used to inoculate promiscuous soybean and enhance N fixation and yield.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacteria of the genera Azorhizobium, Bradyrhizobium, Mesorhizobium, Rhizobium, and Sinorhizobium, collectively known as rhizobia, enter into N2-fixing symbiosis with leguminous plants (Sprent 2001; Willems 2006). Rhizobium–legume interaction is specific and in most cases defined species of legumes may enter into symbiosis with only one (or few) bacterial species (Perret et al. 2000; Willems 2006). This symbiosis benefits the plants through biological nitrogen fixation (BNF) and takes place in specialized organs referred to as nodules, which can form on roots or stems. There are many restrictions to full development of nodules (Tewari et al. 2003); for example, in soybean, nodulation and BNF are readily suppressed by high concentrations of N in the soil (Salvagiotti et al. 2008).
Soybean is considered one of the oldest crops in the world, emerging as a domesticated plant in the eastern half of northern China around the eleventh century BC. The cultivated form of soybean is thought to have been introduced into Korea around the first century AD, and to Japan around the third century AD. A secondary gene center occurs in Indonesia, the Phillipines, Vietnam, Thailand, Malaysia, and Burma (Morse 1950; Hymowitz 1970). Soybean is thought to have been introduced into Europe before 1737 and into South America at the end of the nineteenth century (Hungria et al. 2006b). Soybean is relatively a specific host and does not nodulate when grown in the field for the first time in many parts including Africa (Giller 2001). Inoculation remains an important constraint in the production of soybean genotypes with specific Bradyrhizobium requirements. To overcome this problem, the soybean breeding program at the International Institute for Tropical Agriculture (IITA), Nigeria, developed soybean genotypes, designated TGx (tropical glycine cross), with reduced nodulation specificity and thus can nodulate effectively with indigenous Bradyrhizobium strains populations (Abaidoo et al. 2007). Trials with these promiscuous genotypes indicate that indigenous Bradyrhizobium populations do not always meet the N demand of the tested TGx genotypes in many locations in Nigeria (Okereke and Eaglesham 1993; Sanginga et al. 1996) and eastern and southern Africa (Mpepereki et al. 2000).
In Brazil and USA, inoculation is common practice for almost all soybean varieties (Hungria et al. 2006a; Peoples et al. 2009). According to Ferreira and Hungria (2002), more than 90% of the soils cropped to soybean have been inoculated at one time or another, and contain high populations of the strains used in commercial inoculants and this has led to economic benefit in terms of N-fertilizer saving of over US$ 2.5 billion per year (Alves et al. 2003). In Argentina, Melchiorre et al. (2011) reported estimates of up to 90% of soybean fields being inoculated. In Zimbabwe, yields of above 2 t ha−1 were achieved with systematic soybean inoculation (Mpepereki et al. 2000). However, the similar success has not been reported elsewhere in sub-Saharan Africa; while soybean is an important grain legume produced by smallholder farmers. This may in part be explained by the limited availability of effective rhizobial inoculants.
On the other hand, when many of the soybean promiscuous varieties selected in Nigeria were introduced for the first time in Kenya (western, central, and coastal province), all of them got nodulated by indigenous rhizobia (B. Vanlauwe, personal communication). Musiyima et al. (2005) showed similar results in Zimbabwe with different promiscuous soybean varieties. However, the presence of nodules does not mean that the symbiotic nitrogen fixation is effective and can significantly enhance the growth of soybean plants because of the possible ineffectiveness of native strains contained in the nodules as demonstrated by Zengeni and Giller (2007) in Zimbabwe on promiscuous soybean varieties. The need to practice the rhizobial inoculation to promiscuous soybean plants is still under discussion.
The present study aimed at answering the following question: would promiscuous soybean respond to commercial rhizobial inoculants developed elsewhere and to what level compared to non-promiscuous Nyala. We therefore set to evaluate the versatility of promiscuous soybean in response to commercial inoculants with different rhizobium strains in terms of biomass production, nodulation, and BNF. Rhizobial inoculants were obtained from different commercial producers all with different strains and/or formulations.
Materials and methods
Rhizobium inoculants
Commercial rhizobial inoculants were obtained from Becker Underwood (USA), Legume Technology (UK), and Soygro (South Africa) as shown in Table 1. The strain TSBF442 isolated at TSBF-CIAT (Kenya) (Wasike et al. 2009) and a reference strain (1495MAR) from Marondera Soil Productivity Research Laboratory in Zimbabwe (Zengeni and Giller 2007) were included. In addition, two commercial products (Twin-N and Leguspirflo, produced by Mapleton Inc., Australia and Soygro, South Africa, respectively) that are described to contain microorganisms such as Azorhizobium and Azospirillum as these supposedly also enhance or induce N fixation in crops were also tested. Vault LVL from Becker Underwood also contains Bacillus subtillis as a bio fungicide. Sojapak contains Bradyrhizobium, and Azospirillum, copper, and zinc.
Study soils
Two representative soils were collected from the central and coast provinces of Kenya, and were classified according to the World Reference Base for soil resources as Humic Nitisol and Rhodic Ferralsol soil classes, respectively (FAO 2006). The soils selected were responsive to both N and P fertilizer, contained a moderate level of organic matter, and had a near-neutral pH (Table 2). At the time of sampling, both fields were cultivated with maize without previous application of fertilizer or organic inputs, and had never been cultivated with soybean. The soils were collected from the 0–20 cm soil layer, shade-dried, and sieved to pass 4 mm and thoroughly homogenized.
Greenhouse experiment
A pot experiment was established in a greenhouse at TSBF-CIAT at the World Agroforestry Centre, Nairobi, Kenya. The effect of various rhizobia inoculants were evaluated in the two study soils for two soybean (Glycine max L. Merrill) varieties: Nyala, a variety bred in Zimbabwe was used as a specific nodulating control (Wasike et al. 2009) and TGx1740-2F that nodulates with indigenous Bradyrhizobium spp. populations (Abaidoo et al. 2007) was used as promiscuous test variety. TGx1740-2F is a cross between a specific nodulating North American soybean genotype and a non-specific (cowpea type) Asian soybean genotype (Kueneman et al. 1984) bred for promiscuous nodulation at IITA-Ibadan, Nigeria. The selected varieties have been released in Kenya and are widely adapted and resistant to major diseases. Nyala is characterized by a shorter maturity period (88–93 days to physiological maturity) than TGx1740-2F (95–100 days). Ten treatments were imposed: six with application of rhizobia inoculants, two with addition of products containing plant-growth-promoting rhizobacteria (PGPR), a negative control (without inoculation), and a positive control (with mineral N addition). In addition, maize (Zea mays L., cv. H513) was grown in both soils as a reference for BNF assessment using the 15N isotope natural abundance method (Mariotti et al. 1980; Herridge et al. 2008).
The trial was established in triplicate. Soil was weighed in 8-L PVC tubes (diameter = 15.6 cm), closed at the bottom by a double-nylon 1-mm mesh. Because of the difference in bulk density, tubes contained different soil masses per tube: 9.5 kg of coast soil and 6.4 kg of soil from central Kenya. All nutrients except for N were applied at optimal rates per pot−1 for crop growth (1,500 mg K, 540 mg Ca, 330 mg Mg, 120 mg S, 72 mg Mn, 3.0 mg Zn, 1.2 mg Cu, 1.8 mg B, 0.3 mg Mo, and 0.3 mg Co pot−1) calculated based on optimal nutrient tissue concentrations and an expected maximal biomass yield of 60 g dry matter (DM) plant−1. Because of the difference in P sorption capacity of the two soils (Table 2), P was applied at 20 and 270 mg P kg−1 in the coast and central Kenya soil, respectively, targeting a P concentration in solution of 0.2 mg P L−1 in both soils. In the mineral N positive control, N was applied as NH4NO3 at a rate of 800 mg N pot−1 (split applied at planting and at 3 weeks later). Nutrient solutions were prepared using salts KH2PO4, KCl, KNO3, MgSO4, CaCl2, MgCl2, ZnCl2, CuSO4, MnCl2, CoCl2, Na2MoO4, and Na2B4O7, and thoroughly mixed with the soil, prior to planting. Pots were randomly placed on tables in the greenhouse and rotated daily. The soil moisture content was brought to 18% and 27% (w/w) for the coast and central Kenya soil, respectively (approximately 90% of field capacity). Prior to planting, seeds were surface-sterilized using 3.3% Ca(OCl)2 for 5 min and rinsed five times with sterile distilled water.
Five soybean seeds were planted in each pot. Peat-based inoculants were applied on the seeds, while liquid inoculants were applied 2 days after emergence, at the base of the plantlets. Application rates per plant corresponded to triple the recommended rate in the field, converted using the optimal planting density (270,000 plants ha−1). During plant growth, the moisture content was restored daily by weighing pots and adding distilled water. Average minimum and maximum temperatures recorded in the greenhouse during plant growth were 18°C and 38°C, respectively. Soybean varieties were harvested at the full podding stage (R4), 57 and 71 days after planting for Nyala and TGx1740-2F, respectively. Plants were cut at 1 cm above the soil surface, using a thoroughly cleaned knife. Subsequently, roots were isolated by washing the whole tube contents over a 2-mm sieve. Nodules were isolated, counted, weighed, and stored in glycerol at −20°C. Root and shoot biomass samples were oven-dried (65°C), weighed, and ground.
Biological N fixation
Biological N fixation was determined using the 15N isotope natural abundance method (Boddey et al. 2000; Herridge et al. 2008). It relies on the commonly observed phenomenon that soil mineral N is usually slightly naturally enriched in the heavy isotope of N, 15N, compared to atmospheric N2 (Shearer et al. 1978). The 15N content of the plant shoots was determined using a stable isotope mass spectrometer (Europa Scientific ANCA 20-20/GSL), and the δ15N value and proportion of N fixed from the atmosphere (%Ndfa) were calculated. The relative reliance of the legume on N2 fixation was calculated by comparing the 15N composition of the N2-fixing legume to a non-N2-fixing reference plant such as maize or a non-nodulated soybean control (Shearer and Kohl 1986). The estimation was done using the formula:
Where: δ15N ref is the δ15N value of reference plants (value of N from sources other than atmospheric N2); δ15Nfix is the δ15N value of the total N in the N2-fixing soybean grown under conditions in which atmospheric N2 and N from other sources are available, and δ15N is the isotopic discrimination (B value) of fixed N in the N2-fixing soybean as measured in N2-fixing soybean forced to depend solely on fixed N by growing them hydroponically with N-free nutrient pots (Shearer and Kohl 1986).
For both varieties, a B value of −0.982 ‰ was used (Houngnandan et al. 2008). The δ15N value in maize was compared with values in treatment with non-nodulated soybean, to verify the suitability of the reference crop.
Nodule analysis
Before analysis the nodules were surface sterilized using 70% ethanol (30 s) and 3.3% Ca(ClO)2 (2 min) and rinsed with sterile distilled water thrice. One nodule was crushed in 150 μl of sterile water and DNA was directly extracted (Krasova-Wade et al. 2003; Rouvier et al. 1996; Thiao et al. 2004). Total genomic DNA from 30 (ten per rep) nodules per treatment was extracted separately.
DNA amplification and restriction
Genetic diversity was determined by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) amplification and restriction of the 16S–23S rDNA intergenic spacer region. A 930- to 1,100-bp intergenic region between the 16S and 23S rDNA was amplified by PCR using rhizobia specific primers derived from the 3′ end of the 16S rDNA (FGPS 1490-72; 5′-TGCGGCTGGATCCCCTCCTT-3′) (Navarro et al. 1992) and from the 5′ end of the 23S rDNA (FGPL 132-38; 5′-CCGGGTTTCCCCATTCGG-3′) (Ponsonet and Nesme 1994). PCR amplification was carried out in a 25-μl reaction volume containing 2 μl of total DNA extract, 10 pmol of each primer, and one freeze-dried bead (puReTaq Ready-To-Go PCR beads, GE Healthcare UK Ltd) containing 2.5 U of Taq DNA polymerase, 200 μM in 10 mM Tris–HCl (pH 9 at room temperature) of each dNTP, 50 mM KCl, and 1.5 mM MgCl2. PCR amplification was performed in a Bio-Rad iCyclerTM thermal cycler adjusted to the following program: initial denaturation for 5 min at 94°C, 35 cycles of denaturation (30 s at 94°C), annealing (30 s at 58°C) and extension (30 s at 72°C) and a final extension (7 min at 72°C). PCR products were visualized by electrophoresis of 3 μl of the amplified DNA on 2% horizontal agarose gel in TBE buffer (1.1% Tris–HCl, 0.1% Na2EDTA·2H2O and 0.55% boric acid), pre-stained with 0.033 mg ml−1 of ethidium bromide. The gel was photographed under UV illumination with Gel Doc (BIO-RAD) Software (USA). Aliquots (10 μl) of PCR products were digested with the restriction endonucleases MspI and HaeIII (5 U) in a total volume of 15 μl for 2 h at 37°C. The restriction fragments were separated by horizontal electrophoresis in 1× TBE buffer with 3% agarose gel pre-stained with 0.033 mg ml−1 of ethidium bromide. The gels were run at 100 V for 3 h and photographed under UV illumination with Gel Doc (BIO-RAD, USA) software. Strains with identical restriction fragment profiles (in individual fragment size and number) were classified into the same intergenic spacer (IGS) group.
Statistical analysis
Analysis of variance for dry biomass yield, nodule fresh weight, and nitrogen fixation was done using mixed procedures of the SAS System (SAS 2006) to assess the effects of (and interactions between) products, soils, and varieties. The effects of the various factors and their interactions were compared by computing least square means and standard errors of difference (SED); significance of difference was evaluated at P < 0.05, P < 0.01, and P < 0.001.
Results
Nodulation and biomass yield
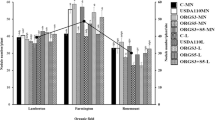
Emergence rates were not affected by treatments. The effects of the inoculants on nodule fresh weights differed between the two soils (independent of the soybean variety) (Fig. 1). There were significant (P < 0.05) two-way interactions between variety × inoculants (Fig. 1) and soil × variety (Fig. 1) and hence both application of Legumefix and Soyflo resulted in significantly (P < 0.05) higher nodule fresh weights in the central soil (3.4 and 3.8 g plant−1, respectively) relative to the soil from coast soil (2.3 and 2.2 g plant−1, respectively). For other inoculants, no differences between soils were observed. The effects of the inoculants on nodule fresh weights also differed significantly (P < 0.05) between the two soybean varieties (Fig. 1). For TGx1740-2F, controls had very low nodulation (Fig. 1) and all inoculants except Leguspirflo significantly increased nodulation. For TGx1740-2F, highest nodulation was observed in treatments with 1495MAR, Legumefix, Sojapak, Soyflo, and Vault LVL. Low nodulation was observed in the treatment with TSBF442. Nodules were generally fewer in Nyala than in TGx1740-2F, and consequently, higher nodule fresh weights were observed for TGx1740-2F (2.9 g plant−1) than for Nyala (0.5 g plant−1), averaged across all nodulated treatments (Fig. 1). In Nyala, no nodules were observed in the control, and only 1495MAR, Legumefix, and Vault LVL resulted in significant increases in nodule fresh weights.
a, b Nodule fresh weight in two soils and varieties as affected by addition of various inoculants. The error bars represents the standard error of the difference (SED) for the inoculant × soil and inoculants × variety interactions. Values marked by an asterisk are significantly (P < 0.05) larger than the control
The effect of the inoculants on shoot DM yield differed significantly between both soybean varieties and inoculants but soil did not significantly (P < 0.05) affect biomass yield (Fig. 2). In Nyala, none of the products increased shoot yield, relative to the control and significant (P < 0.05) negative effects of Soyflo and Twin-N were observed. In TGx1740-2F, addition of Soyflo and 1495MAR increased shoot DM yields, but yield increases were relatively small (13–20%). Average biomass yield for each plant of TGx1740-2F (16 g) was twice as large as of Nyala (7.5 g). Effects of the inoculants were independent of soil effects. Shoot DM yields of both varieties were about 30% higher in the soil from central Kenya than in the coast soil. In the soil from central Kenya, pod yields were similar for both varieties (on average 2.2 g plant−1) while in the coast soil, contrarily, pod yields were larger for Nyala (1.1 g plant−1) than for TGx1740-2F (0.5 g plant−1). Pod yields were not significantly affected by the inoculants (data not shown).
Biological N fixation
The δ15N-value observed in maize (7.0‰) was similar to the values observed in soybean for treatments without nodulation in the central Kenya soil. In the coast soil, however, the value in maize (8.4‰) was significantly lower than in soybean for treatments without nodulation, and the value for TGx1740-2F (12.1‰) was higher than for Nyala (10.0‰). For correct estimation of BNF in soybean using the natural abundance method, δ15N values observed in soybean for treatments without nodulation were used as reference values. There was no three-way interaction between soil × variety × inoculants but there were two-way interactions between soil and variety and hence means are shown for the interaction (Fig. 3a, b). In the control, no significant amount of N was fixed from the atmosphere by any of the varieties in any of the soils (Fig. 3a, b). The effect of the inoculants differed between the two varieties. In Nyala, none of the inoculants significantly increased %Ndfa, relative to the control. In TGx1740-2F, however, addition of 1495MAR, Legumefix, Soyflo, and Vault LVL significantly increased %Ndfa to about 60%, corresponding to 250–350 mg N fixed plant−1. Moderate increases in %Ndfa were observed in treatments with application of Sojapak and TSBF442. Other inoculants did not significantly affect %Ndfa. The effect of the inoculants differed significantly in the two soils and %Ndfa was about 30% higher in the coast soil than in the soil from central Kenya.
a, b Proportion of N derived from the atmosphere (%Ndfa) and quantity of N fixed as affected by addition of various inoculants in two soybean varieties (averaged across both soils). Error bars represent the standard error of the difference (SED) for the inoculants × variety soil interaction. Values marked by an asterisk are significantly (P < 0.05) larger than the control
Nodule analysis
In total five IGS groups were obtained from the inoculants. The nodules in the TGx1740-2F control were ineffective as they had a whitish-cream color when crashed during DNA extraction process and could not give a PCR product with the expected size (930–1,050 bp). Nyala did not nodulate after inoculation with Twin-N and Leguspirflo and nodulation was very low in the coastal soil after inoculation with TSBF442. Nyala only nodulated with commercial strains containing Bradyrhizobium sp.
Figure 4 shows IGS profiles for the PCR products obtained from pure cultures isolated from the inoculants before application and profiles obtained from the nodules after inoculation. Table 3 shows summary nodule occupancy obtained after analysis of the nodules. Nodule occupancy after PCR-RFLP was identical in both soybean varieties. Two profiles (II and V) presented in Fig. 4 did not correspond to any of the profiles from the applied inoculants. IGS group II was obtained from the central Kenya soils while IGS profile V was obtained from the coastal Kenya soil. Legumefix and Vault LVL had similar profiles (IGS group IV), while 1495MAR, Sojapak, and Soyflo had the same IGS profile (III) as expected for Soyflo and Sojapak.
Discussion
Controls for the TGx1740-2F genotype were poorly nodulated (less than ten nodules per plant) in both soils, and the nodules were ineffective as estimation of %Ndfa showed no BNF. This suggests that populations of indigenous rhizobia capable of nodulating soybean were very low in both soils. This was confirmed by MPN tests carried out on the same soils indicating rhizobial populations of <103 CFU g−1 of soil. Weaver and Frederick (1974) reported that a soil population density of at least 103 CFU g−1 of soil is required for maximizing nodule numbers on seedling tap roots, and efficient BNF to satisfy the N demand of soybean. Wasike et al. (2009) showed the occurrence of various native rhizobial populations nodulating promiscuous and non-promiscuous soybean varieties in soils from Mitunguu (central Kenya) and Bungoma (western Kenya) with a long history of soybean cultivation, contrary to the soils from coastal and central Kenya used in our study.
Interestingly, application of Twin-N and Leguspirflo increased nodulation in TGx1740-2F but not in Nyala, and two new profiles were found from IGS groups II and V in the soils from central and coastal Kenya, respectively. It has been demonstrated that nodulation can be enhanced by plant-growth-promoting rhizobacteria (Bashan and de-Bashan 2005; Saxena et al. 2006), and both Twin-N and Leguspirflo contain Azospirillum. PGPR capable of improving in certain conditions root growth and function, often leading to increased uptake of water and mineral nutrients (Matiru and Dakora 2004) and could have led to the better nodulation on TGx1740-2F as has been reported elsewhere (Lynch 1990; Lata and Tilak 2000). This increase in nodulation was however not accompanied by an increase in %Ndfa.
Soybean variety TGx1740-2F inoculated with strain TSBF442 showed an estimable number of nodules occupied by native strains from IGS groups II and V for the central and coastal Kenya soils, respectively, which were also found in treatments with application of Twin-N and Leguspirflo. This suggests that TSBF442 strain is probably less competitive than the strains contained in Legumefix, Soyflo, Sojapak, Vault LVL, and 1495MAR, which gave 100% occupancy for all the nodules analyzed. Analysis by PCR-RFLP and sequencing showed that the strain contained in Vault LVL and Legumefix was identical (Herrmann et al. 2010), and the products only differed in their formulation and the presence of Bacillus subtilis in Vault LVL. This could explain the difference in nodule and biomass yields from both inoculants. Xavier et al. (2004) demonstrated the importance of formulation for commercial production of rhizobial inoculants. It has also been shown that variant strains exhibit differences in N2 fixing efficiency (Barcellos et al. 2009; Bizarro et al. 2011) and partly may be due to adaptation to their new environment changes (Ferreira and Hungria 2002; Galli-Terasawa et al. 2003).
The amount of nitrogen fixed by 1495MAR, Legumefix, Soyflo, and Vault LVL (250–350 mg N fixed plant−1) compared favorably by results reported by Guimarães et al. (2008) who found about 500 mg plant−1 (at about 80% Ndfa) when a plant is grown to maturity. Other studies have reported %Ndfa values ranging between 26% and 87% (Sanginga et al. 1997) for soybean. The amounts of %Ndfa are also comparable to the averages values found in farmer’s fields and researcher managed trials (58% and 68%, respectively) (Herridge et al. 2008; Walley et al. 2007). Soybean variety TGx1740-2F had significantly higher biomass yield and pod numbers and this could be attributed to the higher N content as a result of higher N fixation and thus higher vegetative growth and podding. Sanginga et al. (1997) reported reliable estimates of N2 fixation by promiscuous genotypes in the Southern Guinea Savannah zones of Nigeria, and our results have demonstrated the same effect for the promiscuous variety TGx1740-2F. Nyala, in contrast, showed good nodulation response to inoculation but the %Ndfa was much lower. This could in part be the reason why conventionally bred soybean varieties do not contribute to the soil N status, as the N fixed is removed by the grains (Sanginga et al. 2003).
The wide variation in amounts of N fixed by different genotypes have been reported elsewhere and are attributed to the N-fixing capability of the genotype, the duration to maturity (and duration of N fixation), the relative fertility of the soils, and the bradyrhizobia species (Sanginga et al. 1997). Possibly, N mineralization after water addition to the dry soil released N with a lower δ15N value, which was utilized relatively more by the faster-growing maize than for soybean, and by the earlier-maturing soybean variety Nyala than by TGx1740-2F. It could also be attributed to the higher number of days required for maturity for TGx1740-2F than Nyala. Similar findings have also been reported (Carsky et al. 1997; Sanginga et al. 2000; Yusuf et al. 2008). Although our results are based on a greenhouse study, high correlation has been previously reported between the N2-fixing effectiveness of strains tested in the greenhouse and in the field, based on total plant weight and total N (Bremer et al. 1990). Hence, similar trends in inoculant performance would be expected under field conditions.
Conclusions
We conclude that promiscuous soybean varieties respond to inoculation, and nodulation, BNF, and biomass yield are improved if the strain is infective and effective. The commercial inoculants tested showed variability in their effectiveness with Legumefix, Vault LVL, and 1495MAR being the suitable inoculants for the promiscuous TGx1740-2F soybean variety, but not for non-promiscuous Nyala. Twin-N and Leguspirflo were found to be ineffective both in terms of stimulating BNF and plant growth. Commercial products produced elsewhere can be an important source of effective strains for use in areas where soybean is being introduced or where low populations of indigenous rhizobia hinder BNF. Further evaluation under field conditions is necessary to confirm these findings.
References
Abaidoo RC, Keyser HH, Singleton PW, Dashiell KE, Sanginga N (2007) Population size, distribution and symbiotic characteristics of indigenous Bradyrhizobium spp. that nodulate TGx soybean genotypes in Africa. Appl Soil Ecol 35:57–67
Food and Agricultural Organisation (2006) World reference base for soil resources, a framework for international classification, correlation and communication. 2nd Edition. Rome, Italy
Alves RBJ, Boddey MR, Urquiaga S (2003) The success of BNF in soybean in Brazil. Plant Soil 252:1–9
Barcellos GF, Batista JSS, Menna P, Hungria M (2009) Genetic differences between Bradyrhizobium japonicum variant strains contrasting in N2-fixation efficiency revealed by representational difference analysis. Arch Microbiol 191:113–122
Bashan Y, de-Bashan LE (2005) Bacteria/plant growth-promotion. In: Hillel D (ed) Encyclopedia of soils in the environment, vol. 1. Elsevier, Oxford, pp 103–115
Bizarro MJ, Giongo A, Vargas KL, Roesch LFW, Gano KA, de Sá ELS, Passaglia LMP, Selbac PA (2011) Genetic variability of soybean bradyrhizobia populations under different soil managements. Biol Fert Soils 47:357–362
Boddey RM, Peoples MB, Palmer B, Dart PJ (2000) Use of the 15 N natural abundance technique to quantify biological nitrogen fixation by woody perennials. Nutr Cycl Agroecosy 57:235–270
Bremer E, Van Kessel C, Nelson L, Rennie RJ, Rennie DA (1990) Selection of Rhizobium leguminosarum strains for lentil (Lens culinaris) under growth room and field conditions. Plant Soil 121:47–56
Carsky RJ, Abaidoo R, Dashiel KE, Sanginga N (1997) Effect of soybean on subsequent maize grain yield in Guinea Savannah of West Africa. Afr Crop Sci J 5(416):31–39
Ferreira MC, Hungria MH (2002) Recovery of soybean inoculant strains from uncropped soils in Brazil. Field Crops Res 79:139–152
Galli-Terasawa LV, Glienke-Blanco C, Hungria M (2003) Diversity of soybean rhizobial population adapted to a Cerrados soil. World J Microb Biotechnol 19:933–939
Giller KE (2001) Nitrogen fixation in tropical cropping systems, 2nd edn. CABI, Wallingford, p 423
Guimarães AP, de Morais RF, Urquiaga S, Boddey RM, Alves BJR (2008) Bradyrhizobium strain and the 15 N natural Abundance quantification of biological N2 fixation in soybean. Sci Agric (Piracicaba, Braz) 65:516–524
Herridge DF, Peoples MB, Boddey RM (2008) Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311:1–18
Herrmann L, Atieno OM, Okalebo J, Lesueur D (2010) Molecular identification of the strains in commercial products for improving agriculture in Africa. Poster presented at the 13th international symposium on microbial ecology. 22nd–27th August 2010. Seattle, USA
Houngnandan PR, Yemadje GH, Oikeh SO, Djidohokpin CF, Boeckx P, Van Cleemput O (2008) Improved estimation of biological nitrogen fixation of soybean cultivars (Glycine max L. Merrill) using 15 N natural abundance technique. Biol Fert Soils 45:175–183
Hungria M, Lı’gia MO, Chueire MM, Megı’as M, Lamrabet Y, Probanza A, Guttierrez-Mañero FJ, Campo RJ (2006a) Genetic diversity of indigenous tropical fast-growing rhizobia isolated from soybean nodules. Plant Soil 288:343–356
Hungria M, Campo RJ, Mendes IC, Graham PH (2006b) Contribution of biological nitrogen fixation to the nitrogen nutrition of grain crops in the tropics: the success of soybean (Glycine max L. Merr.) in South America. In: Singh RP, Shankar N, Jaiwal PK (eds) Nitrogen nutrition in plant productivity. Studium, Houston, pp 43–93
Hymowitz T (1970) On the domestication of the soybean. Econ Bot 24:408–421
Krasova-Wade T, Ndoye I, Braconnier S, Sarr B, de Lajuide P, Neyra M (2003) Diversity of indigenous bradyrhizobia associated with three cowpea cultivars (Vigna unguiculata L.) (Walp) grown under limited and favourable water conditions in Senegal (West Africa). Afr J Biotechnol 2:13–22
Kueneman EA, Root WR, Dashiell KE, Hohenberg J (1984) Breeding soybean for the tropics capable of nodulating effectively with indigenous Rhizobium spp. Plant Soil 82:387–396
Lata SAK, Tilak KVBR (2000) Biofertilizers to augment soil fertility and crop production. In: Krishna KR (ed) Soil fertility and crop production. Science Publishers, Enfield, pp 279–312
Lynch JM (1990) Beneficial interactions between microorganisms and roots. Biotech Adv 8:335–346
Mariotti A, Pierre D, Vedy JC, Brukert S, Guillemot J (1980) The abundance of natural 15 N in the organic matter of soils along an altitudinal gradient (Chablais, Haute Savoie, France). Catena 7:293–300
Matiru VN, Dakora FD (2004) Potential use of rhizobial bacteria as promoters of plant growth for increased yield in landraces of African cereal crops. Afr J Biotechnol 3:1–7
Melchiorre M, de Luca MJ, Anta GG, Suarez P, Lopez C, Lascano R, Racca RW (2011) Evaluation of bradyrhizobia strains isolated from field-grown soybean plants in Argentina as improved inoculants. Biol Fert Soils 47:81–89
Morse WJ (1950) History of soybean production. In: Markley KL (ed) Soybeans and soybean products. Interscience Publ. Inc., New York, pp 3–59
Mpepereki S, Javaheri F, Davis P, Giller KE (2000) Soybeans and sustainable agriculture: promiscuous soybeans in southern Africa. Field Crops Res 65:137–149
Musiyima K, Mpepereki S, Giller KE (2005) Symbiotic effectiveness and host ranges of indigenous nodulating promiscuous soybean varieties in Zimbabwean soils. Soil Biol Biochem 37:1169–1176
Navarro E, Simonet P, Normand P, Bardin R (1992) Characterization of natural population of Nitrobacter spp using PCR-RFLP analysis of ribosomal intergenic spacer. Arch Microbiol 157:107–115
Okereke GU, Eaglesham ARJ (1993) Nodulation and nitrogen fixation by 79 promiscuous soybean genotypes in soil in eastern Nigeria. Agro Afrique 2:113–122
Peoples MB, Brockwel J, Herridge DF, Rochester IJ, Alves BRJ, Urquiaga S, Boddey RM, Dakora FD, Bhattarai S, Maskey SL, Sampet C, Rerkasem B, Khans DF, Hauggaard-Nielsen H, Jensen BS (2009) The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 48:1–17
Perret X, Staehelin C, Spaink HP (2000) Molecular basis of symbiotic promiscuity. Microbiol Mol Biol R 64:180–201
Ponsonet C, Nesme X (1994) Identification of agrobacterium strains by PCR-RFLP analysis of pTi and chromosomal regions. Arch Microbiol 16:300–309
Rouvier C, Prin Y, Reddel P, Normand P, Simonet P (1996) Genetic diversity among Frankia strains nodulating members of the family Casuarinaceae in Australia revealed by PCR and restriction fragment length polymorphism analysis with crushed root nodules. Appl Environ Microbiol 62:979–985
Salvagiotti F, Cassman KG, Specht JE, Walters DT, Weiss A, Dobermann (2008) Nitrogen uptake, fixation and response to fertilizer N in soybean: a review. Field Crops Res 108:1–13
Sanginga N, Abaidoo RC, Dashiell K, Carsky RJ, Okogun JA (1996) Persistence and effectiveness of rhizobia nodulating promiscuous soybeans in moist savanna zones of Nigeria. Appl Soil Ecol 3:215–224
Sanginga N, Dashiell K, Okogun JA, Thottappilly G (1997) Nitrogen fixation and N contribution by promiscuous nodulating soybeans in the southern Guinea savanna of Nigeria. Plant Soil 195:257–266
Sanginga N, Thottappilly G, Dashiell KE (2000) Effectiveness of rhizobia nodulating recent promiscuous soybean selections in the moist savannah of Nigeria. Soil Biol Biochem 32:215–224
Sanginga N, Dashiell K, Diels J, Vanlauwe B, Lyasse O, Carsky RJ, Tarawali S, Asafo-Adjei B, Menkir A, Schulz S, Singh BB, Chikoye D, Keatinge D, Rodomiro O (2003) Sustainable resource management coupled to resilient germplasm to provide new intensive cereal–grain legume–livestock systems in the dry savanna. Agr Ecosyst Environ 100:305–314
SAS Institute Inc (2006) SAS user’s guide: statistics. SAS Institute Inc, Cary
Saxena AK, Shende R, Grover M (2006) Interactions among beneficial microorganisms. In: Mukerirji KG, Manoharachary C, Singh J (eds) Microbial activity in the rhizosphere. Springer, Berlin, pp 121–132
Shearer GB, Kohl DH (1986) N2-fixation in field settings: estimations based on natural 15 N abundance. Austr J Plant Physiol 13:699–756
Shearer G, Kohl DH, Chien SH (1978) The 15 N abundance in a wide variety of soils. Soil Sci Soc Am J 42:899–902
Sprent JI (2001) Nodulation in legumes. Royal Botanic Gardens, Kew
Tewari K, Minagawa R, Suganuma T, Fujikake H, Ohtake N, Sueyoshi K, Takahashi Y, Ohyama T, Tsuchida T (2003) Effect of deep placement of slow release nitrogen fertilizers and inoculation of bradyrhizobia on the first cropping of soybean in the field dressed with mountain soil. Soil Sci Plant Nutr 74:183–189
Thiao M, Neyra M, Isidore E, Sylla S, Lesueur D (2004) Diversity and effectiveness of rhizobium from Gliricidia sepium native to Reunion Island, Kenya and New Caledonia. World J Microb Biot 20:703–709
Walley FL, Clayton GW, Miller PR, Carr PM, Lafond GP (2007) Nitrogen economy of pulse production in the northern great plains. Agron J 99:1710–1718
Wasike VW, Lesueur D, Wachira FN, Mungai NW, Mumera LM, Sanginga N, Mburu HN, Mugadi D, Wango P, Vanlauwe B (2009) Genetic diversity of indigenous Bradyrhizobium nodulating promiscuous soybean [Glycine max (L) Merr.] varieties in Kenya: impact of phosphorus and lime fertilization in two contrasting sites. Plant Soil 322:151–163
Weaver RW, Frederick LR (1974) A new technique for most probable-number counts of rhizobia. Plant Soil 36:219–222
Willems A (2006) The taxonomy of rhizobia: an overview. Plant Soil 287:3–14
Xavier IJ, Holloway G, Leggett M (2004) Development of rhizobial inoculant formulations. Online. Crop Management doi:10.1094/CM-2004-0301-06-RV
Yusuf AA, Abaidoo RC, Iwuafor ENO, Olufajo OO (2008) Genotype effects of cowpea and soybean on nodulation, N2 fixation and N balance in the Northern Guinea savannah of Nigeria. Agron J 7:258–264
Zengeni R, Giller KE (2007) Effectiveness of indigenous soybean rhizobial isolates to fix nitrogen under field conditions of Zimbabwe. Symbiosis 43:129–135
Acknowledgment
This work was funded by the Bill and Melinda Gates Foundation through the Tropical Soil Biology and Fertility Institute of CIAT (TSBF-CIAT) project on commercial microbial products. We gratefully acknowledge the contributions of Edwin Mutegi, Phillip Malala, Harrison Mburu, Elias Mwangi, Ruth Mukhongo, and Samuel Mathu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thuita, M., Pypers, P., Herrmann, L. et al. Commercial rhizobial inoculants significantly enhance growth and nitrogen fixation of a promiscuous soybean variety in Kenyan soils. Biol Fertil Soils 48, 87–96 (2012). https://doi.org/10.1007/s00374-011-0611-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-011-0611-z