Abstract
Background and aims
Changes to soil nutrient concentrations following vegetation fire may affect biogeochemical cycling and foliar stoichiometry. Phosphorus (P)-limited plant communities are widespread and may be particularly sensitive to fire, but have received relatively little research attention in this context.
Methods
We measured soil nutrient concentrations, foliar carbon (C), nitrogen (N) and P stoichiometry of understorey plants in a recently, frequently burned eucalyptus forest area in south-east Queensland, Australia, and compared these properties to an adjacent unburned area.
Results
Surface soils in the area subjected to relatively recent, frequent prescribed burning had higher P concentrations than those in the adjacent unburned area, although this did not include the ‘available’ forms of P. All plant species had high foliar N:P ratios, regardless of fire history, consistent with widespread P-limitation. Some species had lower foliar N:P ratios in the burned area, indicating interspecific variation in nutrient requirements and burning responses. The nutrient resorption proficiencies of a grasstree (Xanthorrhoea johnsonii Lee) were lower in the burned area, suggesting that the nutrient cycling of this species was made less conservative by burning.
Conclusions
The stoichiometric patterns observed in the responses of plants to prescribed burning highlight the significance of fire in this P-impoverished plant community, and suggest the potential value of stoichiometric approaches in fire ecology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prescribed burning is a commonly used forest management practice around the world, and given the expected increases in wildfire potential in the future (e.g. Liu et al. 2010) as well as the recent occurrence of catastrophic wildfires globally (e.g. the 2009 Australian ‘Black Saturday’ forest fires), agencies are under pressure to increase the extent and frequency of prescribed burning (e.g. Victorian Government 2010). An improved understanding of the ecological effects of prescribed burning is therefore vital to achieving positive ecological outcomes while simultaneously reducing the risk of wildfires. One aspect to consider is whether and how fire-induced changes in the concentrations and cycling of nutrients in soil affect the stoichiometric balances of nutrients in plant biomass in the long-term, as such changes may reflect considerable perturbation of nutrient cycling within the plant-soil system. Further, plant biomass stoichiometry may be related to productivity (Elser et al. 2010), rates of herbivory (Pérez-Harguindeguy et al. 2003) and litter decomposition (Güsewell and Gessner 2009), and overall ecosystem dynamics (e.g. Sterner and Elser 2002), while interspecific variation in plant responses to altered nutrient supply may influence community composition (Tilman 1982).
Fire may increase the concentrations of soil available nitrogen (N) and phosphorus (P) in the short-term through the deposition of nutrient-rich ash (Certini 2005; Covington and Sackett 1992; Giardina et al. 2000; González-Pérez et al. 2004). This effect is particularly well-known for common measures of available N and P (e.g. KCl-extractable mineral N, Bray-P, Olsen-P) in Australian ecosystems (Romanyà et al. 1994; Tomkins et al. 1991). However, the high temperatures reached during forest fires often result in losses of forest floor N through volatilization, whereas P does not tend to be lost through this process (Certini 2005; Nave et al. 2011; Pivello and Coutinho 1992). The disproportionate volatilization of N versus P suggests that fire may be particularly important for the function of ecosystems where P tends to limit plant growth. Approximately half of all terrestrial ecosystems worldwide are thought to be limited by P (Elser et al. 2007); however N-limited plant communities appear to have received relatively more attention than P-limited communities in previous studies of fire’s relationships with biogeochemical cycling. Further, the results of these studies have not been fully consistent, with some studies reporting reduced foliar C:nutrient ratios following burning (e.g. Cui et al. 2010; Schafer and Mack 2010), and others reporting an absence of stoichiometric effects in plants (e.g. Pellegrini et al. 2015).
A review into fire’s effects on foliar N and P concentrations found that nutrient inputs associated with burning lead to increased foliar N and P concentrations in woody vegetation when N and P were limiting, respectively (Dijkstra and Adams 2015). The authors thus concluded that fire served to ease N and P imbalances in vegetation. However, Dijkstra and Adams (2015) focused on the effects of fire within 4 years of burning, and within that time effects diminished rapidly with time since fire. Further, there were no relationships observed between fire’s effects on soil and foliar nutrients. Thus, it seems plausible that the stoichiometric effects observed by Dijkstra and Adams (2015) were driven by short-term, post-fire pulses of soil nutrient availability and redistribution of plant nutrients stimulated by loss of above-ground biomass, both of which may be relatively transient effects (e.g. Van de Vijver et al. 1999). In comparison, long-term changes in foliar stoichiometry in post-fire environments, particularly those coinciding with fire-altered soil nutrient concentrations or fractionation, may be indicative of significant changes in the nutrient cycling of plant-soil systems. Foliar stoichiometry shifts of this nature may be significant to ecological structure and function over longer time periods.
The influence of fire on plant stoichiometry also encompasses changes to foliar nutrient resorption patterns, and we are aware of only a small number of studies into such effects (e.g. Huang and Boerner 2007, Lü et al. 2011). Huang and Boerner (2007) observed lower N and P resorption efficiencies following fire (the percentage reductions of N and P from green leaves to senescent leaves, hereafter referred to as NRE and PRE respectively), whereas Lü et al. (2011) found that NRE increased for some grassland species after annual burning, while PRE and the resorption proficiencies of N and P (the concentrations of N and P remaining in senescent leaves, with lower concentrations corresponding to higher resorption proficiency; NRP and PRP respectively) were reduced (Lü et al. 2011). Unlike Huang and Boerner (2007); Lü et al. (2011) attributed these effects to fire-altered soil nutrient availabilities. In general, however, the relationships between soil nutrient availability and nutrient resorption are becoming increasingly clear, such that resorption proficiency represents a sensitive measure of plant responses to soil N and P supply (Rejmánková 2005).
Here we present the findings of a preliminary study that compared foliar C:N:P stoichiometry of six species and nutrient resorption of one species between two areas of a subtropical lowland eucalypt forest with differing fire histories in south-east Queensland, Australia. We aimed to determine if fire altered soil nutrient concentrations and foliar C:N:P stoichiometry in the longer-term (i.e. > 4 years since fire), and in doing so we hoped to eliminate the likely short-lived stoichiometric effects associated with ash-bed or nutrient-pulse effects, flushes of new growth and the re-allocation of plant nutrients following losses of above-ground plant biomass. Past studies indicate that soils in our study location are very low in P, to the extent that microbial biomass tends to occlude available P such that increases in P associated with fire may remain longer than those typically associated with the ash-bed effect (Huang et al. 2013). Thus, we anticipated an overall trend of high foliar N:P ratios in all species as evidence of widespread P-limitation, which is often the case in sub-tropical forests (Güsewell et al. 2003; 2004; Vitousek 1984). Further, we hypothesized that repeated prescribed burning would be associated with higher soil P concentrations, particularly in rapidly-cycled labile P pools, and these would be reflected in foliar stoichiometry shifts in favour of P. In addition, we expected foliar N and P resorption proficiencies to be lower as a result of elevated soil N and particularly P concentrations in recently burned areas.
Materials and methods
Study site and study design
Toohey Forest is a remnant fragment of open eucalypt forest in south-east Queensland, Australia, covering an area of 681 ha and surrounded by residential suburbs of the greater Brisbane region. Average annual rainfall for the region is 1030 mm (1981–2015) and average daily mean temperature is 20.5 °C (1981–2015). A study site was selected near the northern perimeter of Toohey Forest (−27.5400, 153.0501) in an area dominated by Eucalyptus crebra F. Muell and Corymbia citriodora (Hook.) K. D. Hill & L. Johnson with Allocasuarina littoralis (Salisb.) L. Johnson and Xanthorrhoea johnsonii Lee prevalent in the understorey. Soils on the site are acidic and have previously been characterized as podzolics associated with the Pullenvale soil landscape (Beckman 1967; Catterall and Wallace 1987).
Within the study site there were two areas of forest with different prescribed burning histories separated by a walking track. The first area was last subjected to a low-intensity prescribed burn in June 1999, and was referred to as the ‘no recent fire’ (NRF) area. The second area was burned at a low intensity in September 2009, and previously in April 2008, May 2003 and (ca.) 1994. As a result, the effects of time since fire on soil and plant properties are confounded with those of fire frequency; therefore the second area was referred to as the recently, frequently burned (RFB) area. It was assumed that the NRF and RFB areas were highly comparable in all properties other than burning regime (e.g. soil profile and pedology, vegetation community) such that differences between them represent the influence of fire history. Five sampling locations were established in both areas, spaced 15–20 m apart along two parallel transects that were separated by the walking track. Sampling locations were paired between the NRF and RFB areas on the basis of proximity. Our study is somewhat limited by this basic design; however comparisons of different fire histories based on what is effectively pseudo-replication are common throughout the fire ecology literature (e.g. Badía et al. 2014; Blank et al. 1994; Giardina and Rhoades 2001).
Sample collection and chemical analysis
Samples of soil and leaves were collected from each of the two areas on 17-Jan.-2014. Composite soil samples consisting of five soil cores (0–10 cm) were obtained from each sampling location using a soil auger (7 cm diameter). Additional samples were collected at each sampling location using a bulk-density ring in order to estimate soil bulk density. Foliar samples were collected from the six dominant understorey species (representing six families) at each sampling location. Each leaf sample consisted of 10–20 mature, green, and intact leaves from an individual plant, such that each individual represented one sample (with five pairs of samples per species and ten individuals per species sampled in total). Individuals of roughly the same size were selected where possible to account for variation in foliar nutrient content with age.
The selected species included the two dominant understorey tree species Acacia leiocalyx (Domin) Pedley (Mimosaceae), Allocasuarina littoralis (Salisb.) L. Johnson (Casuarinaceae), a prevalent shrub species Hakea plurinervia F. Muell. ex. Benth. (Proteaceae), two dominant ground cover species Acrotriche aggregata R. Brown (Epacridaceae) and Entolasia stricta (R. Br.) Hughes (Poaceae), and a highly prevalent grasstree Xanthorrhoea johnsonii Lee (Xanthorrhoeaceae). These species were selected largely based on their prevalence and because they represented a range of functional groups and fire response types which provided some basis for interpretation of potential stoichiometric effects. Further, we focused on understorey species because they may be particularly responsive to changes in fire regime (e.g. Lewis et al. 2012). Acacia leiocalyx and A. littoralis are both N-fixing species, and may therefore be advantaged by the hypothesized changes in soil N and P concentrations associated with increased fire frequencies, given than N-fixation can be P-limited under some circumstances (Augusto et al. 2013). There is some evidence that the post-fire re-growth rate of A. leiocalyx is relatively high due to high rates of N-fixation (Bai et al. 2013), while A. littoralis may instead favour the long-term absence of fire (Lunt 1998). Comparison of post-fire foliar stoichiometry shifts between these species may indicate whether nutrition plays a role in these responses. In addition, Hakea plurinervia is a member of the Proteaceae family, which is well adapted to low-P soils and may be susceptible to P-toxicity (Lamont 2003; Ozanne and Specht 1981). As a result, the expected increases in soil P due to fire may inhibit the growth of H. plurinervia, and reductions in foliar N:P may indicate an increased potential for P-toxicity in the post-fire environment.
To investigate the effects of fire history on foliar resorption patterns, we collected samples of newly senescent leaves from X. johnsonii individuals in addition to mature green leaves. We focused on resorption in X. johnsonii because there is some evidence that fire stimulates grasstree reproduction through a localized ash-bed effect (Bülow-Olsen et al. 1982; Lamont et al. 2004), which would be regulated to some extent by the nutrient concentration of the highly-flammable senescent leaves; however we are not aware of any investigations into such a relationship for this species. The sample size for X. johnsonii was double that of other species due to our focus on foliar resorption, such that there were ten sampling locations along each transect rather than five for both green and senescent leaves, with the additional locations positioned between the main locations. Senescent leaves of X. johnsonii were defined as the dry, brown leaves that comprise the highly flammable ‘skirt’ characteristic of the species, and care was taken to sample only the top layer of senescent leaves on the skirt to ensure similar resorption periods among individuals.
Soil samples were sieved to 2 mm and air-dried for 2 weeks, while leaf samples were oven dried (105 °C for 2 h, then 65 °C for 1 week). Basic properties of soil samples were determined using standard methods (pH and EC measured in water at 1:5 soil to water ratio, clay content determined using the hydrometer method). Soil and leaf samples were finely ground (<150 μm) and analyzed for total C and total N concentrations using a Leco TruMac TCN Determinator. Soil soluble organic C and soluble total N concentrations were determined using hot-water extraction methods (Chen et al. 2005; Sparling et al. 1998; Tutua et al. 2013) wherein samples were incubated in 40 ml water at 70 °C for 16 h, centrifuged at 10,000 rpm for 10 min and filtered with Whatman 42 filter papers. The filtrates were then analyzed using a Shimadzu TOC-VCPH/CPN analyzer (which is also fitted with a TN unit).
The total P, inorganic P and organic P concentrations in soil samples were determined according to the Saunders and Williams (1955) ignition method. In brief, samples were combusted in a furnace at 550 °C for 1 h, then extracted on an end-to-end shaker for 1 h with 50 ml of 0.5 M H2SO4 solution and filtered using Whatman 42 filter papers. Another portion of soil samples were directly extracted with 50 ml of 0.5 M H2SO4 without the ignition. The P concentration in the extracts was determined by the molybdenum-blue colorimetric method (Murphy and Riley 1962). The P concentration in the ignited soils was considered an estimate of total soil P, while that in the non-ignited soils inorganic P. Organic P was calculated as the difference between total soil P and inorganic P. To determine soil soluble total P in the hot water extracts as above, 5 ml aliquots of extracts were autoclaved for 90 min at 121 °C with 0.6 g of K2S2O7 and 10 ml of 0.9 M H2SO4 solution prior to colorimetric analysis. Further, we measured two indices of plant-available P using 0.5 M NaHCO3 (Olsen P) and NH4F in dilute HCl (Bray-1 P) extractants (Rayment and Lyons 2010). Leaf P concentrations of finely ground samples were determined by nitric and perchloric acid digestion followed by spectrophotometry (Jackson 1958).
Calculations and statistical analysis
Foliar C:N:P ratios were calculated on a dry-mass basis. Resorption proficiency was equivalent to the nutrient concentration in senescent leaves. It is important to note that resorption proficiency is a measure of a plant’s ability to reduce nutrients in senescing leaves to minimal levels, regardless of initial green leaf nutrient concentration, with a lower nutrient concentration in senescent leaves corresponding to a higher nutrient proficiency of that nutrient (Killingbeck 1996). Resorption efficiency was calculated as the percentage of nutrient concentration reduction from green leaves to senescent leaves. Data were tested for normality using the Shapiro-Wilk test and were log-transformed prior to subsequent analyses if necessary. Means of NRF and RFB areas were compared using two-tailed Student’s paired t-tests, with each species analysed separately, while correlation analyses (Pearson’s) were used to examine relationships among foliar stoichiometry and patterns of foliar nutrient resorption. Statistical analyses were performed using Statistix 8.0 software (Analytical Software, Tallahassee, FL, USA), with significance determined at P < 0.05.
Results
Overall, there were no significant differences in basic soil properties between the NRF and RFB areas, although the clay content was slightly higher in the RFB area (Table 1). Mean concentrations of total P, organic P, soluble total P and inorganic P in RFB area soil samples were 83 %, 87 %, 45 % and 53 % higher than soils from the NRF area respectively (P-values <0.05; Table 1). Neither form of soil available P had significantly different concentrations between the two areas. The soil total P:clay and organic P:clay ratios were substantially higher in the RFB area than in the NRF area, which suggests that the perceived increase in soil P concentration in the RFB area represents a genuine effect of fire, and is not merely an original effect of clay content and associated P sorption (Table 1). Soil total N and soluble total N concentrations were significantly higher in the RFB area than in the NRF area.
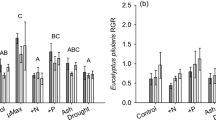
Green leaf N and P concentrations were extremely low in all species studied (total N ranged from 0.5–1.8 %, total P ranged from 0.018–0.039 %). For reference, concentrations of 0.3 % for N and 0.01 % for P are used to represent ‘complete’ resorption in woody perennial species (Killingbeck 1996). We believe this is due to the nutrient-poor soils on the study site which has led to the dominance of species with very low nutrient requirements, or specialized nutrient acquisition strategies (e.g. N-fixation, proteoid roots). Mean foliar N concentration in X. johnsonii in the RFB area was 27 % higher than in the NRF area (P = 0.001), and this corresponded to significantly lower foliar C:N ratios (P = 0.001; Fig. 1a). However, fire history had no effect on the foliar N concentrations and C:N ratios of any other species. The concentrations of P in leaves of A. leiocalyx, A. aggregata and H. plurinervia were substantially higher in the RFB area than in the NRF area (Table 2), but this only corresponded to significantly lower C:P ratios in A. aggregata (P = 0.038; Fig. 1b). Finally, foliar N:P ratios were 22 %, 16 % and 25 % lower in the RFB area for A. leiocalyx, A. aggregata and H. plurinervia, respectively (P-values of Student’s t-test mean comparisons were 0.026, 0.023 and 0.045 respectively; Fig. 1c).
Comparisons of observed means (+SE) for (a) foliar C:N ratios, (b) foliar C:P ratios and (c) foliar N:P ratios between ‘no recent fire’ areas (NRF, dark grey bars) and recently, frequently burned areas (RFB, light grey bars); N = 5 in all cases, except Xanthorrhoea johnsonii where n = 10; significant differences between treatment means denoted * (Student’s paired t-test P-values <0.05)
Foliar N resorption proficiency (NRP) and P resorption proficiency (PRP) of X. johnsonii were extremely low, and the PRP values were an order of magnitude lower than the complete resorption values estimated by Killingbeck (1996). However, Xanthorrhoea are highly-specialized and adapted to very low-nutrient soils, and some species within the genus (e.g. X. preissii) are able to withdraw up to 84 % of the already-low foliar P (Lamont et al. 2004), such that the values given by Killingbeck (1996) may not be appropriate for comparison. Foliar NRP and PRP were 30 % and 27 % lower in the RFB area than in the NRF area (Student’s paired t-test P-values of 0.002 and 0.016, respectively; Fig. 2), and were positively correlated (Fig. 3). In contrast, the resorption efficiencies of N and P were not strongly correlated or affected by fire history (P-values >0.05 in all cases, Fig. S1). Further, resorption proficiencies showed a positive linear relationship with the nutrient concentration of green leaves, while resorption efficiencies did not (Fig. 4). Instead, NRE was positively correlated with foliar N:P (P-value =0.021, r2 = 0.26), but not with foliar P concentration (P-value >0.05), whereas PRE did not display a strong relationship with foliar N:P (Fig. S2a).
Pairwise comparisons of foliar N and P resorption proficiencies (observed means + SE) in Xanthorrhoea johnsonii between ‘no recent fire’ areas (NRF; dark grey bars) and recently, frequently burned areas (RFB; light grey bars); N = 10 in all cases; significant differences between treatment means denoted * (Student’s paired t-test P-values <0.05)
a Pearson’s correlations between foliar N resorption efficiency (NRE; filled circles) and foliar [N] (%; P-value >0.05, N = 20), and foliar N resorption proficiency (NRP; open triangles) and foliar [N] (%; P-value =0.0001, r2 = 0.59, N = 20) in Xanthorrhoea johnsonii; b Pearson’s correlations between foliar P resorption efficiency (PRE; filled circles) and foliar [P] (%; P-value >0.05, N = 20), and log-transformed foliar P resorption proficiency (lnPRP; back-transformed data shown; open triangles) and foliar [P] (%; P-value =0.009, r2 = 0.34, N = 20) in X. johnsonii
Discussion
Soil nutrients and foliar C:N:P stoichiometry
The results of this study were generally consistent with previous studies in that prescribed fire altered both soil nutrient concentrations and foliar C:N:P stoichiometry (e.g. Cui et al. 2010; Payton et al. 1986), although results were inconsistent between species and stronger for C:P and N:P than for C:N. Given the 4 year gap between the most recent fire in the RFB area and our sampling date, it seems unlikely that these stoichiometry shifts were simply a result of nutrient pulses associated with the ash-bed effect, or of the re-allocation of nutrients associated with the loss of above-ground biomass and subsequent re-sprouting, as these tend to be relatively short-term effects (Schafer and Mack 2010; Van de Vijver et al. 1999). Instead, we suggest that the repeated return of P from litter and living plant material to soil in ash has led to altered P fractionation and below-ground P cycling, such that available P in ash was rapidly converted into labile and organic forms by microbial activity. This was supported by the higher organic and soluble total P (i.e. weakly sorbed inorganic and labile organic P, see Tutua et al. 2013) concentrations in the RFB soils and an absence of differences in available P pools between the NRF and RFB soils (Table 1). Although ‘labile’ forms of P may not be immediately available for plant uptake, they are less prone to leaching and are cycled relatively rapidly, making them critical to plant nutrition in P-poor soils on timescales longer than those typically associated with ash-bed effects (e.g. Chambers and Attiwill 1994). Similar patterns have been observed elsewhere in Toohey Forest (Huang et al. 2013), and we argue that these effects contributed to the long-term shifts in foliar stoichiometry in our study. These results were generally consistent with our hypothesis, and indicate that fire can modify biogeochemical cycling in the plant-soil system for several years. Further, our results demonstrate that traditional measurements of soil available P (e.g. Bray, Olsen and Colwell P) are well-complemented by consideration of other P pools when investigating the effect of fire on plant nutrition.
The elevated soil P concentrations associated with relatively recent, frequent prescribed burning represent a crucial role for fire in this ecosystem, given the evidence for a widespread state of P-limitation for biomass production provided by the relatively high foliar N:P ratios of all species (Güsewell et al. 2003). High foliar N:P ratios were consistent with our initial expectations and may reflect the inherently low-P status of the soils in the study site. While the low foliar N:P ratios of the shrub H. plurinervia may indicate N and P co-limitation at this site, the magnitudes of the N:P ratios of all other species (Fig. 1c), along with the N-fixing abilities of A. leiocalyx and A. littoralis, might suggest a prevailing state of P-limitation. By definition, a state of P-limitation means that higher P-availability should lead to enhanced growth and productivity. Thus, although preliminary in nature, our study’s findings support the conclusion of Dijkstra and Adams (2015) that fire is particularly important for P-impoverished vegetation communities. However, definitions of nutrient limitation are largely derived from relatively controlled agricultural studies and may not apply to slow-growing species that are well-adapted to low-nutrient conditions. Further, significant shifts were not present for all species; therefore the relationships between foliar stoichiometry shifts, nutrient limitation and plant growth responses require further research.
The idea that fire-mediated provisions of P impacted the overlying plant community was supported by the significantly reduced foliar N:P ratios of A. leiocalyx, A. aggregata and H. plurinervia in the RFB area. The lower foliar N:P ratios may have been indicative of the higher cellular concentrations of ribosomal RNA associated with enhanced growth rates (Güsewell 2004; Sterner and Elser 2002). In this context the different foliar N:P responses between A. leiocalyx and A. littoralis are somewhat consistent with the previously observed responses of these species to fire history (Bai et al. 2013; Lunt 1998). However, it is still unclear whether the coupling of P with growth rate holds under nutrient-limited conditions, and the reductions in N:P may instead represent increased P storage (Sterner and Elser 2002). Further, numerous authors question the value of nutrient ratios for diagnosing nutrient availability or limitation in general (e.g. Hayati and Proctor 1991; Pegtel et al. 1996). Nevertheless, the significantly lower foliar N:P ratios of A. leiocalyx, A. aggregata and H. plurinervia in the RFB area relative to the NRF area suggest that these species may have come closer to being co-limited by P and N, or may have overcome P-limitation, as a result of recent, frequent burning and associated inputs of P (Fig. 1c). In the case of H. plurinervia, which is well-adapted to nutrient-poor soils, co-limitation seems more likely, in which case the higher foliar P (and lower N:P) in RFB areas may be due to a higher degree of cellular P-storage. This effect may bring H. plurinervia individuals closer to the point of P-toxicity (Shane et al. 2004), suggesting that increases in fire frequency may be detrimental to this species.
If A. leiocalyx, A. aggregata and H. plurinervia were not P-limited, owing to an original state of limitation by some other nutrient (e.g. potassium, sulfur) or to a fire-induced switch away from P-limitation, then the interspecific differences in foliar N:P responses to fire may indicate community-level co-limitation by multiple resources (i.e. N, P or other nutrients; see Bracken et al. 2015; Gehring et al. 1999). Indeed, the apparently unaffected foliar N:P ratios of A. littoralis, E. stricta and X. johnsonii demonstrate that not all species respond to fire and associated changes in soil nutrient availabilities in the same way. Multiple states of nutrient limitation in a plant community have been theoretically linked to greater community stability, particularly in nutrient-poor environments (e.g. Tilman 1982), and the environmental changes induced by different fire regimes may contribute to the stability of alternative stable states of vegetation communities (e.g. Dantas et al. 2015; Wood and Bowman 2011). Thus, the results of our preliminary study indicate that further investigations into the relationships between fire-altered ecosystem stoichiometry, nutrient limitation and plant community composition are warranted.
Foliar nutrient resorption patterns
In the absence of fire on this site, the majority of plant-available nutrients likely come from the recycling of plant-bound nutrients (e.g. Holt and Coventry 1990). Foliar resorption patterns govern the nutrient concentration of senescent leaves and litter to a large extent, which in turn regulates inputs of nutrients to soil (Güsewell and Gessner 2009). However, resorption patterns may also be sensitive to soil nutrient concentrations (Rejmánková 2005). As a result, the changes in the NRP and PRP of X. johnsonii (Fig. 2) indicate that nutrient cycling in the plant-soil system has been altered by fire, and also suggest subsequent changes in nutrient input rates from senescent plant material to soil. The effects of fire on the foliar nutrient resorption patterns of X. johnsonii were consistent with Lü et al. (2011), in that the significantly lower foliar N and P resorption proficiencies in the RFB area were associated with higher concentrations of N and P in soil. Resorption proficiency may be determined by the point where nutrient acquisition from soil becomes less energetically expensive than nutrient acquisition via resorption (Wright and Westoby 2003). We suggest that fire and associated nutrient enrichment may have lowered the energy cost of obtaining soil nutrients and thereby reduced resorption proficiency in this species. This effect may have implications for X. johnsonii reproduction, given that increased nutrient concentration in senescent foliage should increase the nutrient concentration of the localized ash-bed produced by future fires, which may lead to enhanced production of P-rich reproductive structures (Bülow-Olsen et al. 1982; Lamont et al. 2004). This suggests that fire frequency, as much as singular fire events, could be a key factor for grasstree reproduction.
There was some evidence that the state of nutrient limitation influenced the NRE of X. johnsonii (Fig. S2a). This relationship may simply be due to a greater amount of N available for resorption with increasing N:P, or higher N:P ratios because of more efficient N resorption. However, variation in N:P ratios is frequently due to changing P content, rather than N content (Sterner and Elser 2002), and this appears to hold for X. johnsonii foliar N:P (Fig. S2b). Thus, we suggest that increasing NRE with potentially more severe P-limitation reflects the most energy efficient mode of nutrient acquisition for X. johnsonii in this ecosystem, wherein greater NRE under hypothetically increasing P-limitation may allow X. johnsonii to dedicate less energy to N-acquisition via the root system, and proportionally more energy to P-acquisition. Overall, this relationship hints at a complex relationship between N and P resorption patterns and energetic constraints, and indicates that further studies into the controls on nutrient resorption with a consideration of multiple species are warranted.
Conclusions
Our study has provided evidence that relatively recent, frequent fire can have a significant impact on the stoichiometry and nutrient cycling of soil-plant systems. Labile, inorganic and total soil P concentrations were higher in the recently burned area and this coincided with lower foliar N:P ratios in three out of six plant species. Further, the lower N and P resorption proficiencies of X. johnsonii suggest that fire has led to less-conservative nutrient use by this species. The study suggests that potential increases in soil nutrient concentrations associated with fire and the ash-bed effect can have a long-term (>4 years) effect on plant foliar chemistry and resorption patterns. Overall, the results of this preliminary study support the general idea that fire is a crucial driver of nutrient cycling and ecological structure in nutrient-poor ecosystems.
References
Augusto L, Delerue F, Gallet-Budynek A, Achat DL (2013) Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Glob Biogeochem Cycles 27:804–815. doi:10.1002/gbc.20069
Badía D, Martí C, Aguirre AJ, Aznar JM, González-Pérez JA, De la Rosa JM, León J, Ibarra P, Echeverría T (2014) Wildfire effects on nutrients and organic carbon of a rendzic phaeozem in NE Spain: changes at cm-scale topsoil. Catena 113:267–275. doi:10.1016/j.catena.2013.08.002
Bai SH, Sun F, Xu Z, Blumfield TJ (2013) Ecophysiological status of different growth stage of understorey Acacia leiocalyx and Acacia disparrima in an Australian dry sclerophyll forest subjected to prescribed burning. J Soils Sediments 13:1378–1385. doi:10.1007/s11368-013-0747-6
Beckman GG (1967) Soils and land use in the Beenleigh-Brisbane area, south-eastern Queensland. Division of Soils, Commonwealth Scientific and Industrial Research Organisation, Melbourne, Australia
Blank RR, Allen F, Young JA (1994) Growth and elemental content of several sagebrush-steppe species in unburned and post-wildfire soil and plant effects on soil attributes. Plant Soil 164:35–41. doi:10.1007/BF00010108
Bracken MES, Hillebrand H, Borer ET, Seabloom EW, Cebrian J, Cleland EE, Elser JJ, Gruner DS, Harpole WS, Ngai JT, Smith JE (2015) Signatures of nutrient limitation and co-limitation: responses of autotroph internal nutrient concentrations to nitrogen and phosphorus additions. Oikos 124:113–121. doi:10.1111/oik.01215
Bülow-Olsen A, Just J, Liddle MJ (1982) Growth and flowering history of Xanthorrhoea johnsonii Lee (Liliaceae) in Toohey Forest Queensland. Bot J Linn Soc 84:195–207. doi:10.1111/j.1095-8339.1982.tb00534.x
Catterall CP, Wallace CJ (1987) An island in suburbia: the natural and social history of Toohey Forest. Institute of Applied Environmental Research. Griffith University, Brisbane, Australia
Certini G (2005) Effects of fire on properties of forest soils: a review. Oecologia 143:1–10. doi:10.1007/s00442-004-1788-8
Chambers DP, Attiwill PM (1994) The ash-bed effect in Eucalyptus regnans forest: chemical, physical and microbiological changes in soil after heating or partial sterilisation. Aust J Bot 42:739–749
Chen CR, Xu ZH, Keay P, Zhang SL (2005) Total soluble nitrogen in forest soils as determined by persulfate oxidation and by high temperature catalytic oxidation. Aust J Soil Res 43:515–523. doi:10.1071/sr04132
Covington WW, Sackett SS (1992) Soil mineral nitrogen changes following prescribed burning in ponderosa pine. For Ecol Manag 54:175–191. doi:10.1016/0378-1127(92)90011-W
Cui Q, Lü X, Wang Q, Han X (2010) Nitrogen fertilization and fire act independently on foliar stoichiometry in a temperate steppe. Plant Soil 334:209–219. doi:10.1007/s11104-010-0375-5
Dantas V, Hirota M, Oliveira RS, Pausas JG (2015) Disturbance maintains alternative biome states. Ecol Lett 5:1–8. doi:10.1111/ele.12537
Dijkstra FA, Adams MA (2015) Fire eases imbalances of nitrogen and phosphorus in woody plants. Ecosystems 18:769–779. doi:10.1007/s10021-015-9861-1
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142. doi:10.1111/j.1461-0248.2007.01113.x
Elser JJ, Fagan WF, Kerkhoff AJ, Swenson NG, Enquist BJ (2010) Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytol 186:593–608. doi:10.1111/j.1469-8137.2010.03214.x
Gehring C, Denich M, Kanashiro M, Vlek PLG (1999) Response of secondary vegetation in Eastern Amazonia to relaxed nutrient availability constraints. Biogeochem 45:223–241. doi:10.1023/A:1006138815453
Giardina CP, Rhoades CC (2001) Clear cutting and burning affect nitrogen supply, phosphorus fractions and seedling growth in soils from a Wyoming lodgepole pine forest. For Ecol Manag 140:19–28. doi:10.1016/S0378-1127(00)00272-3
Giardina CP, Sanford RL, Døckersmith IC (2000) Changes in soil phosphorus and nitrogen during slash-and-burn clearing of a dry tropical forest. Soil Sci Soc Am J 64:399–405. doi:10.2136/sssaj2000.641399x
González-Pérez JA, González-Vila FJ, Almendros G, Knicker H (2004) The effect of fire on soil organic matter—a review. Environ International 30:855–870. doi:10.1016/j.envint.2004.02.003
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266. doi:10.1111/j.1469-8137.2004.01192.x
Güsewell S, Gessner MO (2009) N : P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct Ecol 23:211–219. doi:10.1111/j.1365-2435.2008.01478.x
Güsewell S, Koerselman W, Verhoeven JTA (2003) Biomass N:P ratios as indicators of nutrient limitation of plant populations in wetlands. Ecol Appl 13:372–384. doi:10.1890/1051-0761.2003.013[0372:BNRAIO]2.0.CO;2
Hayati AA, Proctor MCF (1991) Limiting Nutrients in Acid-Mire Vegetation : Peat and Plant Analyses and Experiments on Plant Responses to Added Nutrients. J Ecol 79:75–95. doi:10.2307/2260785
Holt JA, Coventry RJ (1990) Nutrient cycling in Australian savannas. J Biogeogr 17:427–432. doi:10.2307/2845373
Huang J, Boerner REJ (2007) Effects of fire alone or combined with thinning on tissue nutrient concentrations and nutrient resorption in Desmodium nudiflorum. Oecologia 153:233–243. doi:10.1007/s00442-007-0733-z
Huang W, Xu Z, Chen CR, Zhou G, Liu J, Abdullah KM, Reverchon F, Liu X (2013) Short-term effects of prescribed burning on phosphorus availability in a suburban native forest of subtropical Australia. J Soils Sediments 13:869–876. doi:10.1007/s11368-013-0660-z
Jackson ML (1958) Soil chemical analysis. Prentice-Hall, New Jersey
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecol 77:1716–1727. doi:10.2307/2265777
Lamont B (2003) Structure, ecology and physiology of root clusters–a review. Plant Soil 248:1–19. doi:10.1007/978-94-010-0243-1_1
Lamont B, Wittkuhn R, Korczynskyj D (2004) Ecology and ecophysiology of grasstrees. Aust J Bot 52:561–582. doi:10.1071/BT03127
Lewis T, Reif M, Prendergast E, Tran C (2012) The effect of long-term repeated burning and fire exclusion on above- and below-ground Blackbutt (Eucalyptus pilularis) forest vegetation assemblages. Austral Ecol 37:767–778. doi:10.1111/j.1442-9993.2011.02337.x
Liu Y, Stanturf J, Goodrick S (2010) Trends in global wildfire potential in a changing climate. For Ecol and Manage 259:685–697. doi:10.1016/j.foreco.2009.09.002
Lü X, Cui Q, Wang Q, Han X (2011) Nutrient resorption response to fire and nitrogen addition in a semi-arid grassland. Ecol Eng 37:534–538. doi:10.1016/j.ecoleng.2010.12.013
Lunt ID (1998) Allocasuarina (Casuarinaceae) invasion of an unburnt coastal woodland at Ocean Grove, Victoria: structural changes 1971–1996. Aust J Bot 46:649–656. doi:10.1071/bt97032
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. doi:10.1016/s0003-2670(00)88444-5
Nave LE, Vance ED, Swanston CW, Curtis PS (2011) Fire effects on temperate forest soil C and N storage. Ecol Appl 21:1189–1201. doi:10.1890/10-0660.1
Ozanne PG, Specht RL (1981) Mineral nutrition of heathlands: phosphorus toxicity. In: Specht RL, ed. ecosystems of the world, Vol 9 A. Heathlands and related shrublands. Descriptive studies. Amsterdam, Elsevier Scientific
Payton IJ, Lee WG, Dolby R, Mark AF (1986) Nutrient concentrations in narrow-leaved snow tussock (Chionochloa rigida) after spring burning. N Z J Bot 24:529–537. doi:10.1080/0028825x.1986.10409940
Pegtel D, Bakker J, Verweij L, Fresci L (1996) N, K and P deficiency in chronosequential cut summer-dry grasslands on gley podzol after the cessation of fertilizer application. Plant Soil 178:121–131. doi:10.1007/bf00011170
Pellegrini AFA, Hedin LO, Staver AC, Govender N (2015) Fire alters ecosystem carbon and nutrients but not plant nutrient stoichiometry or composition in tropical savanna. Ecol 96:1275–1285. doi:10.1890/14-1158.1
Pérez-Harguindeguy N, Díaz S, Vendramini F, Cornelissen J, Gurvich DE, Cabido M (2003) Leaf traits and herbivore selection in the field and cafeteria experiments. Austral Ecol 28:642–650. doi:10.1046/j.1442-9993.2003.01321.x
Pivello VR, Coutinho LM (1992) Transfer of macro-nutrients to the atmosphere during experimental burnings in an open cerado (Brazilian savanna). J Trop Ecol 8:487–497. doi:10.1017/s0266467400006829
Rayment GE, Lyons DJ (2010) Soil chemical methods – Australasia. CSIRO Publishing, Australia
Rejmánková E (2005) Nutrient resorption in wetland macrophytes : comparison across several regions of different nutrient status. New Phytol 167:471–482. doi:10.1111/j.1469-8137.2005.01449.x
Romanyà J, Khanna PKK, Raison RJJ (1994) Effects of slash burning on soil phosphorus fractions and sorption and desorption of phosphorus. For Ecol Manag 65:89–103. doi:10.1016/0378-1127(94)90161-9
Saunders WMH, Williams EG (1955) Observations on the determination of total organic phosphorous in soils. J Soil Sci 6:254–267. doi:10.1111/j.1365-2389.1955.tb00849.x
Schafer JL, Mack MC (2010) Short-term effects of fire on soil and plant nutrients in palmetto flatwoods. Plant Soil 334:433–447. doi:10.1007/s11104-010-0394-2
Shane MW, McCully ME, Lambers H (2004) Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae). J Exp Bot 55:1033–1044. doi:10.1093/jxb/erh111
Sparling G, Vojvodić-Vuković M, Schipper LA (1998) Hot-water-soluble C as a simple measure of labile soil organic matter: the relationship with microbial biomass C. Soil Biol Biochem 30:1469–1472. doi:10.1016/s0038-0717(98)00040-6
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton, USA
Tilman D (1982) Resource competition and community structure. Princeton University Press, New Jersey, USA
Tomkins IB, Kellas JD, Tolhurst KG, Oswin DA (1991) Effects of fire intensity on soil chemistry in a Eucalypt forest. Aust J Soil Res 29:25–47. doi:10.1071/sr9910025
Tutua SS, Xu Z, Chen CR, Blumfield TJ (2013) Hot water extractable phosphorus pools as indicators of soil P responses to harvest residue management in an exotic pine plantation of subtropical Australia. J Soils Sediments 13:1573–1578. doi:10.1007/s11368-013-0775-2
Van de Vijver CADM, Poot P, Prins HHT (1999) Causes of increased nutrient concentrations in post-fire regrowth in an East African savanna. Plant Soil 214:173–185. doi:10.1023/A:1004753406424
Victorian Government (2010) 2009 Victorian Bushfires Royal Commission: Final report summary. Victoria, Australia
Vitousek PM (1984) Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecol 65:289–298. doi:10.2307/1939481
Wood SW, Bowman DMJS (2011) Alternative stable states and the role of fire–vegetation–soil feedbacks in the temperate wilderness of southwest Tasmania. Landsc Ecol 27:13–28. doi:10.1007/s10980-011-9677-0
Wright IJ, Westoby M (2003) Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species. Funct Ecol 17:10–19. doi:10.1046/j.13652435.2003.00694.x
Acknowledgments
This work was supported by a grant of Australian Research council Future Fellowship project (FT0990547). We would like to thank the Brisbane City Council for granting permission to conduct work in Toohey Forest Conservation Park and for their assistance with site selection, as well as Bob Coutts, Dr. Haibo Dong, Zhongming Lan, Xian Liu, Maryam Esfandbod and Dr. Mehran Rezaei Rashti for their generous support in the field and in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Rafael S. Oliveira.
Electronic supplementary material
ESM 1 Fig. S1
(DOCX 20 kb)
ESM 2 Fig. S2
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Butler, O.M., Lewis, T. & Chen, C. Prescribed fire alters foliar stoichiometry and nutrient resorption in the understorey of a subtropical eucalypt forest. Plant Soil 410, 181–191 (2017). https://doi.org/10.1007/s11104-016-2995-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2995-x