Abstract
Aims
The objectives of this study were to determine the spatial structure of soil respiration (Rs) in a naturally-regenerated longleaf pine forest and to assess the ecological factors affecting the spatial variability in Rs.
Methods
Soil respiration, soil temperature (Ts), and soil moisture were repeatedly measured over 6 days in summer 2012 in 3 semi-independent plots. Edaphic, forest floor, and root variables were measured. Diameters of 338 trees were mapped. Spatial analysis and regression were applied.
Results
Soil respiration was spatially autocorrelated across plots (66—92 m), but not within plots (6—34 m). Spatial distributions of Rs were relatively stable from morning through early evening and were decoupled from temporal variation of Ts. Ecological covariates (e.g., soil moisture, bulk density and carbon, litter mass, understory cover, roots, nearby trees) related to the spatial variability in Rs; however, models varied between plots.
Conclusions
This study shows the importance of stationary plant and soil factors in determining the spatial, temperature-independent distribution of Rs in a heterogeneous forest. We suggest the need for a better understanding of the complex interactions between the heterotrophic, autotrophic, and physical processes driving Rs in order to better model forest carbon budgets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil respiration (Rs) is the sum evolution of CO2 respired from the activity of roots, mycorrhizae, and microorganisms within both the root-affected rhizosphere and the bulk soil (Raich and Nadelhoffer 1989; Bahn et al. 2010). Autotrophic and heterotrophic activity are affected by biotic and abiotic factors to varying degrees, thus causing temporal and spatial variability in Rs (Lavigne et al. 2003; Ruehr and Buchmann 2010; Chen et al. 2011). Soil temperature (Ts) is generally the most important factor influencing the seasonal changes in Rs as it increases both heterotrophic and autotrophic activity during the warmer growing seasons (Fang et al. 1998; Maier and Kress 2000; Davidson et al. 2006; DeForest et al. 2006; Ruehr and Buchmann 2010), but Ts often fails to explain the spatial variation in Rs within forests (Søe and Buchmann 2005; Vande Walle et al. 2007; Geng et al. 2012). Soil temperature and Rs have also been found to be related on a diel basis. As examples, over a 24-h period, Ts and Rs were related in a Canadian boreal forest (Rayment and Jarvix 2000), in a Zimbabwe miombo forest (Merbold et al. 2011), and in a Siberian Scots pine (Pinus sylvestris L.) forest (Shibistova et al. 2002).
Plant and soil factors, on the other hand, can directly affect the spatial variability of Rs within a forest. The allocation of photosynthates to roots increases autotrophic respiration; root exudates increase heterotrophic respiration within the rhizosphere; and litterfall quantity and quality increases heterotrophic respiration in surface soil layers (Bahn et al. 2010; Metcalfe et al. 2011). Belowground edaphic factors also exert spatial influence upon Rs, such as soil moisture, pH, bulk density, and soil carbon (Vande Walle et al. 2007; Luan et al. 2012; Fóti et al. 2014), and these edaphic variables are not independent of plant, root, and litter distribution in forests. For instance, the interaction between precipitation and trees, specifically throughfall and stemflow, affects the spatial variability of soil moisture in forests (Bryant et al. 2005). Trees can also affect soil nutrients, soil microbial community composition, and soil properties such as pH and bulk density (Zinke 1962; Vetaas 1992; Weber and Bardgett 2011; Lavoie et al. 2012). Suchewaboripont et al. (2015) found that spatial patterns in Rs were related to both edaphic and plant factors in an old-growth beech-oak forest in Japan, highlighting the interplay between autotrophic and heterotrophic mechanisms. In contrast, Ferré et al. (2015) concluded that tree growth maps alone may be enough to accurately stratify the spatial variability in Rs in plantations, and Fang et al. (1998) suggested that heterotrophic respiration alone was the most important factor in predicting spatial distribution of Rs in a slash pine (Pinus elliottii Engelm.) plantation.
After historical degradation from logging, turpentine extraction, grazing and fire suppression (Noss 1988), longleaf pine (Pinus palustris Mill.) ecosystems are now being restored throughout the southeastern United States to provide ecosystem services, such as rare species habitat and biodiversity conservation (Jose et al. 2006; Mitchell et al. 2006). These restoration efforts have given rise to an impetus for research into the environmental and ecological controls of forest and soil carbon dynamics in longleaf pine forests (e.g., Samuelson et al. 2014; ArchMiller and Samuelson 2016). Naturally-regenerated longleaf pine forests are characterized by a spatially heterogeneous structure of the canopy, understory, and soils (Noss 1988; Battaglia et al. 2002; Lavoie et al. 2012). Longleaf pine forests depend upon prescribed fire to reduce succession by other trees, maintain herbaceous understory niches, and expose mineral soil to facilitate seedling regeneration (Noss 1988; Brockway and Lewis 1997). The frequency and intensity of fire can affect the spatial structure and chemistry of soil (e.g., higher soil nutrient loads in relation to tree trunks) and the composition of midstory and understory plant species (Lavoie et al. 2012; Lashley et al. 2014). Canopy gaps in longleaf pine forests affect light availability to the forest floor and results in spatial variability in above- and belowground biomass of understory plants (McGuire et al. 2001). Longleaf pine rooting zones extend well beyond the extent of the canopy (Heyward 1933; Hodgkins and Nichols 1977), which creates a mosaic of woody and herbaceous roots throughout longleaf pine forests. Furthermore, the spatial distribution of lateral roots is related to the competitive position of trees within the forest, and the shape of the rooting zone outward from the stem is influenced by the presence of other trees (Hodgkins and Nichols 1977). Additionally, nitrogen-fixing legumes, which are common in longleaf pine forests, affect the spatial distribution of soil nitrogen and decomposition rates of litter (Vetaas 1992). Thus, spatial variability of Rs in naturally-regenerated longleaf pine forests may be greater than expected based on Rs studies of southeastern conifer plantations (e.g., Fang et al. 1998).
The objectives of this study were to first quantify the spatial structure of Rs in a longleaf pine forest and second explore which ecological factors (Table 1) are key determinants of spatial variability in Rs. The study was conducted in a 64-year-old, naturally-regenerated longleaf pine forest and designed to isolate the temperature-independent, spatial variability in Rs. Soil respiration and related covariates were systematically measured in 75 regularly spaced locations split between 3 semi-independent plots 3 times per day during 6 days. It was expected that Rs would exhibit positive spatial autocorrelation because of the heterogeneous distribution of trees, which was shown to be positively related to Rs in longleaf pine forests (ArchMiller and Samuelson 2016). We tested the hypothesis that multiple linear regression using a comprehensive set of biophysical parameters would be sufficient to explain the spatial variability in Rs. Alternatively, if regression was insufficient, thus leaving spatially autocorrelated residuals and violating linear regression assumptions (Wheeler and Tiefelsdorf 2005), residual kriging, which incorporates spatially autocorrelated residuals with an empirical residual variogram model, would be applied to incorporate the spatial dependence of Rs (e.g., Wu and Li 2013). Furthermore, because the spatial distribution of plant and soil factors influence the autotrophic and heterotrophic components of Rs (e.g., Vande Walle et al. 2007; Luan et al. 2012; Fóti et al. 2014), we hypothesized that stationary ecological factors (e.g., litter mass, root biomass, understory vegetation cover, nearby trees) would positively relate to spatial patterns of Rs within the forest, while daily fluctuating factors (i.e., Ts and soil moisture) would influence the temporal variability in longleaf pine Rs.
Methods
Site description
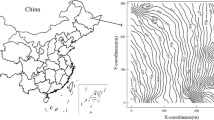
The study site was located at Fort Benning Military Base near Columbus, Georgia (Fig. 1). The climate in this area has a mean maximum temperature of 24.6 °C, a mean minimum temperature of 18.7 °C, and a mean annual precipitation of 1187 mm (1981–2010 Normals) (National Climatic Data 2015a). The stand used for this study was in a naturally-regenerated, even-age 64-year-old longleaf pine forest located on the Alabama property of Fort Benning (32° 19.142’ N, −85° 0.514’ W, 97 m A.S.L.), and was previously described in Samuelson et al. (2014). Soils at this stand were classified by the United States Department of Agriculture as complexes of loamy, kaolinitic, thermic Grossarenic Kandiudults; fine, mixed, semiactive, thermic Typic Hapludults; and fine-loamy, kaolinitic, thermic Typic Kanhapludults (i.e., Troup-Springhill-Luverne complexes) (Soil Survey Staff 2014). These complexes are typically very deep, well to excessively drained loamy fine sand or loamy sand soils on side slopes (10–30 % slopes) (Soil Survey Staff 2014). Soil texture consisted of 79 % sand, 8.5 % silt, and 12.5 % clay (Samuelson et al. 2014), and associated wilting point, field capacity, and saturation were 9.8, 18.2, and 41.5 % volumetric soil moisture, respectively (Oram and Nelson 2014). The stand was last burned in the winter of 2010 and was maintained on an approximately 3-year burning cycle.
(Left) Plots established within a 64-year-old longleaf pine in eastern Alabama (inset map). Each plot contained a 5 by 5 grid of 25 Rs collars spaced 6 m apart. (Right) The location and diameter classes of longleaf pine (LLP), other pine (OP), and hardwood tree species (HWD) within each tree survey extent. Tree diameters are not to scale. Aerial imagery from Bing Maps and 10 m DEM contour lines downloaded from Geospatial Data Gateway (January 16, 2015; www.datagateway.nrcs.usda.gov)
The experimental design consisted of three 24 m by 24 m plots positioned over a gradual 10 m topographical gradient (Fig. 1). Within each plot, 25 1 m2 Rs sampling subplots (hereafter “subplots”) were laid out evenly in a 5 by 5 grid with 6 m spacing between subplot centroids. At the center of each subplot, an Rs collar (PVC, 10 cm diameter, 4.5 cm depth) was installed into the ground through the standing litter and to a consistent 2.5 cm depth of mineral soil. In July 2012, all trees taller than 2 m height with diameter at breast height (DBH, 1.37 m) greater than 1 cm were inventoried within 8 m of each Rs collar and classified as mature (DBH ≥ 10 cm) or saplings (DBH < 10 cm; Fig. 1). The three plots ranged in mature longleaf pine basal area from 8.0 to 10.3 m2 ha−1, sapling longleaf pine basal area from 0.2 to 0.7 m2 ha−1, and in total basal area from 11.5 to 15.1 m2 ha−1 (Table 2). Density of mature and sapling longleaf pine trees ranged from 79 to 125 trees ha−1 and 85 to 373 trees ha−1, respectively, among plots (Table 2). Mean DBH of mature longleaf pine trees ranged from 19.0 to 36.1 cm among plots and mean plot-level DBH of sapling longleaf pine trees ranged from 4.5 to 5.0 cm (Table 2). Other pine species present in these stands included mature and sapling loblolly pine (P. taeda L.) and shortleaf pine (P. echinata Mill.), and hardwood sapling species included oaks (Quercus spp.), hickories (Carya spp.), and sweetgum (Liquidambar styraciflua L.). No mature hardwood trees were observed within the tree survey extents.
Soil respiration measurements
Soil respiration measurements were repeated on all 75 Rs collars in the morning (830—1100 h), midday (1130—1400 h), and afternoon (1430—1700 h) on 6 days in 2012 (July 14, July 24, July 26, August 2, August 14, and August 17). Soil respiration was measured consistently and systematically in each plot, beginning with subplot 1 and ending with subplot 25 before moving to the next plot, and each plot took approximately 45 min to complete. Soil respiration was measured using a LI-6400-09 Soil CO2 Flux Chamber attached to a LI-6400 portable infrared gas analyzer (LI-COR Biosciences, Lincoln, NE, USA). To reduce the temporal variability between measurements within a plot, ambient atmospheric CO2 concentration was measured at the first Rs collar in each plot and used as target CO2 concentration for all 25 subplots within that plot. Within approximately 10 cm of each Rs collar, Ts was measured with a 15-cm depth soil temperature thermocouple (6000–09, LI-COR Biosciences) and soil moisture was measured with a 20-cm depth soil moisture time domain reflectometry probe (Hydrosense II, Campbell Scientific, Logan, UT, USA).
Ecological covariate measurements
Prior to the installation of Rs collars, total percent live understory vegetation cover (<1 m height) was ocularly estimated within the subplots, as well as by plant functional group (woody, forb, legume, vine, and graminoid). Subsequent to all of the Rs measurements on August 17, 2012, standing litter mass was collected from within the Rs collars, dried to a constant weight at 70 °C, and weighed. Standing litter included fallen tree leaf litter, understory leaf litter, and fine woody debris. After litter collection, the Rs collars were removed, and soil samples (10 cm diameter, 15 cm depth) were collected from below the Rs collars, bagged, and kept cool until processing. Processing the soil samples consisted of dry sifting the soil through a 2 mm mesh sieve to retrieve roots. Roots were washed, sorted by type and size, dried to constant weight at 70 °C, and weighed. Roots were sorted into very fine (diameter ≤ 1 mm), fine (1 mm > diameter ≤ 2 mm), coarse (2 mm > diameter ≤ 5 mm), and very coarse (diameter > 5 mm) categories and by live or dead based on texture, resiliency to bending, and coloration.
Air-dried soil samples were sent to the Auburn University Soil Testing Laboratory (Auburn, AL, USA) for measurement of pH and concentrations of carbon (C), organic matter (OM), and soil elements including nitrogen (N), phosphorus (P), calcium (Ca), potassium (K), magnesium (Mg), and aluminum (Al). Soil pH was measured in a water to soil ratio of 1:1 (Hue and Evans 1979). Total soil C and N were analyzed by combustion with an Elementar Vario Macro CNS Analyzer (Elementar Analysensysteme, Germany), and OM was calculated as total C multiplied by 1.72. The concentrations of other soil elements other than N were determined simultaneously with a Varian-MPX Radial Spectrometer (Varian Analytical Instruments, Australia) (Odom and Kone 1997).
Within 10 cm of each Rs collar in undisturbed soil, bulk density samples were taken from 1 to 10 cm depth with a 5.7 cm diameter soil sampler (0200 Soil Core Sampler, Soil Moisture Equipment Corp., Goleta, GA, USA). Bulk density was calculated following the procedure of Law et al. (2008). Briefly, soil was oven-dried (96 h at 105 °C) and sifted, and then rocks and roots were removed, soil was weighed, and rock volume was determined through water displacement. Root volume was negligible. Bulk density was calculated as the rock and root-free soil mass divided by the rock-free soil volume. Soil nutrient concentrations were converted to a per area basis (Mg ha−1) based on soil bulk density.
Data analysis
The locations of the plots and Rs collars were collected with a handheld, decimeter-accurate global positioning system (Trimble GeoXH, Trimble Navigation Limited, Sunnyvale, CA, USA) and downloaded to ArcGIS (Environmental Systems Research Institute, Inc., Redlands, CA, USA). The tree inventory data was digitized in ArcGIS and used to calculate the mean DBH, total DBH, and number of trees within 8 m, 6 m, 4 m, 2 m, and 1 m of each Rs collar, as well as the distance to the nearest tree from each Rs collar. The tree basal area of each Rs collar was calculated using a modified prism technique with a 10-factor prism (Šálek and Zahradník 2008). All spatial analysis was completed in the Universal Transverse Mercator projected coordinate system (Zone 16 N) with the World Geodetic System 1984 geographic coordinate system (ArcGIS projection file WGS_1984_UTM_Zone_16N).
Repeated measures analysis with an autoregressive moving average covariance structure was used to test for plot differences in Rs, Ts, and soil moisture within a mixed-model framework for each time period (i.e., morning, midday, or afternoon) with SAS (version 9.3, SAS Institute Inc., Cary, NC, USA). Then, five measurement days were used for model building with one randomly selected measurement day (August 2, 2012) used for model validation. Soil respiration, Ts, and soil moisture were averaged across the other five measurement days by time period and subplot for geospatial and regression analysis. To explore the temporal variability in Rs, the daily coefficient of variation of Rs (dCVRs) was calculated for each Rs collar. Contour graphs of Rs were created in SigmaPlot (version 13.0, Systet Software Inc., Richmond, VA, USA) with five minor lines, linear scaling, and a consistent scale of Rs from 2.0 to 8.6 μmol m−2 s−1 across graphs.
Spatial analysis, correlation, and regression were conducted independently by time period and for the subplots within each plot (n = 25) and across all three plots (n = 75). First, spatial autocorrelation of Rs was determined with Moran’s I and Geary’s C indices at an α level of 0.05 with PROC VARIOGRAM in SAS (Wu and Li 2013). While these indices are related, Moran’s I provides an evaluation of global spatial autocorrelation while Geary’s C provides an evaluation of local spatial autocorrelation (Moran 1950; Geary 1954). Then, the relationships between Rs and ecological covariates were evaluated with Pearson’s correlation coefficients at α = 0.05. Because of high multicollinearity between stand structural variables, the relationship between Rs and stand structural covariates was examined with principal components analysis following Søe and Buchmann (2005). Multiple linear regression models were built through stepwise model selection with entry and exit α values of 0.15 (default for PROC REG in SAS). Regression analysis between ecological factors and the spatial variability in Rs was conducted with morning Rs measurements only because of the redundancy of the spatial variability in Rs between time periods. Variance inflation factors (VIFs) were used to reduce the impact of multicollinearity (i.e., parameter VIFs were < 2.0), and residuals were visually examined to check for homoscedasticity. Residuals were also checked for spatial dependency with Moran’s I and Geary’s C. If regression model residuals were spatially autocorrelated, residuals kriging analysis was conducted by applying empirical semivariogram models to the residuals (Wu and Li 2013).
Semivariogram analysis was conducted with statistical program R (package gstat; R Foundation for Statistical Computing, Vienna, Austria). Semivariance (γ) measures the spatial relationship of Rs at two locations (x and x + h) separated by a lag distance h:
where n(h) is the number of pairs at distance h and z is the value of residuals at each location (Stoyan et al. 2000; Mitra et al. 2014). Semivariance was graphed versus h in a semivariogram and fit with empirical models (linear, exponential, or spherical). The final semivariogram model was chosen by minimizing the residual error sum of squares (SSE).
Model validation was conducted by comparing the multiple linear regression residuals (hereafter “model residuals”) and the differences between August 2 Rs and model-predicted Rs (hereafter “August 2 residuals”). Residuals were compared with a paired t-test to determine the differences between August 2-observed and model-predicted Rs. Graphs of the spatial structure of the model-predicted Rs values were visually compared with plots of Rs averaged across the five measurement days. Mean absolute error (MAE) was calculated to determine the magnitude of error difference between August 2 Rs and model-predicted Rs, mean biased error (MBE) was calculated to determine error bias, and root mean squared error (RMSE) was calculated to evaluate overall model performance (Wu and Li 2013).
Results
Daily maximum air temperature varied from 27.8 to 38.3 °C during the measurement period from July 14, 2012 through August 17, 2012 at Columbus Metropolitan Airport Weather Station (Fig. 2). Daily minimum air temperature ranged from 26.7 to 17.8 °C and daily precipitation ranged from 0.0 to 25.7 mm day−1 (Fig. 2). Mean soil temperature was different between plots during the morning but not during the midday (F 2,7 = 4.18, p = 0.0638 and F 2,7 = 2.05, p = 0.1991, respectively; Table 3). Mean soil moisture was different between plots during both the morning and the midday (F 2,7 = 31.59, p = 0.0003 and F 2,7 = 31.59, p = 0.0003, respectively; Table 3). Coefficients of variation (CV) for Ts and soil moisture ranged from 2 to 6 % and 31 to 91 %, respectively (Table 3).
Daily maximum and minimum temperatures and total daily precipitation (National Climatic Data 2015b). Triangles represent dates of soil respiration (Rs), soil temperature, and soil moisture sampling
Spatial patterns of soil respiration
Across the five measurement days, mean morning Rs was 5.16, 3.72, and 3.98 μmol m−2 s−1 in Plots 1, 2, and 3, respectively, and was significantly different between plots (F 2,7 = 154.23, p < 0.0001; Fig. 3, Table 3). Mean midday Rs ranged from 3.77 to 4.97 μmol m−2 s−1 and was significantly different between plots (F 2,7 = 32.29, p = 0.0003; Fig. 3, Table 3). Mean afternoon Rs ranged from 3.20 to 5.02 μmol m−2 s−1, but not enough afternoon time periods were measured for cross-plot comparisons (Fig. 3, Table 3). Plot-wise CVs for Rs ranged from 28 to 42 % (Table 3).
Soil respiration was not significantly spatially autocorrelated during any time period in any individual plot (Table 4). Across all three plots, Rs was significantly, positively spatially autocorrelated during each time period, based on both Moran’s I and Geary’s C indices (Table 4). The ranges of Rs autocorrelation were 91.6 m, 67.4 m, and 66.1 m in the morning, midday, and afternoon time periods, respectively (Fig. 4).
Semivariance (γ) of soil respiration versus lag distance measured across all three plots in the morning (a), midday (b), and afternoon (c) within a 64-year-old longleaf pine forest. Observed data points (white dots) are scaled to the number of pairs at each lag distance from 23 pairs (smallest) to 366 pairs (largest). In a, the regression line represents the linear empirical fit with a nugget (y-intercept) of 1.62 (μmol m−2 s−1)2, a partial sill of 0.99 (μmol m−2 s−1)2, and a range of 91.6 m (SSE = 0.10). In b, the regression line represents a spherical empirical fit with a nugget of 1.37 (μmol m−2 s−1)2, a partial sill of 0.73 (μmol m−2 s−1)2, and a range of 67.4 m (SSE = 0.04). In c, the regression line represents a linear empirical fit with a nugget of 1.63 (μmol m−2 s−1)2, a partial sill of 1.31 (μmol m−2 s−1)2, and a range of 66.08 m (SSE = 0.12)
The spatial pattern of Rs did not vary greatly between time periods (Fig. 3). Mean dCVRs was 22.0 ± 2.4 %, 25.0 ± 1.3 %, and 26.0 ± 2.5 % in plots 1, 2, and 3, respectively, and across plots, mean dCVRs was 24.4 ± 1.2 %. Pairwise correlations between morning and midday Rs, midday and afternoon Rs, and morning and afternoon Rs were r = 0.94 (p < 0.0001), r = 0.90 (p < 0.0001), and r = 0.89 (p < 0.0001), respectively.
Relationships between covariates and spatial variability in Rs
Due to the relatively consistent spatial structure of Rs between time periods, the spatial variability in Rs was modeled using the morning Rs data only to prevent redundant analyses across time periods. In Plot 1, morning Rs was significantly and negatively related to bulk density and the total number of trees within 6 m, and positively related to C, woody and graminoid cover, and the mean DBH of trees within 2 m of the Rs collars (Table 5). The models for morning Rs accounted for 58 % of the variation in Rs (Table 5), and linear regression model residuals for Plot 1 were not spatially autocorrelated (Table 4).
In Plot 2, morning Rs was significantly and negatively related to soil moisture, soil bulk density, P, and very coarse root biomass, and positively related to N and litter mass (Table 5). The multiple linear regression model for Plot 2 Rs accounted for 73 % of the variation in Rs (Table 5), and the residuals from this model were not spatially autocorrelated (Table 4).
Soil respiration was significantly and negatively related to soil moisture, and positively related to Al, buried coarse woody debris, and the tree basal area of the Rs collars in Plot 3 (Table 5). The multiple linear regression model accounted for 52 % of the variation in Plot 3 Rs. The residuals from the Plot 3 multiple linear regression model were not spatially autocorrelated (Table 4).
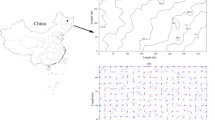
In the morning and across all three plots, Rs was significantly, negatively correlated with soil moisture, Al, and coarse dead root biomass, and positively related to litter mass, forb cover, and fine live root biomass (Tables 3 and 6). Principal components analysis with Rs and forest structural variables indicated that spatial variability in Rs across plots was most closely related to the DBH of trees within 1 or 2 m of the Rs collar (Fig. 5). The multiple linear regression model built across plots for morning Rs included negative relationships with soil moisture, soil bulk density, very coarse root biomass, and the number of trees within 4 m of the Rs collars, and positive relationships with woody cover and the total DBH of trees within 8 m of the Rs collars (Table 5). This model accounted for 24 % of the variability in Rs (Table 5), and the residuals from this model were not spatially autocorrelated (Table 4).
The first rotated principal component (PCA 1) versus the second rotated principal component (PCA 2) from principal components analysis for stand structural variables and morning soil respiration (Rs) across all three plots measured in a 64-year-old longleaf pine forest (n = 75). These two components account for 53 % of the variability in the dataset. Stand structural variables included the total diameter at breast height of trees at radius r (tD-r), mean diameter at breast height of trees at radius r (mD-r), and the total number of trees at radius r (tN-r). Inventoried trees were taller than 2 m in height with diameter at breast height (DBH) greater than 1 cm
Spatial Rs model validation
Spatial patterns in Rs on August 2, 2012, which was used as a validation dataset, were similar to the patterns averaged across the five other days as indicated both graphically (Fig. 3 and Supplementary Material, Fig. S1) and with pairwise correlations between August 2, 2012 Rs and Rs from the other 5 days (r = 0.94, p < 0.0001). Mean absolute error, MBE, and RMSE calculated with the August 2 validation dataset did not show consistent patterns between plots (Table 5). Mean absolute error ranged from 0.8 to 1.0 μmol m−2 s−1, MBE ranged from 0.1 to 0.2 μmol m−2 s−1, and RMSE ranged from 1.0 to 1.4 μmol m−2 s−1. When compared graphically, the model-predicted Rs from plot-specific multiple linear regression models (Supplementary Material, Fig. S2) better captured spatial variability in Rs than the multiple linear regression models built across all three plots (Supplementary Material, Fig. S3). Finally, based on descriptive statistics and t-tests, the August 2 residuals (i.e., difference between August 2 Rs and model-predicted Rs) were always more positive (underestimated) than the model residuals, and the model residuals had a smaller range than the August 2 residuals (Supplementary Material, Table S1).
Discussion
Capturing spatial-dependent variability in Rs in new ecosystems is difficult without a priori knowledge of spatial patterns of Rs and related covariates in that specific ecosystem, and in this case no a priori information was available on which to base the Rs collar locations or sampling density. Techniques to capture the spatial-dependent variability with chamber-based sampling have been proposed by others (e.g., Rodeghiero and Cescatti 2008; Dore et al. 2014; Ferré et al. 2015); however, in most cases a previous field-based measurement campaign is needed to correctly stratify samples to fully capture the spatial variability in Rs. For example, Rodeghiero and Cescatti (2008) recommend taking high-density Rs samples for three periods spaced 15 days apart in order to determine how to spatially sample Rs based on stratifying the range in measured Rs values. Without a priori knowledge in this study, Rs collars were spaced 6 m apart and spatial autocorrelation was not detected at the plot scale (from 6 to 34 m) scale. Because of a large semivariogram nugget, Søe and Buchmann (2005) inferred that Rs was spatially autocorrelated at scales less than 6 m in a mixed broad-leaf German forest, and we suggest that the large nugget (y-intercept) indicates that Rs may have been spatially autocorrelated at smaller scales than we measured (i.e., less than 6 m). Soil respiration can be spatially autocorrelated at the sub-meter scale due to variability in plant-driven autotrophic respiration (Stoyan et al. 2000). For example, Rs was spatially autocorrelated at scales less than 1 m in black spruce (Picea mariana (Mill.) Britton) forests (Rayment and Jarvix 2000) and up to 3 m in sandy grasslands (Fóti et al. 2014). On the other hand, Rs did exhibit spatial autocorrelation at the larger, across-plot scale with semivariogram ranges from 66 to 92 m, which may have been caused by the influence of large-scale edaphic or topographic gradients on heterotrophic respiration, as reported by La Scala, Jr. et al. (2000) who measured spatial autocorrelation in CO2 emissions resultant of heterotrophic activity (i.e., bare soil) at scales from 29 to 58 m, and Herbst et al. (2012) who detected spatial autocorrelation in heterotrophic respiration at scales of 35 m in agricultural bare soil. Because the scales of spatial autocorrelation of Rs appear to be ecosystem and species-specific, nested sampling designs (i.e., two or more sampling intervals) in situations such as this one with no a priori expectation of the scale of spatial dependence in Rs (Fox et al. 2015) are recommended, although the scales that spatial autocorrelation was measured and inferred (less than 6 m) in this study may be used as a guide to stratify future sampling schemes in longleaf pine forests or similar low-density pine forests.
Spatial variability in Rs was isolated by limiting the temporal influence of Ts and the substrate-induced influence of phenological changes on Rs, and Ts exhibited low spatial variability compared to Rs. Similarly, Ohashi et al. (2008) found that Rs measurements in a tropical dripterocarp forest were correlated between consecutive days in November and December, but not between the 2 months. Soil respiration measured on 3 days in November from bare soil in Brazil was found to exhibit varying spatial structures depending on the day and proximity to rainfall events (La Scala et al. 2000), and Rs spatial autocorrelation ranged from 2.5 to 8.8 m in a sagebrush-steppe in Wyoming when measured on 4 days in June and July (Mitra et al. 2014). Although the overall range in Rs measurements varied seasonally, the spatial patterns of Rs were found to remain stable regardless of measurement date in deciduous forests (Martin and Bolstad 2009). The relationships illustrated with this study advance our understanding of the ecological variables that affect spatial patterns of Rs within a forest, which is necessary for enhancing mechanistic Rs models that include plant-soil interactive effects (Martin and Bolstad 2009; Bahn et al. 2010; Chen et al. 2011; Martin et al. 2012; Mitra et al. 2014).
As hypothesized, spatial relationships were found between Rs and edaphic, forest floor, root, and forest structural variables, but models were not identical across plots, suggesting that the autotrophic and heterotrophic components of Rs may have responded to ecological covariates at varying spatial and temporal scales, creating a complex pattern of Rs not easily modeled. The edaphic properties of soil can directly influence the heterotrophic component of Rs. For instance, saturated soil conditions can cause anaerobic conditions and decrease heterotrophic respiration (Moyano et al. 2013), and high bulk density correlates with low C and OM rates, which can limit substrate supply for soil microorganisms and decrease heterotrophic activity (Verlinden et al. 2013). Soil substrate availability was reported to be more important than microbial community composition in explaining spatial variability in heterotrophic respiration across four subtropical forests in China (Wei et al. 2015). Rodeghiero and Cescatti (2005) observed that soil C content was positively related to spatial variability in Rs between sites along an elevation gradient in the Italian Alps. Similarly, soil C was the only measured ecological variable that could account for within-plot spatial variability in Rs in Zambabwe miombo forests (Merbold et al. 2011). Edaphic characteristics such as bulk density and soil moisture can also influence the physical drivers of Rs. For instance, high bulk density or saturated soil conditions can limit the diffusion rate of CO2 through soil (Raich and Schlesinger 1992). As observed in this study, bulk density was inversely related to Rs in burned mixed-conifer forests in California (Dore et al. 2014), in naturally regenerated oak forests and monoculture pine plantations in China (Luan et al. 2012), and in Sitka spruce stands varying in age (Saiz et al. 2006). Soil moisture, which is influenced by topography, soil depth, transpiration, and tree basal area (Tromp-van Meerveld and McDonnell 2006), has been shown to moderate the spatial structure of Rs by reducing CO2 diffusion. Specifically, high soil moisture conditions have low spatial variability (Tromp-van Meerveld and McDonnell 2006), which can cause a concomitant homogenizing effect on Rs. For instance, high soil moisture reduced the spatial variability in Rs in sandy grasslands (Fóti et al. 2014), and heavy rainfall resulted in spatially independent Rs measured on bare soil compared to highly autocorrelated Rs on drier days (La Scala et al. 2000).
Heterotrophic respiration is also directly affected by litter mass (Reinke et al. 1981; Irvine and Law 2002; Taneva and Gonzalez-Meler 2011), so we expected litter mass to strongly relate to Rs. Litter mass was positively related to Rs in Plot 2 and across the three plots. Spatial variability in litter mass can be influenced by fire intensity (Brockway and Lewis 1997) or through interactions with overstory trees (Zinke 1962; Vetaas 1992). In mature longleaf pine forests with a range in stand basal areas, litter mass was found to be positively related to monthly and annual variability in Rs (Samuelson and Whitaker 2012). Litter mass was also found to be specifically related to spatial variability in Rs in a Florida slash pine plantation (Fang et al. 1998) and in unmanaged California mixed-conifer forests (Dore et al. 2014).
Above and belowground vegetation directly influence the autotrophic component of Rs (Mäkiranta et al. 2008; Hojjati and Lamersdorf 2010; Prolingheuer et al. 2014), and we thus expected that areas with higher understory cover, root biomass, and more nearby trees would relate to pockets of higher Rs. However, woody, forb, and graminoid cover were positively related to Rs only in some specific circumstances, and no consistent relationship was found between root biomass and the spatial variability of Rs. Fine root biomass has been demonstrated to be positively related to spatial variability in Rs, such as in control mixed-conifer forests (Dore et al. 2014), in an unmanaged beech forest (Søe and Buchmann 2005), and in a slash pine plantation (Fang et al. 1998). We separated roots by diameter because of the potential for varying specific root respiration rates between root size classes (e.g., very coarse roots have been found to exhibit lower specific respiration rates than fine roots; Chen et al. 2009). However total root biomass may have been confounded by varying specific root respiration rates between plant functional groups. For example, fine pine root biomass, but not non-pine root biomass, was positively related to temperature-independent variability in Rs across four longleaf pine stands varying in age and structure (ArchMiller and Samuelson 2016), and root respiration rates have been shown to vary between different understory functional groups (Tjoelker et al. 2005).
The positive relationship between Rs and the DBH of nearby trees (i.e., within 1 and 2 m of Rs collars) was likely a result of the priming effect that photosynthates can have on autotrophic and heterotrophic respiration near tree roots (Farrar et al. 2003). Proximity to trees has been shown to increase Rs in other studies, such as across longleaf pine stands varying in age and structure (ArchMiller and Samuelson 2016) and in a 22-year-old longleaf pine forest (Clinton et al. 2011). In loblolly pine plantations varying in age, Rs was significantly higher at the base of loblolly trees than 1.5 m away from trees (Wiseman and Seiler 2004). On the other hand, tree density within 4 or 6 m of the Rs collars was negatively related with Rs on a plot-by-plot basis. Gap sizes significantly affect the spatial structure of light availability to the floor of longleaf pine forests (Battaglia et al. 2002), such that shading effects may have reduced understory productivity and autotrophic respiration in dense areas of the forest.
This study of the spatial variability in Rs indicated that residuals kriging was unnecessary in this case (i.e., spatially independent residuals). Likewise, Luan et al. (2012) detected no spatial variability in Rs at 10 m scales in Chinese forests and concluded that observations were spatially independent and that regular regression analysis was adequate and statistically valid. Aiken et al. (1991) detected no spatial autocorrelation in residual Rs variation after detrending the Rs data with regression models that included positional coordinates. Soil respiration was spatially autocorrelated at large scales (i.e., 66 to 92 m) in this study, but this spatial structure was accounted for by the detrending multiple linear regression analyses with ecological covariates. However, these multiple linear regression models at the scale across plots were not as good as the individual plot models at predicting finer-scale variation in Rs. Ferré et al. (2015) determined that ecological covariates that vary at larger scales (77 to 100 m; e.g., tree diameter or soil texture) better correlated with Rs than shorter scale variables (26 to 46 m; e.g., available phosphorus) in a poplar (Populus x euroamericana) plantation. Similar to our predicted Rs spatial patterns, the corresponding spatial patterns of the larger scale covariates did not capture fine-scale patterns within the forest in Ferré et al. (2015).
Conclusions
Soil respiration, which was measured systematically in gridded plots over a restricted temporal extent in a 64-year-old longleaf pine forest, exhibited positive spatial autocorrelation with ranges of 66 to 92 m (i.e., across plots), but did not exhibit spatial autocorrelation at the plot (i.e., < 34 m) scale. Spatial variability in Rs was decoupled from soil temperature, and multiple linear regression with stationary ecological covariates (e.g., edaphic, forest floor, root, and stand structural characteristics) accounted for 52 to 73 % of the spatial variability in Rs at the plot scale. Across plots, stationary factors accounted for 24 % of the spatial variability in Rs and successfully accounted for the positive spatial autocorrelation in Rs, as indicated by spatially independent residuals. Although we expected to detect diurnal variation in Rs from early morning through early evening, we observed only negligible changes in the spatial distribution of Rs throughout the day, indicating the importance of the interrelationship between stationary plant and soil factors on the spatial, temperature-independent distribution of Rs in heterogeneous longleaf pine forests. Models based on within-plot plant-soil interactions better-captured fine scale patterns in Rs than across-plot models, highlighting the need for a better understanding of the complexity of interactions between ecological factors and the autotrophic and heterotrophic components of Rs.
References
Aiken RM, Jawson MD, Grahammer K, Polymenopoulos AD (1991) Positional, spatially correlated and random components of variability in carbon dioxide efflux. J Environ Qual 20:301–308
ArchMiller AA, Samuelson LJ (2016) Intra-annual variation of soil respiration across four heterogeneous longleaf pine forests in the southeastern United States. Forest Ecol Manag (in press). doi: 10.1016/j.foreco.2015.05.016
Bahn M, Janssens IA, Reichstein M, Smith P, Trumbore SE (2010) Soil respiration across scales: towards an integration of patterns and processes. New Phytol 186:292–296
Battaglia MA, Mou P, Palik BJ, Mitchell RJ (2002) The effect of spatially variable overstory on the understory light environment of an open-canopied longleaf pine forest. Can J For Res 32:1984–1991
Brockway DG, Lewis CE (1997) Long-term effects of dormant season prescribed fire on plant community diversity, structure and productivity in a longleaf pine wiregrass ecosystem. For Ecol Manag 96:167–183
Bryant ML, Bhat S, Jacobs JM (2005) Measurements and modeling of throughfall variability for five forest communities in the southeastern US. J Hydrol 312:95–108. doi:10.1016/j.jhydrol.2005.02.012
Chen D, Zhang Y, Lin Y, Chen H, Fu S (2009) Stand level estimation of root respiration for two subtropical plantations based on in situ measurement of specific root respiration. For Ecol Manag 257:2088–2097
Chen X, Post WM, Norby RJ, Classen AT (2011) Modeling soil respiration and variations in source components using a multi-factor global climate change experiment. Clim Chang 107(3–4):459–480
Clinton BD, Maier CA, Ford CR, Mitchell RJ (2011) Transient changes in transpiration, and stem and soil CO2 efflux in longleaf pine (Pinus palustris Mill.) following fire induced leaf area reduction. Trees 25:997–1007
Davidson EA, Janssens IA, Luo Y (2006) On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Glob Chang Biol 12:154–164
DeForest JL, Noormets A, McNulty SG, Sun G, Tenney G, Chen J (2006) Phenophases alter the soil respiration-temperature relationship in an oak dominated forest. Int J Biometeorol 1(2):135–144
Dore S, Fry DL, Stephens SL (2014) Spatial heterogeneity of soil CO2 efflux after harvest and prescribed fire in a California mixed conifer forest. For Ecol Manag 319:150–160
Fang C, Moncrieff JB, Gholz HL, Clark KL (1998) Soil CO2 and its spatial variation in a Florida slash pine plantation. Plant Soil 205:135–146
Farrar J, Hawes M, Jones D, Lindow S (2003) How roots control the flux of carbon to the rhizosphere. Ecology 84:827–837
Ferré C, Castrignanò A, Comolli R (2015) Assessment of multi-scale soil-plant interactions in a poplar plantation using geostatistical data fusion techniques: relationships to soil respiration. Plant Soil 390:95–109. doi:10.1007/s11104-014-2368-2
Fóti S, Balogh J, Nagy Z, Herbst M, Pintér K, Péli E, Koncz P, Bartha S (2014) Soil moisture induced changes on fine-scale spatial pattern of soil respiration in a semi-arid sandy grassland. Geoderma 213:245–254
Fox GA, Negrete-Yankelevich S, Sosa VJ (eds) (2015) Ecological statistics: contemporary theory and application, first edn. Oxford University Press, Oxford
Geary RC (1954) The contiguity ratio and statistical mapping. Inc Stat 5:115–146
Geng Y, Wang Y, Yang K et al (2012) Soil respiration in Tibetan alpine grasslands: belowground biomass and soil moisture, but not soil temperature, best explain the large-scale patterns. PLoS ONE 7(4):1–12
Herbst M, Bornemann L, Graf A, Welp G, Vereecken H, Amelung W (2012) A geostatistical approach to the field-scale pattern of heterotrophic soil CO2 emission using covariates. Biogeochemistry 111:377–392. doi:10.1007/s10533-011-9661-4
Heyward F (1933) The root system of longleaf pine on the deep sands of Western Florida. Ecology 14(2):136–148
Hodgkins EJ, Nichols NG (1977) Extent of main lateral roots in natural longleaf pine as related to position and age of the trees. For Sci 23(2):161–166
Hojjati SM, Lamersdorf NP (2010) Effect of canopy composition on soil CO2 emission in a mixed spruce-beech forest at Solling, Central Germany. J For Res 21(4):461–464. doi:10.1007/s11676-010-0098-8
Hue NV, Evans CE (1979) Procedures used by the Auburn University Soil Testing Laboratory. http://aurora.auburn.edu/handle/11200/1413. Accessed 27 November 2015
Irvine J, Law BE (2002) Contrasting soil respiration in young and old-growth ponderosa pine forests. Glob Chang Biol 8:1183–1194
Jose SJ, Jokela E, Miller DL (eds) (2006) The longleaf pine ecosystem: ecology, silviculture and restoration. Springer, New York
La Scala N Jr, Marques J Jr, Pereira GT, Cora JE (2000) Short-term temporal changes in the spatial variability model of CO2 emissions from a Brazilian bare soil. Soil Biol Biochem 32:1459–1462
Lashley MA, Chitwood MC, Prince A et al (2014) Subtle effects of a managed fire regime: a case study in the longleaf pine ecosystem. Ecol Indic 38:212–217. doi:10.1016/j.ecolind.2013.11.006
Lavigne MB, Boutin R, Foster RJ, Goodine G, Bernier P, Robitaille G (2003) Soil respiration responses to temperature are controlled more by roots than by decomposition in balsam fir ecosystems. Can J For Res 33:1744–1753
Lavoie M, Mack MC, Hiers JK, Pokswinski S (2012) The effect of restoration treatments on the spatial variability of soil processes under longleaf pine trees. Forests 3:591–604
Law BE, Arkebauer T, Campbell JL et al (2008) Terrestrial carbon observations: protocols for vegetation sampling and data submission. Available from http://www.fao.org/gtos, Global Terrestrial Observing System, Rome, Italy
Luan J, Liu S, Zhu X, Wang J, Liu K (2012) Roles of biotic and abiotic variables in determining spatial variation of soil respiration in secondary oak and planted pine forests. Soil Biol Biochem 44:143–150
Maier CA, Kress LW (2000) Soil CO2 evolution and root respiration in 11 year-old loblolly pine (Pinus taeda) plantations as affected by moisture and nutrient availability. Can J For Res 30:347–359
Mäkiranta P, Minkkinen K, Hytönen J, Laine J (2008) Factors causing temporal and spatial variation in heterotrophic and rhizospheric components of soil respiration in afforested organic soil croplands in Finland. Soil Biol Biochem 40:1592–1600. doi:10.1016/j.soilbio.2008.01.009
Martin JG, Bolstad PV (2009) Variation of soil respiration at three scales: components within measurements, intra-site variation, and patterns on the landscape. Soil Biol Biochem 41:530–543
Martin JG, Phillips CL, Schmidt A, Irvine J, Law BE (2012) High-frequency analysis of the complex linkages between soil CO2 fluxes, photosynthesis and environmental variables. Tree Physiol 32:49–64
McGuire JP, Mitchell RJ, Moser EB, Pecot SD, Gjerstad DH, Hedman CW (2001) Gaps in a gappy forest: plant resources, longleaf pine regeneration, and understory response to tree removal in longleaf pine savannas. Can J For Res 31:765–778
Merbold L, Ziegler W, Mukelabai MM, Kutsch WL (2011) Spatial and temporal variation of CO2 efflux along a disturbance gradient in a miombo woodland in Western Zambia. Biogeosciences 8:147–164. doi:10.5194/bg-8-147-2011
Metcalfe DB, Fisher RA, Wardle DA (2011) Plant communities as drivers of soil respiration: pathways, mechanisms, and significance for global change. Biogeosciences 8:2047–2061
Mitchell RJ, Hiers JK, O’Brien JJ, Jack SB, Engstrom RT (2006) Silviculture that sustains: the nexus between silviculture, frequent prescribed fire, and conservation of biodiversity in longleaf pine forests of the southeastern United States. Can J For Res 36:2714–2736
Mitra B, Mackay DS, Pendall E, Ewers BE, Clearly MB (2014) Does vegetation structure regulate the spatial structure of soil respiration within a sagebrush steppe ecosystem? J Arid Environ 103:1–10
Moran PAP (1950) Notes on continuous stochastic phenomena. Biometrika 37:17–23
Moyano FE, Manzoni S, Chenu C (2013) Responses of soil heterotrophic respiration to moisture availability: an exploration of processes and models. Soil Biol Biochem 59:72–85. doi:10.1016/j.soilbio.2013.01.002
National Climatic Data Center (2015a) 1981–2012 normals. Columbus Metropolitan Airport, GA. Station GHCND:USW00093842. http://www.ncdc.noaa.gov/cdo-web/datatools/normals. Accessed 15 January 2015
National Climatic Data Center (2015b) Daily summaries. Columbus Metropolitan Airport, GA. Station GHCND:USW00093842. http://www.ncdc.noaa.gov/cdo-web/datatools/findstation. Accessed 12 February 2015
Noss RF (1988) The longleaf pine landscape of the southeast: almost gone and almost forgotten. Endanger Species Updat 5(5):1–8
Odom J, Kone M (1997) Elemental analysis procedures used by the Auburn University Department of Agronomy & Soils. Department Series Publication 203, Auburn University Department of Agronomy and Soils
Ohashi M, Kumagai T, Kume T et al (2008) Characteristics of soil CO2 efflux variability in an aseasonal tropical rainforest in Borneo Island. Biogeochemistry 90:275–289
Oram B, Nelson R (2014) Soil texture triangle: hydrolic properties calculator. Sourced from Saxton et al. (1986), Soil Sci Soc Amer J 50(4):1031–1036. http://hydrolab.arsusda.gov/soilwater/Index.htm. Accessed 21 November 2014
Prolingheuer N, Scharnagl B, Graf A, Vereecken H, Herbst M (2014) On the spatial variation of soil rhizospheric and heterotrophic respiration in a winter wheat stand. Agric For Meteorol 195–196:24–31
Raich JW, Nadelhoffer KJ (1989) Belowground carbon allocation in forest ecosystems: global trends. Ecology 70(5):1346–1354
Raich JW, Schlesinger WH (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 44B:81–99
Rayment MB, Jarvix PG (2000) Temporal and spatial variation of soil CO2 efflux in a Canadian boreal forest. Soil Biol Biochem 32:35–45
Reinke JJ, Adriano DC, McLeod KW (1981) Effects of litter alteration on carbon dioxide evolution from a South Carolina pine forest floor. Soil Sci Soc Am J 45:620–623
Rodeghiero M, Cescatti A (2005) Main determinants of forest soil respiration along an elevation/temperature gradient in the Italian Alps. Glob Chang Biol 11:1024–1041
Rodeghiero M, Cescatti A (2008) Spatial variability and optimal sampling strategy of soil respiration. For Ecol Manag 255:106–112
Ruehr NK, Buchmann N (2010) Soil respiration fluxes in a temperate mixed forest: seasonality and temperature sensitivities differ among microbial and root-rhizosphere respiration. Tree Physiol 30(2):165–176
Saiz G, Green C, Butterbach-Bahl K, Kiese R, Avitabile V, Farrell EP (2006) Seasonal and spatial variability of soil respiration in four Sitka spruce stands. Plant Soil 287:161–176
Šálek L, Zahradník D (2008) Wedge prism as a tool for diameter and distance measurement. J For Sci 54(3):121–124
Samuelson LJ, Whitaker WB (2012) Relationships between soil CO2 efflux and forest structure in 50-year-old longleaf pine. For Sci 58(5):472–484
Samuelson LJ, Stokes TA, Butnor JR et al (2014) Ecosystem carbon stocks in Pinus palustris forests. Can J For Res 44(5):476–486
Shibistova O, Lloyd J, Evgrafova S et al (2002) Seasonal and spatial variability in soil CO2 efflux rates for a central Siberian Pinus sylvestris forest. Tellus 54B:552–567
Søe ARB, Buchmann N (2005) Spatial and temporal variations in soil respiration in relation to stand structure and soil parameters in an unmanaged beech forest. Tree Physiol 25(11):1427–1436
Soil Survey Staff (2014) Web soil survey. http://websoilsurvey.nrcs.usda.gov. Downloaded January 2012
Stoyan H, De-Polli H, Böhm S, Robertson GP, Paul EA (2000) Spatial heterogeneity of soil respiration and related properties at the plant scale. Plant Soil 222:203–214
Suchewaboripont V, Ando M, Iimura Y, Yoshitake S, Ohtsuka T (2015) The effect of canopy structure on soil respiration in an old-growth beech-oak forest in central Japan. Ecol Res 1–11
Taneva L, Gonzalez-Meler MA (2011) Distinct patterns in the diurnal and seasonal variability in four components of soil respiration in a temperate forest under free-air CO2 enrichment. Biogeosciences 8:3077–3092
Tjoelker MG, Craine JM, Wedin D, Reich PD, Tilman D (2005) Linking leaf and root trait syndromes among 39 grassland and savanna species. New Phytol 167:493–508
Tromp-van Meerveld HJ, McDonnell JJ (2006) On the interrelations between topography, soil depth, soil moisture, transpiration rates and species distribution at the hillslope scale. Adv Water Resour 29:293–310. doi:10.1016/j.advwatres.2005.02.016
Vande Walle I, Samson R, Looman B, Verheyen K, Lemeur R (2007) Temporal variation and high-resolution spatial heterogeneity in soil CO2 efflux in a short-rotation pine plantation. Tree Physiol 27(6):837–848
Verlinden MS, Broeckx LS, Wei H, Ceulemans R (2013) Soil CO2 efflux in a bioenergy plantation with fast-growing Populus trees – influence of former land use, inter-row spacing and genotype. Plant Soil 369:631–644
Vetaas OR (1992) Micro-site effects of trees and shrubs in dry savannas. J Veg Sci 3:337–344
Weber P, Bardgett RD (2011) Influence of single trees on spatial and temporal patterns of belowground properties in native pine forest. Soil Biol Biochem 42:1372–1378
Wei H, Xiao G, Guenet B, Janssens IA, Shen W (2015) Soil microbial community composition does not predominately determine the variance of heterotrophic soil respiration across four subtropical forests. Sci Rep 5:7854. doi:10.1038/srep07854
Wheeler D, Tiefelsdorf M (2005) Multicollinearity and correlation among local regression coefficients in geographically weighted regression. J Geogr Sci 7:161–187. doi:10.1007/s10109-005-0155-6
Wiseman PE, Seiler JR (2004) Soil CO2 efflux across four age classes of plantation loblolly pine (Pinus taeda L.) on the Virginia Piedmont. For Ecol Manag 192:297–311
Wu T, Li Y (2013) Spatial interpolation of temperature in the United States using residual kriging. Appl Geogr 44:112–120
Zinke PJ (1962) The pattern of influence of individual forest trees on soil properties. Ecology 43(1):130–133
Acknowledgments
This research was supported in part by the United States Department of Defense, through the Strategic Environmental Research and Development (SERDP), and by the School of Forestry and Wildlife Sciences, Auburn University, Auburn, Alabama. This work was also supported by the USDA National Institute of Food and Agriculture, Award #2011-68002-30185. James Parker and Brian Waldrep from Fort Benning, Land Management Division provided invaluable support and site access. The authors would also like to thank the following people for field and laboratory assistance: Tom Stokes, Jake Blackstock, Justin Rathel, Ann Huyler, Lorenzo Ferrari, and Michael Gunter, and we also thank A. Christopher Oishi and two anonymous reviewers for their thorough review of earlier drafts of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Per Ambus.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1012 kb)
Rights and permissions
About this article
Cite this article
ArchMiller, A.A., Samuelson, L.J. & Li, Y. Spatial variability of soil respiration in a 64-year-old longleaf pine forest. Plant Soil 403, 419–435 (2016). https://doi.org/10.1007/s11104-016-2817-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2817-1