Abstract
Aims
Maize is exposed to the combined stresses of water deficiency and soil salinity within its natural habitat, particularly in irrigated and dry land agricultural areas. Hence, the effect of these combined stresses on the metabolic response of maize plants was determined to improve understanding of stress tolerance mechanisms of maize in the field.
Methods
Maize plants were either singly or simultaneously exposed to soil water deficiency and high salinity for 7 d. Physiological characteristics were analyzed and metabolic changes were quantified by conducting 1H NMR-based analysis of polar and non-polar fractions of maize leaf extracts.
Results
The response of maize plants to the combined stresses was distinct from that of plants subjected to either drought stress or salt stress alone at both the metabolic and physiological level. Maize plants showed a new pattern of metabolic response to the combined stresses. Some metabolites specifically responded to combined stresses and differed from those caused by each stress applied individually. The global metabolic response of maize to the combined stresses was related to the physiological processes.
Conclusions
Our results provide valuable insights into the response of maize to combined drought and salt stress by linking stress-related physiological responses to changes in metabolites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil drought and high salinity are two of the most crucial and common environmental stress factors that influence plant growth and development. Both factors mainly limit crops productivity in cultivated areas worldwide; furthermore, these conditions are likely to worsen in the coming decades (Boyer 1982; Rozema and Flowers 2008; Chaves et al. 2009). In fact, crops in their natural habitats are routinely subjected to a combination of drought and salt stresses. Due to irrigation on cultivated land and the expansion of agriculture to semi-arid and arid regions by applying intensive irrigation, secondary salinization increases possibly (Oki and Kanae 2006; Cramer et al. 2007; Rozema and Flowers 2008). Thus, the combined water deficiency and salt stress represents an excellent example of two different abiotic stress conditions that simultaneously occur in the field, particularly in irrigated and dry land agricultural areas. To overcome these limitations and improve stress tolerance in crops by implementing effective engineering strategies, researchers should determine and understand the effects of these stress factors on crop biochemistry and physiological adaptive mechanisms at system level (Flowers 2004; Verslues and Juenger 2011; Carreno-Quintero et al. 2013).
Drought and salt stresses in different plants species have been extensively studied as isolated stress factors (Mahajan and Tuteja 2005; Chaves et al. 2009; Obata and Fernie 2012; Opitz et al. 2014). Under each condition, early metabolism effects include the activation of stress-induced molecular networks (Cheeseman 1988; Zhu 2002); in later phases, signal transduction pathways trigger the production of different proteins and compounds; these compounds then restore or achieve a new state of metabolic homeostasis, which had been disrupted and altered under the stress conditions (Shulaev et al. 2008). Metabolomics is a powerful tool to obtain a comprehensive perspective of the mechanisms by which metabolic networks are regulated and metabolic pathways respond to stress-induced perturbations (Fiehn 2002). Furthermore, nuclear magnetic resonance (NMR) is an efficient method to quantitatively and qualitatively analyze metabolites present in a plant under certain conditions (Kim et al. 2010; Obata and Fernie 2012), and therefore has been successfully applied in studies focusing on plant responses to various abiotic stress conditions, including drought and salinity (Krishnan et al. 2005; Kim et al. 2007; Ward et al. 2007; Gavaghan et al. 2011; Zhang et al. 2011). However, these previous studies focused only on the polar metabolites of plants; studies on the metabolite profile of non-polar extracts by NMR are limited (Choi et al. 2004; Sidhu et al. 2011; Rivas-Ubacha et al. 2012). Non-polar constituents exhibit different physiological and ecological functions in interactions with abiotic stress. Therefore, both non-polar and polar metabolic profiles should be determined to understand the global metabolic alteration under stress conditions.
It is acknowledged that drought and salt stresses result in both general and specific effects on plants growth and development. In the short-term, both stresses cause dehydration of plants. However, under prolonged salt stress, in addition to water deficits, plants respond to ion toxicity (Munns 2002; Chinnusamy et al. 2004; Munns and Tester 2008). In addition to basic differences existing between plant responses to each single stress condition, conflicting or antagonistic responses are triggered when both stress conditions are combined (Rizhsky et al. 2004; Mahajan and Tuteja 2005; Mittler 2006; Chaves et al. 2009). Pérez-Pérez et al. (2007) showed that the interaction of salt and water stress reduces the ability of Carrizo citrange seedlings to recover photosynthetic activity after stress is alleviated compared with plants subjected to a single stress. Another comparative analysis has revealed that significant differences and overlaps are observed in the transcript and metabolite profiles of wine grape shoot tissues exposed to water deficit and salinity stress (Cramer et al. 2007). Despite these findings, studies have focused only on the differences in plant responses to drought stress and salinity stress; limited information is available regarding the effects of the combined drought and salinity stress condition on plants.
Maize (Zea mays L.) is the third most important cereal in the world and grows under a wide range of climate conditions. With the reliance on maize for food, fiber, and fuel, continuous improvement in maize grain yields is a substantial challenge. However, maize is very sensitive to drought; among cereals, maize is also considered the most sensitive to salt (Ashraf and McNeally 1990; Mahajan and Tuteja 2005; Zhang et al. 2014). The tolerance of maize to the combined stresses of drought and salt is a desirable breeding target (Mittler 2006). Therefore, discovering the physiological and metabolic mechanism and exploring new strategies conferring stress combination tolerance to maize are of agronomic and economic interest. However, an integrative overview of the physiological status and metabolic regulation taking place remains limited.
Therefore, we initially analyzed the physiological and metabolic responses of maize plants to combined drought and salinity stress. Both polar and non-polar metabolic profiles were analyzed to gain a holistic understanding of the effects of the combined stresses on metabolic perturbations in maize plants by utilizing NMR-based metabolomic technology in conjunction with multivariate data analysis methods. We also conducted this study to determine the possible association between physiological processes and metabolomic responses to drought, salt, and co-occurring stress conditions.
Materials and methods
Plant growth conditions and treatments
The maize (Zea mays L.) variety studied was ‘DY 606’. The seeds were surface-sterilized in 70 % (v/v) ethanol for 15 min and then rinsed three times with sterile distilled water. The seeds were sown in pots (40 cm × 25 cm × 15 cm) filled with topsoil from the brunisolic region of northeast China and then grown side-by-side in an outdoor growing area at the Experimental Station of Northeastern University, Shenyang (123° 4ʹ E, 41° 8ʹ N), Liaoning, from May 2012 to June 2012. All of the treatments were performed in parallel. Plant culture was performed according to standard commercial practices. After 1.5 months of growth (sixth leaf stage), the seedlings were administered with different treatments.
Preliminary tests were performed to assess the optimal water content and salt concentration to be used in the experiment. The degree of soil drying (60 %; soil water content) was controlled by measuring pot weight. An open rainproof shed was used to protect from rain when it’s necessary. Pots for single salt and combined stress treatments received saline solution (5 mL 250 mM NaCl per pot each day), and then the water content was adjusted with supplementing water based on pot weight (Fig. 1a). As mentioned above, in order to manage the water content in combined stresses of drought and salt, high concentration and small volume of NaCl solution were applied specially in this experiment. Electrical conductivity (EC) of soil and Na+ content in maize shoots were measured to monitor the level of salt stress, respectively (Supplementary Table S1 and Fig. 1b). Both monitors were almost at similar level as literatures, in which moderate level of salt stress was applied (Shani and Dudley 2001; Ding et al. 2009; Tavakkoli et al. 2010). These moderate-intensity stress conditions were chosen because they induced a significant stress response in maize at the same level of growth reduction compared with each stress applied individually. Using this procedure (Fig. 1a), we established three different stress conditions: a combination of water deficiency and salt supplement; water deficiency; and salt supplement (subsequently abbreviated as D + S, D and S, respectively). Control maize plants were fully irrigated with water only (subsequently abbreviated as CK). Each treatment was prepared with 12 replicates. After 7 d of treatments, the net photosynthetic rate based on living leaf was determined; the plants were then harvested. All of the tissues were collected, divided into groups, and used for the physiological and metabolic analyses. In NMR analysis, fresh maize leaves were obtained from different treatments, immediately frozen, and stored at – 80 °C. Afterward, the leaves were ground in liquid nitrogen for metabolomic analysis.
Na + content of maize shoots subjected to a combination of drought and salt stress. A, The experimental design for applying a combination of drought and salt stress to maize is described in “Materials and methods”. The treatments of control, drought, salt and a combination of drought and salt stress are showed as CK, D, S and D + S in Fig. 1 b, respectively. Na+ content of maize plant (b) was measured after 7 days treatment. Results are presented as mean and standard deviation of twelve individual measurements. Different letters above the bars indicate significant differences between treatments (P < 0.05)
Physiological characteristics analysis
Net photosynthetic rate (P N ) of a single leaf was determined between 09:00 h and 11:00 h on a cloudless day by using a portable photosynthesis system LI-6400XT (LI-COR, Lincoln, NE, USA) equipped with a standard 2 cm × 3 cm leaf cuvette and integral temperature and humidity sensors. In this experiment, the light intensity of photosynthetically active reaction was 1,100 μmol m−2 s−1 to 1,500 μmol m−2 s−1, atmospheric CO2 concentration was 395 ± 5 μmol mol−1, air temperature was 25 ± 5 °C, and relative air humidity was 50–65 %. At the start of the measurement, the infrared gas analyzers of LI-6400XT were calibrated according to the manufacturer’s instructions. The shading of the measured leaves was prevented as much as possible. The first young fully mature leaves from the top of the stem from different plants were analyzed.
After harvesting, electrolyte leakage was determined. Leaf disks of 5-mm diameter were collected and washed in sterile water and introduced to test tubes containing 10 mL of deionised water. After gently shaking at 20 °C for 4 h in the dark, the electrical conductivity of the bathing solution (EL1) was measured with an Elmenton Conductometer (Shanghai, China). Then, the solution with leaf disks was boiled for 15 min. After cooling to a temperature of 20 °C, electrical conductivity of the bathing solution was measured (EL2). Relative electrolyte leakage (REL) was calculated as (EL1/EL2) × 100 %.
Then, we recorded the fresh weights (FW) of the shoots. These shoots were then dried in oven at 80 °C for 24 h. The dry weights (DW) were also recorded. Afterwards, the same samples were ground and divided for Na+, C, and N analysis. Twelve biological replicate samples were used to analyze REL, FW, DW, Na+, C, and N.
Na+ was analyzed by acid digestion. In brief, 0.50 g of leaf powder was weighed and placed in a Teflon tube. Then 5 mL of nitric acid and 2 mL of H2O2 were added. Temperature was increased to 130 °C over a 10 min ramp. The samples were maintained at this temperature for 5 min. Afterwards, temperature was increased to 200 °C over a 10 min ramp, and the samples were maintained at 200 °C for 20 min. Temperature was further increased to 220 °C over a 5 min ramp and maintained at this temperature for 20 min to complete digestion. All of the digested samples were dissolved in 50 mL of 1 % nitric acid. After digestion was performed, Na+ content was determined using an atomic absorption spectrophotometer (Model TAS-990, Purkinje General, Peking, China).
To determine C and N contents, for each sample, 0.010 g of powder was weighed and transferred to a tin microcapsule. C and N contents were determined by elemental analysis using an elemental analyzer (Elementar Analysensysteme GmbH, Germany) in a C-N operation mode.
NMR and metabolites
Five hundred milligrams of plant sample was ground in liquid nitrogen; the ground sample was then transferred to a centrifuge tube. Afterwards, 2 mL of pre-cooled water–methanol (1:1) mixture and 2 mL of chloroform were added to the tube, vortexed for 30 s, and sonicated in an ice bath for 1 min. The sample was then centrifuged at 4 °C for 20 min. This procedure was performed 3 times; aqueous (polar phase) and organic (nonpolar phase) fractions were combined and collected separately. For aqueous samples, methanol was removed under vacuum (to reduce the methanol percentage to approximately 5 %); afterward, the supernatants were frozen at – 80 °C and lyophilized in a freeze-drier for at least 24 h. Organic samples were dried under reduced pressure in a rotary vacuum evaporator. Finally, 800 μL of 100 % D2O and 160 μL of phosphate-buffered saline (pH 7) containing 10 % D2O and 0.02 mM sodium 3-trimethlysilyl [2,2,3,3-D4] propionate (TSP) were added to the dried aqueous fractions. 1 mL of chloroform-D containing 0.03 % tetramethylsilyl (TMS) was then added to the dried organic fractions. TSP and TMS were used as internal standards. All of the contents were transferred to Eppendorf tubes and then centrifuged at 12,000 g for 5 min. For each sample, 0.65 mL of supernatant was transferred to 5 mm NMR sample tubes. Twelve biological replicates of each sample were used for NMR analysis.
Samples were scanned through high-resolution 1D 1H NMR spectroscopy (1H frequency, 600.13 MHz) generating polar and nonpolar metabolic profiles using a Bruker Avance 600 spectrometer (Bruker Biospin, Germany). Sample handling, automation, and acquisition were controlled using TopSpin 2.1 software (Bruker Biospin). For both kinds of samples, a standard 1H 90° pulse sequence was used, and residual water resonance was suppressed in the aqueous samples. After the probe was introduced, the samples were allowed to equilibrate for 1 min. Each spectrum was obtained as 32 k data points at a spectral width of 16 ppm and as the sum of 128 transients at a relaxation delay of 2 s. For D2O samples (polar phase), frequency-domain spectra were phase- and baseline-corrected automatically and manually referenced to the TSP residual resonance at δH 0.00 ppm. For the CDCl3 samples (non-polar phase), the spectra were phase- and baseline-corrected automatically and referenced manually to the TMS residual resonance at δH 0.00 ppm. For resonance assignment purposes, 1H-1H total correlation spectroscopy (TOCSY) and 1H-1H correlation spectroscopy (COSY) 2D NMR spectra were obtained for selected samples. In the COSY and TOCSY experiments, 48 transients were collected into 2,048 data points for each of the 256 increments with a spectral width of 10 ppm for both dimensions. A phase insensitive mode was used with gradient selection in the COSY experiments, whereas common MLEV-17 was utilized as a spin-lock scheme in the phase-sensitive TOCSY experiment (TPPI) with a mixing time of 100 ms.
Data and statistics analysis
1H-NMR spectra were automatically reduced to ASCII files by using AMIX (version 3.7, Bruker Biospin). Spectral intensities were scaled to TMS for the CDCl3 extract and to TSP for the water–methanol extract. The spectral intensities were then reduced to integrated regions with an equal width (0.04 ppm) corresponding to the region of δ 10.00 to δ – 0.04 (nonpolar phase) or δ 9.00 to δ – 0.04 (polar phase). For the polar phase, the regions of δ 5.00 – δ 4.70 and δ 3.38 to δ 3.30 were excluded from analysis because of the residual signals of water and methanol. For the non-polar phase, the region of δ 7.40 – δ 7.20 was discarded from analysis because of the residual signal of chloroform. The integrated NMR spectral data were exported into the SIMCA-P + (version 11.5, Umetrics, Umea, Sweden) software package. The projection to partial least squares-discriminant analysis (PLS-DA) was performed to analyze the effects of different stress factors on metabolic profiles. Similar to all supervised multivariate methods, overfitting of data is a possibility; therefore, permutation testing (with 100 permutations) was used to validate the model of each data set. The results were visualized with score plots in which each point represented a sample’s metabolome. Loading plots from the PLS-DA results showed the variables that contribute to group differences. Heat map analysis combined with hierarchical cluster analysis of the same data set, as used in PLS-DA analyses, was performed using MeV version 4.2. Euclidean distance was used to calculate the distance matrix and an average linkage method was used in hierarchical clustering to define the similarities between clusters. The relative levels of metabolites were calculated from the least overlapping NMR signals of the metabolites and TSP and TMS with known concentrations under the assumption of minimal intersample variations in spin–lattice relaxation time of the same protons. These semi-quantitative data were expressed as a mean value. Differences between CK and each treatment were considered significant when P < 0.05 (SPSS for Windows 17.0, SPSS Inc., Chicago).
One-way ANOVA was also used to test the overall differences among the treatments of the physiological variables (calculated using SPSS 17.0). If the ANOVA results showed an overall significant effect, individual pairs of treatment means were compared by Student-Newman-Keuls analysis. Differences were considered significant at P < 0.05. Linear regression was performed to determine the relationship between the physiological characteristics and metabolomic shifts under different stress conditions.
Results
Physiological characterization
Na+ concentrations were used to monitor the physiological response of maize plants to the presence of saline in soil. Na+ accumulated to a high level (2.659 mg g−1 DW) in plants subjected to salt stress, and it also accumulated significantly in plants subjected to a combination of drought and salt stress (3.165 mg g−1 DW), in which both maize plants were treated with the same NaCl concentrations (Fig. 1b and Supplementary Table S2). The Na+ content of shoots growing under drought without any added NaCl did not accumulate Na+ compared to the control plants. In the presence of NaCl, the leakage of electrolyte increased by three times or more compared to the controls, whereas in drought condition, only by less two times (Fig. 2a). C assimilation decreased to 67 %, 86 %, and 50 % in plants subjected to drought, salinity, and combined stress conditions, respectively (Fig. 2c). Figure 2b shows that the maize plants grown under drought and salinity stress conditions exhibited considerably lower FW and DW than those grown under the control condition. These effects markedly increased under the combined stresses. Although each stress condition (drought and salinity) significantly reduced FW, the magnitude of the decrease in FW under drought conditions was larger than that under saline conditions (Fig. 2b). Figure 2d shows that all of the stress conditions significantly increased N content per shoot but slightly decreased C content per shoot. Only the decrease in C content was significant when both stress conditions were simultaneously applied compared with that when each stress condition was applied individually. Although the N content of maize significantly increased under drought and salt stress conditions compared with that of the control group, the difference was not significant between the single drought treatment and combined treatments (Fig. 2d).
Physiological characterization of maize plants subjected to a combination of drought and salt stress. Relative electrolyte leakage (a), fresh and dry biomass (b), net photosynthetic rate (c) based on single leave of maize, and contents of total carbon and nitrogen in maize plants (d) were measured after 7 days treatment. The treatments of control, drought, salt and a combination of drought and salt stress are showed as CK, D, S and D + S, respectively. Results are presented as mean and standard deviation of twelve individual measurements. Different letters above the bars indicate significant differences between treatments (P < 0.05)
Overall, the effects of drought and salinity on REL, FW, DW, and P N were synergistic and additive but not absolutely the same (Supplementary Table S3). However, the same additive effects of water and salt stress conditions were not observed in N and C contents (Fig. 2d and Supplementary Table S3).
Assignments of metabolites
1H NMR was performed to determine the response pattern of the stress-associated metabolites in maize leaves, and also to identify the global metabolite profile in polar (water–methanol) and non-polar (chloroform) extracts of maize leaves subjected to drought, salt stress, or combined stresses. Twelve biological replicates were performed and similar results were obtained (Supplementary Data, Fig. S1). Therefore, representative spectra of both polar and non-polar extracts are shown (Fig. 3). Metabolite resonances were assigned on the basis of previous studies (Fan 1996; Tate et al. 2001) and publicly available databases (e.g., Spectral Database for Organic Compounds, Biological Magnetic Resonance Data Bank, and Madison Metabolomics Consortium Database). These assignments were further confirmed with extensive 2D NMR data from COSY and TOCSY spectra. The identified chemical shifts of the compounds were listed in Table S4. The maize metabolome was dominated by 39 polar metabolites and 15 non-polar metabolites or regions (Fig. 3 and Supplementary Table S4).
Typical 600 MHz 1H NMR spectra of poplar (water–methanol) and non-polar (chloroform) extracts of maize leaves. In polar profile, the regions δ 8.5 to δ 5.9 and δ 3.1 to δ -0.1 were vertically expanded five times compared with the region δ 5.6 to δ 3.1, respectively. Assignments of signals to metabolites were indicated. Leu, leucine; Ile, isoleucine; Val, valine; IB, isobutyrate; β-HB, β-hydroxybutyrate; Thr, threonine; Lac, lactate; Ala, alanine; AA, acetate; Glu, glutamate; Gln, glutamine; Pyr, pyruvate; Succ, succinate; CA, citrate; Asp, aspartate; MA, malate; DMA, dimethylamine; Asn, asparagine; GABA, γ-amino-butyrate; Mal, malonate; Ch, chloine; Ino, inositol; Fru, fructose; Pro, proline; Suc, sucrose; β-Gluc, β-glucose; α-Gluc, α-glucose; CMP, cytidine monophosphate; SA, shikimate; Up, unknown compound; Fum, fumarate; Trans-Ac, trans-aconitic acid; Tyr, tyrosine; His, histidine; Trp, tryptophan; Phe, phenylalanine; Uri, uridine; Ade, adenine; For, formate. In non-polar profile, the regions δ 11.5 to δ 7.8 and δ 7.8 to δ 2.6 were vertically expanded four and two times compared with the region δ 2.6 to δ 0.1, respectively. Assignments of signals to metabolites or regions were indicated. SS, sterols; FA, fatty acid; LFA, linoleyl fatty acid; TTP, triterpenoids; Unp, unknown compound; FAl, fatty alcohols; Ter, terpene; UFA, unsaturated fatty acid; FFA, free fatty acids; PUFA, polyunsaturated fatty acids; DAG, diacylglyceride; FAME, fatty acid methyl ester; TAG, triacylglyceride; PP, polyphenols region; Ald, aldehydes group
PLS-DA using the obtained NMR data
To examine the metabolic changes in maize leaves in response to different stresses and the significance of various metabolites contributing to these alterations, the data were analyzed by a supervised chemometric method, partial least squares discriminant analysis (PLS-DA). Three separate PLS-DA models were constructed using two components between the control condition and different stress treatments. The first model was separated from the second principal component for control and drought; but the last two models were separated from the first principal component for control and salinity, control and the combined stresses (Fig. 4a, d and g). A permutation test with 100 permutations was performed to establish the statistical significance of the models. Fig. 4b, e, and h showed the permutation plots, revealing that the original PLS-DA models were valid. The S loading plots showed the metabolites with evident changes under drought stress were Suc, Ala, Leu, Glu, Gln, Asn, Phe, Trp, Tyr, SA, Fum, AA, β-HB, and IB (Fig. 4c). The most evident metabolic perturbations differentiating saline-treated samples from control samples were observed in Suc, Gluc, Asp, Glu, Gln, Leu, Ala, Thr, Ch, MA, Lac, and one unknown compound (designated as Up; Fig. 4f). The results also suggested that Suc, Gluc, Fru, Pro, Ala, Tyr, Asp, Asn, Glu, Gln, AA, Ch, and Up in the maize leaves were changed after the plants were exposed to the combined stresses (Fig. 4i). The 1H NMR spectra of the non-polar extracts of maize leaves were also analyzed by PLS-DA (Supplementary Data, Fig. S2).
Scores plots of PLS-DA analysis showing the differences between maize polar extracts of (a) control and drought stress, (d) control and salt stress, and (g) control and a combination of drought and salt stress treatment groups. (c), (f) and (i) are corresponding loadings plots of (a), (d) and (g), where ▲ represents variables and values represent chemical shifts. (b), (e) and (h) are permutations testing plots of model (a), (d) and (g), separately
A considerable overlap was found among metabolites involved in the metabolomic separation between treatments of drought, salinity, and combined stress. The most important conserved metabolites that significantly contributed to the discrimination between the control and treated plants (salt and/or drought stress) were Suc and amino acids, particularly Ala, Glu, and Gln, as well as TAG. Furthermore, the maize plants showed unique metabolites in response to drought, salinity, and combined stress. In this study, “unique” refers to those metabolites that were statistically significant in one stress compared with the control condition but not in other stress conditions. For example, Phe, Trp, and SA evidently responded to drought stress. However, MA and DAG were up regulated under salt stress. Pro was up regulated after maize plants were exposed to combined stress. This result highlighted the plasticity of the plant metabolome and demonstrates the plant’s ability to respond to complex environmental conditions.
Heat map and cluster analysis
The considerable signal overlap in the NMR spectra could be considered as a limitation in metabolomic analysis. In our heat map analysis, this overlap can be solved to a great extent by applying global segment integration instead of the assigned metabolite integration, which is often used in the analysis of the targeted metabolome. The form of a heat map in Fig. 5 provides a global perspective of the changes in metabolite profiles in response to different stress treatments. In general, a distinct cluster related to treatment was found in the samples after the plants were exposed to the combined stress for 7 d; this result demonstrated that the amplitude of metabolic changes in maize leaves was greater due to combined stress than single stress. Moreover, the treatment of drought stress was clustered in a group that was distinct from the control plants and salt stress-exposed plants. The metabolome of maize plants exposed to salt stress showed a lower response because its cluster was closer to the control cluster.
Heat map visualization and cluster tree representations of change of metabolite profiles in response to different stress treatments. Each metabolite shown as chemical shift is represented by a single row. Each treatment is shown as a single column obtained by averaging the replicate measurements of each stress treatment. Red or green indicates metabolites with the high or low abundance in a single column, respectively. The data set used in this analysis was same as that in Fig. 4 and Fig. S3
Targeted metabolomic analysis
To investigate the metabolic states of maize plants under different stress treatments, we subjected the major metabolites to targeted metabolomic analysis. These metabolites and their structures were listed in Supplementary Table S4. However, formate could not be determined in most of the samples subjected to salt stress alone and combined stress. On the basis of the characteristics of metabolites, we further classified the metabolites listed in Table 1 into 11 groups (Group A to Group K). Group A consisted of sugars. The amounts of Suc and Fru in this group significantly increased under different stress conditions, whereas the amount of glucose significantly decreased. Group B comprised various metabolites, such as those involved in glycolysis and other metabolites linking glycolysis to the tricarboxylic acid (TCA) cycle. The metabolites in group C were organic acids involved in the TCA cycle. In this study, drought-treated leaf extracts showed increased amounts of Pyr, Ino, For, Succ, and MA; by comparison, the extracts exposed to combined stresses revealed increased amounts of CA and Ino but decreased amount of Fum. However, all of these metabolites were detected at similar levels in the salt stress-exposed extracts compared with the control plants. Group D comprised various amino acids. The results showed that branched-chain amino acids (BCAAs), the glutamate family, and the aspartate family were up regulated. Aromatic amino acids produced by the shikimic acid pathway were down regulated under combined stresses except for phenylalanine. The increases in N content under different stress conditions were similar to the differences observed in the abundance of BCAAs and amino acids of the glutamate family and aspartate family; this result suggested that N content was correlated with the abundance of group D metabolites whose synthesis or protein degradation was promoted by different stress conditions. Among the analyzed amino acids, only aromatic amino acids were decreased when maize plants were exposed to salt stress or water deficiency (Table 1). Abiotic stress conditions often affect cellular gene expression machinery. Therefore, the molecules involved in the processing of nucleic acids classified in group E were also possibly affected. The characteristics of metabolites in group K remain unknown. The metabolites clustered in groups F, G, H, I, and J were non-polar extracts. The levels of FAs and secondary metabolites generally increased when maize plants were exposed to stress treatments.
Among the 54 metabolites that we analyzed, 4 sugars, 6 organic acids, 11 amino acids, 2 nucleotides, eight non-polar metabolites, and 2 unknown compounds were significantly changed by combined stresses and showed differences compared with those in plants exposed to each single stress. These metabolites could be distributed into shared and stress-specific responses by using a Venn diagram (Fig. 6). The results revealed that the leaves of three different treated plants shared eight metabolites that were significantly different from the control leaves. An overlap of nine metabolites was observed in the leaves of both water-deficient and salt-treated plants, and 9 metabolites were changed by salt stress and combined stress. Furthermore, 19 metabolites were common in maize plants exposed to water deficiency and combined stress. Approximately 73 % and 25 % of the metabolites that were altered because of combined stresses were also changed because of drought stress and salt stress, respectively; this result indicated a stronger relationship between the responses to drought and combined stresses than between the responses to salt and combined stress. However, approximately 38 % of metabolites were specifically changed after maize plants were exposed to drought, salinity, and combined stress. Interestingly, exposure to combined stresses resulted in the unique response of six metabolites compared with drought stress (6 metabolites) or salt stress (2 metabolites); thus, maize elicited more specific responses to the drought and combined stresses than the salt stress.
Relationship between physiological response and metabolome shift
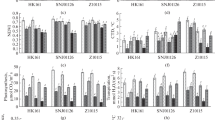
To link the physiological process to metabolome, we investigated the relationships of principal component 1 (PC1) scores of PLS-DA analysis with the elemental contents of N and C as well as DW and P N . Fig. 7 showed that the PC1 scores were significantly correlated with N and C contents of maize plants and with DW and P N . Thus, the highest N content and the lowest C content were associated with low P N and biomass production under the combined stresses condition. Moreover, these changes in the physiological characteristics coincided with the metabolomic separation based on polar metabolic alterations of maize plants in response to different stress conditions. Furthermore, these physiological characteristics were significantly correlated with the PC1 scores obtained from the non-polar metabolomic data (Supplementary Data, Fig. S3). These results provide strong evidence of a relationship between the changes in plant physiology and the changes in the plant metabolome during environmental stress. Therefore, environmental changes could force maize plants to adjust their physiological processes and their metabolomes in an interrelated manner to maintain optimal performance under each stress condition.
Discussion
Physiological responses to combined drought and salt stress
The reduction in biomass production and photosynthesis of plants under drought or salt stress is a common response (Mahajan and Tuteja 2005; Chaves et al. 2009). In the present study, FW, DW, and P N were reduced by either single stress or the combined stresses (Fig. 2). Several authors evaluated the combined effects of salinity and PEG-induced water stress; their results suggested that these sources of stress likely elicit an additive effect on plant development (Shalhevet 1994; Shani and Dudley 2001). Similar to these results, our findings demonstrated that drought and salt stress individually elicit an additive effect on DW and P N ; thus, combined stresses caused severe outcomes (Supplementary Data, Table S3). Interestingly, either water limitation or salinity alone did not significantly reduce the total C content of maize, but combined stresses significantly decreased the total C content (Fig. 2d). Although the total N content of plants was increased significantly under drought and salt stress conditions compared with the control condition, the difference was not significant between drought and combined treatments (Fig. 2d). These results indicate that the complex nature of plant responses to salt and water stress may result in a metabolic response that is not simply equal or additive when the two stress factors are imposed simultaneously. The Na+ concentration was significantly higher than expected in plants simultaneously exposed to drought and salinity compared with plants exposed to saline stress only; this result suggested that Na+ accumulation was enhanced in maize plants by the combined stresses (Fig. 1 and Supplementary Data, Table S3). Martínez et al. (2005) demonstrated that specific salinity levels may improve the ability of plants to cope with water stress. Na+ in plants may positively induce response to water stress (Parida and Das 2005; Slama et al. 2008; Sucre and Suárez 2011; Alla et al. 2012). However, studies have yet to elucidate the exact mechanisms by which plants are favored when they grow under simultaneous saline and water stress conditions compared with those subjected to individual stress.
Metabolic changes in response to combined drought and salt stress
Heat map and cluster analysis results revealed that changes between the metabolic states of control maize plants and drought-stressed plants were greater than those between the metabolic states of control plants and salt-stressed plants (Fig. 5). Cramer et al. (2007) concluded that water deficiency elicits a more severe effect than salinity on the growth, gene expression, and specific metabolites of grapevines at equivalent water potentials. Alla et al. (2012) also found that the accumulation of Na+ inside the leaves of A. halimus improved the water status of the leaves and positively affected metabolic and osmotic processes. The combined stress treatment was clustered in a group distinct from the controls and each single stress; this result demonstrated that the extent of the metabolic changes in maize plants was greater under combined stresses compared to individual stress; this metabolic response may be a new type that clearly differs from other responses (Fig. 5). On the basis of targeted metabolites and Venn diagram analysis results, we studied the differences and crosstalk of metabolites of maize in response to drought, salt, and combined stresses (Table 1 and Fig. 6).
Common metabolic changes in response to all of the stress conditions
Despite differences by which metabolism was perturbed, all of the stress conditions were related to the accumulation of Suc, Gln, Glu, Ala, Ch, TAG and Ter, decline in Gluc content, and alteration of a metabolite with unknown function, although the extent of these regulatory processes varied among different stress conditions (Table 1 and Fig. 6). This extensive overlap among the three stress treatments showed the commonalities among stress responses, suggesting cross talk among responses triggered by these stresses. The components of drought and salt stress exhibit a crosstalk with one another might be these stress conditions both result in cell dehydration, osmotic imbalance, and associated oxidative stress when plants are exposed to drought and salinity (Apel and Hirt 2004; Mahajan and Tuteja 2005; Wong et al. 2006). Our results in maize were consistent with previous observations that showed an overlap of the responses of Arabidopsis (Wong et al. 2006) and rice (Rabbani et al. 2003) to individual drought and high salinity stress, although these studies were based on the comparison of each individual stress.
The accumulation of sugar and certain metabolites involved in the biosynthesis of these sugars is a common response to drought or salinity in many plant species (Gupta and Kaur 2005; Rolland et al. 2006; Wingler and Roitsch 2008). Under stress conditions, sugars act as osmoprotectants to help maintain osmotic balance and stabilize macromolecules (Iyer and Caplan 1998); sugars also provide an immediate energy source for plants restarting growth after they experience a period of stress-induced dormancy (Chaves and Oliveira 2004; Yancey 2005; Widodo et al. 2009). Furthermore, sugars are important signaling molecules and may be implicated in the adaptive mechanisms to different stress conditions (Hanson and Smeekens 2009; Ramel et al. 2009; Pinheiro and Chaves 2011; Alla et al. 2012). The interplay of sugars with other stress elicitors, such as redox and hormone signals, is also well established (Rolland et al. 2006; Hanson and Smeekens 2009). In addition to an increase in the abundance of some well-known stress-responsive sugars, such as Suc and Fru, a decrease in Gluc content was observed in maize exposed to the three stress conditions used in this study (Table 1). This finding suggests that a precise defense strategy was used under the stress conditions. However, the source of Suc in cells subjected to a combination of drought and salt stress remains unknown.
A major consequence of drought and NaCl stress is the loss of intracellular water, as observed in a decrease in fresh matter (Fig. 2b). To prevent this water loss from cells and protect cellular proteins, plants accumulate many metabolites that are also known as “compatible solutes” (McNeil et al. 2001; Chen and Murata 2011; Obata and Fernie 2012). The accumulation of Ch during stress treatments suggests that flux toward Ch biosynthesis is enhanced. Ch accumulation may also occur when Ch is released from membrane phospholipids by phospholipase D (Mattoo et al. 2006). In the presented dataset, glutamate family amino acids, namely, Gln and Glu, and aspartate family amino acids, including Ala, were accumulated under all of the stress conditions (Table 1). The amino acids of the glutamate family and aspartate family are the major N forms in plant leaves (Glass et al. 2002; Mattoo et al. 2006). Gln, Asp, and other amino acids possibly function as sensors of N status in maize and participate in a signal transduction pathway involving Asp aminotransferase and Gln synthetase (Seebauer et al. 2004; Mattoo et al. 2006; Sanchez et al. 2011). Ansari et al. (2005) proposed that increased Glu and Gln concentrations are related to senescence and cell damage in rice because these amino acids become increasingly available during leaf senescence.
Thus far, many metabolome-related studies have focused on polar metabolites; however, our results suggested that polar and non-polar metabolites should be considered to understand the metabolic response of plants to stress conditions. We found that TAG increased significantly when maize plants were subjected to the three stress treatments. Neutral lipid (DAGs and TAGs) accumulation, previously observed in soybean, rape, and wheat plants, is considered a defense mechanism of plants against drought stress (Martin et al. 1986; Dakhma et al. 1995). For example, Gomez-Merino et al. (2005) found that DAG was responsible for opening of stomata in Arabidopsis. Released during water stress by the action of lipases on polar lipids, FFAs can be stored in TAGs to avoid oxidation by free radicals and activated oxygen forms (Gigon et al. 2004). Membrane phospholipids constitute a dynamic system that generates a multitude of signaling molecules, such as inositol 1,4,5-triphosphate and DAGs, which acts as a second messenger. Our results showed that increased DAG levels in maize plants were correlated with increased Ch levels under different stress conditions (Table 1); this result is consistent with that in previous studies, in which Ch is the precursor of membrane phospholipids and the intracellular messenger DAG.
Specific metabolic changes in response to combined drought and salt stress
A stress-specific change in the metabolite level possibly resulted from an inhibition/activation of a specific metabolic pathway. Therefore, such a metabolite should be a good candidate for analysis to elucidate the specific effects of a given abiotic stress and adaptive mechanisms against this stress. In this study, a total of 14 metabolites were specifically changed in association with at least one stress treatment. Among these metabolites, 6 responded to the combined treatment (Fig. 6). This result suggested that the combined stress-responsive mechanism in maize is divergent for each of the stress conditions. Among 6 metabolites, 3 of the amino acids belong to branched chain amino acids (BCAAs) and one member of the aromatic amino acids reacted differently from the majority of aromatic amino acids in the same group (Table 1 and Fig. 6). Joshi et al. (2010) suggested that BCAAs function as compatible osmolytes because these amino acids increased in various plant tissues under drought stress. Under stress conditions, BCAAs also function as an alternative electron donor in the mitochondrial electron transport chain (Araújo et al. 2010). BCAAs accumulate as a result of protein degradation and activated synthetic pathway, which is observed under drought stress conditions. Thus, our analysis emphasized the importance of BCAA metabolism generally under abiotic stress conditions. Interestingly, the pattern of phenyl-Ala accumulation is similar to that of BCAAs (Table 1), although the reason why they are related under stress conditions remains unclear. A previous study suggested that aromatic amino acids in Arabidopsis thaliana increase during a short period after this plant is subjected to NaCl treatment while decreasing in the long-term (Kim et al. 2007). The induction of aromatic amino acids implies that lignin biosynthesis may be induced by stress. Increased lignin levels may contribute to the enhancement of cell wall strength. Among aromatic amino acids, Trp possibly functions as a positive feedback activator of Phe and Tyr biosynthesis (Bentley 1990; Zhao et al. 1998). Under combined drought and salt stress, Phe content increased, whereas other aromatic amino acids, including Trp and Tyr decreased. Otherwise, an unknown metabolite was observed in plants subjected to the combined drought and salt stress. Some studies have demonstrated that the mitochondrial electron transport chain can be supplied with electrons from the degradation of BCAAs and chlorophyll in addition to the direct electron supply from the TCA cycle or the GABA shunt (Shelp et al. 1999; Bouché and Fromm 2004; Araújo et al. 2010). Our results also showed that BCCAs and aromatic amino acids were co-reduced under the combined drought and salt stress. Thus, the exact mechanistic functions of BCAAs and aromatic amino acids under various conditions should be clarified in future studies.
Pyr, For, Succ, and MA accumulated in maize leaves subjected to drought. GMP and TTP accumulated in maize leaves subjected to salt stress. CA and BCAAs accumulated in maize leaves subjected to combined drought and salt stress; in this treatment, Fum was also decreased (Table 1). Such difference may suggest that the combined drought and salt stress imposed on plants exhibited a different type of internal stress (compared with either drought or salt stress) that requires BCAAs rather than organic acids as an osmoprotectant of cells during stress. Under combined stress, the TCA cycle intermediates CA and Fum were changed and negatively correlated with each other, indicating that the genetic regulation of biosynthetic routes enhances the abundance of these intermediates in opposite directions. Similar observations were reviewed by Hill et al. (2013), who concluded that negative genetic correlations are observed in metabolites that share common pathways. In contrast to these previous studies, another study (Guevara et al. 2012) demonstrated that organic solutes accumulate in plants under osmotic stress to perform various functions, such as scavenging free radicals, detoxifying, and adjusting osmotic conditions. However, a metabolite profile does not elucidate whether the related metabolic pathway is upregulated or downregulated because both the upregulation of upstream reactions and the downregulation of downstream reactions can lead to the accumulation of a metabolite. Our result also suggests that the response of maize plants to complex environmental conditions is plastic.
Association between physiology and metabolome under combined drought and salt stress
Studies have provided comprehensive information on alterations in metabolic profiles in response to drought, salt, and other stress conditions. However, the main problem involves the association of such changes with meaningful effects on physiological processes. We evaluated the physiological parameters related to stress treatments and the response of plants at the metabolic level simultaneously under controlled stress. Changes in C concentrations mirrored the general C status of plants under stress conditions. Under natural conditions, assimilated C in a photosynthetically active leaf is primarily used to form Suc or incorporated into starch used as an internal C reserve. However, the assimilated C is preferentially partitioned to Suc under single drought or salt conditions, that is, soluble sugar contents increase, whereas starch content decreases under these stresses (Chaves et al. 2009; Krasensky and Jonak 2012). We found that the carbon balance was disturbed under drought or salt stress, particularly more significantly under the combined stresses (Fig. 2 and Table 1). Because elements function mostly as parts of molecular compounds, changes in the elemental stoichiometry of plants in response to changing environmental conditions should be related to metabolomic changes (Rivas-Ubacha et al. 2012; Carreno-Quintero et al. 2013). The significant relationship between metabolome and C content also showed that in response to reduced carbon substrate under stress conditions, related changes in leaf biochemistry occurred (Fig. 7). Non-polar metabolites, such as FA, Ter, PP, and other C-rich components, increased under stress conditions (Table 1). These changes might be assimilated C is allocated more to anti-stress or defensive mechanisms than to growth and energy supply under stress conditions (Rivas-Ubacha et al. 2012).
N metabolism is strongly interconnected with C metabolism (Nunes-Nesi et al. 2010; Schlüter et al. 2013). We also found that N content increased significantly under drought and salt stresses compared with that under control conditions; this result differed from C content (Fig. 2d). Moreover, metabolome change was significantly related to N content in maize plants subjected to different stresses. Our results are partly consistent with those of (Rivas-Ubacha et al. 2012), who found that the change in N content is related to the change in the metabolome of Erica multiflora plants under moderate experimental field conditions of drought and warming. N assimilation and photosynthesis are closely interdependent (Lawlor 2002; Sato and Yanagisawa 2014). A large portion of assimilated N is devoted to maintaining photosynthesis. An increase in N concentration is partly attributed to the decrease in growth rate that results from stress (Munns 2002; Widodo et al. 2009; Schlüter et al. 2013). In the present study, the N content in maize leaves increased under stress conditions, and this result is consistent with a decrease in P N and DW. Therefore, this N accumulation may partly be a secondary consequence of reduced growth and photosynthesis. Among the 56 metabolites, 48 increased after maize plants were exposed to three different stress conditions or at least to one stress treatment (Table 1). Accumulation is probably related to a cessation of growth, thereby reducing metabolite consumption. However, resource balancing theory predicts that plants may respond to stress by re-allocating mineral nutrients in their systems (Ericsson 1995; Weih et al. 2011). In that case, the growth of maize plants was likely limited predominantly by water or salinity; as a result, higher N contents were allocated per unit leaf area to increase assimilation at a reduced total leaf area and stomatal conductance. Therefore, increased leaf N content shows not only a passive consequence of, and but also a functional adaptation to, water shortage or higher salinity. Cramer et al. (2007) reported that the transcript abundance for nitrate transport and assimilation is stimulated; they further indicated that the pathway produces additional N inputs in grapevines subjected to salt stress or water deficiency. Amino acid accumulation under stress conditions is possibly attributed to the decline in protein biosynthesis (Jacob and Lawlor 1991). We found that the content of Gln and Glu, key compounds for N assimilation, and Ala increased significantly under the three stress conditions. These findings may imply that the abundance of metabolites that have storage function associated with N assimilation is highly variable and that Gln, Glu and Ala are involved in these processes in maize plants.
Photosynthetic carbon fixation provides carbon skeletons for nutrient metabolism and structural components (Wulff-Zottele et al. 2010). Photosynthesis and cellular metabolism are closely linked to optimize plant performance (Wong et al. 2006). Our results demonstrated that P N decreased significantly under combined drought and salinity stress compared with other stress conditions and control conditions (Fig. 2c). A reduction of photosynthetic activity under abiotic stress possibly limited the availability of energy and photoassimilation for metabolism; as a result, the catabolism of stored carbohydrates increases via respiration to provide cells with energy (Widodo et al. 2009). Therefore, a significant relationship was observed between metabolome and P N (Fig. 7). We also observed that the relationship between metabolome and biomass was significant; this result showed that leaf biochemistry was changed in response to biomass reduction under stress conditions (Fig. 7).
Conclusions
We describe a new type of stress-related response in maize plants induced by combined drought and salt stress. This response was characterized by increased N content, decreased C content, photosynthesis and biomass production, and a complex metabolic response pattern. 1H NMR-based metabolomic analysis of polar and non-polar fractions of maize leaf extracts revealed the pattern of metabolic response to the combined stresses. In this response, eight metabolites changed significantly in response to the combined stress; these metabolites also changed significantly in each stress treatment. Furthermore, six metabolites changed significantly in response to combined stress, but these metabolites remained unchanged in each individual stress treatment. These results also revealed a greater crosstalk between the metabolic pathways of drought and combined stresses than between the metabolic pathways of drought and salt or between the metabolic pathways of salt and combined stress. Our studies provide valuable insights into plant responses to combined stresses. Considering physiology and metabolomics, we found that the metabolic response of maize to environmental stress was associated with plant C-N homeostasis and physiological processes, such as photosynthesis and growth. Thus, these results improve our understanding of stress-related physiological responses and changes in metabolites. It is worth noting that the responses of maize to drought and salt stresses may differ with genotypes. Therefore, further compares and analyses of these metabolic responses between tolerant versus sensitive genotypes of maize will promote our understanding of the metabolic mechanisms underlying adaptation to water deficit and high salinity and enable targeted breeding strategies.
References
Alla MMN, Khedr AHA, Serag MM, Abu-Alnaga AZ, Nada RM (2012) Regulation of metabolomics in Atriplex halimus growth under salt and drought stress. Plant Growth Regul 67:281–304. doi:10.1007/s10725-012-9687-1
Ansari MI, Lee R-H, Chen S-CG (2005) A novel senescence associated gene encoding γ-aminobutyric acid (GABA): pyruvate transaminase is upregulated during rice leaf senescence. Physiol Plant 123:1–8. doi:10.1111/j.1399-3054.2004.00430.x
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. doi:10.1146/annurev.arplant.55.031903.141701
Araújo WL, Ishizaki K, Nunes-Nesi A, Larson TR, Tohge T, Krahnert I, Witt S, Obata T, Schauer N, Graham IA, Leaver CJ, Fernie AR (2010) Identification of the 2-Hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell 22:1549–1563. doi:10.1105/tpc.110.075630
Ashraf M, McNeally T (1990) Improvement of salt tolerance in maize for selection and breeding. Plant Breed 104:101–107. doi:10.1111/j.1439-0523.1990.tb00410.x
Bentley R (1990) The shikimate pathway- a metabolic tree with many branches. Crit Rev Biochem Mol Biol 25:307–384. doi:10.3109/10409239009090615
Bouché N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9:110–115. doi:10.1016/j.tplants.2004.01.006
Boyer JS (1982) Plant productivity and environment. Sci 218:443–448. doi:10.1126/science.218.4571.443
Carreno-Quintero N, Bouwmeester HJ, Keurentjes JJB (2013) Genetic analysis of metabolome- phenotype interactions: from model to crop species. Trends Genet 29:41–50. doi:10.1016/j.tig.2012.09.006
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. doi:10.1093/aob/mcn125
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55:2365–2384. doi:10.1093/jxb/erh269
Cheeseman JM (1988) Mechanisms of salinity tolerance in plants. Plant Physiol 87:547–550. doi:10.1104/pp. 87.3.547
Chen THH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34:1–20. doi:10.1111/j.1365-3040.2010.02232.x
Chinnusamy V, Schumaker K, Zhu JK (2004) Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J Exp Bot 55:225–236. doi:10.1093/jxb/erh005
Choi YH, Tapias EC, Kim HK, Lefeber AWM, Erkelens C, Verhoeven JT, Brzin J, Zel J, Verpoorte R (2004) Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiol 135:2398–2410. doi:10.1104/pp. 104.041012
Cramer G, Ergűl A, Grimplet J, Tillett R, Tattersall E, Bohlman M, Vincent D, Sonderegger J, Evans J, Osborne C, Quilici D, Schlauch K, Schooley D, Cushman J (2007) Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct Integr Genomic 7:111–134. doi:10.1007/s10142-006-0039-y
Dakhma WS, Zarrouk M, Cherif A (1995) Effects of drought-stress on lipids in rape leaves. Phytochem 40:1383–1386. doi:10.1016/0031-9422(95)00459-K
Ding D, Zhang L, Wang H, Liu Z, Zhang Z, Zheng Y (2009) Differential expression of miRNAs in response to salt stress in maize roots. Ann Bot 103:29–38. doi:10.1093/aob/mcn205
Ericsson T (1995) Growth and shoot:root ratio of seedlings in relation to nutrient availability. Plant Soil 168:205–214. doi:10.1007/BF00029330
Fan TWM (1996) Metabolite profiling by one- and two-dimensional NMR analysis of complex mixtures. Prog Nucl Magn Reson Spectrosc 28:161–219
Fiehn O (2002) Metabolomics-the link between genotypes and phenotypes. Plant Mol Biol 48:155–171. doi:10.1007/978-94-010-0448-0_11
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319. doi:10.1093/jxb/erh003
Gavaghan CL, Li JV, Hadfield ST, Hole S, Nicholson Je K, Wilson ID, Howe PWA, Stanley PD, Holmes E (2011) Application of NMR-based metabolomics to the investigation of salt stress in maize (Zea mays). Phytochem Anal 22:214–224. doi:10.1002/pca.1268
Gigon A, Matos AR, Laffray D, Zuily-Fodil Y, Pham-Thi AT (2004) Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (Ecotype Columbia). Ann Bot 94:345–351. doi:10.1093/aob/mch150
Glass ADM, Britto DT, Kaiser BN, Kinghorn JR, Kronzucker HJ, Kumar A, Okamoto M, Rawat S, Siddiqi MY, Unkles SE, Vidmar JJ (2002) The regulation of nitrate and ammonium transport systems in plants. J Exp Bot 53:855–864. doi:10.1093/jexbot/53.370.855
Gomez-Merino FC, Arana-Ceballos FA, Trejo-Tellez LI, Skirycz A, Brearley CA, Dormann P, Mueller-Roeber B (2005) Arabidopsis AtDGK7, the smallest member of plant diacylglycerol kinases (DGKs), displays unique biochemical features and saturates at low substrate concentration: the DGK inhibitor R59022 differentially affects AtDGK2 and AtDGK7 activity in vitro and alters plant growth and development. J Biol Chem 280:34888–34899. doi:10.1074/jbc.M506859200
Guevara DR, Champigny MJ, Tattersall A, Dedrick J, Wong CE, Li Y, Labbe A, Ping CL, Wang Y, Nuin P, Golding GB, McCarry BE, Summers PS, Moffatt BA, Weretilnyk EA (2012) Transcriptomic and metabolomic analysis of Yukon Thellungiella plants grown in cabinets and their natural habitat show phenotypic plasticity. BMC Plant Biol 12:175-192. doi:10.1186/1471-2229-12-175
Gupta AK, Kaur N (2005) Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J Biosci 30:761–776. doi:10.1007/BF02703574
Hanson J, Smeekens S (2009) Sugar perception and signaling - an update. Curr Opin Plant Biol 12:562–567. doi:10.1016/j.pbi.2009.07.014
Hill CB, Taylor JD, Edwards J, Mather D, Bacic A, Langridge P, Roessner U (2013) Whole-genome mapping of agronomic and metabolic traits to identify novel quantitative trait loci in bread wheat grown in a water-limited environment. Plant Physiol 162:1266–1281. doi:10.1104/pp. 113.217851
Iyer S, Caplan A (1998) Products of proline catabolism can induce osmotically regulated genes in rice. Plant Physiol 116:203–211. doi:10.1104/pp. 116.1.203
Jacob J, Lawlor DW (1991) Stomatal and mesophyll limitations of photosynthesis in phosphate deficient sunflower, maize and wheat plants. J Exp Bot 42:1003–1011. doi:10.1093/jxb/42.8.1003
Joshi V, Joung JG, Fei Z, Jander G (2010) Interdependence of threonine, methionine and isoleucine metabolism in plants: accumulation and transcriptional regulation under abiotic stress. Amino Acids 39:933–947. doi:10.1007/s00726-010-0505-7
Kim HK, Choi YH, Verpoorte R (2010) NMR-based metabolomic analysis of plants. Nat Protoc 5:536–549. doi:10.1038/nprot.2009.237
Kim JK, Bamba T, Harada K, Fukusaki E, Kobayashi A (2007) Time-course metabolic profiling in Arabidopsis thaliana cell cultures after salt stress treatment. J Exp Bot 58:415–424. doi:10.1093/jxb/erl216
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63:1593–1608. doi:10.1093/jxb/err460
Krishnan P, Kruger NJ, Ratcliffe RG (2005) Metabolite fingerprinting and profiling in plants using NMR. J Exp Bot 56:255–265. doi:10.1093/jxb/eri010
Lawlor DW (2002) Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. J Exp Bot 53:773–787. doi:10.1093/jexbot/53.370.773
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: An overview. Arch Biochem Biophys 444:139–158. doi:10.1016/j.abb.2005.10.018
Martin BA, Schoper JB, Whinne R (1986) Change in soybean (Glycin max [L.] Merr.) glycerolipids in response to water stress. Plant Physiol 81:798–801. doi:10.1104/pp. 81.3.798
Martínez JP, Kinet JM, Bajji M, Lutts S (2005) NaCl alleviates polyethylene glycol induced water stress in the halophyte species Atriplex halimus L. J Exp Bot 56:2421–2131. doi:10.1093/jxb/eri235
Mattoo AK, Sobolev AP, Neelam A, Goyal RK, Handa AK, Segre AL (2006) Nuclear magnetic resonance spectroscopy-based metabolite profiling of transgenic tomato fruit engineered to accumulate spermidine and spermine reveals enhanced anabolic and nitrogen-carbon interactions. Plant Physiol 142:1759–1770. doi:10.1104/pp. 106.084400
McNeil SD, Nuccio ML, Ziemak MJ, Hanson AD (2001) Enhanced synthesis of choline and glycine betaine in transgenic tobacco plants that overexpress phosphoethanolamine N-methyltransferase. Proc Natl Acad Sci U S A 98:10001–10005. doi:10.1073/pnas.171228998
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19. doi:10.1016/j.tplants.2005.11.002
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250. doi:10.1046/j.0016-8025.2001.00808.x
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. doi:10.1146/annurev.arplant.59.032607.092911
Nunes-Nesi A, Fernie AR, Stitt M (2010) Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant 3:973–996. doi:10.1093/mp/ssq049
Obata T, Fernie AR (2012) The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol Life Sci 69:3225–3243. doi:10.1007/s00018-012-1091-5
Oki T, Kanae S (2006) Global hydrological cycles and world water resources. Sci 313:1068–1072. doi:10.1126/science.1128845
Opitz N, Paschold A, Marcon C, Malik WA, Lanz C, Piepho HP, Hochholdinger F (2014) Transcriptomic complexity in young maize primary roots in response to low water potentials. BMC Genomics 15:741–754. doi:10.1186/1471-2164-15-741
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349. doi:10.1016/j.ecoenv.2004.06.010
Pérez-Pérez JG, Syvertsen JP, Botía P, García-Sánchez F (2007) Leaf water relations and net gas exchange responses of salinized Carrizo citrange seedlings during drought stress and recovery. Ann Bot 100:335–345. doi:10.1093/aob/mcm113
Pinheiro C, Chaves MM (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62:869–882. doi:10.1093/jxb/erq340
Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Monitoring expression profiles of rice genes under cold, crought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol 133:1755–1767. doi:10.1104/pp. 103.025742
Ramel F, Sulmon C, Gouesbet G, Couee I (2009) Natural variation reveals relationships between pre-stress carbohydrate nutritional status and subsequent responses to xenobiotic and oxidative stress in Arabidopsis thaliana. Ann Bot 104:1323–1337. doi:10.1093/aob/mcp243
Rivas-Ubacha A, Sardansa J, Pérez-Trujillob M, Estiartea M, Peñuelasa J (2012) Strong relationship between elemental stoichiometry and metabolome in plants. PNAS 109:4181–4186. doi:10.1073/pnas.1116092109
Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696. doi:10.1104/pp. 103.033431
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709. doi:10.1146/annurev.arplant.57.032905.105441
Rozema J, Flowers T (2008) Ecology: crops for a salinized world. Sci 322:1478–1480. doi:10.1126/science.1168572
Sanchez DH, Pieckenstain FL, Escaray F, Erban A, Kraemer U, Udvardi MK, Kopka J (2011) Comparative ionomics and metabolomics in extremophile and glycophytic Lotus species under salt stress challenge the metabolic pre-adaptation hypothesis. Plant Cell Environ 34:605–617. doi:10.1111/j.1365-3040.2010.02266.x
Sato S, Yanagisawa S (2014) Characterization of metabolic states of Arabidopsis thaliana under diverse carbon and nitrogen nutrient conditions via targeted metabolomic analysis. Plant Cell Physiol 55:306–319. doi:10.1093/pcp/pct192
Schlüter U, Colmsee C, Scholz U, Bräutigam A, Weber APM, Zellerhoff N, Bucher M, Fahnenstich H, Sonnewald U (2013) Adaptation of maize source leaf metabolism to stress related disturbances in carbon, nitrogen and phosphorus balance. BMC Genomics 14:442–467. doi:10.1186/1471-2164-14-442
Seebauer JR, Moose SP, Fabbri BJ, Crossland LD, Below FE (2004) Amino acid metabolism in young maize earshoots: implications for assimilate movement and nitrogen signaling. Plant Physiol 136:4326–4334. doi:10.1104/pp. 104.043778
Shalhevet J (1994) Using water of marginal quality for crop production: major issues. Agric Water Manag 25:233–269. doi:10.1016/0378-3774(94)90063-9
Shani U, Dudley LM (2001) Field studies of crop response to water and salt stress. Soil Sci Soc Am J 65:1522–1528. doi:10.2136/sssaj2001.6551522x
Shelp BJ, Bown AW, McLean MD (1999) The metabolism and functions of gamma aminobutyric acid. Trends Plant Sci 4:446–452. doi:10.1016/S1360-1385(99)01486-7
Shulaev V, Cortes D, Miller G, Mittler R (2008) Metabolomics for plant stress response. Plant Physiol 132:199–208. doi:10.1111/j.1399-3054.2007.01025.x
Sidhu OP, Annarao S, Chatterjee S, Tuli R, Roy R, Khetrapal CL (2011) Metabolic alterations of Withania somnifera (L.) dunal fruits at different developmental stages by NMR spectroscopy. Phytochem Anal 22:492–502. doi:10.1002/pca.1307
Slama I, Ghnaya T, Savouré A, Abdelly C (2008) Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation of Sesuvium portulacastrum. CR Biol 331:442–451. doi:10.1016/j.crvi.2008.03.006
Sucre B, Suárez N (2011) Effect of salinity and PEG-induced water stress on water status, gas exchange, solute accumulation, and leaf growth in Ipomoea pes-caprae. Environ Exp Bot 70:192–203. doi:10.1016/j.envexpbot.2010.09.004
Tate AR, Damment SJP, Lindon JC (2001) Investigation of the metabolite variation in control rat urine using 1H NMR. Anal Biochem 291:17–26. doi:10.1006/abio.2001.5008
Tavakkoli E, Rengasamy P, McDonald GK (2010) The response of barley to salinity stress differs between hydroponic and soil systems. Funct Plant Biol 37:621–633. doi:10.1071/FP09202
Verslues PE, Juenger TE (2011) Drought, metabolites, and Arabidopsis natural variation: a promising combination for understanding adaptation to water-limited environments. Curr Opin Plant Biol 14:240–245. doi:10.1016/j.pbi.2011.04.006
Ward JL, Baker JM, Beale MH (2007) Recent applications of NMR spectroscopy in plant metabolomics. FEBS J 274:1126–1131. doi:10.1111/j.1742-4658.2007.05675.x
Weih M, Bonosi L, Ghelardini L, Rönnberg-Wästljung AC (2011) Optimizing nitrogen economy under drought: increased leaf nitrogen is an acclimation to water stress in willow (Salix spp.). Ann Bot 108:1347–1353. doi:10.1093/aob/mcr227
Widodo PJH, Newbigin E, Tester M, Bacic A, Roessner U (2009) Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J Exp Bot 60:4089–4103. doi:10.1093/jxb/erp243
Wingler A, Roitsch T (2008) Metabolic regulation of leaf senescence: interactions of sugar signalling with biotic and abiotic stress responses. Plant Biol 10:50–62. doi:10.1111/j.1438-8677.2008.00086.x
Wong CE, Li Y, Labbe A, Guevara D, Nuin P, Whitty B, Diaz C, Golding GB, Gray GR, Weretilnyk EA, Griffith M, Moffatt BA (2006) Transcriptional profiling implicates novel interactions between abiotic stress and hormonal responses in Thellungiella, a close relative of Arabidopsis. Plant Physiol 140:1437–1450. doi:10.1104/pp. 105.070508
Wulff-Zottele C, Gatzke N, Kopka J, Orellana A, Hoefgen R, Fisahn J, Hesse H (2010) Photosynthesis and metabolism interact during acclimation of Arabidopsis thaliana to high irradiance and sulphur depletion. Plant Cell Environ 33:1974–1988. doi:10.1111/j.1365-3040.2010.02199.x
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830. doi:10.1242/jeb.01730
Zhang JT, Zhang Y, Du YY, Chen SY, Tang HR (2011) Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J Proteome Res 10:1904–1914. doi:10.1021/pr101140n
Zhang W, Han Z, Guo Q, Liu Y, Zheng Y, Wu F, Jinet W (2014) Identification of maize long non-coding RNAs responsive to drought stress. PLoS ONE 9:e98958. doi:10.1371/journal.pone.0098958
Zhao J, Williams CC, Last RL (1998) Induction of Arabidopsis tryptophan pathway enzymes and camalexin by amino acid starvation, oxidative stress, and an abiotic elicitor. Plant Cell 10:359–370. doi:10.1105/tpc.10.3.359
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273. doi:10.1146/annurev.arplant.53.091401.143329
Acknowledgments
This study was financially supported by National Natural Science Foundation of China (No. 31300331) and Fundamental Research Funds for the Central University (N120405008), P. R. of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Frans J.M Maathuis.
Rights and permissions
About this article
Cite this article
Sun, C., Gao, X., Fu, J. et al. Metabolic response of maize (Zea mays L.) plants to combined drought and salt stress. Plant Soil 388, 99–117 (2015). https://doi.org/10.1007/s11104-014-2309-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2309-0