Abstract
Aims
The aim of this study was to quantify and understand the driving factors of the spatial variation of soil respiration (R S) in an old-growth mixed broadleaved-Korean pine forest in northeastern China.
Methods
All woody stems ≥1 cm diameter at breast height (DBH) were measured in the 9 ha plot. Simultaneous measurements of R S, soil temperature (T S) and soil water content (W S) were conducted for 256 sampling points on a regular 20-m grid refined with 512 additional sampling points randomly placed within each of the 20-m blocks in May, July and September of 2014.
Results
The variogram analyses revealed 87–91 % of the sample variance was explained by autocorrelation over a range of 15 to 23 m during the observation periods. The R S were highly correlated among the measurements made in May, July and September. The model indicated that the W S, bulk density (BD) and maximum DBH for trees within 3 m (radius) of the measurement collars explained 46 % of the spatial variation in R S seasonally averaged across three observations.
Conclusions
The spatial patterns of R S remained constant across the three measurement campaigns. The spatial variation in R S was primarily controlled by the W S and forest stand structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forest ecosystems are major terrestrial carbon (C) sinks that sequester large amounts of atmospheric CO2 and offer the potential for the mitigation of climate change (Lorenz and Lal 2010). The C balance of old growth forests has traditionally received little attention because they are believed to be C neutral (Odum 1969). However, recent study reported that old growth forests act as net sinks of atmospheric CO2 (Luyssaert et al. 2008; Hudiburg et al. 2009; Lewis et al. 2009). The quantification of the major components of forest ecosystem C cycling and the understanding of their control factors are prerequisites for the estimation of C sources or sinks. Soil respiration (R S) is the second largest C flux in the forest ecosystem after gross primary production and thus plays a crucial role in ecosystem C cycling (Davidson et al. 2006).
R S originates from rhizospheric respiration (respiration from live roots and their associated mycorrhizal fungi and rhizosphere microorganisms) and heterotrophic respiration (respiration from microorganisms decomposing litter and soil organic matter), two components involving various biological and ecological processes and responding differently to environmental changes (Scott-Denton et al. 2006; Song et al. 2015). Therefore, there is substantial temporal and spatial variation in R S. The temporal variation of R S is straightforward to capture by using continuous automated measurements and is known to be affected by the soil temperature (T S) and soil water content (W S) (Wang et al. 2013). In contrast, the spatial variation of R S remains under-researched, which is mostly uncertain due to the limited measurement methods and the high labour and time costs (Dore et al. 2014; Prolingheuer et al. 2014). Geostatistics is an appropriate methodology for the capture, representation and interpretation of the spatial patterns of R S and the soil properties and has been applied over the last few decades (Robertson and Gross 1994; Teixeira et al. 2013; Yuan et al. 2013; Ferré et al. 2015).
In addition to the quantification of spatial variation in R S, identifying key factors that regulate the spatial variation in R S is essential for designing an optimal sampling approach and accurately estimating R S at the ecosystem scale. Biotic factors, such as the stand structure, fine root biomass and leaf litterfall, all contribute to the spatial variation of R S at the ecosystem scale (Katayama et al. 2009; Barba et al. 2013). Trees are involved directly in root respiration and indirectly in root-derived rhizosphere respiration and the decomposition of aboveground and belowground litter by microorganisms (Bréchet et al. 2011). Therefore, the tree diameter may be a good proxy for the biotic factors that would explain the spatial variation of R S (Bréchet et al. 2011; Luan et al. 2012). The main abiotic factors influencing the identified spatial variation of R S are the soil water content, which controls gas diffusivity (CO2 and O2) and microbial activity (Herbst et al. 2009; Martin and Bolstad 2009; Yoon et al. 2014), and the soil substrate quantity and quality, such as the soil organic carbon content (SOC), soil total nitrogen content (TN) and soil C:N ratio (Luan et al. 2012; Ngao et al. 2012). Nonetheless, the contribution of these factors is highly variable in different ecosystems and needs to be verified.
The Asian temperate mixed forest is predominantly found in northeastern China (40°15′–50°20′N and 126°–135°30′E) and is one of the three largest areas of temperate mixed forest in the world (i.e., northeastern North America, Europe, and Eastern Asia) (Wang et al. 2006). The temperate forest in northeastern China accounts for approximately one-third of the forested land area and forest stock in China (State Forestry Administration (SFA) 2005). The primary mixed broadleaved-Korean pine (Pinus koraiensis) forest is the zonal climax vegetation in northeast China. Previous studies by our laboratory and other researchers have reported the seasonal dynamic of R S and its components in this forest (Wang et al. 2010; Shi et al. 2015). However, thus far, no studies have been conducted in this important forest to elucidate the mechanisms and quantification of the spatial variation of R S, which are critical for estimating the C budgets on the scales of the ecosystems to the regional scale. Our study attempts to fill in this gap of knowledge, and our specific objectives were to (1) quantify and visualize the spatial variation of R S and its temporal changes in an old-growth temperate mixed forest in northeastern China and (2) identify the roles of biotic and abiotic factors, such as the forest structure (size and spatial distribution of the trees), T S, W S, bulk density (BD), SOC, TN, soil C:N ratio and soil pH, that are used to determine the spatial variation in R S at the ecosystem scale. We hypothesized that (1) the spatial variation of R S remained constant during the growing season and (2) the spatial variation of R S would mainly be determined by the W S, BD and the spatial distribution of the tree size.
Materials and methods
Study sites and experimental design
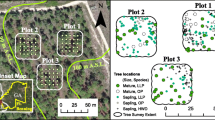
The study site was in the Liangshui National Reserve (47°10′50″N, 128°53′20″E) in northeastern China. The region lies on the eastern part of Eurasia, and the climate is classified as continental monsoon. The mean annual temperature is −0.3 °C, with a frost-free period of 100 to 120 days and snow period of 130 to 150 days. The daily air temperature revealed distinct seasonal variations throughout the growing season (Shi et al. 2015). The mean annual precipitation is 676 mm. The soil is dark-brown forest soil by the Chinese soil classification, which is equivalent to Humaquepts or Cryoboralfs based on the American Soil Taxonomy (Soil Survey Staff 1999). The total area of the reserve is 12,133 ha, with 1.88 million m3 of growing stock and an average canopy cover of 98 %. The mixed broadleaved-Korean pine forest accounts for 63.7 % and 77.4 % of the forested area and standing tree volume in the whole reserve, respectively, which has not been disturbed for more than 300 years. The forest is primarily composed of Pinus koraiensis, Betula costata, Tilia amurensis, Acer ukurunduense, Abies nephrolepis, Ulmus laciniata, Acer tegmentosum, Fraxinus mandshurica, and Acer mono.
The 9-ha (300 × 300 m) survey plot was established in the mixed broadleaved-Korean pine forest in 2005 (Fig. 1). A 20 × 20 m square grid was placed within the plot, and 256 intersections were considered to be R S measurement base points. To capture the spatial variation in the R S at a finer scale, two additional sample points (2, 5, or 8 m) were selected in a randomly assigned cardinal direction (N, NE, E, SE, S, SW, W or NW) from the base point (Webster and Oliver 2007). Thus, we selected a total of 768 sample locations in the 9-ha plot (Fig. 2).
Soil respiration and soil climate
R S was measured using an LI-6400 portable CO2 infrared gas analyser (IRGA) (LI-COR Inc., Lincoln, NE, USA) in spring (May), summer (July) and autumn (September) of 2014 (one measurement in each season). No measurements were conducted in winter because the analysers cannot run at low temperatures. Each measurement campaign lasted for about one week and was performed from 10:00 am to 16:00 pm. To reduce the time required for each measurement campaign, two LI-6400 analysers (IRGA) carried out the field work simultaneously. R S was measured on rainless days, and when a rain event occurred, the measurements were interrupted and resumed the next day to reduce the effect of rainfall on R S. PVC collars (10.4-cm diameter × 6 cm height) were installed at each sampling point at the beginning of May, which was one week before the first measurement campaign. The collars were inserted 4 cm into the soil (including the litter layer). Simultaneous with each R S measurement, the T S was measured at a depth of 5 cm using a thermocouple penetration probe (Li-6000-09 TC, LI-COR, Inc.). The W S at 0–10 cm soil depth was measured using a time-domain reflectometry (TDR) probe (IMKO, Ettlingen, Germany) at two points next to each collar.
Stand structural parameters and soil properties
In July 2010, the diameter at breast height (DBH) of each tree with a DBH greater than 1 cm was measured, as well as its position in the 9-ha plot (Fig. 2). Based on these data, we calculated a series of stand structural parameters, including the total basal area (BA), maximum DBH (max. DBH) of the trees, and mean DBH of the trees within 1–10 m of each R S measurement point. One soil sample was collected from the top of the soil near each R S measurement base point using 100-ml (50.46-mm diameter, 50-mm height) cylinders to analyse the BD. In July 2013, three soil subsamples were collected using a soil corer (5-cm diameter) at 0–10 cm of soil approximately 0.5 m from each sample location (collar). These three subsamples were mixed as one sample to analyse the soil nutrients, including the SOC and TN. The SOC was determined by a multi N/C 2100 analyser (Analytik Jena AG, Jena, Germany). The TN was measured using a Hanon K9840 auto Kjeldahl analyser (Jinan Hanon Instruments Co., Ltd., China). The SOC and TN were also used to calculate the C:N ratio. The soil pH was measured in water (1: 2.5 w/v).
Statistical analysis
Geostatistical analyses (variogram calculation, semivariogram model fitting and kriging) were performed with GS+ version 7.0 for Windows (Gamma Design Software 2004). Before the geostatistical analysis, the data were logarithmically transformed to normalise skewed frequency distributions. The experimental semivariance γ (h) for the distance interval h was calculated as follows:
where n (h) is the number of observation pairs separated by the distance h and z (x i ) and z (x i + h) are the variable values at the locations x i and x i + h. An exponential variogram was used to model the experimental variograms obtained.
where c 0 is the nugget, c 0 + c is the sill, and a is the correlation length. The practical range for the exponential model is 3a. In the following, the proportion of the model sample variance (c 0 + c) explained by the structural variance (c) was used as a normalized measure of spatial dependence (Robertson et al. 1993). The experimental variogram was calculated using an active lag distance of 212.13 m (slightly less than half of the maximum separation distance between the sampling points) and a lag class distance of 14.14 m.
To construct the interpolation maps, we used ordinary block kriging with a block size of 3.23 m across the field and a 2 × 2 discretization grid within each block. The block kriging and the exponential semivariance models were used for mapping the spatial patterns of the R S and W S. According to previous studies (Herbst et al. 2009) and our hypothesis, the relationship between R S and the W S could be closer when compared to the T S, thus, the map was not created for the T S. The maps were produced using Surfer spatial analysis software (Version 11, Golden Software, Inc., CO, USA).
The regression analyses were used to examine the similarities in the spatial patterns of the R S among the measurement campaigns. The relationships between the R S and the soil climate, the stand structural parameters and the soil properties were also examined by linear regression (Pearson correlation coefficient). A backward multiple regression analysis was carried out on the selected variables that could control the spatial variation of the R S during the observation periods. Logarithmic transformation of R S was performed as needed to achieve linearity and homoscedasticity. The raw data supporting the paper’s main results are available as electronic supplementary material, in an MS Excel file. These statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, USA). The scatter and line graphs were generated using Sigmaplot 12.5 software (Systat Software, Inc., San Jose, CA, USA).
Results
Spatial variation of soil respiration
The seasonal pattern of the spatial mean R S was similar to that of T S, peaking in summer (Table 1). The spatial coefficient of variance (CV) of T S was lower (7–19 %) than that of R S (42–55 %) during the observation periods. In contrast, the CV for W S was between 32 and 34 %, which was higher than that of T S. Kriged maps were used to provide a visual impression of the spatial patterns of R S and W S (Fig. 3). W S was relatively high in the north and the west of the plot during the observation periods, whereas R S was low at these points. The areas of lower R S in the spring remained low during the summer and autumn and did not vary with the season. We found a significantly positive correlation between the R S in spring and summer (r = 0.711, P < 0.01), summer and autumn (r = 0.738, P < 0.01) and spring and autumn (r = 0.604, P < 0.01).
The proportion of the structural variance (c) to the sill (c 0 + c) and the spatial autocorrelation ranges for R S differed little between the sampling periods, ranging between 87 and 91 % and between 15 and 23 m, respectively (Table 2, and Fig. 4). The proportion and autocorrelation ranges for T S differed substantially between the sampling periods. The proportion for T S was higher in summer (89 %) and in autumn (88 %) than in spring (52 %), whereas the autocorrelation ranges were lower in summer (27 m) than in spring (285 m) and autumn (508 m). The proportion for W S was lower in autumn (69 %) than in spring (90 %) and summer (91 %), and the autocorrelation ranges varied from 17 m (spring) to 67 m (autumn). The autocorrelation range for the T S-average (105 m) was higher than for the R S-average (18 m) and the W S-average (42 m).
Factors affecting the spatial variation of soil respiration
The spatial variation of R S was positively related to T S and was negatively correlated with W S and BD during the observation periods (P < 0.05, Table 3). We found significant correlations in the relationships between R S and the forest structural parameters (mean DBH, BA and max. DBH, P < 0.05), and the correlations depended on the distance from the measurement locations (Table 3, and Fig. 5). The correlations between the mean DBH1, BA4, max. DBH3 and R S were strongest during the observation periods, and they were introduced for backward multiple regression analysis (Table 3, and Fig. 5). With the spatial variation of R S, the SOC had a weak significant correlation in all of the observation periods except for spring (P < 0.05), whereas the pH had a significant correlation in all of the observation periods except for autumn (P < 0.001). No significant correlation of the R S with the TN and C:N was found (P > 0.05, Table 3). Subsequently, all of the correlated variables that showed significant relationships with R S were used in a backward multiple regression analysis (Table 3, Table 4). The model indicated that the W S-average, BD and max. DBH3 explained 46 % of the spatial variation in the R S-average. The regression model in summer showed an R 2 of 0.45, which was higher than for spring (0.27) and autumn (0.36).
Discussion
Spatial variation of soil respiration
Our estimated CV of R S, T S and W S correspond well with the CV of temperate forests in previous studies. Ngao et al. (2012) reported that the spatial CV of R S varied throughout the measurement period, ranging from 9 to 62 % in a temperate beech forest. A strong spatial heterogeneity of R S was observed in the regenerated temperate forest, with coefficients of variance of 25 to 40 % (Luan et al. 2012). Consistent with the previous findings (Ngao et al. 2012; Dore et al. 2014), we found that the CV of T S (7–8 %) was much lower than the CV of R S (51–55 %) and W S (33–34 %) in summer and autumn (Table 1). These findings indicated that the T S was not an important variable for explaining the spatial variation of R S in summer and autumn. Moreover, Wang et al. (2006) reported that the CV of R S within the plots (20 × 30 m) varied from 20 to 27 % for four secondary forests and two plantations in northeastern China, which were lower than those reported in our study. A study by Wang et al. (2006) and our study have adjacent geographical positions, the same original vegetation in the history, climate and soil types. As such, the differences in the CV of R S between the two studies may be attributed to the fact that the stand structure of the primary forest in our study was more complex in contrast to the secondary forests and plantations studied by Wang et al. (2006), and the plot spatial extent was larger in contrast to that of the latter.
The autocorrelation range of R S depended on the plot spatial extent and the distance between the samples (Western and Blöschl 1999; Prolingheuer et al. 2014). In this study, R S shows a pronounced spatial autocorrelation, and 87–91 % of the sample variance was explained by an autocorrelation over a range of 15 to 23 m (Table 2, and Fig. 4). The range for the R S of a 72 × 72 m plot in a broad-leaved mixed temperate forest was smaller than 6 m, which could be explained by the smaller spatial extent than in this study (Søe and Buchmann 2005). However, our results should be interpreted with caution, as geostatistics involves uncertainties and subjective decisions for estimating the semivariance (Webster and Oliver 2007).
Sources of soil respiration variation
Kriged maps of R S and the significant correlations among the R S of the three measurement campaigns confirmed the hypothesis that the spatial patterns of R S remained constant across the three measurement campaigns. Our result is consistent with other studies (Søe and Buchmann 2005; Martin and Bolstad 2009; Luan et al. 2012). As reported in six northern hardwood sites and two aspen sites (Martin and Bolstad 2009), the pattern of a consistently high or consistently low R S over time indicates the existence of a mechanism or mechanisms that consistently produce more or less CO2 in the soil profile. The above reasoning was also supported by the results inferred from our final multiple linear regressions (Table 4). The regression model suggested that the spatial variation in R S was mainly controlled by the W S, BD and max. DBH3, which were relatively stable. However, T S was also an important determinate of the spatial variation of R S in spring, which may be attributed to the fact that R S was limited by the low soil temperature and that the CV of T S in spring (19 %) was higher than those (7–8 %) in summer and autumn. The effects of the W S on the spatial variation in R S can be direct or indirect and involve physical or biological mechanisms. The relationship between R S and W S usually shows a threshold value (approximately 20 %; Xu and Qi 2001; Herbst et al. 2009; Moyano et al. 2013; Cartwright and Hui 2015). Below this threshold, a positive linear relationship between R S and W S was found (Xu and Qi 2001; Chang et al. 2014; Escolar et al. 2015), and this is due to the low W S that causes a decrease in the rate of diffusion of soluble substrates, which can limit soil microbial respiration (Davidson et al. 2006). Above the threshold, R S is negatively correlated with W S (Xu and Qi 2001; Cartwright and Hui 2015). The reason for this is that a high W S not only reduces CO2 transport but can also limit O2 availability and microbial activity (Smith et al. 2003). However, our study did not find a threshold value. It is possible that the R S measurement points (W S-average > 20 %) account for 90 % of the total due the fact that our site had plenty of rainfall in the growing season and that the water holding capacity of the soil was high. Thus, W S was always relatively high at our study site, and it was never low enough to limit R S.
The C assimilated by a tree is transported to the roots and is used to support the roots and the associated mycorrhizal fungi and rhizosphere microorganism respiration, which further affects the total soil respiration because root respiration accounts for approximately 50 % of the total (Bond-Lamberty et al. 2004; Kuzyakov and Gavrichkova 2010). Thus, the spatial distribution of tree size may explain the spatial variation of R S. The significant correlations between R S and the forest structural parameters were found in previous studies (Søe and Buchmann 2005; Katayama et al. 2009; Luan et al. 2012). Katayama et al. (2009) reported that the mean DBH within 6 m of the measurement points had a significant linear relationship with R S in a Bornean tropical rainforest (R 2 = 0.60). Luan et al. (2012) also found that the stand structure parameters (including the BA, max. DBH and mean DBH) within 4–5 m of the measurement points had a significant influence on the spatial variation of R S in a regenerated temperate forest. Our results showed that the mean DBH1, BA4 and max. DBH3 were significantly correlated to the spatial variation of R S. This confirms the assumption that the forest stand structure determines the spatial variation in R S. Furthermore, the final model indicated that the max. DBH3 was one of the three most important parameters for explaining the spatial variation of the R S-average. This result suggested that the largest trees may influence the local spatial variation of R S, which may be attributed to the fact that large trees may have a greater belowground C allocation than small trees or that the spatial distribution of emergent trees may affect the root distribution of surrounding smaller trees, resulting in the spatial variation of R S (Søe and Buchmann 2005; Bréchet et al. 2011; Luan et al. 2012; Ohashi et al. 2015).
Despite the promising results from the model fits discussed above, there was considerable variation (54 %) in the spatial heterogeneity of R S that could not be explained. Several other ecologically driven processes and potential biases could contribute to unexplained variation. First, there was a significant, but weak correlation between R S and the SOC during all of the observation periods, except for spring. The light fraction organic carbon may explain the spatial variation of R S better when compared to the SOC, as it can partly reflect the substrate availability or the microbial activity (Laik et al. 2009; Luan et al. 2012). Future studies are needed to confirm this inference. Second, the stand structure and R S were not investigated simultaneously, which may be a potential bias. However, this old-growth forest has not been recently disturbed and the stand structure was relatively stable. Thus, it was appropriate to estimate the relationship between the stand structure parameters and R S in this study, even though the two variables were investigated in the different year. Finally, the relationship between R S and the root biomass and soil microbial biomass may be close. However, obtaining these driving factors is difficult due to high labour and time costs. Our results showed that the forest stand structure contributed to the spatial variation of R S at the ecosystem scale. This is an important finding to extrapolate R S spatially using forest stand structural parameters at the ecosystem scale where the tree diameter are more easily obtained than root mass (Katayama et al. 2009).
Conclusion
Our results demonstrated that R S had a strong spatial heterogeneity and spatial autocorrelation. These results have implications for an optimum sampling setup. For example, at a plot or ecosystem scale, it is possible to generate biased average R S estimates if the number of replicates is small (<the number of measurements required to obtain an average R S within 20 % of its actual value at the 95 % confidence level) or measurement points are located close to each other (<spatial autocorrelation length). Additionally, the spatial patterns of R S did not remarkably vary from season to season. The spatial variation of R S was tightly linked to the forest stand structure and soil parameters, such as the W S and BD, which were easily obtained. These findings enable us to understand the mechanisms underlying R S and estimate the net ecosystem C exchange at the ecosystem scale in an old-growth mixed broadleaved-Korean pine forest in northeastern China.
References
Barba J, Yuste JC, Martínez-Vilalta J, Lloret F (2013) Drought-induced tree species replacement is reflected in the spatial variability of soil respiration in a mixed Mediterranean forest. For Ecol Manag 306:79–87
Bond-Lamberty B, Wang CK, Gower ST (2004) A global relationship between the heterotrophic and autotrophic components of soil respiration? Glob Chang Biol 10(10):1756–1766
Bréchet L, Ponton S, Alméras T, Bonal D, Epron D (2011) Does spatial distribution of tree size account for spatial variation in soil respiration in a tropical forest? Plant Soil 347:293–303
Cartwright J, Hui DF (2015) Soil respiration patterns and controls in limestone cedar glades. Plant Soil 389:157–169
Chang CT, Sabaté S, Sperlich D, Poblador S, Sabater F, Gracia C (2014) Does soil moisture overrule temperature dependency of soil respiration in mediterranean riparian forests? Biogeosci Discuss 11:7991–8022
Davidson EA, Janssens IA, Luo YQ (2006) On the variability of respiration in terrestrial ecosystems: moving beyond Q 10. Glob Chang Biol 12:54–164
Dore S, Fry DL, Stephens SL (2014) Spatial heterogeneity of soil CO2 efflux after harvest and prescribed fire in a California mixed conifer forest. For Ecol Manag 319:150–160
Escolar C, Maestre FT, Rey A (2015) Biocrusts modulate warming and rainfall exclusion effects on soil respiration in a semi-arid grassland. Soil Biol Biochem 80:9–17
Ferré C, Castrignanò A, Comolli R (2015) Assessment of multi-scale soil-plant interactions in a poplar plantation using geostatistical data fusion techniques: relationships to soil respiration. Plant Soil 390:95–109
Herbst M, Prolingheuer N, Graf A, Huisman JA, Weihermüller L, Vanderborght J (2009) Characterisation and understanding of bare soil respiration spatial variability at plot scale. Vadose Zone J 8:762–771
Hudiburg T, Law B, Turner DP, Campbell J, Donato D, Duane M (2009) Carbon dynamics of Oregon and northern California forests and potential land-based carbon storage. Ecol Appl 19:163–180
Katayama A, Kume T, Komatsu H, Ohashi M, Nakagawa M, Yamashita M, Otsuki K, Kumagai T (2009) Effect of forest structure on the spatial variation in soil respiration in a Bornean tropical rainforest. Agric For Meteorol 149:1666–1673
Kuzyakov Y, Gavrichkova O (2010) Time lag between photosynthesis and carbon dioxide efflux from soil: a review of mechanisms and controls. Glob Chang Biol 16:3386–3406
Laik R, Kumar K, Das DK, Chaturvedi OP (2009) Labile soil organic matter pools in a calciorthent after 18 years of afforestation by different plantations. Appl Soil Ecol 42:71–78
Lewis SL, Lopez-Gonzalez G, Sonké B, et al. (2009) Increasing carbon storage in intact African tropical forests. Nature 457:1003–1007
Lorenz K, Lal R (2010) Carbon sequestration in forest ecosystems. Springer, New York
Luan JW, Liu SR, Zhu XL, Wang JX, Liu K (2012) Roles of biotic and abiotic variables in determining spatial variation of soil respiration in secondary oak and planted pine forests. Soil Biol Biochem 44:143–150
Luyssaert S, Schulze ED, Börner A, Knohl A, Hessenmöller D, Law BE, Ciais P, Grace J (2008) Old-growth forests as global carbon sinks. Nature 455:213–215
Martin JG, Bolstad PV (2009) Variation of soil respiration at three spatial scales: components within measurements, intra-site variation and patterns on the landscape. Soil Biol Biochem 41:530–543
Moyano FE, Manzoni S, Chenu C (2013) Responses of soil heterotrophic respiration to moisture availability: an exploration of processes and models. Soil Biol Biochem 59:72–85
Ngao J, Epron D, Delpierre N, Bréda N, Granier A, Longdoz B (2012) Spatial variability of soil CO2 efflux linked to soil parameters and ecosystem characteristics in a temperate beech forest. Agric For Meteorol 154–155:136–146
Odum EP (1969) The strategy of ecosystem development. Science 164:262–270
Ohashi M, Kume T, Yoshifuji N, Kho LK, Nakagawa M, Nakashizuka T (2015) The effects of an induced short-term drought period on the spatial variations in soil respiration measured around emergent trees in a typical Bornean tropical forest, Malaysia. Plant Soil 387:337–349
Prolingheuer N, Scharnagl B, Graf A, Vereecken H, Herbst M (2014) On the spatial variation of soil rhizospheric and heterotrophic respiration in a winter wheat stand. Agric For Meteorol 195:24–31
Robertson GP, Gross KL (1994) Assessing the heterogeneity of below ground resources: quantifying pattern and scale. In: Caldwell MM, Pearcy RW (eds) Exploitation of environmental heterogeneity by plants: ecophysiological processes above-and belowground. Academic Press, New York, pp. 237–252
Robertson GP, Crum JR, Ellis BG (1993) The spatial variability of soil resources following long-term disturbance. Oecologia 96:451–456
Scott-Denton LE, Rosenstiel TN, Monson RK (2006) Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Glob Chang Biol 12:205–216
Shi BK, Gao WF, Jin GZ (2015) Effects on rhizospheric and heterotrophic respiration of conversion from primary forest to secondary forest and plantations in northeast China. Eur J Soil Biol 66:11–18
Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A (2003) Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur J Soil Sci 54:779–791
Søe ARB, Buchmann N (2005) Spatial and temporal variations in soil respiration in relation to stand structure and soil parameters in an unmanaged beech forest. Tree Physiol 25:1427–1436
Software GD (2004) GS+: geostatistics for the environmental sciences. Gamma Design Software, Plainwell, Michigan
Soil Survey Staff (1999) Soil Taxonomy: A basic system of soil classification for making and interpreting soil surveys. USDA Natural Resources Conservation Service, Washington, D.C
Song W, Chen S, Wu B, Zhu Y, Zhou Y, Lu Q, Lin G (2015) Simulated rain addition modifies diurnal patterns and temperature sensitivities of autotrophic and heterotrophic soil respiration in an arid desert ecosystem. Soil Biol Biochem 82:143–152
State Forestry Administration (SFA) (2005) China forestry yearbook 2005. China Forestry Press, Beijing(in Chinese)
Teixeira DDB, Bicalho ES, Cerri CEP, Panosso AR, Pereira GT, La Scala N (2013) Quantification of uncertainties associated with space-time estimates of short-term soil CO2 emissions in a sugar cane area. Agric Ecosyst Environ 167:33–37
Wang CK, Yang JY, Zhang QZ (2006) Soil respiration in six temperate forests in China. Glob Chang Biol 12:2103–2114
Wang X, Jiang Y, Jia B, Wang F, Zhou G (2010) Comparison of soil respiration among three temperate forests in changbai mountains, China. Can J For Res 40:788–795
Wang CK, Han Y, Chen JQ, Wang XC, Zhang QZ, Bond-Lamberty B (2013) Seasonality of soil CO2 efflux in a temperate forest: biophysical effects of snowpack and spring freeze-thaw cycles. Agric For Meteorol 177:83–92
Webster R, Oliver MA (2007) Geostatistics for environmental scientists. John Wiley & Sons Ltd, Chichester
Western AW, Blöschl G (1999) On the spatial scaling of soil moisture. J Hydrol 217:203–224
Xu M, Qi Y (2001) Soil-surface CO2 efflux and its spatial and temporal variations in a young ponderosa pine plantation in northern California. Glob Chang Biol 7(6):667–677
Yoon TK, Noh NJ, Han S, Lee J, Son Y (2014) Soil moisture effects on leaf litter decomposition and soil carbon dioxide efflux in wetland and upland forests. Soil Sci Soc Am J 78(5):1804–1816
Yuan ZQ, Gazol A, Lin F, Ye J, Shi S, Wang XG, Wang M, Hao ZQ (2013) Soil organic carbon in an old-growth temperate forest: spatial pattern, determinants and bias in its quantification. Geoderma 195:48–55
Acknowledgments
This work was financially supported by the Ministry of Science and Technology of the People’s Republic of China (No. 2011BAD37B01) and the Program for Changjiang Scholars and Innovative Research Team in Universities (IRT_15R09). We thank Prof. Chuankuan Wang for useful suggestions with the experimental design. We also would like to thank the responsible editor and the anonymous reviewers for their constructive and helpful comments that helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Per Ambus.
Electronic supplementary material
ESM 1
(XLSX 474 kb)
Rights and permissions
About this article
Cite this article
Shi, B., Gao, W., Cai, H. et al. Spatial variation of soil respiration is linked to the forest structure and soil parameters in an old-growth mixed broadleaved-Korean pine forest in northeastern China. Plant Soil 400, 263–274 (2016). https://doi.org/10.1007/s11104-015-2730-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2730-z