Abstract
The crucial roles played by arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria (PGPR) in enhancing plant nutrient uptake and soil quality are widely recognized across various plant species. This study explored the effects and potential of Bacillus velezensis S141 as a plant growth-promoting rhizobacterium on promoting a symbiotic relationship of AMF, Rhizophagus irregularis with Lotus japonicus. B. velezensis S141 inoculation positively influenced fungal growth and development. B. velezensis S141 promoted fungal abundance, such as AM root colonization and spore number. It also boosted plant nutrient uptake, enhancing the nitrogen and phosphorus concentration by 1.65 and 1.51 times, respectively, under tripartite interaction conditions. However, the indole-3-acetic acid (IAA) producing capability of B. velezensis S141, based on the inoculation experiment test of S141 mutants defective in IAA synthesis, was not the key mechanism for promoting this symbiotic interaction. Interestingly, the S141 strain, originating from rhizospheric soil fields of soybeans, was found to penetrate plant root cells and establish itself as an endophyte. The presence of B. velezensis S141 not only triggered the expression of marker genes associated with early stages of AMF colonization and nutrient uptake in the host plant, but it also led to an upregulation of AMF genes responsible for cell cycle regulation. These results suggest that B. velezensis S141 holds promise as a helper bacterium in promoting plant-AMF symbiosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plant growth promoting rhizobacteria (PGPR) are a group of beneficial bacteria that enhance plant growth and development (Oleńska et al. 2020; Vocciante et al. 2022; Bhat et al. 2023). These beneficial bacteria, including various species of Alcaligenes, Arthrobacter, Azospirillum, Azotobacter, Pseudomonas, Klebsiella, Enterobacter, Burkholderia, and Bacillus, are found in association with plant roots (Figueiredo et al. 2010; Bashan et al. 2014; Singh et al. 2019), and some of them have been identified for their potential applications as biofertilizers (Sun et al. 2020; Barin et al. 2022). The growth-promoting effects contributed by these bacteria are mediated through diverse mechanisms, for example, nitrogen (N) fixation (Liu et al. 2019; Matse et al. 2019), phosphate solubilization (Prabhu et al. 2019; Amy et al. 2022), production of plant growth hormones, and biocontrol of plant pathogens (Odoh et al. 2019; Chauhan et al. 2021).
Arbuscular mycorrhizal fungi (AMF) significantly benefit plant growth through their symbiotic relationships with plant roots (Genre et al. 2018). They enhance plant resistance to environmental stress. They do so by helping in osmolyte accumulation and ion absorption and fostering soil fertility through the enhancement of water and mineral nutrition via their mycelial hyphae (Kempel et al. 2010; Mitra et al. 2021). The symbiotic relationship is established by a complex and sophisticated mechanism between AMF and the host plants. The interaction begins with the release of signaling molecules, such as chitooligosaccharides and lipochitooligosaccharides from the AMF, which are then recognized by plant receptors on the root surface (Feng et al. 2019; Allen Rush et al. 2020). This recognition event activates symbiotic signaling pathways in the plant that result in the development of specialized fungal structures, called arbuscules, within the cortical cells of the plant root. Arbuscules contribute to the supply of nutrients, such as phosphorus (P) and N, to the plant (Smith 1988; George et al. 1995; Smith et al. 2011).
In the rhizosphere, synergistic interactions between PGPR and AMF have been observed to enhance plant growth and performance through an increase in AMF colonization, regulation of plant hormones, such as auxin and cytokinin, and inhibition of plant pathogens (Tsukanova et al. 2017; Emmanuel & Babalola 2020). For example, co-inoculation of soybean with B. amyloliquefaciens and AMF led to an increase in AMF colonization, which is postulated to have contributed to improvements in plant growth and field yield (Sheteiwy et al. 2021). Our previous research also confirms that the combined application of PGPR Brevibacillus sp. SUT47 with AMF (Acaulospora tuberculata) resulted in increased AMF colonization and spore production in maize. This was in comparison to single AMF inoculation in sterilized sand, which was conducted under laboratory conditions (Yuttavanichakul et al. 2018; Kiddee et al. 2020). Recently, Begum et al. (2022) showed that Bacillus methylotrophicus co-inoculated with AMF produced an increase in photosynthetic activity and mineral, osmolyte, and phytohormone content in tobacco in the field. These increases positively affected secondary metabolite content and antioxidant system under drought stress conditions and resulted in enhanced tobacco growth.
B. velezensis is a recently characterized PGPR species that has shown potential as a biocontrol agent (Adeniji et al. 2019; Rabbee et al. 2019). For example, B. velezensis FZB42 has been reported to produce secondary metabolites with antimicrobial properties (Fan et al. 2018). Yet another strain, B. velezensis S141, efficiently mitigated cercospora leaf spots in mung beans when isolated from soybean-adjoining rhizospheric soil in Thailand and sprayed on leaf surfaces (Prakamhang et al. 2015; Songwattana et al. 2023). In soybean-Bradyrhizobium symbiosis, B. velezensis S141 colonized the nodule and root surface and induced the production of larger nodules and enhanced nitrogen-fixing efficiency by B. diazoefficiens USDA110 (Sibponkrung et al. 2020). B. velezensis S141 has a set of genes for auxin biosynthesis in its genome (Sibponkrung et al. 2017). After inoculating the disrupted strains of those genes into soybean-Bradyrhizobium, the disrupted of auxin biosynthesis attenuated the effects of B. velezensis S141 on increasing nodule size and promotion of N-fixing activity. These findings suggest that this specific PGPR strain may play an important role in enhancing the symbiotic functionality between rhizobia and legumes through its phytohormone producing properties.
In contrast, it remains to be elucidated whether B. velezensis S141 exerts an influence on plant–AMF symbiosis. Phytohormones, including auxins, have been shown to modulate the development of the mycorrhizal symbiosis (Gutjahr 2014; Pozo et al. 2015; Liao et al. 2018). Etemadi et al. (2014) demonstrated that overexpression of miR393, an miRNA that specifically targets a plant auxin receptor gene, resulted in suppression of arbuscule formation, suggesting a necessary role for auxin perception in arbuscule development. In this situation, we hypothesized that B. velezensis S141 could affect the symbiotic relationship between plants and AMF through its potential to produce auxins. To test this hypothesis, we investigated the effects of B. velezensis S141 and its mutant strains containing disrupted auxin biosynthesis genes on AMF development both intra- and extra-radically. Since the interactions between L. japonicus and R. irregularis has been intensively studied in the molecular level due to the available of genome information, L. japonicus and R. irregularis were selected as a model to investigate the effect of B. velezensis S141 on promoting plant-AMF symbiosis. In addition, we analyzed the gene expression of symbiosis-related genes in both AMF and host plant.
2 Materials and methods
2.1 Biological materials and growth conditions
L. japonicus B-129 seeds were surface-sterilized with a 3% (w/v) sodium hypochlorite solution for 10 min and then thoroughly rinsed with sterile distilled water. The sterilized seeds were germinated on moist paper in a Petri dish and incubated at 26 °C in the dark for 2 days followed by 3 more days under light. Seedlings were transplanted into 50-mL centrifuge tubes (one plant per tube), each with a drainage cavity at the bottom, which contained filter paper with a pore size of 2 μm and filled with autoclaved river sand (particle size, 0.5–2.0 mm) as planting material. This filter allows the bacteria to remain in the system while allowing water to flow out. The sand was washed several times until the water ran clear and sterilized at 121 °C for 45 min by autoclaving and repeating the sterilization twice within 24 h. The concentration of commercial AMF inoculum R. irregularis DAOM197198 (Mycorise, Premier Tech, Rivière-du-Loup, Canada) at 4,000 spores/mL was diluted using sterilized water to achieve a final spore concentration of 250 spores/mL. Subsequently, a hole was carefully made at the center of the pot containing planting material with sufficient depth to cover the roots of L. japonicus. The AMF spores were then evenly distributed around the hole, followed by transplanting the plant and covering it with river sand.
The plant growth-promoting rhizobacterium Bacillus velezensis S141 (Sibponkrung et al. 2017) was cultured in Luria–Bertani (LB) broth at 28 °C for 20 h and used as inoculum. Derivative mutant strains of B. velezensis S141 (∆IPyAD, ∆yhcX and ∆dhaS), which have a reduced production of indole-3-acetic acid (IAA), were cultured under the same conditions but in the presence of antibiotics [1 µg/mL mg erythromycin, 10 µg/mL kanamycin, and 100 µg/mL spectinomycin] to ensure that only the desired bacteria were grown, while the B. velezensis S141 GFP-tagged strain was cultured in LB medium supplemented with 8 µg/mL phleomycin (Sibponkrung et al. 2020). Bacterial cells were separated by centrifugation and washed twice with 0.85% (w/v) NaCl. The cell density was then adjusted to OD600 = 1 (approximately 108 CFU/mL). The bacterial cells at different cell concentrations (105, 106, and 107 CFU in 50 mL) were then inoculated or co-inoculated with R. irregularis in sand. Non-inoculated control plants were also included in the experiment by adding them with 50 mL of 0.85% (w/v) NaCl.
Plants were grown in a growth chamber at 28 °C under a 16/8-h light and dark cycle and a light intensity of 150 µmol m−2 s−1. The plants were supplied with a half-strength Hoagland’s solution containing a low concentration of KH2PO4 (100 µM) every 2 days since high P concentration could inhibit AMF infection (Sugimura and Saito 2017). Plants were harvested at either 45 or 60 days after inoculation (DAI), depending on the specific data collection goals, such as assessing plant growth, nutrient uptake, colonization efficiency, spore count, or the localization of arbuscular mycorrhizal fungi (AMF) or bacteria. Each experiment was conducted with triplicate samples. For plant growth data collection, the plants were harvested and separated into shoot and root components. Subsequently, these plant parts were dried at 70 °C for 48 h and weighed following the method outlined by Shipley and Vu (2002). Plant height above ground level was measured at 45 or 60 DAI, as specified in each experiment. Chlorophyll content in fully expanded leaves from the top was estimated in SPAD units using the SPAD-502 Plus instrument (Konica Minolta Optics, Japan). Each treatment was replicated four times in the experiment.
2.2 Analysis of plant P and N contents
Plant shoots were dried at 70 °C for 48 h, finely ground, and weighed. Subsequent analysis of phosphorus (P) involved acid digestion in a solution of nitric and perchloric acids (HNO3 + HClO4). P concentration was then established using the vanadomolybdate blue method (Watanabe and Olsen 1965). Pursuing the nitrogen (N) analysis, plant samples were heated in a Kjeldahl digestion apparatus, gradually increasing the temperature to 200 °C, then further to 350–375 °C for 1 h until transparent. N concentrations were ascertained using the Kjeldahl method (Yash and Kalra 1998).
2.3 Localization of bacteria in roots
L. japonicus were inoculated with B. velezensis S141 at a concentration of 105 CFU/plant or co-inoculated with both B. velezensis S141 and 250 spores/plant of R. irregularis. These plants were grown under the conditions described above. At 7, 15, 30 and 45 DAI, roots were harvested and thoroughly washed with sterilized water to remove sand particles. These roots were then cut into fine segments, each 1–2 cm in length. The segments were embedded in 5% agarose gel and sectioned into 40 μm thick slices using a Leica vibratome (VT1000S, Leica Microsystems, Germany). The sections were stained for 10 min with 5 μM SYTO 9 (Thermo Fisher Scientific, USA) to visualize bacterial cells and 0.01% (w/v) Calcofluor (Bonaldi et al. 2011) to emphasize plant roots and AMF cell walls. We did not use the GFP-tagged strain in this study due to low fluorescence expression. However, the experiment was conducted under sterile conditions, enabling comparisons with the non-inoculated control. No bacterial cells or fungus were detected under the microscope in the control group, eliminating the possibility of seed endophyte contamination. The stained sections were washed with phosphate-buffered saline (PBS), mounted on a glass slide with 10% PBS-glycerol solution, and covered with a coverslip. The Calcofluor was excited at 405 nm, and its emission signal was detected using a 460–500 nm filter for image acquisition. SYTO 9 fluorescence was detected by exciting the samples with a 488 nm laser line and collecting the emission signal at 490 to 522 nm. Bacterial localization was observed using a Nikon inverted Eclipse Ti-E Confocal Laser Scanning Microscope (Nikon, Japan).
2.4 Enumeration of endophytic bacteria
The roots of L. japonicus, inoculated with and without the S141 GFP-tagged strain (Sibponkrung et al. 2020), were surface-sterilized 30 days after infection. This process involved immersing the roots in a 3% NaClO solution for 5 min and then soaking them in 70% ethanol for another 5 min. Once sterilized, the roots were thoroughly rinsed at least five times with sterile distilled water (Pongdet et al. 2015). The sterilized plant roots were then ground with 2 mL of 0.85% NaCl. Serial dilutions were prepared from this mixture, ranging from 100 to 104. From each dilution, 100 µL was plated onto LB agar, which was augmented with 8 µg/mL of phleomycin. The plates were then incubated at a temperature of 28 °C for a period of 20 h to allow for the enumeration of the target strain. The number of endophytic bacteria present was calculated as CFU/plant.
2.5 Assessment of AMF colonization
The root system of L. japonicus was collected and carefully cleaned by removing all soil particles. The cleaned roots were cut into 1–2 cm segments and washed with tap water. To remove the cytoplasmic content of the plant root cells, these root segments were treated with 10% (w/v) potassium hydroxide (KOH) at 90 °C for 10 min followed by acidification with 2% (v/v) hydrochloride acid (HCl) for 5 min. The segments were subsequently stained with 0.05% trypan blue in lactic acid at 90 °C for 10 min. The degree of AMF colonization was assessed using the method described by Trouvelot et al. (1986) with some modifications. Briefly, approximately 10 root fragments from each plant were mounted on glass slides and observed under a light microscope with a 10 × objective. Each slide was observed over a total of 100 microscopic fields. The intensity of AMF colonization and the abundance of arbuscules in a field of view were categorized into six and three classes, respectively. Based on the scores obtained from 100 fields of view, five parameters of AMF colonization (F%, M%, m%, A%, and a%) were calculated according to Trouvelot et al. (1986). F% represents the frequency of mycorrhiza in the root system, M% represents the intensity of the mycorrhizal colonization in the root system, m% represents the intensity of the mycorrhizal colonization in the root fragments, A% represents the arbuscule abundance in the root system, and a% represents the arbuscule abundance in the mycorrhizal parts of the root fragments.
2.6 Quantification of AMF in roots by quantitative PCR
Inoculated root samples of L. japonicus were collected, and total DNA was extracted using the Dneasy® Plant Mini Kit (Qiagen, USA) according to the manufacturer’s instructions. The abundance of fungi was estimated through quantitative real-time polymerase chain reactions (qPCR), using approximately 50 ng of each DNA sample on a StepOne Real-Time PCR System (Thermo Fisher Scientific). Four biological replicates were performed in this experiment. A primer set specific for R. irregularis was used for qPCR. The set consisted of a forward primer (CCCACCAGGGCAGATTAATC), a reverse primer (TGGCTTTGTACAGGCAACAG), and a TaqMan-MGB probe (197198P: FAM-CCCTGGAGTATCTG-MGBEQ synthesized by Eurofins Genomics, Japan). This primer set was designed based on the single-copy cox3-rnl intergenic region in mitochondrial DNA (Badri et al. 2016). As an internal control, a primer set designed for genomic DNA of the L. japonicus ubiquitin-conjugating enzyme E2 gene (LotjaGi1g1v0401300.1) was used. The primer set for this gene consisted of a forward primer (AAATGGACGGCTCTTATCAAGGT), a reverse primer (GACCGGTCGAACATCTTACACA), and a TaqMan-MGB probe (FAM-TGCTGGCTAATATGC-MGBEQ). Data were normalized relative to this internal control and analyzed according to the 2−ΔΔCt method with four replications (Livak and Schmittgen 2001).
2.7 Enumeration of R. irregularis spores in soil
AMF spores were extracted by wet sieving and decanting (Gerdemann and Nicolson 1963; Daniels and Skipper 1982). All soil contained in a 50 mL tube was collected and mixed with 100 mL of tap water in a 500 mL beaker. The soil sample was agitated vigorously to disperse the AMF spores and left to stand for 5 min to allow the heavier soil particles to settle to the bottom. The supernatant was decanted through standard sieves with pore sizes of 250, 106, 75, and 38 µm. The AMF spores and soil particles retained on the 106, 75, and 38 µm sieves were collected in 15 mL centrifuge tubes and centrifuged at 4,000 rpm for 5 min. After removing the supernatant, 40% (w/v) sucrose solution was added and centrifuged at 5,000 rpm for 5 min. The supernatant was carefully removed and poured into sieves to retain the AMF spores. The spores on the sieves were rinsed with tap water and collected in Petri dishes. AMF spores were counted under a stereomicroscope.
2.8 Gene expression analysis
Total RNA was extracted using RNA Prep Pure Plant Plus Kit (TIANGEN Biotech, China). To remove DNA contamination, the RNA samples were treated with Dnase I (Thermo Fisher Scientific). cDNA was synthesized from the RNA using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) following the manufacturer’s instructions. Quantitative RT-PCR was conducted using Luna Universal qPCR Master Mix (New England Biolabs, USA) and the CFX Opus 96 Real-Time PCR System (Bio-Rad, USA). The primers for this system are listed in Supplementary Tables S1 and S2. As an internal control for gene expression, we used elongation factor 2 (EF2) in L. japonicus and EF1β in R. irregularis (Kobae et al. 2015). We evaluated three biological samples of the plant at 45 DAI due to resource limitations and experimental practicality, each using approximately 5 ng of cDNA template. We calculated relative gene expression using the 2-ΔΔCT method (Livak and Schmittgen 2001).
2.9 Statistical analyses
We conducted statistical analyses using R software (version 4.2.2). To identify specific differences among samples belonging to three or more groups, we used Tukey’s honestly significant difference (HSD) test whenever ANOVA pointed to a significant discrepancy in group means. We also applied the Levene test to verify normal distribution and demonstrate equal variances across the groups. To compare the effects of single inoculation with R. irregularis against co-inoculation with R. irregularis and B. velezensis S141, we used Student’s t-test. We visualized all data using GraphPad Prism (v.9.1.1).
3 Results
3.1 B. velezensis S141 enhances AMF abundance in the interior and exterior of L. japonicus roots
To investigate whether B. velezensis S141 could enhance the development of R. irregularis during its symbiosis with L. japonicus, we assessed AMF abundance in roots and quantified spore production in soil. We co-inoculated AMF with B. velezensis S141 using different cell densities on L. japonicus and measured the extent of AMF colonization at 60 DAI. The presence of B. velezensis S141 caused a slight but significant increase in the intensity of AMF colonization (M% and m%) compared to the absence of B. velezensis S141 (Fig. 1A). This enhancement was consistently observed across all B. velezensis S141 inoculum cell densities. In contrast, the frequency of AMF colonization (F%) and arbuscular abundances (A% and a%) were not affected by inoculation with B. velezensis S141. AMF abundance in roots was further quantified using a hydrolysis probe-based qPCR assay specifically targeting R. irregularis DAOM197198 mitochondrial DNA as described by Badri et al. (2016). The relative copy number of the cox3-rnl intergenic region consistently increased more than twofold in the co-inoculation treatments compared to AMF single inoculation (Fig. 1B). Notably, B. velezensis S141 also promoted AMF spore production in soil (Fig. 1C). Regarding the inoculum cell density of B. velezensis S141 at 105, 106, and 107 CFU/plant, the co-inoculation produced an increase in the number of spores produced in soil by 39%, 19%, and 27%, respectively when compared to single inoculation with R. irregularis. These results show that B. velezensis S141 significantly promoted AMF development both inside and outside the roots.
The abundance of R. irregularis at 60 days after inoculation (DAI). L. japonicus was either inoculated with R. irregularis alone (AMF) or co-inoculated with both R. irregularis and B. velezensis S141 at different cell densities: 105 (AMF + S141(105)), 106 (AMF + S141(106)), 107 (AMF + S141(107)) CFU/plant. (A) AMF colonization in L. japonicus roots. F(%), the frequency of mycorrhiza in the root system; M(%), the intensity of the mycorrhizal colonization in the root system; m(%), the intensity of the mycorrhizal colonization in the root fragments; a(%), the arbuscule abundance in the mycorrhizal parts of the root fragments; and A(%), the arbuscule abundance in the root system. (B) The relative abundance of R. irregularis in L. japonicus roots estimated by quantitative PCR (qPCR) using a hydrolysis probe designed from the cox3-rnl intergenic region in R. irregularis mitochondrial DNA. The abundance of R. irregularis in roots is normalized based on the copy number of the genomic region of the L. japonicus ubiquitin-conjugating enzyme E2 gene and expressed relative to that in plants singly inoculated with R. irregularis (AMF). (C) The number of R. irregularis spores produced in the soil. Values are means ± s.e.m. (n = 4). Bars marked with the same letter are not significantly different at p < 0.05 on Tukey's HSD test. None of AMF were detected in the non-inoculated control plant
3.2 B. velezensis S141 affects mycorrhizal plant growth
To determine the direct effect of B. velezensis S141 on L. japonicus growth, the plant was inoculated with different densities of the bacteria in the absence of AMF. At 60 DAI, B. velezensis S141 did not affect the plant growth of L. japonicus. However, the bacterial cells at a density of 107 CFU/plant produce a significant increase in the shoots’ dry weights by 2.1-fold compared to the non-inoculated plants (Table S3). Although inoculation only AMF did not notably enhance plant growth at day 60 compared to non-inoculated plants, when B. velezensis S141 was paired with AMF at a density of 105 CFU/plant, there was a significant rise in plant height compared to just AMF inoculation.
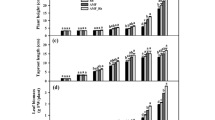
The 60-day growth period was probably too long to evaluate L. japonicus growth in a 50-mL pot. Therefore, we assessed plant growth at 45 DAI. A density of 105 CFU/plant was selected for inoculation with B. velezensis S141, a density that was shown to be effective in enhancing plant height at 60 DAI (Table S3), and it is the lowest density that significantly promote AMF spore production (Fig. 1C). Even at 45 DAI, a modest increase in AMF colonization, M% and m%, due to co-inoculation (Fig. 2A) was found. No direct effect of S141 on L. japonicus growth was observed at 45 DAI (Fig. 2B, C). Conversely, the effect of AMF inoculation was pronounced, namely plant heights and dry weights of AMF-inoculated plants were significantly greater than those of non-inoculated plants. In particular, the co-inoculation of B. velezensis S141 and R. irregularis led to a maximal shoot dry weight approximately 1.2-fold higher than that of the single AMF inoculation (Fig. 2C). In addition, shoot nitrogen (N) and phosphorus (P) concentrations significantly increased with co-inoculation yielding 1.7- and 1.5-fold increases in N and P, respectively, relative to the single AMF inoculation (Fig. 2D). These results indicate that B. velezensis S141 promotes plant growth and nutrient acquisition when AMF is present.
R. irregularis abundance and L. japonicus growth at 45 days after inoculation (DAI) under four inoculation conditions: non-inoculation (NI), inoculation with B. velezensis S141 (S141), inoculation with R. irregularis (AMF), and co-inoculation with both R. irregularis and B. velezensis S141 (AMF + S141). Cell density of B. velezensis S141 was 105 CFU/plant. (A) AMF colonization in L. japonicus roots. F(%), the frequency of mycorrhiza in the root system; M(%), the intensity of the mycorrhizal colonization in the root system; m(%), the intensity of the mycorrhizal colonization in the root fragments; a(%), the arbuscule abundance in the mycorrhizal parts of the root fragments; and A(%), the arbuscule abundance in the root system. Plant height (B) and dry weight (C) of L. japonicus. (D) The concentration of nitrogen (N) and phosphorus (P) in L. japonicus shoot. The values presented are means ± s.e.m. (n = 3). Student's t-test (n.s., not significant; *, p < 0.05) was conducted in (A) and (D). Bars marked with the same letter are not significantly different at p < 0.05 on Tukey's HSD test in (B) and (C)
3.3 B. velezensis S141 stimulates root elongation at early growth stage
To explore the possibility of a direct growth-promoting effect from B. velezensis S141 on L. japonicus during the early growth stages, we assessed shoot and root elongation at 7 and 15 DAI. B. velezensis S141 produced no influence on shoot elongation (Fig. 3). However, the length of tap roots inoculated with the bacteria was approximately double than that of non-inoculated plants at 15 DAI.
Growth of L. japonicus inoculated with 105 CFU/plant of B. velezensis S141 (S141) or not inoculated (NI) at 7 and 15 days after inoculation (DAI). Graphs show shoot length (A) and tap root length (B). Values are means ± s.e.m. (n = 4). The statistical analysis was performed using Student's t-test (n.s., not significant; ***, p < 0.001). (C) Appearance of L. japonicus roots. Scale bars: 1 cm
3.4 IAA production-related genes in B. velezensis S141 are not involved in promoting tap root elongation and AMF colonization
Given that B. velezensis S141 produces IAA (Sibponkrung et al. 2020), and this IAA could stimulate root elongation in L. japonicus and consequently, AMF colonization. Therefore, we investigated whether the IAA produced by B. velezensis S141 supported root elongation using derivative mutants of B. velezensis S141 (ΔdhaS, ΔyhcX, and ΔIPyAD) that yielded lower amounts of IAA than the wild type (Sibponkrung et al. 2020). The lengths of shoot and tap root of the plants that were inoculated with these mutant strains were comparable to those of plants inoculated with the wild type at both 7 and 15 DAI, indicating that the B. velezensis S141 dhaS, yhcX, and IPyAD genes do not significantly affect root elongation (Fig. 4A, B). In addition, we evaluated the abundance of AMF when co-inoculated with these mutant strains. We observed no significant differences in AMF colonization between inoculations of the wild type and mutant strains at 60 DAI (Fig. 4C). Overall, IAA produced by dhaS, yhcX, and IPyAD of B. velezensis S141 does not appear to be a major factor in promoting tap root elongation and AMF colonization in L. japonicus.
The growth promotion of L. japonicus at 7 (A) and 15 (B) days after inoculation (DAI) when inoculation with B. velezensis S141 wide type (S141) and its derivative mutants (ΔdhaS, ΔyhcX, and ΔIPyAD) decreases in the indole-3-acetic acid (IAA) production. (C) The measurement of AMF colonization at 60 DAI (AMF root colonization was defined as F(%), the frequency of mycorrhiza in the root system, M(%), the intensity of the mycorrhizal colonization in the root system, and m(%), the intensity of the mycorrhizal colonization in the root fragments). Values are means ± s.e.m. (n = 4). Bars marked with the same letter are not significantly different at p < 0.05 on Tukey's HSD test
3.5 B. velezensis S141 affects gene expression in both L. japonicus and R. irregularis during symbiosis
We investigated the changes in gene expression associated with the symbiotic response of L. japonicus and R. irregularis after inoculation with B. velezensis S141. L. japonicus marker genes involved in the regulation of AMF accommodation within roots and nutrient exchange were assessed at 45 DAI. The expression of the subtilisin-like serine protease gene, SbtM1, which participates in the early phases of AMF colonization (Takeda et al. 2009), was enhanced sixfold after co-inoculation with B. velezensis S141 compared to the single AMF inoculation (Fig. S2A). The expression of the transcription factor gene RAM1, which is mainly responsible for arbuscule formation, was doubled. The mycorrhiza-specific H+-ATPase HA1, the phosphate transporter (PT4) and the ammonium transporter (AMT2;2), all of which are involved in P and N uptake in arbuscule-containing cortical cells (Javot et al. 2007; Guether et al. 2009; Krajinski et al. 2014; Wang et al. 2014), showed elevated expression levels in the presence of B. velezensis S141. For genes implicated in fatty acid biosynthesis and lipid transport in arbuscular mycorrhizal symbiosis, B. velezensis S141 produced enhancement of expression of the glycerol-3-phosphate acyltransferase gene RAM2 (Wang et al. 2012) while the acyl-ACP thioesterase FatM (Bravo et al. 2017; Brands et al. 2018) and the ABC transporter gene STR (Zhang et al. 2010) did not undergo enhancement.
We also analyzed transcript levels of cell cycle-related genes and phophorus (P) and carbon (C) nutrition-related genes in R. irregularis colonizing L. japonicus roots. Five out of the seven cell cycle-related genes tested showed significantly higher expression in roots with B. velezensis S141 than in those without the bacteria (Fig. S2B). For genes involved in P nutrition in R. irregularis, we focused on those related to polyphosphate synthesis and degradation (Ezawa and Saito 2018). The expression levels of genes encoding the polyphosphate-synthesizing enzymes, VTC1, VTC2, and VTC4 (Nguyen et al. 2022), were found to have decreased upon co-inoculation with B. velezensis S141. On the other hand, the transcript levels of the endopolyphosphatase genes PPN1–4 (Nguyen & Saito 2021) showed no significant differences. The expression of the monosaccharide transporter gene MST2, which plays a crucial role in sugar uptake in AMF, was reduced in the presence of B. velezensis S141.
3.6 B. velezensis S141 is an endophytic bacterium
The localization of B. velezensis S141 was examined using confocal microscopy to confirm the presence of the bacteria in L. japonicus roots during the symbiotic association with AMF. No bacterial cells were observed in the longitudinal root sections of non-inoculated plants at 7 and 45 DAI (Fig. 5A, C). Intriguingly, bacterial cells were observed within root cells at 7 DAI (Fig. 5B) although they did not colonize within intraradical hyphae and arbuscules of AMF (Fig. 5D). The amount of living endophytic bacteria was detected at 30 DAI at a density of approximately in log10 value as 2.15 ± 0.7 and 1.9 ± 0.6 CFU/plant in mycorrhizal and non-mycorrhizal plant roots, respectively, using a plate count technique.
Confocal laser scanning microscopy images illustrating the bacterial localization in L. japonicus roots at 7 (A, B) and 45 (C, D) days after-inoculation (DAI). (A, C) Single inoculation of R. irregularis. (B, D) Co-inoculation of R. irregularis with B. velezensis S141. Longitudinal sections depict mycorrhizal roots stained with calcofluor (blue) to visualize cell walls of plants and arbuscular mycorrhizal fungi, and with SYTO 9 (green) to detect live bacterial cells (white arrowheads). Arbuscules of R. irregularis are highlighted by the yellow arrowhead. Scale bars denote 10 μm
4 Discussion
PGPRs and AMF can coexist in roots, and certain combinations of these microorganisms produce enhancement of both AMF abundance and plant growth (Meyer & Linderman 1986; Yuttavanichakul et al. 2018; Kiddee et al. 2020; Sheteiwy et al. 2021). In this study, we demonstrated that the endophytic PGPR B. velezensis S141 produced an increase in AMF colonization in L. japonicus roots and promoted spore production (Fig. 1). Although B. velezensis S141 was originally isolated as a PGPR for soybean, cell concentrations of 105 and 106 cells/plant showed no effect on the biomass production of L. japonicus. However, in the presence of AMF, B. velezensis S141 augmented the plant biomass in addition to shoot N and P concentrations (Fig. 2). This finding suggests that B. velezensis S141 can exert its PGPR effect in L. japonicus when coexisting with AMF. At 15 days after inoculation with B. velezensis S141, the root length more than doubled compared to that without the bacterial strain (Fig. 3). Similar plant growth promotion at the seedling stage has also been observed in peppers inoculated with B. velezensis BBC047 (Stoll et al. 2021). This accelerated initial root elongation may cause enlargement of the zone of AMF colonization in the early phase, leading to greater AMF abundance in roots. The increase in AMF colonization due to the co-inoculation could lead to enhancement in biomass and N and P concentrations of L. japonicus. Alternatively, the synergistic effects of B. velezensis S141 and AMF in the roots might have altered the gene expression patterns in both the plant and AMF, resulting in activation of plant and AMF growth.
Auxin is involved in the initiation of arbuscular mycorrhizal symbiosis and the development of arbuscules (Ho-Plágaro & García-Garrido 2022). B. velezensis S141 produces auxins, and disruption of the IpyAD, yhcX, and dhaS genes reduces auxin production to 4% to 30% of the wild-type strain (Sibponkrung et al. 2020). Using these mutant strains, we tested the potential role of auxins from B. velezensis S141 with respect to producing an increase in AMF abundance, but AMF colonization was similar to wild-type inoculation (Fig. 4). Such levels of auxin reduction might not affect AMF abundance, possibly due to compensatory effects from other auxin-producing genes. Another explanation for this finding could be that the auxin-producing capability of B. velezensis S141 is not a primary mechanism for promoting plant growth or AMF colonization. Nevertheless, B. velezensis S141 might employ other mechanisms that facilitate AMF–plant symbiosis, and these mechanisms should be further investigated.
B. velezensis S141 enhanced mycorrhizal formation in L. japonicus, a finding that is consistent with the activation of plant marker genes (SbtM1 and RAM1) associated with mycorrhizal symbiosis (Fig. S2A). Similarly, the expression of the HA1, PT4, and AMT2:1 genes, all of which are essential for nutrient uptake during mycorrhizal symbiosis, was also upregulated by inoculation with B. velezensis S141. This finding mirrors the observed increase in shoot N and P concentrations. These results suggest that the presence of endophytic B. velezensis S141 amplifies the benefits of mycorrhizal symbiosis. In contrast, the expression of genes involved in lipid transfer from plants to AMF (except for RAM2) remained unchanged after B. velezensis S141 inoculation. The different gene expression responses related to carbon supply and symbiotic nutrient uptake when exposed to B. velezensis S141 might influence the cost–benefit balance in mycorrhizal symbiosis.
Expression of AMF cell cycle-related genes is related to AMF proliferation in roots in response to environmental changes (Sugimura and Saito 2017). AMF cell cycle-related genes were found to be downregulated during the suppression of AMF colonization in roots caused by high phosphate exposure. In our experiments, the presence of B. velezensis S141 led to an increase in the expression of cell cycle-related genes in R. irregularis (Fig. S2B). This finding is consistent with the increase in abundance of R. irregularis in L. japonicus roots after inoculation with B. velezensis S141, thus supporting the occurrence of activation of AMF proliferation by the PGPR strain in terms of gene expression. We also analyzed gene expression related to P metabolism, an important function in AM symbiosis, as an effect of the coexistence of B. velezensis S141 on AMF. Polyphosphate synthesized in AMF is an important source of P for plants, and the amount of polyphosphate in AMF is thought to be regulated through a metabolic balance between polyphosphate synthesis by the vacuolar transporter chaperone (VTC) complex and degradation by endopolyphosphatases (PPNs) (Ezawa and Saito 2018). Based on our gene expression analysis, the amount of PPN transcripts in AMF was not affected by B. velezensis S141 inoculation, but VTC transcripts had decreased (Fig. S2B). Although polyphosphate levels were not determined, polyphosphate may progress in the direction of degradation in intraradical hyphae and arbuscules of AMF. Since short-chain polyphosphate acts as a pool of P supply to the plant (Takanishi et al. 2009), the higher shoot P content of plants inoculated with B. velezensis S141 may be related to modulation of polyphosphate metabolism genes in AMF. In addition, the expression of MST2, which is involved in sugar transport to AMF cells, was downregulated after co-inoculation with B. velezensis S141.
Intriguingly, B. velezensis S141 was localized within plant cells, with no bacteria detected in the intercellular space (Fig. 5). This observation suggests a specific and targeted mode of communication, with the bacteria potentially relying on obtaining nutrients from living plant cells, indicative of a mutualistic relationship. These results shed light on the intricate mechanisms involved in nutrient and sugar acquisition, as well as delivery within the plant-AMF partnership. They also underscore the potential role of co-inoculation with an endophytic PGPR in these processes, warranting further investigation.
5 Conclusions
To summarize, our study sheds light on the beneficial effects of B. velezensis S141 on the plant-AMF symbiosis. The results suggest that B. velezensis S141 leads to enhancement of root growth in the early stages and later promotes the abundance of AMF. However, the production of a plant growth hormone, IAA, is not the key mechanism used by B. velezensis S141 to promote mycorrhizal symbiosis. Surprisingly, strain B. velezensis S141 was identified as an endophyte in L. japonicus, and this strain had the capability of inducing the expression of plant marker genes mainly involved in mycorrhizal formation and nutrient uptake. Moreover, induction of the expression of marker genes related to the cell cycle of R. irregularis when co-inoculated with B. velezensis S141 strongly supports its potential for promoting fungal growth. These results highlight the importance of exploring the potential of beneficial microbes in enhancing AMF growth and promoting plant growth through the nutrient uptake pathway.
References
Adeniji AA, Loots DT, Babalola OO (2019) Bacillus velezensis: phylogeny, useful applications, and avenues for exploitation. Appl Microbiol Biotechnol 103:3669–3682. https://doi.org/10.1007/S00253-019-09710-5
Allen Rush T, Puech-Pagès V, Bascaules A et al (2020) Lipo-chitooligosaccharides as regulatory signals of fungal growth and development. Nat Commun 11:3897. https://doi.org/10.1038/s41467-020-17615-5
Amy C, Avice JC, Laval K, Bressan M (2022) Are native phosphate solubilizing bacteria a relevant alternative to mineral fertilizations for crops? part I. when rhizobacteria meet plant P requirements. Rhizosphere 21:100476. https://doi.org/10.1016/J.RHISPH.2022.100476
Badri A, Stefani FOP, Lachance G et al (2016) Molecular diagnostic toolkit for Rhizophagus irregularis isolate DAOM-197198 using quantitative PCR assay targeting the mitochondrial genome. Mycorrhiza 26:721–733. https://doi.org/10.1007/s00572-016-0708-1
Barin M, Asadzadeh F, Hosseini M et al (2022) Optimization of biofertilizer formulation for phosphorus solubilizing by Pseudomonas fluorescens Ur21 via response surface Methodology. Processes 10:650. https://doi.org/10.3390/pr10040650
Bashan Y, de Bashan LE, Prabhu SR, Hernandez JP (2014) Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 378:1–33. https://doi.org/10.1007/S11104-013-1956-X
Begum N, Wang L, Ahmad H et al (2022) Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb Ecol 83:971–988. https://doi.org/10.1007/S00248-021-01815-7
Bhat MA, Mishra AK, Jan S et al (2023) Plant growth promoting rhizobacteria in plant health: A perspective study of the underground interaction. Plants 12:629. https://doi.org/10.3390/PLANTS12030629
Bonaldi K, Gargani D, Prin Y et al (2011) Nodulation of Aeschynomene afraspera and A. indica by photosynthetic Bradyrhizobium sp. strain ORS285: the nod-dependent versus the nod-independent symbiotic interaction. Mol Plant-Microbe Interact 24:1359–1371. https://doi.org/10.1094/MPMI-04-11-0093
Brands M, Wewer V, Keymer A et al (2018) The Lotus japonicus acyl-acyl carrier protein thioesterase FatM is required for mycorrhiza formation and lipid accumulation of Rhizophagus irregularis. Plant J 95:219–232. https://doi.org/10.1111/tpj.13943
Bravo A, Brands M, Wewer V et al (2017) Arbuscular mycorrhiza-specific enzymes FatM and RAM2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol 214:1631–1645. https://doi.org/10.1111/NPH.14533
Chauhan A, Saini R, Sharma JC (2021) Plant growth promoting rhizobacteria and their biological properties for soil enrichment and growth promotion. J Plant Nutr 45:273–299. https://doi.org/10.1080/01904167.2021.1952221
Daniels BA, Skipper H (1982) Methods for the recovery and quantitative estimation of propagules from soil. In: Schneck NC (ed) American Phytopathological Society. St Paul, Minnesota, p 244
Emmanuel OC, Babalola OO (2020) Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiol Res 239:126569. https://doi.org/10.1016/J.MICRES.2020.126569
Etemadi M, Gutjahr C, Couzigou JM et al (2014) Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol 166:281–292. https://doi.org/10.1104/PP.114.246595
Ezawa T, Saito K (2018) How do arbuscular mycorrhizal fungi handle phosphate? New insight into fine-tuning of phosphate metabolism. New Phytol 220:1116–1121. https://doi.org/10.1111/NPH.15187
Fan B, Wang C, Song X et al (2018) Bacillus velezensis FZB42 in 2018: the gram-positive model strain for plant growth promotion and biocontrol. Front Microbiol 9:2491. https://doi.org/10.3389/FMICB.2018.02491
Feng F, Sun J, Radhakrishnan GV et al (2019) A combination of chitooligosaccharide and lipochitooligosaccharide recognition promotes arbuscular mycorrhizal associations in Medicago truncatula. Nat Commun 10:5047. https://doi.org/10.1038/s41467-019-12999-5
Figueiredo M do VB, Seldin L, de Araujo FF, Mariano R de LR (2010) Plant growth promoting rhizobacteria: fundamentals and applications. In: Kaheshwari DK (ed) Plant Growth and Health Promoting Bacteria, Springer, 21–43. https://doi.org/10.1007/978-3-642-13612-2_2
Genre A, Balestrini R, Reinhardt D et al (2018) Beneficial services of arbuscular mycorrhizal fungi from ecology to application. Front Plant Sci 9:1270. https://doi.org/10.3389/fpls.2018.01270
George E, Marschner H, Jakobsen I (1995) Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil. Crit Rev Biotechnol 15:257–270. https://doi.org/10.3109/07388559509147412
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244. https://doi.org/10.1016/S0007-1536(63)80079-0
Guether M, Neuhäuser B, Balestrini R et al (2009) A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol 150:73–83. https://doi.org/10.1104/PP.109.136390
Gutjahr C (2014) Phytohormone signaling in arbuscular mycorrhiza development. Curr Opin Plant Biol 20:26–34. https://doi.org/10.1016/j.pbi.2014.04.003
Ho-Plágaro T, García-Garrido JM (2022) Molecular regulation of arbuscular mycorrhizal symbiosis. Int J Mol Sci 23:5960. https://doi.org/10.3390/ijms23115960
Javot H, Penmetsa RV, Terzaghi N et al (2007) A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A 104:1720–1725. https://doi.org/10.1073/PNAS.0608136104
Kempel A, Schmidt AK, Brandl R, Schädler M (2010) Support from the underground: induced plant resistance depends on arbuscular mycorrhizal fungi. Funct Ecol 24:293–300. https://doi.org/10.1111/J.1365-2435.2009.01647.X
Kiddee S, Yuttavanichakul W, Boonkerd N et al (2020) Secretion compounds from Brevibacillus sp. SUT47 promote spore propagation of Acaulospora tuberculata colonizing maize roots (Zea mays L. cultivar Suwan 5). Sci Asia 40:634–638. https://doi.org/10.2306/scienceasia1513-1874.2020.073
Kobae Y, Kawachi M, Saito K et al (2015) Up-regulation of genes involved in N-acetylglucosamine uptake and metabolism suggests a recycling mode of chitin in intraradical mycelium of arbuscular mycorrhizal fungi. Mycorrhiza 25:411–417. https://doi.org/10.1007/s00572-014-0623-2
Krajinski F, Courty PE, Sieh D et al (2014) The H+-ATPase HA1 of Medicago truncatula is essential for phosphate transport and plant growth during arbuscular mycorrhizal symbiosis. Plant Cell 26:1808–1817. https://doi.org/10.1105/TPC.113.120436
Liao D, Wang S, Cui M et al (2018) Phytohormones regulate the development of arbuscular mycorrhizal symbiosis. Int J Mol Sci 19:3146. https://doi.org/10.3390/IJMS19103146
Liu X, Li Q, Li Y et al (2019) Paenibacillus strains with nitrogen fixation and multiple beneficial properties for promoting plant growth. PeerJ 7:e7445. https://doi.org/10.7717/PEERJ.7445/SUPP-4
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Matse DT, Huang CH, Huang YM, Yen MY (2019) Effects of co-inoculation of rhizobium with plant growth promoting rhizobacteria on the nitrogen fixation and nutrient uptake of Trifolium repens in low phosphorus soil. J Plant Nutr 43:739–752. https://doi.org/10.1080/01904167.2019.1702205
Meyer JR, Linderman RG (1986) Response of subterranean clover to dual inoculation with vesicular-arbuscular mycorrhizal fungi and a plant growth-promoting bacterium, Pseudomonas putida. Soil Biol Biochem 18:185–190. https://doi.org/10.1016/0038-0717(86)90025-8
Mitra D, Djebaili R, Pellegrini M et al (2021) Arbuscular mycorrhizal symbiosis: plant growth improvement and induction of resistance under stressful conditions. J Plant Nutr 44:1993–2028. https://doi.org/10.1080/01904167.2021.1881552
Nguyen CT, Saito K (2021) Role of cell wall polyphosphates in phosphorus transfer at the arbuscular interface in mycorrhizas. Front Plant Sci 12:725939. https://doi.org/10.3389/fpls.2021.725939
Nguyen CT, Ezawa T, Saito K (2022) Polyphosphate polymerizing and depolymerizing activity of VTC4 protein in an arbuscular mycorrhizal fungus. Soil Sci Plant Nutr 68:256–267. https://doi.org/10.1080/00380768.2022.2029220
Odoh CK, Nwadibe C, Kalu AU, Unah UV (2019) Plant growth promoting rhizobacteria (PGPR): a novel agent for sustainable food production. Am J Agric Biol Sci 14:35–54. https://doi.org/10.3844/ajabssp.2019.35.54
Oleńska E, Małek W, Wójcik M et al (2020) Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci Total Environ 743:140682. https://doi.org/10.1016/J.SCITOTENV.2020.140682
Pongdet P, Teerana G, Neung T et al (2015) Preferential association of endophytic bradyrhizobia with different rice cultivars and its implications for rice endophyte evolution. Appl Environ Microbiol 81(9):3049–3061. https://doi.org/10.1128/AEM.04253-14
Pozo MJ, López-Ráez JA, Azcón-Aguilar C, García-Garrido JM (2015) Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol 205:1431–1436. https://doi.org/10.1111/nph.13252
Prabhu N, Borkar S, Garg S (2019) Phosphate solubilization by microorganisms: Overview, mechanisms, applications and advances. In: Meena SN, Naik MM (eds) Advances in Biological Science Research: A Practical Approach, 161–176.https://doi.org/10.1016/B978-0-12-817497-5.00011-2
Prakamhang J, Tittabutr P, Boonkerd N et al (2015) Proposed some interactions at molecular level of PGPR coinoculated with Bradyrhizobium diazoefficiens USDA110 and B. japonicum THA6 on soybean symbiosis and its potential of field application. Appl Soil Ecol 85:38–49. https://doi.org/10.1016/j.apsoil.2014.08.009
Rabbee MF, Sarafat Ali M, Choi J et al (2019) Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules 24:1046. https://doi.org/10.3390/MOLECULES24061046
Sheteiwy MS, Abd Elgawad H, Xiong YC et al (2021) Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol Plant 172:2153–2169. https://doi.org/10.1111/PPL.13454
Shipley B, Vu TT (2002) Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytol 153:359–364. https://doi.org/10.1046/J.0028-646X.2001.00320.X
Sibponkrung S, Kondo T, Tanaka K et al (2017) Genome sequence of Bacillus velezensis S141, a new strain of plant growth-promoting rhizobacterium isolated from soybean rhizosphere. Genome Announc 5:e01312-e1317. https://doi.org/10.1128/genomea.e01312-17
Sibponkrung S, Kondo T, Tanaka K et al (2020) Co-inoculation of Bacillus velezensis strain S141 and Bradyrhizobium strains promotes nodule growth and nitrogen fixation. Microorganisms 8:678. https://doi.org/10.3390/microorganisms8050678
Singh M, Singh D, Gupta A et al (2019) Plant growth promoting rhizobacteria: application in biofertilizers and biocontrol of phytopathogens. In: Singh AM, Kumar A, Singh PK (eds) PGPR Amelioration in Sustainable Agriculture, Woodhead Publishing, 41–66.https://doi.org/10.1016/B978-0-12-815879-1.00003-3
Smith S (1988) Physiological interactions between symbionts in vesicular-arbuscular mycorrhizal plants. Annu Rev Plant Physiol Plant Mol Biol 39:221–244. https://doi.org/10.1146/annurev.arplant.39.1.221
Smith SE, Jakobsen I, Grønlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057. https://doi.org/10.1104/PP.111.174581
Songwattana P, Boonchuen P, Piromyou P et al (2023) Insights into antifungal mechanisms of Bacillus velezensis S141 against Cercospora leaf spot in mungbean (V. radiata). Microbes Environ 38:ME22079. https://doi.org/10.1264/JSME2.ME22079
Stoll A, Salvatierra-Martínez R, González M et al (2021) Importance of crop phenological stages for the efficient use of PGPR inoculants. Sci Rep 11:19548. https://doi.org/10.1038/s41598-021-98914-9
Sugimura Y, Saito K (2017) Transcriptional profiling of arbuscular mycorrhizal roots exposed to high levels of phosphate reveals the repression of cell cycle-related genes and secreted protein genes in Rhizophagus irregularis. Mycorrhiza 27:139–146. https://doi.org/10.1007/S00572-016-0735-Y
Sun B, Gu L, Bao L et al (2020) Application of biofertilizer containing Bacillus subtilis reduced the nitrogen loss in agricultural soil. Soil Biol Biochem 148:107911. https://doi.org/10.1016/j.soilbio.2020.107911
Takanishi I, Ohtomo R, Hayatsu M, Saito M (2009) Short-chain polyphosphate in arbuscular mycorrhizal roots colonized by Glomus spp.: A possible phosphate pool for host plants. Soil Biol Biochem 41:1571–1573. https://doi.org/10.1016/J.SOILBIO.2009.04.002
Takeda N, Sato S, Asamizu E et al (2009) Apoplastic plant subtilases support arbuscular mycorrhiza development in Lotus japonicus. Plant J 58:766–777. https://doi.org/10.1111/J.1365-313X.2009.03824.X
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and Genetical Aspects of Mycorrhizae. INRA press, Paris, pp 217–221
Tsukanova KA, Chebotar V, Meyer JJM, Bibikova TN (2017) Effect of plant growth promoting rhizobacteria on plant hormone homeostasis. S Afr J Bot 113:91–102. https://doi.org/10.1016/J.SAJB.2017.07.007
Vocciante M, Grifoni M, Fusini D et al (2022) The role of plant growth-promoting rhizobacteria (PGPR) in mitigating plant’s environmental stresses. Appl Sci 12:1231. https://doi.org/10.3390/APP12031231
Wang E, Schornack S, Marsh JF et al (2012) A common signaling process that promotes mycorrhizal and oomycete colonization of plants. Curr Biol 22:2242–2246. https://doi.org/10.1016/J.CUB.2012.09.043
Wang E, Yu N, Bano SA et al (2014) A H+-ATPase that energizes nutrient uptake during mycorrhizal symbioses in rice and Medicago truncatula. Plant Cell 26:1818–1830. https://doi.org/10.1105/TPC.113.120527
Watanabe FS, Olsen SR (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci Soc Am pro 2:677–678. https://doi.org/10.2136/SSSAJ1965.03615995002900060025X
Yash P and Kalra (1998) Handbook of reference methods for plant analysis. Soil and plant analysis council, Inc. CRC-Press. 76–88
Yuttavanichakul W, Teamtisong K, Teaumroong N (2018) Brevibacillus sp. promotes maize root colonization by Acaulospora tuberculata and the alteration of associated plant protein responses. J Plant Interact 13:543–554. https://doi.org/10.1080/17429145.2018.1547844
Zhang Q, Blaylock LA, Harrison MJ (2010) Two Medicago truncatula half-ABC transporters are essential for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Cell 22:1483–1497. https://doi.org/10.1105/TPC.110.074955
Acknowledgements
This work was financially supported by The Royal Golden Jubilee (RGJ) Ph.D. Program, The Thailand Research Fund (TRF), Suranaree University of Technology (SUT), The Program Management Unit for Human Resources & Institutional Development and Innovation (PMU-B) under grant number B16F640113, Thailand Science Research and Innovation (TRS), National Science, Research and Innovation Fund (NSRF) (NRIIS number 179326), and the Grant-in-Aid for Scientific Research (JSPS 22H02226) from the Japan Society for the Promotion of Science. We express our gratitude to the National BioResource Project for providing L. japonicus B-129 seeds through the Frontier Science Research Center of the University of Miyazaki, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The biosecurity concerns of this study were reviewed and approved by Suranaree University of Technology (approval number: SUT-IBC-05/ 2021) and are in accordance with the levels of risk in pathogens and animal toxins listed in “the Risk Group of Pathogen and Animal Toxin (2017)” issued by the Department of Medical Sciences, Ministry of Public Health, the Pathogen and Animal Toxin Act (2015) and Biosafety Guidelines for Modern Biotechnology, BIOTEC (2016).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kiddee, S., Wongdee, J., Piromyou, P. et al. Unveiling the tripartite synergistic interaction of plant-arbuscular mycorrhizal fungus symbiosis by endophytic Bacillus velezensis S141 in Lotus japonicus. Symbiosis 92, 355–367 (2024). https://doi.org/10.1007/s13199-024-00975-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-024-00975-7