Abstract
Background and aims
Understanding the interactions between endophytic bacteria, rhizobia, free living root associated bacteria and their host plants under stressed conditions remains a significant challenge for proposing strategies to improve the efficacy of PGPR. In this study we analyzed the role of the endophytic bacterium Stenotrophomonas rhizophila in alleviating salinity stress in plants. The nodulation efficiency, plant growth, nitrogen and phosphorus uptake of soybean under hydroponic salt stress conditions were determined.
Methods
Soybean seedlings were inoculated with Bradyrhizobium japonicum BDYD1 and S. rhizophila ep-17 were grown in hydroponic plastic pots containing 2 l of Hoagland solution for 42 days. Salinity conditions were established by adding 50 and 75 mM NaCl to the nutrient solution.
Results
The results showed that the salinity decreased the colonization of B. japonicum BDYD1 in the rhizosphere of soybean, inhibited shoot, root growth, and nodulation compared with those of unstressed plants. We found synergistic interactions between compatible salt tolerant S. rhizophila ep-17 and B. japonicum BDYD1 strains which were manifested themselves as improved root, shoot length, dry weight, N and P uptake and number of nodules compared with the uninoculated plants grown under 75 mM NaCl condition.
Conclusions
S. rhizophila and Bradyrhizobium build beneficial association in the rhizosphere and can act synergistically on promoting plant growth, nutrient uptake and fitness of hydroponically grown soybean under salt stress condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic stresses such as drought and salinity are one of the main consequences of climate change and remain as a big challenge while addressing the problem of food insecurity, hunger and malnutrition (Egamberdiyeva et al. 2007; Parvaiz and Satyawati 2008). It is estimated that 33 % of the potentially arable land area of the world are affected by salinity (UNEP 2008). Major factors increasing salinity include irrigation of cultivated lands with saline water, poor cultural practices and low precipitation, which results in a yield loss of various crop plants (Egamberdieva et al. 2010a, 2014; Ondrasek et al. 2009). Most legumes are sensitive to salinity (Bruning and Rozema 2013), where significant reductions in germination, root and shoot growth and nodulation were reported for faba bean (Vicia faba L.) (Rabie and Almadini 2005), goat’s rue (Galega officinalis Linn.) (Egamberdieva et al. 2013), lentil (Lens culinaris Medik.) (Golezani and Yengabad 2012), chickpea (Cicer arietinum L.) (Egamberdieva et al. 2014), and common bean (Phaseolus vulgaris L.) (Kaymakanova 2009).
Soybean (Glycine max) is an important legume, source of flour, protein, edible oil, animal feed, fatty acids, soaps and biodiesel in many countries in the world (Dwevediand Kayastha 2011) with an annual production of 276.4 Mio t world-wide (FAOSTAT 2013). Soybean is a sensitive plant to salinity stress, and reduced plant growth, number of pods, and yield was observed under salt stress (Essa 2002; Hamayun et al. 2010). Salinity inhibits root growth and the number of hairs, which is necessary for nodule formation, and also reduces colonization of rhizobia in the root (Hashem et al. 1998). Garg and Chandel (2011) reported that nodulation of chickpea was reduced with increasing salt concentrations that resulted in reduction N and P levels in the leaves and roots. It has been also reported that salinity, particularly sodium chloride, negatively affects the composition and activities of rhizosphere bacteria, probably through its influence on the root exudates and by high osmotic strength and toxic effects by salts (Ofek et al. 2006; Nelson and Mele 2007). Root associated bacteria such as Pseudomonas, Bacillus, Stenotrophomonas, Alcaligenes, Enterobacter and Rhizobium that are tolerant to salt stress may colonize and proliferate in the rhizosphere of plants grown under saline conditions (Nabti et al. 2007; Shahzad et al. 2010; Berg et al. 2010, 2013; Egamberdieva et al. 2012; 2013). A synergistic interaction of those bacteria in the rhizosphere has been shown to improve plant growth and nutrition of common bean (Dardanelli et al. 2008), chickpea (Rokhzadi et al. 2008), faba bean (Rabie and Almadini 2005), and soybean (Argaw 2012) under salt stress condition. Stenotrophomonas species are endophytes and also free living bacteria that were reported as dominant member of the plant-associated bacterial community (Berg et al. 2010; Zhu et al. 2012; Naz and Bano 2012). They are capable of fixing nitrogen, promote plant growth, protect plants from various soil borne pathogens and confer resistance in plants to salt stress (Park et al. 2005; Egamberdieva et al. 2011; Berg et al. 2013). In response to plant exudates, S. rhizophila produces and excretes spermidine and glucosylglycerol; both substances are able to protect plant roots as well as the rhizosphere-associated microbiome against abiotic stress (Alavi et al. 2013). Although it was shown that Stenotrophomonas treatment induces a shift within the fungal community that eliminate minor pathogens (Schmidt et al. 2012), there are few reports on the mutualistic interactions of Stenotrophomonas with other PGPR strains and their effect on plant physiology. Several studies showed that strains of S. maltophilia as well as S. rhizophila are salt tolerant and actively colonize the rhizosphere of plants (Berg et al. 2010; Egamberdieva et al. 2011). Understanding the interactions between endophytic bacteria, rhizobia, free living root associated bacteria and their host plants under stressed conditions remains a significant challenge for proposing strategies to improve the efficacy of PGPR
This study addresses the question whether salt tolerant Stenotrophomonas rhizophila could play a role within the legume-rhizobium symbioses under salinity stress condition. We therefore analyzed nodulation efficiency, plant growth, nitrogen and phosphorus uptake of soybean under hydroponic salt stress conditions.
Material and methods
Microorganisms
The salt tolerant Stenotrophomonas rhizophila strain ep-17 (Wolf et al. 2002) was obtained from the culture collection of Graz University of Technology, Graz, Austria. The strains were isolated from the rhizosphere of oilseed rape growing in weakly loamy sand near Rostock (Germany). Bradyrhizobium japonicum strain BDYD1 was received from the culture collection of the Root Biology Centre, South China Agricultural University, Guangzhou, China. S. rhizophila strain ep-17 was grown on nutrient broth (NB, Oxoid) and B. japonicum strain BDYD1 on tryptone yeast extract agar (TY, Oxoid) (Beringer 1974) at 28 °C. Stock cultures were stored at −80 °C in 20 % glycerol. Before being used, the strains were grown overnight at 30 °C with 120 rpm in NB (S. rhizophila) and in TY broth (B. japonicum) for 24 h.
Germination of seeds
Seeds of soybean (cultivar YCo3-3) were received from the Root Biology Centre, South China Agricultural University, Guangzhou, China. The seeds were first sorted to eliminate broken, small seeds and then they were surface-sterilized in 10 % v/v NaOCl for 1 min and rinsed five times with sterile, distilled water. Surface-sterilized seeds were transferred on paper tissue towels soaked in 0.5 mM CaSO4 and germinated for 7 days in a dark room at 25 °C.
Colonization of introduced bacteria in the rhizosphere
The colonization of soybean roots by S. rhizophila ep-17 and B. japonicum BDYD1 were investigated under gnotobiotic conditions using test tubes (25 mm in diameter, 200 mm in length) as described by Simons et al. (1996). The tubes contained 60 g of a sterilized mixture of washed sand and vermiculite (1:1) soaked with 6 ml of diluted nitrogen-free Jensen nutrient solution (Vincent 1970). Salinity conditions were established by adding 50 and 75 mM NaCl into the Jensen nutrient solution. For the seed inoculation, B. japonicum BDYD1 was grown overnight in TY broth and S. rhizophila ep-17 in NB. One ml of each culture was pelleted by centrifugation (13 000 xg) and cell pellets were washed with 1 ml phosphate buffered saline (PBS) (20 mM sodium phosphate, 150 mM NaCl, pH 7.4) and re-suspended into PBS. The suspension used for the inoculation was adjusted to the final concentration of approximately 108 CFU mL1. The cell suspensions containing two strains were prepared by mixing them in a ratio 1:1 and vortexed vigorously to achieve a homogenous suspension. Uniform seedlings were first placed with sterile forceps into bacterial suspension for 15 min and were then transplanted into gnotobiotic tubes filled with sand and vermiculite.
The treatments were as follows: i) seeds inoculated with B. japonicum BDYD1 alone, ii) B. japonicum BDYD1 combined with kanamycin resistant derivative of S. rhizophila ep-17. The seedlings were grown for 18 days in a growth cabinet with a 16-h light period at 22 °C and an 8-h dark period at 16 °C. The seedlings were removed from the sand and 1 cm of root tip was cut from the plantlets and transferred into a tube containing 1 ml of PBS. Bacterial cells were removed from the root tip by vortexing root tip in PBS. The homogenates were serially diluted and appropriate dilutions, 10−3 and 10−4, were spread on two agar plates. Nutrient agar supplemented with kanamycin (50 μg/ml) was used to select for kanamycin resistant S. rhizophila ep-17 strain and B. japonicum enumerated on YEM agar supplemented with congo red. The number of bacterial cells colonized soybean root was calculated as CFU per 1 cm of root tip. The length of shoots and roots, the dry weight of whole plants and the number of nodules were also determined.
Plant growth under hydroponic conditions
Effects of bacterial inoculation treatments on the growth, nodulation and the content of nitrogen and phosphorus in whole plant tissues in salt-stressed soybean were studied under hydroponic conditions in a greenhouse. The modified, low-nitrogen containing (50 μM nitrogen) Hoagland plant nutrient solution was prepared according to Lynch et al. (1990). During the plant experiment, the pH was maintained between 5.8 and 6.0 with additions of KOH or HCl. Bacterial inoculants were prepared and the seeds were inoculated as described above. Inoculated seedlings were transplanted into hydroponic plastic pots containing 2 l of Hoagland solution. Salinity conditions were established by adding 50 and 75 mM NaCl to the nutrient solution. Two soybean seedlings were transplanted into each pot, but later one seedling was removed.
The inoculation treatments were as follows: i) uninoculated seedlings, ii) seedlings inoculated with B. japonicum BDYD1 alone, and iii) seedlings co-inoculated together with B. japonicum BDYD1 and S. rhizophila ep-17. Six replicate pots were used per treatment (N = 6). Plants were grown for 42 days in a greenhouse with an average temperature of 29/20 °C (day/night), and relative humidity of 48/83 % v/v (day/night). The average photosynthetically active radiation varied between 500 and 1000 μmol photons m−2 s−1 during the day.
At harvest, soybean shoots and roots were separated and the length of shoots and roots and the number of nodules were recorded. Shoots and roots were oven-dried to a constant weight at 75 °C for 48 h and weights were recorded. The number of nodules per plant root was counted with Leica MZF LIII stereomicroscope.
Determination of nitrogen and phosphorus content
For the determination of nitrogen and phosphorus content, oven-dried shoots and roots were powdered separately and then roots and shoots of each plant were combined and six replicate measurements were done for each treatment (N = 6). The powder was digested with 98 % H2SO4 and 30 % H2O2. The total nitrogen content in plant tissues was determined following the semi-micro Kjedahl procedure using a nitrogen analyzer (Kjedahl 2300; FOSS, Hoganas, Sweden). The phosphorus content of plant samples were determined spectrophotometrically using molybdenum blue method, developed by Murphy and Riley (1962).
Statistical analysis
Data were tested for statistical significance using the analysis of variance package included in Microsoft Excel 2007. Comparisons were done using Student’s t-test. Mean comparisons were conducted using a least significant difference (LSD) test (P = 0.05).
Results
Root colonization of introduced bacteria in the rhizosphere
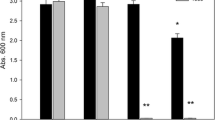
Gnotobiotic sand-vermiculite system was used to preliminary determine the colonization of B. japonicum BDYD1 and S. rhizophila ep-17 in the rhizosphere of soybean grown under non saline and saline conditions, and the response of plant to the bacterial inoculation. The results showed that the salinity decreased the colonization of B. japonicum BDYD1 in the rhizosphere of soybean (Fig. 1). The bacterial cell numbers (CFU) were decreased from 7.2 × 103 at 0 mM NaCl to 2.7 × 103 CFU cm−1 of root tip at 75 mM NaCl. The presence of S. rhizophila ep-17 in the rhizosphere of soybean enhanced colonization of root tips by B. japonicum BDYD1 under non saline and saline conditions, being 10.4 × 103 under non saline condition and 3.5 × 103 at 75 mM NaCl. In general the proportion of Stenotrophomonas cells exceeded that of Bradyrhizobium cells.
Colonization behavior of bacterial strains in the root tips of soybean seedlings grown under salt stress for 18 days in gnotobiotic sand-vermiculite system. Seedlings were inoculated either with Bradyrhizobium japonicum BDYD1 alone or combined with Stenotrophomonas rhizophila ep-17 in a ratio 1:1., a BDYD1 – (the number of CFU counts (cm−1 of root tip) of B. japonicum BDYD1, seedling inoculated with BDYD1 alone), b BDYD1 + (the number of CFU counts of B. japonicum BDYD1, seedlings co-inoculated together with B. japonicum BDYD1 and S. rhizophila e-p17), c ep-17 (the number of CFU counts of S. rhizophila ep-17, seedlings co-inoculated together with B. japonicum BDYD1 and S. rhizophila ep-17). Error bars show standard deviation
The number of CFU of S. rhizophila ep-17 was higher under non saline (11.5 × 103 CFU cm−1) and saline condition (75 mM NaCl) (5.1 × 103 CFU cm-1) compared to B. japonicum BDYD1 (Fig. 1).
The higher salt concentration (75 mM NaCl) impaired plant growth and nodulation of soybean inoculated with Bradyrhizobium strain alone compared with those of unstressed plants (Table 1). The co-inoculation of B. japonicum BDYD1 symbiont with the PGPR S. rhizophila ep-17 enhanced root, shoot length, dry weight and nodulation of both salt-stressed and unstressed soybean seedlings (Table 1). The root length was increased by 27 and 18 % at 50 and 75 mM NaCl, respectively, and the shoot length was increased on average by 32 % at 50 and 13 % at 75 mM NaCl compared with those of plants inoculated with B. japonicum BDYD1 alone. The combined inoculation also induced significantly more nodules on soybean roots. The co-inoculation of soybean with B. japonicum BDYD1 and S. rhizophila ep-17 resulted in twice more nodules at 75 mM NaCl than the inoculation with B. japonicum BDYD1 alone (Table 1).
Plant growth and nodulation
The response of soybean to the co-inoculation of B. japonicum BDYD1 with the salt tolerant S. rhizophila ep-17 and B. japonicum BDYD1 alone was further investigated in the greenhouse. Our study showed that after the soybeans were grown for 42 days in 0, 50 and 75 mM NaCl under hydroponic conditions, the root and shoot lengths as well the dry weights, nodule numbers and nutrient uptake responded to salt and inoculation treatments (Figs. 1, 2, 3, 4 and 5).
The root (a) and shoot (b) length of soybean when seedlings were inoculated with Bradyrhizobium japonicum strain BDYD1 alone and together with Stenotrophomonas rhizophila strain ep-17. Plants were grown hydroponically in a greenhouse for 42 days under two NaCl concentrations (50 and 75 mM NaCl) in the plant nutrient solution. Columns represent means for six plants (N = 6) with error bars showing standard deviation. Columns marked with an asterisk differed significantly from uninoculated plants at P < 0.05
The root (a) and shoot (b) dry weight of soybean when seedlings were inoculated with Bradyrhizobium japonicum strain BDYD1 alone and together with Stenotrophomonas rhizophila strain ep-17. Plants were grown hydroponically in a greenhouse for 42 days under two NaCl concentrations (50 and 75 mM NaCl) in the plant nutrient solution. Columns represent means for six plants (N = 6) with error bars showing standard deviation. Columns marked with an asterisk differed significantly from uninoculated plants at P < 0.05
The nodule number of soybean when seedlings were inoculated with Bradyrhizobium japonicum strain BDYD1 alone and together with Stenotrophomonas rhizophila strain ep-17. Plants were grown hydroponically in a greenhouse for 42 days under two NaCl concentrations (50 and 75 nM NaCl) in the plant nutrient solution. Non-inoculated controls were devoid of nodules. Columns represent means for six plants (N = 6) with error bars showing standard deviation. Columns marked with an asterisk differed significantly from single inoculated plants with B. japonicum BDYD1 at P < 0.05
The nitrogen (a) and phosphorus (b) content of soybean when seedlings were inoculated with Bradyrhizobium japonicum strain BDYD1 alone and together with Stenotrophomonas rhizophila strain ep-17. Plants were grown hydroponically in a greenhouse for 42 days under two NaCl concentrations (50 and 75 nM NaCl) in the plant nutrient solution. Columns represent means for six plants (N = 6) with error bars showing standard deviation. Columns marked with an asterisk differed significantly from uninoculated plants at P < 0.05
The inoculation of soybean with B. japonicum BDYD1 improved plant growth compared with that of uninoculated plants under non saline conditions. Shoot, root length (Fig. 2a and b), and shoot dry weight (Fig. 3b) slightly increased, and the dry weigh of root increased up to 29 % (Fig. 3a). Co-inoculation of B. japonicum BDYD1 with S. rhizophila ep-17 was increased shoot and root length, on average by 13 % compared with the single-strain inoculation. Shoot and root weights were increased slightly, but there was no significant difference compared to plants inoculated with B. japonicum BDYD1 alone. Only the root weight of co- inoculated soybean were statistically significantly increased (35 %) compared with those of uninoculated plants (Fig. 3a and b). The co-inoculation of B. japonicum BDYD1 with S. rhizophila ep-17 significantly improved (by twofold) the nodulation of unstressed soybean (Fig. 4).
The root and shoot dry weight of uninoculated soybean was decreased by 55 % at 50 mM NaCl but this decrease was 75 and 72 % at 75 mM NaCl respectively. The salt concentration of 75 mM NaCl also impaired shoot and root length by 10 and 75 % compared with those of unstressed plants respectively (Fig. 2a and b). The nodulation of soybean was more adversely affected by salinity than root and shoot growth.
Higher salt concentration inhibited plant growth promoting abilities of B. japonicum BDYD1, whereas under both saline conditions (50 and 75 mM NaCl), single inoculated soybean plants with B. japonicum BDYD1 grow almost similarly as untreated control plants. Nodulation of single-inoculated salt-stressed soybeans were inhibited compared with that of non-stressed plants (by 78 % in 50 mM NaCl), and did not nodulate at all at 75 mM NaCl condition (Fig. 4). The salt tolerant PGPR S. rhizophila ep-17 strain could improve the symbiotic interaction between soybean and B. japonicum BDYD1 under salt stress conditions.
The root dry weight was increased significantly by 22 and 54 % at 50 and 75 mM NaCl, whereas shoot dry weight was increased by 31 and 15 % at 50 and 75 mM NaCl, respectively (Fig. 3a and b). The co-inoculation of S. rhizophila ep-17 with B. japonicum BDYD1 induced significantly more nodules on soybean roots at 50 mM NaCl than single-inoculated ones, especially at 75 mM NaCl, whereas nodulation of plants inoculated with the symbiont B. japonicum BDYD1 was totally inhibited (Fig. 4).
Plant nitrogen (N) and phosphorus (P) uptake
The effect of bacterial inoculation on nitrogen (N) and phosphorus (P) uptake of soybean was determined under non saline and saline conditions. In general, the nitrogen content in soybean tended to increase in response to bacterial and salt treatments. Under non-stressed conditions, single inoculation increased N content in plant by 7 %, and the co-inoculation by 87 % compared with that of uninoculated plants (Fig. 5a).
Salt-affected uninoculated plants contained on average 24 % more nitrogen than unstressed uninoculated ones. When soybean was inoculated either with B. japonicum BDYD1 alone, or combined with S. rhizophila ep-17 the nitrogen content was increased in salt-affected plants (by 64 and 42 %) in those grown under 50 mM NaCl and (by 70 and 40 %) 75 mM NaCl plant solutions, compared unstressed plants. After single-strain inoculation of soybean with B. japonicum BDYD1 grown in 50 and 75 mM NaCl plant solution contained on average 43 % more nitrogen than uninoculated plants (Fig. 5a).
When the symbiont B. japonicum BDYD1 was co-inoculated with S. rhizophila ep-17, the nitrogen content of salt-affected soybean grown in 50 mM NaCl solution was significantly increased on average by 52 %, and in 75 mM NaCl by 45 % compared that plants inoculated with B. japonicum BDYD1 alone (Fig. 5a).
The phosphorus content in soybean tended to decrease in response to salt treatments. Among uninoculated soybean and inoculated with B. japonicum BDYD1 alone, phosphorus content decreased at the same level on average by 36 % for plants grown in 75 mM NaCl plant solution (Fig. 5b).
The P content was increased significantly by 13 and 39 % when unstressed soybean was inoculated either with B. japonicum BDYD1 alone or combined with the S. rhizophila ep-17, respectively (Fig. 5b). Soybean contained 18 % more P than plants were inoculated with B. japonicum BDYD1 alone grown in 75 mM NaCl solution, compared that uninoculated plants. The increase in plant P content was significant for soybean grown in 50 mM NaCl solution and co-inoculated with B. japonicum BDYD1 and S. rhizophila ep-17 by 48 %, compared that plants inoculated with B. japonicum BDYD1 alone.
Discussion
Here we report a symbiotic tripartite interaction of rhizobia, the endophytic Stenotrophomonas and the host plant soybean under salt stress condition. We observed significant growth benefit of the synergistic association of soybean with rhizobia and Stenotrophomonas in hydroponic culture under salt stress. Treatments by biological inoculants, which were shown here, offer a high potential for agriculture under abiotic stress conditions and can compensate problems due to climate change. Although there are several reports in which a positive effect of dual inoculation with different Rhizobium strains and PGPR in legume growth has been found e.g., rhizobia with Pseudomonas on chickpea (Khurana and Sharma 2000; Siddiqui et al. 2001; Qureshi et al. 2009; Shahzad et al. 2010), soybean and alfalfa (Rosas et al. 2006), and fodder galega (Egamberdieva et al. 2010b), less is known about combination with endophytes as well as about the underlying mechanisms. We know that the stress protection agent (SPA) S. rhizophila produces and excretes spermidine and glucosylglycerol (Alavi et al. 2013). Spermidine is a well-known plant growth regulator and has been revealed to play a critical role in plant embryo development (Imai et al. 2004). Spermidine was found to prolong the life span of several eukaryotic model organisms including yeasts, nematodes, flies, and plants as well as significantly reduce age-related oxidative protein damage in mice which could indicate a potential universal anti-aging drug for eukaryotes. In addition, spermidine has been recently shown to strongly promote the growth of Eruca sativa plants (Al-Whaibi et al. 2012). Glucosylglycerol (GG) is a well-known and highly efficient osmoprotectant (Roder et al. 2005). In the type strain of S. rhizophila DSM14405T, ggpS and ycaD are both strongly up-regulated under 3 % salt and are essential for the gene synthesis and transport of GG (Alavi et al. 2013); a finding which corresponds with both the general role of GG as a cell protector and previous findings that the amount of GG excreted into the medium increases substantially in comparison with intracellular GG content resulting from a shift of lower (less than 2 %) to higher salt concentrations (Roder et al. 2005). Colonization of plant root system by microbes is the very first step in nearly all interactions and it is affected by abiotic factors. For example, salinity leads to a failure in the establishment of rhizobia in the rhizosphere of legumes which result decreased nodulation (Hashem et al. 1998; Zahran 1999). The lower colonization rates of roots by rhizobia under salt stress agree with our previous studies in goat’s rue that show the decreased number of nodules under saline condition (Egamberdieva et al. 2013). We have observed that salinity decreased the colonization of B. japonicum in the rhizosphere of soybean and the presence of S. rhizophila enhanced colonization of root tips by rhizobia.
Co-inoculation of B. japonicum and S. rhizophila recorded significantly higher nodule number, root and shoot dry weight than B. japonicum alone. This result indicates that S. rhizophila generally does not interfere with the ability of B. japonicum to form nodules in soybean roots, it may enhance nodulation and plant growth. The increase in nodulation might be due to synergistic effect of the two types of microbes such as symbiotic and endophytic for biological nitrogen fixation. In previous studies Stenotrophomonas isolates were reported as free-living nitrogen-fixing bacteria, which promote plant growth under N-limiting conditions (Park et al. 2005).
Increased N contents have been observed in soybean grown under salt stress condition. According Hiz et al. (2014) when the growth of plant inhibited by salt stress, the consumption of nitrogen decreases and nitrogen starts to accumulate inside plant tissues. The higher N content was observed in pigeon peas (Cajanus cajan) (Subbarao et al. 1990) and mung bean (Phaseolus aureus) (Imamul Huq and Larher 1983). The combined inoculation of B. japonicum and S. rhizophila resulted besides long roots and good nodulation, a high N, and P content in soybean in both non saline and saline conditions. An explanation for the improved nutrient uptake could be that S. rhizophila facilitated to absorb more nutrients through increased root system, - and formation of nitrogen-fixing nodules.
Previous works indicated that nodule biomass is strongly correlated to P availability in plant, and when plants grow under limited P concentration, the nodulation and its size were reduced (Gunawardena et al. 1992; Hellsten and Huss Danell 2001).
According Spaepen et al. (2007) auxin levels in the host legume plants are necessary for nodule formation. The higher nodulation of soybean inoculated with Stenotrophomonas than with the Bradyrhizobium alone is most likely due to improved root system of the plants by IAA producing Stenotrophomonas. Strain S. rhizophila is able to produce the phytohormone indole-3-acetic acid (Suckstorff and Berg 2003), and salinity (up to 4 % NaCl) did not inhibit its auxin production (Egamberdieva et al. 2011). Microbial phytohormones increase hormone level in plants and partially modify the plant cell metabolism (Bano et al. 2010). Moreover, bacterial IAA increases root surface area, which increase nutrient absorbing surfaces, and thereby facilitate greater absorption of water and essential nutrients from the soil through which plant growth will increase significantly (Haas and Défago 2005; Berg et al. 2010; Egamberdieva 2009, 2012). Bianco and Defez (2009) reported that IAA enhancing different cellular defence systems for protection plants from external adverse conditions. It has been observed that co-inoculation of Bradyrhizobium with PGPR strains possessing ACC-deaminase activity enhanced the nodulation in mung bean compared with inoculation with Bradyrhizobium alone (Shaharoona et al. 2006). Similar results were observed by Ma et al. (2003), where ACC-deaminase producing R. leguminosarum could lower ethylene production in pea roots and improved nodulation. S. rhizophila strain ep-17 is able to utilize ACC as N source indicating the presence of ACC deaminase and increased salt tolerance of soybean, stimulating shoot, and root growth under saline condition. The exopolysaccharides protect the plant from desiccation, through formation of protective layer around soil aggregates (Tisdall and Oades 1982). According to Wolf et al. (2002) S. rhizophila was shown to produce glucosylglycerol (GG) in addition to trehalose, which contributes to survival under changing osmotic conditions. EPS-producing rhizobacteria can bind cations including Na+, thereby alleviate salt stress in plants grown under saline conditions (Upadhyay et al. 2011).
The combined inoculation of B. japonicum with S. rhizophila resulted in the best root growth, nodulation and N uptake under saline compared to non saline condition. This would agree with previous studies showing highest microbial benefits to plant growth under salt stress condition, compared adequate environment (Egamberdieva and Kucharova 2009). For example, Estevez et al. (2009) observed that co-inoculation of Rhizobium tropici CIAT899 with Chryseobacterium balustinum Aur9 improved growth and symbiotic performance of salt-stressed soybeans and beans compared with the single inoculation (CIAT899). Similar observation reported by Molla et al. (2001), where the total root length, root number, dry matter, root hair development, number of nodules, and shoot dry matter of soybean were significantly increased by Azospirillum lipoferum and Bradyrhizobium japonicum. In our previous work the greenhouse experiment with potting soil demonstrated that the salt tolerance of goat’s rue was clearly improved when the plant was inoculated with Rhizobium galegae sv. officinalis and Pseudomonas trivialis (Egamberdieva et al. 2013). In other study co-inoculation of common bean with Rhizobium and Azospirillum strains improved rhizobia legume symbioses under saline conditions through reduction of the negative effects of salt stress (Dardanelli et al. 2008).
One of the most important issues is a risk assessment for potential biocontrol and stress protection agents. B. japonicum is a symbiotic plant bacterium and applied for a long time as bacterial fertilizer without any risk. In contrast to potentially pathogenic S. maltophilia, strains of S. rhizophila are considered as promising candidates for stress protection on plants without risk for human health (rev. in Berg and Martinez 2015).
Conclusion
In summary, S. rhizophila and Bradyrhizobium build beneficial association in the rhizosphere and can act synergistically on promoting plant growth, nutrient uptake and fitness of hydroponically grown soybean under salt stress condition. The successful tripartite bacterial-legume symbioses under saline condition can be an effective approach for enhancement of plant’s tolerance to adverse environmental stresses.
References
Alavi P, Starcher MR, Zachow C, Müller H, Berg G (2013) Root-microbe systems: the effect and mode of interaction of stress protecting agent (SPA) Stenotrophomonas rhizophila DSM14405T. Front Plant Sci 4:141
Al-Whaibi MH, Siddiqui MH, Al-Munqadhi BMA, Sakran AM, Ali HM, Basalah MO (2012) Influence of plant growth regulators on growth performance and photosynthetic pigments status of Eruca sativa Mill. J Med Plants Res 6:1948–1954
Argaw A (2012) Evaluation of co-inoculation of Bradyrhizobium japonicum and phosphate solubilizing Pseudomonas spp. effect on soybean (Glycine max L. (Merr.)) in Assossa Area. J Agric Sci Tech 14:213–224
Bano A, Yasmeen S (2010) Role of phytohormones under induced drought stress in wheat. Pak J Bot 42(4):2579–2587
Berg G, Martinez JL (2015) Friends or foes: can we make a distinction between beneficial and harmful strains of the Stenotrophomonas maltophilia complex? Front Microbiol 6:241
Berg G, Egamberdieva D, Lugtenberg B, Hagemann M (2010) Symbiotic plant-microbe interactions: stress protection, plant growth promotion and biocontrol by Stenotrophomonas. In: Seckbach J, Grube M (eds) Symbioses and stress, cellular origin, life in extreme habitats and astrobiology, Springer-Verlag 17(4):445–460
Berg G, Alavi M, Schmidt CS, Zachow C, Egamberdieva D, Kamilova F, Lugtenberg B (2013) Biocontrol and osmoprotection for plants under saline conditions. In: Frans J. de Bruijn (ed), Molecular microbial ecology of the rhizosphere, Wiley -Blackwell, USA
Beringer JB (1974) R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188–198
Bianco C, Defez R (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60:3097–3107
Bruning B, Rozema J (2013) Symbiotic nitrogen fixation in legumes: perspectives for saline agriculture. Environ Exp Bot 92:134–143
Dardanelli MS, De Cordoba FJF, Espuny MR, Carvajal MAR, Diaz MES, Serrano AMG, Okon Y, Megias M (2008) Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol Biochem 40:2713–2721
Dwevedi A, Kayastha AM (2011) Soybean: a multifaceted legume with enormous economic capabilities, soybean - biochemistry, chemistry and physiology, Tzi-Bun Ng (Ed.) In Tech
Egamberdieva D (2009) Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Phys Plant 31:861–864
Egamberdieva D (2012) Pseudomonas chlororaphis: a salt tolerant bacterial inoculant for plant growth stimulation under saline soil conditions. Acta Phys Plant 34:751–756
Egamberdieva D, Kucharova Z (2009) Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol Fertil Soils 45:561–573
Egamberdieva D, Berg G, Lindstrom K, Rasanen L (2010a) Root colonizing Pseudomonas spp. improve growth and symbiosis performance of fodder galega (Galega orientalis LAM) grown in potting soil. Eur J Soil Biol 46(3–4):269–272
Egamberdieva D, Renella G, Wirth S, Islam R (2010b) Secondary salinity effects on soil microbial biomass. Biol Fertil Soils 46(5):445–449
Egamberdieva D, Kucharova Z, Davranov K, Berg G, Makarova N, Azarova T, Chebotar V, Tikhonovich I, Kamilova F, Validov S, Lugtenberg B (2011) Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol Fertil Soils 47:197–205
Egamberdieva D, Berg G, Lindström K, Räsänen LA (2013) Alleviation of salt stress of symbiotic Galega officinalis L. (goat's rue) by co-inoculation of Rhizobium with root colonizing Pseudomonas. Plant Soil 369(1):453–465
Egamberdieva D, Shurigin V, Gopalakrishnan S, Sharma R (2014) Growth and symbiotic performance of chickpea (Cicer arietinum) cultivars under saline soil conditions. J Biol Chem Res 31(1):333–341
Egamberdiyeva D, Gafurova L, Islam KR (2007) Salinity effects on irrigated soil chemical and biological properties in the syrdarya basin of Uzbekistan. In: Lal R, Sulaimanov M, Stewart B, Hansen D, Doraiswamy P (eds) Climate change and terrestrial c sequestration in Central Asia. Taylor-Francis, New York, pp 147–162
Essa TA (2002) Effect of salinity on growth and nutrient composition of three soybean (Glycine max L.) cultivars. J Agric Crop Sci 188:86–93
Estévez J, Dardanelli MS, Megias M, Rodríguez-Navarro DN (2009) Symbiotic performance of common bean and soybean co inoculated with rhizobia and Chryseobacterium balustinum Aur9 under moderate saline conditions. Symbiosis 49(1):29–36
FAOSTAT (2013) FAOSTAT database, Food and Agriculture Organization of the United Nations. http://faostat.fao.org/
Garg N, Chandel S (2011) Effect of mycorrhizal inoculation on growth, nitrogen fixation and nutrient uptake in Cicer arietinum L. under salt stress. Turk J Agric For 35:205–214
Golezani KG, Yengabad FM (2012) Physiological responses of lentil (Lens culinaris Medik.) to salinity. Int J Agric Crop Sci 4(20):1531–1535
Gunawardena SFBN, Danso SKA, Zapata F (1992) Phosphorus requirement and nitrogen accumulation by three mung bean (Vigna radiata (L.) Welzek) cultivars. Plant Soil 147:267–274
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Hamayun M, Khan SA, Shinwari ZK, Khan AK, Ahmad N, Lee IJ (2010) Effect of polyethylene glycol induced drought stress on physio-hormonal attributes of soybean. Pak J Bot 42:977–986
Hashem FM, Swelim DM, Kuykendall LD, Mohamed AI, Abdel-Wahab SM, Hegazi NI (1998) Identification and characterization of salt and thermo-tolerant Leucaena nodulating Rhizobium strains. Biol Fertil Soil 27:335–341
Hellsten A, Huss-Danell K (2001) Interaction effects of nitrogen and phosphorus on nodulation in red clover (Trifolium pretense L.). Acta Agric Scand Sect B Soil Plant Sci 50:135–142
Hiz MC, Canher B, Niron H, Turet M (2014) Transcriptome analysis of salt tolerant common bean (Phaseolus vulgaris L.) under saline conditions. PLoS One 9(3), e92598
Imai A, Matsuyama T, Hanzawa Y, Akiyama T, Tamaoki M, Saji H (2004) Spermidine synthase genes are essential for survival of Arabidopsis. Plant Physiol 135:1565–1573
Imamul Huq SM, Larher F (1983) Osmoregulation in higher plants. Effect of NaCl salinity on non-nodulated Phaseolus aureus L. II. Changes in orgnaic solutes. New Phytol 93:209–216
Kaymakanova M (2009) Effect of salinity on germination and seed physiology in bean (Phaseolus vulgaris L.). Biotechnol Equip 23:326–329
Khurana AS, Sharma P (2000) Effect of dual inoculation of phosphate solubilizing bacteria, Bradyrhizobium sp. and phosphorus on nitrogen fixation and yield of chickpea. Indian J Pulses Res 13:66–67
Lynch JP, Epstein E, Lauchli A, Weight GE (1990) An automated greenhouse sand culture system suitable for studies of P nutrition. Plant Cell Environ 13:547–554
Ma W, Guinel FC, Glick BR (2003) Rhizobium leguminosarum biovar. viciae 1-amino cyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Appl Environ Microbiol 69:4396–4402
Molla AH, Shamsuddin ZH, Halimi MS, Morziah M, Puteh AB (2001) Potential for enhancement of root growth and nodulation of soybean coinoculated with Azospirillum and Bradyrhizobium in laboratory systems. Soil Biol Biochem 33:457–463
Murphy J, Riley J (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chem Acta 27:31–36
Nabti E, Sahnoune M, Adjrad S, Van Dommelen A, Ghoul M, Schmid M, Hartmann A (2007) A halophilic and osmotolerant Azospirillum brasilense strain from Algerian soil restores wheat growth under saline conditions. Eng Life Sci 7(4):354–360
Naz I, Bano A (2012) Assessment of phytohormones producing capacity of Stenotrophomonas maltophilia SSA and its interaction with Zea mays l. Pak J Bot 44(1):465–469
Nelson DR, Mele PM (2007) Subtle changes in the rhizosphere microbial community structure in response to increased boron and sodium chloride concentrations. Soil Biol Biochem 39:340–351
Ofek M, Ruppel S, Waisel Y (2006) Effects of salinity on rhizosphere bacterial communities associated with different root types of Vicia faba L. In: Ozturk M, Waisel Y, Khan A, Gork G (eds) Biosaline agriculture and salinity tolerance in plants. Birkhauser Verlag, Basel, pp 1–21
Ondrasek G, Rengel Z, Romic D, Poljak M, Romic M (2009) Accumulation of non/essential elements in radish plants grown in salt-affected and cadmium contaminated environment. Cereal Res Commun 37:9–12
Park M, Kin C, Yang J, Lee Y, Shin W, Kim S, Sa T (2005) Isolation and characterization of diazotrophic growth promoting bacteria from rhizosphere of agricultural crops of Korea. Microbiol Res 160:127–133
Parvaiz A, Satyawati S (2008) Salt stress and phyto-biochemical responses of plants - a review. Plant Soil Environ 54(3):89
Qureshi MA, Shakir MA, Naveed M, Ahmad MJ (2009) Growth and yield response of chickpea to co-inoculation with Mesorhizobium ciceri and Bacillus megaterium. J Anim Plant Sci 19(4):205–211
Rabie GH, Almadini AM (2005) Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr J Biotechnol 4(3):210–222
Roder A, Hoffmann E, Hagemann M, Berg G (2005) Synthesis of the compatible solutes glucosylglycerol and trehalose by salt-stressed cells of Stenotrophomonas strains. FEMS Microb Lett 243:219–226
Rokhzadi A, Asgharzadeh A, Darvish F, Nour-Muhammadi G, Majidi E (2008) Influence of plant growth promotingrhizobacteria on dry matter accumulation and yield of chickpea (Cicer arietinum L.) under field conditions. Am Eur J Agric Environ Sci 3(2):253–257
Rosas SB, Andres JA, Rovera M, Correa N (2006) Phosphate-solubilizing Pseudomonas putida can influence the rhizobia-legume symbiosis. Soil Biol Biochem 38:3502–3505
Schmidt CS, Alavi M, Cardinale M, Müller H, Berg G (2012) Stenotrophomonas rhizophila DSM14405T promotes plant growth probably by altering fungal communities in the rhizosphere. Biol Fertil Soils 48:947–960
Shaharoona B, Arshad M, Zahir ZA (2006) Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.). Lett Appl Microbiol 42(2):155–159
Shahzad SM, Khalid A, Arshad M, Rehman K (2010) Improving nodulation, growth and yield of Cicer arietinum L. through bacterial ACC-deaminase induced changes in root architecture. Eur J Soil Biol 46(5):342–347
Siddiqui ZA, Mahmood I (2001) Effects of rhizobacteria and root symbionts on the reproduction of Meloidogyne javanica and growth of chickpea. Bioresour Technol 79(1):41–45
Simons M, van der Bij AJ, Brand I, de Weger LA, Wijffelman CA, Lugtenberg B (1996) Gnotobiotic system for studying rhizosphere colonization by plant growth-promoting Pseudomonas bacteria. Mol Plant-Microbe Interact 9:600–607
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic-acid in microbial and microorganism-plant signaling. FEMS Microb Rev 31:425–448
Subbarao GV, Johansen C, Jana MK, Rao JVDKK (1990) Physiological basis of differences in salinity tolerance of pigeonpea and its related wild species. J Plant Physiology 137(1):64–71
Suckstorff I, Berg G (2003) Evidence for dose-dependent effects on plant growth by Stenotrophomonas strains from different origins. J Appl Microbiol 95(4):656–663
Tisdall JM, Odes JM (1982) Organic matter and water stable aggregates in soils. J Soil Sci 33:141–163
UNEP (2008) In Dead Water. Merging of climate change with pollution, over-harvest, and infestations in the world’s fishing grounds. UNEP/GRID-Arendal, Arendal, Norway. Available online at: http://www.grida.no/_res/site/file/publications/InDeadWater_LR.pdf
Upadhyay SK, Singh JS, Singh DP (2011) Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2:214–222
Vincent JM (1970) A manual for the practical study of root nodule bacteria LBP Handbook No.15. Blackwell, Oxford, p 83
Wolf A, Fritze A, Hagemann M, Berg G (2002) Stenotrophomonas rhizophila sp. nov., a novel plant-associated bacterium with antifungal properties. Int J Syst Evol Microbiol 52:1937–1944
Zahran HH (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63:968–989
Zhu B, Liu H, Tian WX, Fan XY, Li B, Zhou XP, Jin GL, Xie GL (2012) Genome Sequence of Stenotrophomonas maltophilia RR-10, Isolated as an endophyte from rice root. J Bacteriol 194(5):1280–1281
Acknowledgments
This study was supported by the UNESCO/CHINA Fellowship for DJ and Alexander von Humboldt Fellowship for DE. We thank Prof. Hong Liao for providing us necessary research facilities at Root Biology Centre, South China Agricultural University, and Muhammad Adam for technical assistance in the greenhouse.
Author contribution
DE and GB did experimental design work, DJ conducted experiments. DE analyzed the data. DE and GB wrote the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stéphane Compant.
Rights and permissions
About this article
Cite this article
Egamberdieva, D., Jabborova, D. & Berg, G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, and nodulation of soybean under salt stress. Plant Soil 405, 35–45 (2016). https://doi.org/10.1007/s11104-015-2661-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2661-8