Abstract
Fire may have different effects on the relative availability of nitrogen (N) and phosphorus (P) because N volatilization occurs at lower temperatures than P volatilization, and fire-mediated changes in soil nutrient availability may affect foliar nutrient concentrations. We assessed the short-term effects of fire on soil and plant nutrients and 15N isotopic signatures in a palmetto flatwoods ecosystem in central Florida. Fire caused a short-term increase in extractable ammonium (NH4 +) and phosphate (PO4 3-). The increase in PO4 3- was greater than the increase in NH4 +, resulting in a decrease in the soil extractable N:P ratio shortly after fire. Similarly, foliar %P of the palmetto Serenoa repens (W. Bartram) Small increased more than foliar %N, resulting in a decrease in foliar N:P ratios shortly after fire. Soil δ15N and the difference between foliar and soil δ15N did not vary with time since fire; however, foliar δ15N of S. repens decreased after fire. Foliar %N of Quercus geminata Small and ericaceous shrubs was positively correlated with soil extractable inorganic N, while foliar %P of S. repens was positively correlated with soil extractable PO4 3-. Variation in foliar δ15N after fire and the positive relationship between soil and foliar nutrients suggest that both increased soil nutrient availability and reallocation of nutrients from below- to aboveground can be important for plant nutrient status after fire in palmetto flatwoods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fire, a natural disturbance in many shrubland ecosystems (Little 1979; Abrahamson et al. 1984; Christensen 1985; Keeley and Keeley 1988; Moreno and Oechel 1994; Bradstock et al. 2001), has profound impacts on nutrient cycling and availability. Fire consumes plant biomass, litter, and soil organic matter, converting organic nutrients into inorganic forms (Certini 2005) that may be lost to the atmosphere or returned to the ecosystem in ash. Although fire often has no detectable effects on total soil nitrogen (N) (Christensen and Muller 1975; Jensen et al. 2001; Wan et al. 2001; Britton et al. 2008; Boerner et al. 2009) or phosphorus (P) pools (Kauffman et al. 1993), numerous studies have measured increases in concentrations of soil ammonium (NH4 +), nitrate (NO3 -), and/or phosphate (PO4 3-) after fire (e.g., Lewis 1974; Wilbur and Christensen 1983; Stock and Lewis 1986; Schmidt and Stewart 1997; Giardina et al. 2000; Grogan et al. 2000; Wan et al. 2001; Smithwick et al. 2005; Turner et al. 2007).
Fire can have different effects on the relative availability of N and P because N volatilization occurs at temperatures as low as 200°C (White et al. 1973), whereas P is volatilized at temperatures above 774°C (Raison et al. 1985a). Regardless of ecosystem type or fire intensity, approximately twice as much N as P is lost to the atmosphere during fire (Gillon and Rapp 1989; Pivello and Coutinho 1992; Cook 1994; Mackensen et al. 1996). Thus, ash on the soil surface contains high concentrations of P and low concentrations of N (DeBano and Conrad 1978; Raison et al. 1985b), suggesting that fire affects both the absolute and relative availability of soil N and P.
Plant species in pyrogenic habitats have evolved a variety of mechanisms that allow them to persist and recover after fire (Sousa 1984; Christensen 1985). While some species are killed by fire and recolonize via seedling recruitment, other species are resilient and resprout after burning (Keeley 1977; Keeley and Zedler 1978; Menges and Kohfeldt 1995; Weekley and Menges 2003). Plant species that recruit from seed after fire rely on nutrients made available by fire; whereas, plant species that resprout after fire may similarly utilize nutrients made available by fire or reallocate nutrients from below- to aboveground tissues (El Omari et al. 2003). The effect of fire-induced changes in soil nutrient availability on plant nutrition, however, remains unclear. Several studies have found increases in foliar N and P after fire (Gilliam 1988; Franco-Vizcaíno and Sosa-Ramirez 1997), while others have found no effect of fire on foliar nutrients (Bennett et al. 2002; Ferran et al. 2005). Understanding the effects of fire on foliar nutrient concentrations is important because variation in foliar N:P ratios with time after fire may indicate changes in plant nutrient status and nutrient limitation, as foliar N:P ratios have been used to indicate N limitation, P limitation, or co-limitation by N and P (Koerselman and Meuleman 1996; Güsewell 2004).
Soil and plant δ15N values have been used as indicators of ecosystem nitrogen cycling (Martinelli et al. 1999). Fire consumes surface soil layers and volatilizes N, which can leave post-fire soils enriched in 15N (Högberg 1997) because soil δ15N tends to increase with depth (Nadelhoffer et al. 1996; Frank and Evans 1997). Foliar δ15N signatures are related to plant N sources, mycorrhizal status, rooting depth, N assimilation, and within-plant N reallocation (Högberg 1997; Evans 2001). Thus, taken together, soil and plant δ15N values may provide insight into integrated fire effects on plant and soil N dynamics and the causes of increased foliar N concentrations after fire.
We examined the effects of fire on plant and soil nutrient dynamics in flatwoods ecosystems of the Lake Wales Ridge in central peninsular Florida, where fire has historically maintained shrub-dominated habitats (Abrahamson et al. 1984; Menges 1999). Although N and P are essential plant nutrients that limit plant growth in most, if not all, terrestrial ecosystems (Vitousek and Howarth 1991), few studies have investigated the effects of fire on both soil and plant N, P, and N:P ratios. Understanding nutrient dynamics in flatwoods ecosystems is important because nutrient availability is low and fires occur relatively frequently. We assessed the short-term effects of fire on soil and plant nutrients and 15N isotopic signatures. We hypothesized that soil extractable N and P would increase immediately post-fire, but that the ratio of soil extractable N:P would decrease immediately post-fire due to the differential effects of fire on N and P. Furthermore, we hypothesized that foliar N and P concentrations of resprouting plants would increase after fire. We investigated 15N isotopic signatures to differentiate among mechanisms that can cause increased foliar N concentrations.
Materials and methods
This study was conducted at Archbold Biological Station (ABS) in Highlands County, Florida, USA (27º10′50″N, 81º21′0″ W), near the southern tip of the Lake Wales Ridge. The Lake Wales Ridge supports fire adapted Florida scrub ecosystems characterized by deep sandy soils derived from paleo dunes (Abrahamson et al. 1984), and high endemism, with many endangered and threatened species (Menges 1999). Archbold Biological Station typically has warm wet summers and cool dry winters (Abrahamson et al. 1984). Mean annual precipitation is 136.5 cm (ABS weather records, 1932–2004), and mean annual temperature is 22.3°C (ABS weather records, 1952–2004). The ABS preserve is divided into burn units that have been managed with prescribed fires for over 35 years. Archbold Biological Station comprises a mosaic of plant communities including seasonal ponds, flatwoods, scrubby flatwoods, oak-hickory scrub, and sand pine scrub.
Our research focused on the palmetto flatwoods plant community. Palmetto flatwoods are dominated by saw palmetto (Serenoa repens (W. Bartram) Small), a repent shrub that reaches heights of 1–2 m, and scattered shrubs with occasional to dense slash pines (Pinus elliottii Engelm.). Palmetto flatwoods often occur as a distinct zone around seasonal ponds on entisols, inceptisols, and spodosols that are poorly drained and can have standing water during times of high rainfall (Abrahamson et al. 1984). Flatwoods typically burn every 2–9 years (Main and Menges 1997). Palmettos and other dominant shrubs resprout after fire, while slash pines survive by resisting fire and recruit from seed after fire (Menges and Kohfeldt 1995). Fires are intense and leave few areas unburned due to the high flammability of palmettos and pine duff (Abrahamson et al. 1984). Maximum sustained fire temperatures in flatwoods range from 373°C to 688°C, while absolute maximum temperatures have been measured as high as 796°C (E. Menges, unpublished data).
On 4 August 2006, we randomly selected five sampling locations within the palmetto flatwoods vegetation association in a 19 acre burn unit that had previously burned in 2003, 1996, 1993, and 1972. At all sampling locations, which were separated by at least 5 m, we marked a soil sampling site and the nearest individual of five common flatwoods species (when present within 1 m of the soil sampling location). Our focal species, all of which resprout after fire, were the palmetto S. repens, the shrubby oak Quercus geminata Small, and the ericaceous shrubs Lyonia fruticosa (Michx.) G.S. Torr., Lyonia lucida (Lam.) K. Koch, and Vaccinium myrsinities Lam. (Wunderlin and Hanson 2003). On 4 August 2006, several hours before ignition of a prescribed fire, we collected five soil samples (0–15 cm depth, 8 cm diameter core), one at each sampling location, and thirteen foliar samples, two to four at each sampling location depending on the species present. Serenoa repens was present at all sampling locations, while Q. geminata was present at four of the five sampling locations. Eleven of the thirteen plants sampled were completely consumed by the fire. The first post-fire soil samples (n = 5) were collected on the afternoon of 4 August 2006, within 3 h after the fire had burned through the unit. Subsequent post-fire soil samples (n = 5) were collected on 24 August, 5 October, and 11 December 2006 and 11 December 2007. We collected post-fire foliar samples (n = 13) on 6 October 2006, 12 December 2006, and 10 December 2007. At all sampling times, we collected the newest leaves from the upper portion of shrub stems. To collect foliar samples of the palmetto S. repens, we clipped a small portion of the newest leaves (1 to 3 depending on total leaf number), thereby permanently marking the leaves. Thus, at all sampling times post-fire, we collected a portion of only the new leaves that had been produced after the previous sampling event.
Within 24 h of collection, we passed soil samples through a 2-mm sieve and sub-sampled for determination of gravimetric soil moisture, pH, total percentages of N and C, inorganic P concentration, inorganic N concentration, N mineralization rates, and soil δ15N. Gravimetric moisture content was determined on samples dried at 105°C for 48 h. For soil pH, 10 g of air dried soil was added to 10 mL of deionized water, shaken for 30 s, allowed to stand for 10 min (Thomas 1996), then pH was determined with an electronic pH meter (Thermo Orion 250A+, Orion Research, Inc., Boston, Massachusetts, USA). A subsample of soil was dried at 60°C for 48 h, ground to a fine powder on a spex mill (8000D dual mixer/mill, Spex Certiprep Inc., Metuchen, New Jersey) at the MacArthur Agro-Ecology Research Center (MAERC), and analyzed for percentages of N, C, and 15N natural abundance at the University of Florida on an elemental analyzer (ECS 4010, Costech Analytical, Valencia, California, USA) coupled with an isotope ratio mass spectrometer (Delta Plus XL, ThermoFinnigan, Brenen, Germany). Abundances of 15N were measured using delta (δ) notation with atmospheric N2 as the standard.
To measure inorganic P concentrations, 30 mL of 0.05 M hydrochloric acid (HCl) and 0.0125 M hydrogen sulfate (H2SO4) was added to 15 g of field moist soil, shaken for 5 min, then filtered through Whatman #42 filter paper. We stored filtered samples in a refrigerator for up to three weeks before analysis for phosphate (PO4 3-) concentrations on a spectrophotometer microplate reader (μQuant Microplate Spectrophotometer, Bio-Tek Instruments, Inc., Winooski, Vermont, USA) using the malachite green method (D’Angelo et al. 2001) at the MAERC.
To measure inorganic N concentrations, 50 mL of 0.5 M potassium sulfate (K2SO4) was added to 10 g of field moist soil, shaken for 30 s, and allowed to stand overnight. We filtered solutions through Whatman #42 filter paper that was pre-leached with 0.5 M K2SO4. Filtered samples were frozen then taken to the University of Florida where ammonium (NH4 +) and nitrate (NO3 -) concentrations were determined colorimetrically on a segmented flow autoanalyzer (Astoria-Pacific, Inc., Clackamas, Oregon, USA). For N mineralization rates, 10 g of field moist soil was contained in a specimen cup and stored in the dark at room temperature (∼ 24°C). After one week, 50 mL of 0.5 M K2SO4 was added to the soil, shaken for 30 s, and allowed to stand overnight. We filtered, stored, and analyzed solutions as described above. Net rates of N mineralization were calculated from the difference in μg N-(NH4 + + NO3 -)·g soil−1 of initial and one week extractions.
Leaf samples were dried at 60°C for 48 h and ground on a spex mill (8000D dual mixer/mill, Spex Certiprep Inc., Metuchen, New Jersey) at the MAERC. All foliar samples were analyzed for percentages of N and C and 15N natural abundance at the University of Florida on an elemental analyzer (ECS 4010, Costech Analytical, Valencia, California, USA) coupled with an isotope ratio mass spectrometer (Delta Plus XL, ThermoFinnigan, Brenen, Germany). Abundances of 15N were measured using delta (δ) notation with atmospheric N2 as the standard. We determined foliar phosphorus for all samples of Serenoa repens. Subsamples of 0.2–0.5 g were weighed into crucibles, ashed in a muffle furnace at 500°C for 5 h, extracted with 6 M HCl, then brought to volume so that the solution was 0.6 M. Extracts were stored in the refrigerator for several days then analyzed colorimetrically on a spectrophotometer microplate reader (PowerWave XS Microplate Reader, Bio-Tek Instruments, Inc., Winooski, Vermont, USA) at the University of Florida using the ascorbic acid molybdenum-blue method (Murphy and Riley 1962). Standard NIST peach leaves were used to determine the efficiency of the digestion.
Statistical analyses
To examine changes in soil variables over time after fire, we used a one-way mixed analysis of variance model with repeated measures with time as the within-subjects factor (SAS 9.1 2003; Littell et al. 2006). Differences in soil variables among times were determined with post-hoc pairwise comparisons with Bonferroni confidence interval adjustments. Soil NH4 + concentrations, total inorganic N, PO4 3- concentrations, N:P ratios, soil δ15N, and soil %C were natural log transformed before analyses. Soil %N was square root transformed before analysis. Soil NO3 - concentrations could not be transformed to fit normality because of many zeros and were analyzed using Friedman’s analysis of variance (SPSS 11.5 for Windows 2000).
To examine changes in foliar nutrients (%N, %P, and N:P ratios) and foliar δ15N over time after fire, we used one-way repeated measures analysis of variance with time as the within-subjects factor (SPSS 11.5 for Windows 2000; Field 2009). Differences in foliar nutrients and foliar δ15N among times were determined with post-hoc pairwise comparisons with Bonferroni confidence interval adjustments. In addition, we calculated the absolute difference between foliar δ15N and soil δ15N (Chang and Handley 2000; Schuur and Matson 2001) for each plant at each soil sampling location and analyzed differences over time after fire with a one-way repeated measures analysis of variance. Foliar nutrient variables were analyzed separately for each species/family (Serenoa repens, Quercus geminata, and Ericaceae (ericaceous shrubs include Lyonia lucida, Lyonia fruticosa, and Vaccinium myrsinities)).
We used linear regression to assess the relationship between soil and foliar nutrients (Sigma Plot for Windows 11.0 2008). We correlated the foliar %N of each individual at each site with total soil extractable inorganic N at each site. Analyses were conducted separately for each species/family. We correlated foliar %P of S. repens with soil extractable PO4 3-, which was natural log transformed. Because the first post-fire foliar sample collection corresponded with the third post-fire soil sample collection, data from only four time points (pre-fire and 62/63, 129/130, and 493/494 days post-fire) were used in the regression analyses.
Results
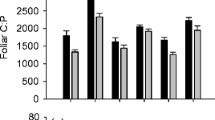
Three hours post-fire, NH4 + concentrations were 5.5 times higher than pre-fire values, and NH4 + remained higher through at least 20 days after fire (F5,20 = 6.16, p = 0.001; Fig. 1a). Soil extractable NO3 - was undetectable pre-fire, but was greater than zero 3 h, 20 days, and 62 days after fire (χ 2(5) = 9.81, p = 0.081; Fig. 1a). Three hours post-fire, PO4 3- concentrations were 30 times higher than pre-fire values, and 62 days after fire, PO4 3- concentrations were 21 times higher than pre-fire values (F5,20 = 15.45, p < 0.001; Fig. 1b). Soil extractable N:P ratios were significantly lower 62 days after fire than 129 and 494 days after fire (F5,20 = 5.85, p = 0.001, Fig. 1c). Soil pH increased over time after fire, and by 494 days, was significantly higher than pre-fire values (Table 1). There were no differences in soil %N, %C, C:N ratios, or soil δ15N over time after fire (Table 1).
Mean (± SE) soil extractable NH4 + and NO3 - (a), soil extractable PO4 3- (b), and soil extractable N:P ratios (c) in palmetto flatwoods pre-fire and 0.125, 20, 62, 129, and 494 days post-fire. Different letters represent significantly different means at α = 0.05 determined by repeated measures analysis of variance with post-hoc pairwise comparisons with Bonferroni confidence interval adjustments
Foliar %N of Serenoa repens was higher 63 days after fire than 493 days after fire (Fig. 2a), and foliar %P of S. repens increased after fire (Fig. 2b). Foliar N:P ratios decreased after fire because of the larger increase in %P (1.39 times pre-fire values) than %N (1.15 times pre-fire values). Foliar %P and N:P ratios of S. repens were similar to pre-fire values by 493 days post-fire (Fig. 2). Foliar %N of Quercus geminata and ericaceous species increased shortly after fire then decreased to pre-fire values by 493 days post-fire (Fig. 3).
Mean (± SE) foliar %N (a), foliar %P (b), and foliar N:P ratios (c) for Serenoa repens (n = 5) in palmetto flatwoods pre-fire and 63, 130, and 493 days post-fire. Different letters represent significantly different means at α = 0.05 determined by repeated measures analysis of variance with post-hoc pairwise comparisons with Bonferroni confidence interval adjustments
Mean (± SE) foliar %N and δ15N for Serenoa repens (n = 5) (a), Quercus geminata (n = 4) (b), and three ericaceous species (Lyonia fruticosa, Lyonia lucida, and Vaccinium myrsinities; n = 4) (c) pre-fire and 63, 130, and 493 days post-fire in palmetto flatwoods. Uppercase letters represent significant differences in foliar %N and lowercase letters represent significant differences in foliar δ15N at α = 0.05 determined by repeated measures analysis of variance with post-hoc pairwise comparisons with Bonferroni confidence interval adjustments. There were no post-hoc differences in foliar %N or foliar δ15N among times after fire for Q. geminata or ericaceous species
Foliar δ15N of S. repens decreased significantly over time after fire, while foliar δ15N of Q. geminata increased then decreased after fire (Fig. 3), although this change was only marginally significant (Table 2). Foliar δ15N of ericaceous species did not change with time since fire (Table 2). The absolute difference between foliar δ15N and soil δ15N did not vary over time after fire for any plant species/family (Table 2). Over the entire study period, the mean (+ SE) difference between foliar and soil δ15N was −3.06 (+ 0.29) for S. repens, −5.14 (+ 0.27) for Q. geminata, and −5.83 (+ 0.15) for ericaceous shrubs.
Total extractable inorganic N was positively correlated with foliar %N of Q. geminata (F1,14 = 3.77, p = 0.073, R2 = 0.21) and ericaceous shrubs (F1,14 = 5.49, p = 0.034, R2 = 0.28) (Fig. 4). Total extractable inorganic N was not correlated with foliar %N of S. repens (F1,18 = 0.85, p = 0.368, R2 = 0.04). Foliar %P of S. repens was positively correlated with soil extractable PO4 3- (F1,18 = 10.57, p = 0.004, R2 = 0.37; Fig. 5).
Relationship between total extractable inorganic N and foliar %N of Serenoa repens (p = 0.368, R2 = 0.04; foliar %N = 1.29 + (0.05 * inorganic N)), Quercus geminata (p = 0.073, R2 = 0.21; foliar %N = 1.09 + (0.16 * inorganic N)), and ericaceous shrubs (p = 0.034, R2 = 0.28; foliar %N = 0.82 + (0.22 * inorganic N))
Discussion
Fire caused a short-term increase in soil extractable nutrients in the palmetto flatwoods ecosystem investigated in our study. While soil N concentrations remained elevated above pre-fire levels for at least 1 month after fire, soil P, in contrast, remained elevated above pre-fire levels for at least 2 months after fire (Fig. 1). Thus, flatwoods shrubs, which resprouted within a month after fire, experienced a sustained increase in P availability, but only a short pulse of N availability. Regardless, both foliar %N and %P increased over the short-term after fire. The relative magnitude of the soil P increase was greater than that of soil N, leading to a decrease in the soil extractable N:P ratio shortly after fire (Fig. 1). Similarly, for the palmetto Serenoa repens, the relative increase in foliar %P was greater than the increase in foliar %N, causing a decrease in the foliar N:P ratio shortly after fire (Fig. 2). Soil δ15N did not vary with time since fire (Table 1), and only S. repens showed significant variation in foliar δ15N with time after fire (Table 2).
Soil ammonium (NH4 +) concentrations increased immediately after fire and decreased to pre-fire levels within 2 months after fire (Fig. 1). High concentrations of NH4 + and NO3 - in burned sites may be related to high N mineralization and nitrification rates (DeLuca et al. 2002; DeLuca and Sala 2006); however, Turner et al. (2007) found that NH4 + increased during the first year after severe stand-replacing fire in pine forests, while net N mineralization rates were negative. Although net N mineralization rates were affected by fire in our study (Table 1), they were negative throughout the study, indicating that post-fire increases in inorganic N availability are not due to increased mineralization, but rather due to microbial or ash derived N. Increased soil temperatures associated with fire (Ewel et al. 1981) kill soil microbes, indicated by a decrease in microbial C and N after fire (Prieto-Fernández et al. 1998), which causes the release of N from ruptured microbial cells (Dunn et al. 1985; Serrasolsas and Khanna 1995). In addition, ash can contain high concentrations of N (Ewel et al. 1981; Kauffman et al. 1993), which can cause an increase in soil N after fire.
Similarly to NH4 +, soil extractable phosphate (PO4 3-) increased immediately after fire; however, in contrast to NH4 +, PO4 3- decreased to pre-fire levels within 4 months after fire (Fig. 1). High concentrations of PO4 3- post-fire are related to high concentrations of P in ash (Raison et al. 1985b; Kauffman et al. 1993). Loss of high nutrient ash can occur by wind (Giardina et al. 2000) or water (Ewel et al. 1981), suggesting that post-fire weather contributes to variation in PO4 3- concentrations. In our study, the first rain event occurred 2 days after fire, and 25.9 cm of rain fell between the first and second post-fire sampling dates (ABS weather records), which likely limited loss of wind blown ash and could have contributed to high concentrations of soil PO4 3- after fire (Tomkins et al. 1991).
Soil extractable PO4 3- increased more than total inorganic N after fire, causing a decrease in soil extractable N:P ratios (Fig. 1). This result is consistent with the findings that more N than P is volatilized by fire (Gillon and Rapp 1989; Pivello and Coutinho 1992; Cook 1994; Mackensen et al. 1996) and that ash has higher concentrations of P than N (Raison et al. 1985b; Marcos et al. 2009). In a wetland with acidic, sandy soils, the soil extractable N:P ratio after fire was similar to our flatwoods site, but the pre-fire soil extractable N:P ratio was greater than in our site, so the magnitude of the decline was greater (Wilbur and Christensen 1983). While post-fire soil extractable N:P ratios may be similar across sites, differences in soil properties such as organic matter quantity or differences in fire temperature may affect the magnitude of fire-induced changes in soil extractable N:P ratios.
The post-fire pulse of PO4 3- persisted twice as long as the post-fire pulse of NH4 + (Fig. 1). Although fire can kill soil microbes, the effects of fire on soil temperature decrease with depth (Ewel et al. 1981; Giardina et al. 2000; Jensen et al. 2001), so growth of microbes below the soil surface may be stimulated by post-fire increases in nutrient availability (Singh et al. 1991) or root exudates (Blagodatskaya et al. 2009) from damaged roots (Scott-Denton et al. 2006). Considering that soil microbial biomass N:P ratios average 7:1 at the global scale (Cleveland and Liptzin 2007), increased microbial growth would cause a faster decrease in soil N than soil P. Alternatively, sandy soils have lower sorption capacity than clayey soils (Villani et al. 1998), and high concentrations of potassium (K+), calcium (Ca2+) (Ewel et al. 1981; Kauffman et al. 1993), and chloride (Cl-) (Khanna and Raison 1986) in ash may affect the mobility of NH4 + and PO4 3- after fire. Leaching of Cl- may be accompanied by leaching of NH4 + (Khanna and Raison 1986), and K+ can compete with NH4 + for surface exchange sites (Chappell and Evangelou 2000); both of these interactions may contribute to high leaching losses of NH4 + after fire. Phosphate (PO4 3-) can form minerals with Ca2+, and limited leaching of Ca2+ after fire (Khanna and Raison 1986) suggests that leaching losses of PO4 3- may be low after fire. Regardless of the mechanism that leads to a shorter pulse of NH4 + than PO4 3-, plants experience a greater period of elevated P; however, if microbial uptake, rather than leaching, reduces extractable NH4 +, N is retained in the ecosystem, rather than lost from the ecosystem, which may prevent or slow N limitation of primary productivity.
In contrast to the effects of fire on inorganic nutrients, fire had no effect on total soil N, C, or C:N ratios (Table 1). DeBano and Conrad (1978) reported decreases in total N in the top 2 cm of soil after fire, which was associated with high soil surface temperatures and a loss of soil organic matter; however, any change in soil N or C in the top 0–2 cm of soil would likely be small relative to the total amount of N and C in the 0–15 cm of soil collected in our study. Other studies have found no effect of fire on total soil N (Christensen and Muller 1975; Jensen et al. 2001; Wan et al. 2001; Britton et al. 2008; Boerner et al. 2009), C, or C:N ratios (Boerner et al. 2009). Although fire often has limited effects on bulk soil properties, soil organic matter content and fire severity may mediate fire effects on total soil N and C.
Soil pH increased over time after fire in our study (Table 1). The presence of ash may increase soil pH (Grogan et al. 2000; Badía and Martí 2003; Molina et al. 2007) due to the high pH of ash (Jensen et al. 2001; Goforth et al. 2005; Molina et al. 2007; Marcos et al. 2009) and the high concentration of cations, such as Ca2+ and K+, in ash (Raison et al. 1985b; Arocena and Opio 2003). The majority of aboveground biomass in our flatwoods site was consumed by fire, leaving large amounts of ash on the soil surface. Soil pH increases with % base saturation (Magdoff and Bartlett 1985), and leaching of ash covered soils increases soil pH (Molina et al. 2007), so integration of cation rich ash through the top 15 cm of soil after rain events likely contributed to the increase in soil pH over time. Soils are not well buffered between pH 4 and 7 (Magdoff and Bartlett 1985; James and Riha 1986), so even a small increase in sorption of Ca2+ and K+ could have caused an increase in soil pH (Skyllberg et al. 2001). In addition, microbial biomass N, total microbial respiration, and total phospholipid fatty acids are lower in soils at pH 4.17 than at pH 4.65 (Aciego Pietri and Brookes 2009), suggesting that the increase in pH over time after fire in our study, from 4.09 to 4.41, could have significant effects on the microbial community.
Foliar %N and %P of the dominant flatwoods species, Serenoa repens, increased shortly after fire, and were similar to pre-fire values within 4 months after fire (Fig. 2). The increase in foliar %N occurred after soil extractable N was similar to pre-fire levels; whereas, the increase in foliar %P persisted over the same time scale as the increase in soil extractable P. Foliar %N of Quercus geminata and ericaceous shrubs also tended to be higher shortly after fire than pre-fire (Fig. 3). Several hypotheses could explain the increase in foliar %N and %P after fire. First, plants may be increasing foliar nutrients post-fire due to increased availability of N and P. Increases in foliar nutrients in our study tended to mirror changes in extractable N and P. Foliar %N of Q. geminata and ericaceous shrubs was positively correlated with soil extractable N (Fig. 4), and foliar %P of S. repens was positively correlated with soil extractable P. Similarly, other studies have found that foliar nutrients of resprouting species are correlated with soils nutrients after fire (Gilliam 1988; Franco-Vizcaíno and Sosa-Ramirez 1997). Second, N and P stored in belowground tissues of resprouting plants may be retranslocated to aboveground tissues. For example, to support new shoot growth, the resprouting shrub Quercus ilex first remobilizes N from belowground reserves then uses available N resources (El Omari et al. 2003). Decreases in retranslocation over time are suggested to occur as resource supply and root biomass increase (Salifu and Timmer 2001); however, for species that resprout after fire, an extensive root system already exists. Percent nonsoluble sugars in belowground structures of Q. geminata and Vaccinium myrsinities increase and decrease, respectively, with time after fire (Olano et al. 2006), suggesting that these species vary in their capacity to resprout after fire and that time after fire may affect the ability of shrubs to reallocate nutrients to aboveground tissues. In our study, foliar %N of S. repens was not correlated with soil extractable N, suggesting that retranslocation of N from below to aboveground may be more important for S. repens than for other species. Third, higher foliar nutrient concentrations after fire could be related to leaf age, increased leaf:shoot ratios, or the concentration of nutrients in a smaller amount of aboveground biomass post-fire. New leaves tend to have higher N concentrations than old leaves (Hikosaka et al. 1994; Anten et al. 1998, Han et al. 2008). Although we have not investigated the effects of age on foliar %N of flatwoods species, we collected the newest leaves at each sampling time to minimize the effects of ontogeny on foliar nutrient concentrations. A decline in foliar N concentrations with leaf age can result from dilution of N (Han et al. 2008), but when plants are grow at high soil NO3 - concentrations, there is less difference in foliar N content with leaf age (Hikosaka et al. 1994), suggesting that an increase in extractable N and P after fire may contribute to more similar nutrient concentrations among leaves of different ages. In a study of savanna grasses, Van de Vijver et al. (1999) determined that higher foliar N concentrations after fire were due to higher leaf:stem ratios after fire, higher N concentrations in young rather than old leaves, and the distribution of N over the lower amount of post-fire biomass; however, higher foliar P concentrations after fire were not easily explained. In our study, the number of palmetto leaves and the size of resprouting shrubs increased over the sampling period, so dilution of nutrients through more biomass could have occurred. Van de Vijver et al. (1999), however, did not find an effect of fire on soil nutrient availability, so their results do not rule out the possibility that higher foliar nutrient concentrations could be related to higher soil nutrient concentrations post-fire when they occur.
In our study, the relative increase in foliar %P was greater than the relative increase in foliar %N, so foliar N:P ratios of Serenoa repens decreased 20% from 15.8 pre-fire to 13.2 two months post-fire (Fig. 2). Over the same time period, soil N:P ratios decreased 83% from 8.4 pre-fire to 1.4 two months post-fire, suggesting that changes in plant nutrition post-fire are related to changes in soil extractable nutrients; however, since foliar N:P ratios did not decrease as much as soil extractable N:P ratios, reallocation of nutrients, particularly N, to aboveground tissue, increased leaf:shoot ratios, and concentration of nutrients in a smaller amount of aboveground biomass post-fire also likely contribute to the post-fire increase in foliar %N and %P. In addition, the foliar N:P ratios of S. repens suggests that flatwoods species are co-limited by N and P, because across habitats, N limitation occurs at foliar N:P ratios of 6.7 to 16 and P limitation occurs at foliar N:P ratios of 12.5 to 26.3 (Tessier and Raynal 2003).
Fire had no effect on soil δ15N (Table 1). Saito et al. (2007) found that soils had to be burned at 400°C for at least 5 min to cause a significant enrichment of soil δ15N, suggesting that high, sustained fire temperatures cause a greater loss of 14N compared to 15N. In addition, if fire consumes surface soils, volatilization of N may cause soils to become enriched in 15N (Högberg 1997). Temperatures recorded throughout a flatwoods fire usually exceeded 400°C for only 1 or 2 min (E. Menges, unpublished data). Thus, low sustained fire temperatures in flatwoods concomitant with low soil organic matter could explain the lack of an effect of fire on soil δ15N. Flatwoods plants were depleted in 15N compared to the soil, which is common in ecosystems with mycorrhizal species (Michelsen et al. 1998; Schmidt and Stewart 2003); however, there was no change in the absolute difference between foliar δ15N and soil δ15N over time after fire (Table 2). Fire did, however, affect the foliar δ15N signatures of flatwoods species; Quercus geminata tended to be more enriched in 15N 63 and 130 days post-fire compared to pre-fire and 493 days post-fire, while Serenoa repens became more depleted in 15N over time (Fig. 3). Grogan et al. (2000) found that all species in a pine forest were enriched in 15N after fire, but this corresponded with an enrichment of soil δ15N after fire (Grogan et al. 2000). Considering that we found no change in soil δ15N after fire, changes in foliar δ15N signatures post-fire could be caused by: (1) use of a different N source or change in discrimination of the same N source, (2) a change in the soil depth at which nutrient uptake occurs, (3) increased or decreased dependence on mycorrhizae for nutrient acquisition, and/or (4) within plant reallocation of N (Högberg 1997, Evans 2001).
Nitrogen sources (e.g. NH4 + versus NO3 -) vary in their isotopic signatures (Dawson et al. 2002), which can affect foliar δ15N signatures (Evans 2001); however, extractable NO3 - was low throughout our study, suggesting that use of NO3 - as a N source did not change with time after fire. Discrimination against 15N during N uptake can occur at high concentrations of NO3 - and NH4 + (Kolb and Evans 2003); however, even the increased concentrations of NH4 + after fire were likely not high enough to cause discrimination against 15N. In addition, the lack of change in the difference in foliar and soil δ15N over time suggests that neither a change in N sources nor greater discrimination occurred in our study.
Soil δ15N values tend to increase with depth (Nadelhoffer et al. 1996), and can increase 3‰ over 0–50 cm (Frank and Evans 1997). A shift in N uptake from surface (0–15 cm) to deeper (>15 cm) roots could explain the increase in foliar δ15N of Q. geminata immediately after fire, while a shift in uptake from deeper to surface roots could cause the foliar δ15N of S. repens to become more depleted over time. In a coastal Florida scrub-oak ecosystem, where CO2 enrichment caused a decrease in soil extractable inorganic N, both Q. geminata and S. repens took up N from the water table, but S. repens showed a greater use of deep soil N (McKinley et al. 2009). Thus, S. repens may shift uptake of N from deep soil to surface soil in response to an increase in extractable N in surface soils after fire.
Fire causes an increase in temperature (Ewel et al. 1981; Giardina et al. 2000; Jensen et al. 2001) and a decrease in moisture (Tomkins et al. 1991) of surface soils, which likely affects nutrient uptake by surface roots. Little is known about the root distribution of Q. geminata and S. repens in palmetto flatwoods, but root biomass has been investigated in other ecosystems where these species occur. In a coastal Florida scrub-oak ecosystem, where Q. geminata and S. repens comprise approximately 20% of the plant community, slightly less than half of roots <0.25 mm in diameter occur in the top 10 cm of soil (Brown et al. 2007). In scrubby flatwoods, a less mesic shrubland ecosystem often occurring at slightly higher elevations than palmetto flatwoods, approximately 85% of palmetto roots and 60% of oak roots in the top 50 cm of soil are ≤2 mm in diameter (Saha et al. in review). Thus, it seems unlikely that Q. geminata and S. repens would differ in fire-related root damage, suggesting that a shift in uptake from surface to deeper roots, or vice versa, could occur in response to changes in availability of or competition for soil nutrients.
Quercus geminata has associations with ectomycorrhizae (Langely et al. 2002), ericaceous species have associations with ericoid mycorrhizae (Pearson and Read 1973), and S. repens has associations with arbuscular mycorrhizae (Fisher and Jayachandran 1999); fractionation during the transfer of N from mycorrhizal fungi to a host plant results in plant tissue depleted in 15N relative to the N source (Evans 2001; Hobbie and Colpaert 2003). Foliar δ15N of Q. geminata could be more enriched shortly after fire if species reduced nutrient uptake through mycorrhizae, while foliar δ15N of S. repens of could become more depleted over time if nutrient uptake through AM increased. Soil δ15N and the difference between foliar and soil δ15N did not change over time for any species, however, suggesting that dependence on mycorrhizae for N uptake did not change after fire. In addition, Anderson and Menges (1997) and Eom et al. (1999) found that fire had no effect on colonization of roots by arbuscular mycorrhizae. Schmidt and Stewart (1997) found that plant δ15N signatures were more similar among species with the same mycorrhizal status than among species with the same post-fire response (e.g. resprouter vs. seeder species), so mycorrhizal status may play at least a small role in affecting changes in foliar δ15N after fire.
Foliar δ15N signatures decrease over time after leaf initiation (Bergersen et al. 1988), suggesting that reallocation of N within a plant can affect foliar δ15N signatures (Evans 2001). Although we sampled the newest leaves, as leaf number increased, we were more likely to sample leaves of varying ages, which could cause a decline in foliar δ15N over time after fire. In addition, leaves may be enriched in 15N compared to roots (Evans et al. 1996), and the foliar δ15N of S. repens could have decreased over time due to reallocation of N depleted in 15N from belowground to aboveground tissues. We hypothesize that changes in foliar 15N signatures after fire are influenced by N reallocation and leaf age and that a change in N uptake from roots at different levels in the soil may also contribute to variation in foliar δ15N.
One limitation of our experimental design is that we did not measure soil or plant nutrients in an unburned control site over the same time period that we measured soil and plant nutrients after fire in our flatwoods site; however, fire effects are the most likely explanation for our results for several reasons. First, across a scrubby flatwoods time-since-fire chronosequence, resin exchangeable NH4 + and PO4 3- was 2.7 and 1.5 times higher, respectively, during September through December compared to June through September (J. Schafer, unpublished manuscript). In our flatwoods site, extractable NH4 + and PO4 3- were 5.5 and 30 times higher, respectively, 3 h after fire than before fire. This change is much greater, and occurred over a much shorter time period, than seasonal variation in nutrient availability; thus, it is unlikely that the increases in extractable nutrients measured in this study are due to seasonal variation. Second, in the same palmetto flatwoods site used in this study, soil pH did not vary between September and November 2009 (J. Schafer, unpublished data). In addition, in oak and saw palmetto scrub, an ecosystem similar to flatwoods, Schmalzer and Hinkle (1991) found that in the first year after fire, soil pH was greater in December (12 months after fire) than in June (6 months after fire); whereas, in the second year after fire, soil pH was greater in June (18 months after fire) than in December (24 months after fire). Thus, changes in soil pH are likely due to fire effects rather than seasonal variation. Third, foliar %N and %P of oaks, ericaceous shrubs, and palmettos is higher six weeks after fire than before fire or one year after fire in scrubby flatwoods sites burned in March and July (J. Schafer, unpublished data), suggesting that the pattern of increased foliar nutrients after fire is consistent across sites and does not depend on burn season. We did not measure foliar nutrient concentrations of clipped plants over the same time scale that we measured foliar nutrient concentrations of burned plants, but burning and clipping can have similar effects on plant nutrient concentrations (Van de Vijver et al. 1999).
In our study, fire caused a short-term increase in soil extractable NH4 + and PO4 3- in a palmetto flatwoods ecosystem (Fig. 1); PO4 3- remained elevated above pre-fire levels twice as long as NH4 +, possibly due to differences in microbial uptake and mobility of NH4 + and PO4 3-. Both foliar %N and %P of resprouting plants increased over the short-term after fire (Table 2). The relative increase in soil extractable P and foliar P was greater than that of soil extractable N and foliar N, leading to a decrease in the soil extractable N:P ratio (Fig. 1) and the foliar N:P ratio of the palmetto Serenoa repens (Fig. 2) shortly after fire. The relationships between soil and foliar nutrients coupled with measurements of soil and foliar δ15N suggest that both an increase in soil extractable nutrients and reallocation of nutrients from belowground to aboveground tissue contribute to the increase in foliar %N and %P shortly after fire. Previous research in Florida scrub ecosystems has found limited effects of fire on soil nutrient availability (Abrahamson 1984, Schmalzer and Hinkle 1991). We found that a pulse of nutrients is detectable if soils are sampled soon enough after fire. Furthermore, our results suggest that even a short-term increase in soil extractable nutrients can be important for plant nutrient status, especially in ecosystems with low nutrient availability.
References
Abrahamson WG (1984) Post-fire recovery of Florida Lake Wales Ridge vegetation. Am J Bot 71:9–21
Abrahamson WG, Johnson AF, Layne JN, Peroni PA (1984) Vegetation of the Archbold Biological Station, Florida: An example of the southern Lake Wales Ridge. Florida Sci 47:209–250
Aciego Pietri JC, Brookes PC (2009) Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol and Biochem 41:1396–1405
Anderson RC, Menges ES (1997) Effects of fire on sandhill herbs: Nutrients, mycorrhizae, and biomass allocation. Am J Bot 84:938–948
Anten NPR, Miyazawa K, Hikosaka K, Nagashima H, Hirose T (1998) Leaf nitrogen distribution in relation to leaf age and photon flux density in dominant and subordinate plant in dense stands of a dicotyledonous herb. Oecologia 113:314–324
Arocena JM, Opio C (2003) Prescribed fire-induced changes in properties of sub-boreal forest soils. Geoderma 113:1–16
Badía D, Martí C (2003) Plant ash and heat intensity effects on chemical and physical properties of two contrasting soils. Arid Land Res Management 17:23–41
Bennett LT, Judd TS, Adams MA (2002) Growth and nutrient content of perennial grasslands following burning in semi-arid, sub-tropical Australia. Plant Ecol 164:185–199
Bergersen FJ, Peoples MB, Turner GL (1988) Isotopic discriminations during the accumulation of nitrogen by soybeans. Aust J Plant Physiol 15:407–420
Blagodatskaya EV, Blagodatsky SA, Anderson T-H, Kuzyakov Y (2009) Contrasting effects of glucose, living roots and maize straw on microbial growth kinetics and substrate availability in soil. Eur J Soil Sci 60:186–197
Boerner REJ, Huang J, Hart SC (2009) Impacts of fire and fire surrogate treatments on forest soil properties: a meta-analytical approach. Ecol Appl 19:338–358
Bradstock RA, Williams JE, Gill AM (2001) Flammable Australia: the fire regimes and biodiversity of a continent. Cambridge University Press, New York
Britton AJ, Helliwell RC, Fisher JM, Gibbs S (2008) Interactive effects of nitrogen deposition and fire on plant and soil chemistry in an alpine heathland. Environ Pollution 156:409–416
Brown ALP, Day FP, Hungate BA, Drake BG, Hinkle CR (2007) Root biomass and nutrient dynamics in a scrub-oak ecosystem under the influence of elevated atmospheric CO2. Plant Soil 292:219–232
Certini G (2005) Effects of fire on properties of forest soils: a review. Oecologia 143:1–10
Chang SX, Handley LL (2000) Site history affects soil and plant 15N natural abundances (δ15N) in forests of northern Vancouver Island, British Columbia. Funct Ecol 14:273–280
Chappell MA, Evangelou VP (2000) Influence of added K+ on ammonium selectivity/mobility by soils with vermiculitic behavior. Soil Sci 11:858–868
Christensen NL (1985) Shrubland fire regimes and their evolutionary consequences. In: Pickett STA, White PS (eds) The ecology of natural disturbance and patch dynamics. Academic, New York, pp 85–100
Christensen NL, Muller CH (1975) Effects of fire on factors controlling plant growth in Adenostoma chaparral. Ecol Monographs 45:29–55
Cleveland CC, Liptzin D (2007) C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochem 85:235–252
Cook GD (1994) The fate of nutrients during fires in a tropical savanna. Aust J Ecol 19:359–365
D’Angelo E, Crutchfield J, Vandiviere M (2001) Rapid, sensitive, microscale determination of phosphate in water and soil. J Environ Quality 30:2206–2209
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Ann Rev Ecol Syst 33:507–509
DeBano LF, Conrad CE (1978) The effect of fire on nutrients in a chaparral ecosystem. Ecology 59:489–497
DeLuca TH, Sala A (2006) Frequent fire alters nitrogen transformations in ponderosa pine stands of the inland northwest. Ecology 87:2511–2522
DeLuca TH, Nilsson M-C, Zackrisson O (2002) Nitrogen mineralization and phenol accumulation along a fire chronosequence in northern Sweden. Oecologia 133:206–214
Dunn PH, Barro SC, Poth M (1985) Soil moisture affects survival of microorganisms in heated chaparral soil. Soil Biol Biochem 17:143–148
El Omari B, Aranda X, Verdaguer D, Pascual G, Fleck I (2003) Resource remobilization in Quercus ilex L. resprouts. Plant Soil 252:349–357
Eom A-H, Hartnett DC, Wilson GWT, Figge DAH (1999) The effect of fire, mowing and fertilizer amendment on arbuscular mycorrhizas in tallgrass prairie. Am Midl Nat 142:55–70
Evans RD (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6:121–126
Evans RD, Bloom AJ, Sukrapanna SS, Ehleringer JR (1996) Nitrogen isotope composition of tomato (Lycopersicon esculentum Mill. Cv. T-5) grown under ammonium or nitrate nutrition. Plant Cell Environ 19:1317–1323
Ewel J, Berish C, Brown B, Price N, Raich J (1981) Slash and burn impacts on a Costa Rican wet forest site. Ecology 62:816–829
Ferran A, Delitti W, Vallejo VR (2005) Effects of fire recurrence in Quercus coccifera L. shrublands of the Valencia region (Spain): II. plant and soil nutrients. Plant Ecol 177:71–83
Field A (2009) Discovering statistics using SPSS, 3rd edn. SAGE
Fisher JB, Jayachandran K (1999) Root structure and arbuscular mycorrhizal colonization of the palm Serenoa repens under field conditions. Plant Soil 217:229–241
Franco-Vizcaíno E, Sosa-Ramirez J (1997) Soil properties and nutrient relations in burned and unburned Mediterranean-climate shrublands of Baja California, Mexico. Acta Oecologica 18:503–517
Frank DA, Evans RD (1997) Effects of native grazers on grassland N cycling in Yellowstone National park. Ecology 78:2238–2248
Giardina CP, Sanford RL Jr, Døckersmith IC (2000) Changes in soil phosphorus and nitrogen during slash-and-burn clearing of a dry tropical forest. Soil Sci Soc Am J 64:399–405
Gilliam FS (1988) Interactions of fire with nutrients in the herbaceous layer of a nutrient-poor Coastal Plant forest. Bull Torrey Bot Club 115:265–271
Gillon D, Rapp M (1989) Nutrient losses during a winter low-intensity prescribed fire in a Mediterranean forest. Plant Soil 120:69–77
Goforth BR, Graham RC, Hubbert KR, Zanner CW, Minnich RA (2005) Spatial distribution and properties of ash and thermally altered soils after high-severity forest fire, southern California. Int J Wildland Fire 14:353–354
Grogan P, Burns TD, Chapin FS III (2000) Fire effects on ecosystem nitrogen cycling in a Californian bishop pine forest. Oecologia 122:537–544
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Han Q, Kawasaki T, Nakano T, Chiba Y (2008) Leaf-age effects on seasonal variability in photosynthetic parameters and its relationships with leaf mass per area and leaf nitrogen concentration within a Pinus densiflora crown. Tree Physiol 28:551–558
Hikosaka K, Terashima I, Katoh S (1994) Effects of leaf age, nitrogen nutrition and photo flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia 97:451–457
Hobbie EA, Colpaert JV (2003) Nitrogen availability and colonization by mycorrhizal fungi correlate with nitrogen isotope patterns in plants. New Phytol 157:115–126
Högberg P (1997) Tansley Review No. 95. 15N natural abundance in soil-plant systems. New Phytol 137:179–203
James BR, Riha SJ (1986) pH buffering in forest soil organic horizons: relevance to acid precipitation. J Environ Quality 15:229–234
Jensen M, Michelsen A, Gashaw M (2001) Responses in plant, soil inorganic and microbial nutrient pools to experimental fire, ash and biomass addition in a woodland savanna. Oecologia 128:85–93
Kauffman JB, Sanford RL, Cummings DL, Salcedo IH, Sampaio EVSB (1993) Biomass and nutrient dynamics associated with slash fires in Neotropical dry forests. Ecology 74:140–151
Keeley JE (1977) Seed production, seed populations in the soil, and seedling production after fire for two congeneric pairs of sprouting and non-sprouting chaparral shrubs. Ecology 58:820–829
Keeley JE, Zedler PH (1978) Reproduction of chaparral shrubs after fire: a comparison of sprouting and seeding strategies. Am Midl Nat 99:142–161
Keeley JE, Keeley SC (1988) Chaparral. In: Barbour MG, Billings WD (eds) North American terrestrial vegetation. Cambridge University Press, New York, pp 166–207
Khanna PK, Raison RJ (1986) Effect of fire intensity on solution chemistry of surface soil under a Eucalyptus pauciflora forest. Aust J Soil Res 24:423–434
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Kolb KJ, Evans RD (2003) Influence of nitrogen source and concentration on nitrogen isotopic discrimination in two barley genotypes (Hordeum vulgare L.). Plant Cell Environ 26:1431–1440
Langely JA, Drake BG, Hungate BA (2002) Extensive belowground carbon storage supports roots and mycorrhizae in regenerating scrub oaks. Oecologia 131:542–548
Lewis WM Jr (1974) Effects of fire on nutrient movement in a South Carolina pine forest. Ecology 55:1120–1127
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute Inc., Cary
Little S (1979) Fire and plant succession in the New Jersey pine barrens. In: Forman RTT (ed) Pine barrens: Ecosystem and landscape. Academic, New York, pp 297–314
Mackensen J, Hölscher D, Klinge R, Fölster H (1996) Nutrient transfer to the atmosphere by burning of debris in eastern Amazonia. For Ecol Management 86:121–128
Magdoff FR, Bartlett RJ (1985) Soil pH buffering revisited. Soil Sci Soc Am J 49:145–148
Main KN, Menges ES (1997) Archbold biological station, station fire management plan. Land Management Publ. 97-1
Marcos E, Villalón C, Calvo L, Luis-Calabuig E (2009) Short-term effects of experimental burning on soil nutrients in the Cantabrian heathlands. Ecol Engineering 35:820–828
Martinelli LA, Piccolo MC, Townsend AR, Vitousek PM, Cuevas E, McDowell W, Robertson GP, Santos OC, Treseder K (1999) Nitrogen stable isotopic composition of leaves and soil: Tropical versus temperate forests. Biogeochem 46:45–65
McKinley DC, Romero JC, Hungate BA, Drake BG, Megonigal JP (2009) Does deep soil N availability sustain long-term ecosystem responses to elevated CO2? Global Change Biol 15:2035–2048
Menges ES (1999) Ecology and conservation of Florida scrub. In: Anderson RC, Fralish JS, Baskin JM (eds) Savannas, barrens, and rock outcrop plant communities of North America. Cambridge University Press, USA, pp 7–22
Menges ES, Kohfeldt N (1995) Life history strategies of Florida scrub plants in relation to fire. Bull Torrey Bot Club 122:282–297
Michelsen A, Quarmby C, Sleep D, Jonasson S (1998) Vascular plant 15N natural abundance in heath and forest tundra ecosystems is closely correlated with presence and type of mycorrhizal fungi in roots. Oecologia 115:406–418
Molina M, Fuentes R, Calderón R, Escudey M, Avendaño K, Gutiérrez M, Chang AC (2007) Impact of forest fire ash on surface charge characteristics of andisols. Soil Sci 172:820–834
Moreno JM, Oechel WC (1994) The role of fire in Mediterranean-type ecosystems. Springer-Verlag, Berlin
Murphy J, Riley JP (1962) A modified single solution method for determination of phosphate in natural waters. Analytica Chimica Acta 26:31–36
Nadelhoffer K, Shaver G, Fry B, Giblin A, Johnson L, McKane R (1996) 15N natural abundance and N use by tundra plants. Oecologia 107:386–394
Olano JM, Menges ES, Martínez E (2006) Carbohydrate storage in five resprouting Florida scrub plants across a fire chronosequence. New Phytol 170:99–106
Pearson V, Read DJ (1973) The biology of mycorrhiza in the Ericaceae. I. The isolation of the endophyte and synthesis of mycorrhizas in aseptic cultures. New Phytol 72:371–379
Pivello VR, Coutinho LM (1992) Transfer of macro-nutrients to the atmosphere during experimental burnings in an open cerrado (Brazilian savanna). J Tropical Ecol 8:487–497
Prieto-Fernández A, Acea MJ, Carballas T (1998) Soil microbial and extractable C and N after wildfire. Biol Fert Soils 27:132–142
Raison RJ, Khanna PK, Woods PV (1985a) Mechanisms of element transfer to the atmosphere during vegetation fires. Can J For Res 15:132–140
Raison RJ, Khanna PK, Woods PV (1985b) Transfer of elements to the atmosphere during low-intensity prescribed fires in three Australian subalpine eucalypt forests. Can J For Res 15:657–664
Saha S, Menges ES, Catenazzi A (in review) Does time since fire explain plant biomass allocation in the Florida scrub ecosystem? Fire Ecology
Saito L, Miller WM, Johnson DW, Qually RG, Provencher L, Carroll E, Szameitat P (2007) Fire effects on stable isotopes in a Sierran forested watershed. J Environ Quality 36:91–100
Salifu KF, Timmer VR (2001) Nutrient retranslocation response of Picea mariana seedlings to nitrogen supply. Soil Sci Soc Am J 65:905–913
SAS 9.1 (2003) SAS institute Inc. Cary, North Carolina
Schmalzer PA, Hinkle CR (1991) Dynamics of vegetation and soils of oak/saw palmetto scrub after fire: observations from permanent transects. NASA Technical Memorandum 103817, Kennedy Space Center, Florida, pp 149
Schmidt S, Stewart GR (1997) Waterlogging and fire impacts on nitrogen availability and utilization in a subtropical wet heathland (wallum). Plant Cell Environ 20:1231–1241
Schmidt S, Stewart GR (2003) δ15N values of tropical savanna and monsoon forest species reflect root specialisations and soil nitrogen status. Oecologia 2003:569–577
Schuur EAG, Matson PA (2001) Net primary productivity and nutrient cycling across a mesic to wet precipitation gradient in Hawaiian montane forest. Oecologia 128:431–442
Scott-Denton LE, Rosenstiel TN, Monson RK (2006) Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Glob Chang Biol 12:205–216
Serrasolsas I, Khanna PK (1995) Changes in heated and autoclaved forest soils of S.E. Australia. I. Carbon and nitrogen. Biogeochem 29:3–24
Sigma Plot for Windows 11.0 (2008) Systat Software, Inc. Chicago, Illinois
Singh RS, Srivastava SC, Raghubanshi AS, Singh JS, Singh SP (1991) Microbial C, N and P in dry tropical savanna: effects of burning and grazing. J Appl Ecol 28:869–878
Skyllberg U, Raulund-Rasmussen K, Borggaard OK (2001) pH buffering in acidic soils developed under Picea abies and Quercus robur—effects of soil organic matter, adsorbed cations and soil solution ionic strength. Biogeochem 56:51–74
Smithwick EAH, Turner MG, Mack MC, Chapin FS III (2005) Postfire soil N cycling in northern conifer forests affected by severe, stand-replacing wildfires. Ecosystems 8:163–181
Sousa WP (1984) The role of disturbance in natural communities. Ann Rev Ecol Syst 15:353–391
SPSS 11.5 for Windows (2000) SPSS, Inc. Chicago, Illinois
Stock WD, Lewis OAM (1986) Soil nitrogen and the role of fire as a mineralizing agent in a South African coastal fynbos ecosystem. J Ecol 74:317–328
Tessier JT, Raynal DJ (2003) Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J Appl Ecol 40:523–534
Thomas GW (1996) Soil pH and soil acidity. In: Bartels JM et al (eds) Methods of soil analysis Part 3 chemical methods. Soil Science Society of America, Inc., Madison, pp 475–490
Tomkins IB, Kellas JD, Tolhurst KG, Oswin DA (1991) Effects of fire intensity on soil chemistry in a Eucalypt forest. Aust J Soil Res 29:25–47
Turner MG, Smithwick EAH, Metzger KL, Tinker DB, Romme WH (2007) Inorganic nitrogen availability after severe stand-replacing fire in the Greater Yellowstone ecosystem. PNAS 104:4782–4789
Van de Vijver CADM, Poot P, Prins HHT (1999) Causes of increased nutrient concentrations in post-fire regrowth in an East African savanna. Plant Soil 214:173–185
Villani EMA, Barros NF, Novais RF, Comerford NB, Costa LM, Neves JCL, Alvarez VH (1998) Phosphorus diffusive flux as affected by phosphate source and incubation time. Soil Sci Soc Am J 62:1057–1061
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea—How can it occur? Biogeochem 13:87–115
Wan S, Hui D, Luo Y (2001) Fire effects on nitrogen pools and dynamics in terrestrial ecosystems: a meta-analysis. Ecol Appl 11:1349–1365
Weekley CW, Menges ES (2003) Species and vegetation responses to prescribed fire in a long-unburned, endemic-rich Lake Wales Ridge scrub. J Torrey Bot Soc 130:265–282
White EM, Thompson WW, Gartner FR (1973) Heat effects on nutrient release from soils under Ponderosa pine. J Range Management 26:22–24
Wilbur RB, Christensen NL (1983) Effects of fire on nutrient availability in a North Carolina coastal plain pocosin. Am Midl Nat 110:54–61
Wunderlin RP, Hanson BF (2003) Guide to the vascular plants of Florida, 2nd edn. University Press of Florida
Acknowledgements
We thank Catherine Pociask and Megan Larson for help in the field and lab, Patrick Bohlen and Adam Peterson for assistance processing plant and soil samples at the MacArthur Agro-Ecology Research Center, Grace Crummer for assistance with δ15N measurements, and Martin Lavoie for help with statistical analyses. We also thank Kevin Main and others who have contributed to effective fire management at Archbold Biological Station. This manuscript was improved by comments from Doria Gordon, Carl Weekley, and two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Elizabeth M. Baggs.
Rights and permissions
About this article
Cite this article
Schafer, J.L., Mack, M.C. Short-term effects of fire on soil and plant nutrients in palmetto flatwoods. Plant Soil 334, 433–447 (2010). https://doi.org/10.1007/s11104-010-0394-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-010-0394-2