Abstract
Background and aims
The ionome (elemental composition) of grassland species has rarely been studied at the level of individual organs and little is known about effects of soil chemical properties on the ionome. Using the model oxalate plant Rumex obtusifolius, we asked how its biomass production and the distribution of elements between its organs is affected by soil chemical properties.
Methods
We established a pot experiment with R. obtusifolius planted in acidic non-contaminated control and in slightly acidic and alkaline soils anthropogenically contaminated by the risk elements As, Cd, Pb, and Zn. Both contaminated soils were untreated and treated by lime and superphosphate. We determined biomass production and the concentrations of elements in its organs.
Results
Biomass production was negatively related to the mobility of micro- and risk elements. Restricted transport of micro- and risk elements from belowground organs into leaves was recorded in untreated contaminated soils. In both lime-treated soils and in superphosphate-treated alkaline soil, elevated transport of micro- and risk elements from belowground organs into leaves was recorded in comparison to untreated contaminated soils. The lowest concentrations of micro- and risk elements were recorded in stems and seeds, followed by belowground organs and leaves.
Conclusions
R. obtusifolius is an As-, Cd-, Pb-, and Zn-excluder and is sensitive to high availability of micro- and risk elements in the soil. Soil chemical properties affect the distribution of essential elements within the plant greatly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ionome is the elemental composition of cells, tissues, organs or whole organisms (Salt et al. 2008). The elemental composition of many agricultural crops has been investigated (Šrek et al. 2012; Zhao et al. 2013). In the case of grassland species, the ionome has predominantly been studied in aboveground organs (White et al. 2012; Lindström et al. 2013). Therefore, insufficient information is available concerning the elemental composition of belowground organs. Even in aboveground organs, the elemental composition of grassland species is frequently determined only in bulk biomass or in leaves of individual species, to determine their forage quality or nutritional status (Thompson et al. 1997; Hejcman et al. 2012a). Hence, little information is available to describe the distribution of elements in individual aboveground organs, particularly in the case of micro- and risk elements (Barman et al. 2000; Guleryuez et al. 2008; Gaweda 2009).
It has long been recognised that the elemental composition of plant tissues is influenced by environmental conditions and in particular by the availability of different elements in the soil (Barman et al. 2000; Anton and Mathe-Gaspar 2005). However, it is still not well known to what degree the concentrations of elements vary in different organs and how much of this variability is determined by soil chemical properties. Using the model oxalate plant Rumex obtusifolius subsp. obtusifolius (broad-leaved dock), which is a common weedy species in temperate grasslands (Hann et al. 2012; Strnad et al. 2012; Hujerová et al. 2013), the aim of this study was to test the extent to which concentrations of macroelements (N, P, K, Ca, and Mg), microelements (Cu, Fe, Mn, Ni, and Zn) and risk elements (As, Cd, Cr, Pb, and Zn) in its organs are affected by soil chemical properties. Zinc can be classified as both a microelement and a risk element, depending upon its availability in the soil and its concentration in plant biomass. R. obtusifolius belongs to the group of ‘oxalate plants’, which regulates excessive Ca concentrations in tissues by Ca-oxalate precipitation (White and Broadley 2003). Organic acids play an important role in heavy metal(loid) tolerance and detoxification in plants because of their external or internal chelation with risk metal(loid)s (Sytar et al. 2013). In oxalate plants with low exudation rates of di- and tri-carboxylic acids (Tyler and Ström 1995), the main detoxification mechanism is probably internal chelation. The uptake and transport of micro- and risk elements by plants can be characterised by the bioaccumulation factor (BF), which is calculated as the plant-to-soil concentration ratio of a particular micro- or risk elements (Zhuang et al. 2007; Gupta et al. 2008). The leaf-to-root concentration ratio of particular micro- or risk elements is termed the translocation factor (TF; Gupta et al. 2008; Barrutia et al. 2009). According to Baker (1981), plants that accumulate micro- and risk elements are characterised by a BF and TF > 1, indicator plants by a BF and TF = 1 and plants that exclude these elements by a BF and TF < 1. Questions that have not yet been addressed are which BF and TF values are characteristic for oxalate plants like R. obtusifolius and to what extent are BF and TF values affected by the micro- and risk elements availability in the soil.
In this study, we asked how (1) biomass production of R. obtusifolius, (2) concentrations of elements in its organs and (3) BF and TF were affected by soil chemical properties.
Materials and methods
Design of the experiment
Two long-term heavily contaminated soils (‘Litavka’ by As, Cd, Pb, and Zn and ‘Malín’ by As, Cd, and Zn) and one control soil without any contamination (‘Mšec’) were used for a pot experiment in an outdoor university vegetation hall in Prague–Suchdol, with natural temperature and light conditions. Details concerning the history and sources of Litavka and Malín soil contamination are given in Borůvka et al. (1996) and Horák and Hejcman (2013). The physicochemical properties of all used soils are provided in Table 1.
In contaminated soils, we manipulated the availability of elements by application of lime and superphosphate according to previous findings of Vondráčková et al. (2013, 2014). We applied 7.3 g lime (CaO) per 1 kg of soil containing 686 g Ca kg−1 with pHCaCl2 12.0 ± 0.01 and 1.3 g superphosphate (Ca(H2PO4)2 · H2O) per 1 kg of soil containing 246 g P kg−1 and 159 g Ca kg−1 with pHCaCl2 2.2 ± 0.003. The pot experiment was established in May 2011 with seven treatments replicated five times: LC—Litavka control soil without any additive; LCa—Litavka soil with lime; LP—Litavka soil with superphosphate; MC—Malín control soil without any additive; MCa—Malín soil with lime; MP—Malín soil with superphosphate; and McC—Mšec, non-contaminated control soil. Five kg of air dried soil were passed through a 10 mm sieve and put in 5-L pots (20 cm in diameter and height). In each pot, the whole soil profile was mixed with nutrient solution, consisting of 0.5 g N as NH4NO3, 0.16 g P and 0.4 g K as K2HPO4. Application of nutrient solution was performed, to ensure that N, P, and K availability was non-limiting for growth of R. obtusifolius in all treatments. The lime and superphosphate additives were mixed with the soil after application of the nutrient solution. One hundred seeds of R. obtusifolius were sown (1–2 cm deep) in each pot (see Hejcman et al. 2012b for details about emergence and survival of seedlings in contaminated soils) and after 1 month of growth, the seedlings were thinned to three plants per pot. The pots were regularly watered with de-ionised water to maintain the optimal moisture condition for plant growth during the vegetative period. Positions of pots were changed weekly to avoid any side effect on the collected data. The plants were harvested after a growth period of 6 months and their biomass divided into belowground organs, stems, leaves, and seeds (i.e., achenes with a perianth). The belowground organs were first washed thoroughly with tap water to remove soil adhered to belowground organs. Then, the belowground organs were washed in ultrasound-assisted bath filled with deionised water (ELMASONIC S30, Elma Ultrasonic Technology).
Chemical analyses
Soil samples were collected from the whole soil profile at the end of the experiment and were analysed for pH, plant-available and acid-extractable concentrations of elements (Table 2). For chemical analyses, soil samples were air dried at 25 °C and sieved to <2 mm. Soil pH was measured in a 1:5 (w/v) suspension of soil and 0.01 mol L−1 CaCl2. Mobile (plant-available) and mobilisable (acid-extractable) portions of elements in soils were determined using 0.01 mol L−1 CaCl2 and 0.11 mol L−1 CH3COOH (hereafter abbreviated as Ca and AA, Tlustoš et al. 1994; Quevauviller 1998). The element concentrations in soil extracts were determined using inductively coupled plasma-optical emission spectrometry (ICP-OES, VARIAN Vista Pro, Varian, Australia).
Fresh biomass was air-dried at 60 °C to total desiccation, dry matter biomass was determined and then plant samples were ground using a stainless-steel mill and subsequently analysed. The total concentrations of elements in organs were determined by ICP-OES (for P, As, Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn) and flame atomic absorption spectroscopy (FAAS, VARIAN SpectrAA-280, Australia, for K, Ca, and Mg) after microwave-assisted high-pressure acid-digestion (65 % HNO3:30 % H2O2 4:1, Ethos 1, MLS GmbH, Germany). Certified reference material (CTA-OTL-1 oriental tobacco leaves) was mineralised under the same conditions for quality assurance of the total element concentrations in experimental plants. The concentration of N in the plant organs was determined by the Kjeldahl method using a Vapodest 50s (Gerhardt, Königswinter, Germany) after wet-digestion with concentrated H2SO4 (98 %).
Data analysis
All statistical analyses were performed using the Statistica 9.0 (www.statsoft.com) and CANOCO 4.5 (ter Braak and Smilauer 2002) programs. All data were checked for homogeneity of variance and normality (Levene and Shapiro-Wilk tests). Soil and biomass data did not meet assumptions for the use of ANOVA and were thus evaluated by non-parametric Kruskal-Wallis test. We assessed the effects of 1) treatment on the concentrations of elements in the soil and biomass and on the BFs and TFs, 2) organ on concentration of elements in the biomass and 3) soil on the soil properties. After obtaining significant results from the Kruskal-Wallis test, we used multiple comparisons of mean ranks for the detection of significant differences between different soils, treatments or organs. The relationship between concentrations of elements in the biomass and biomass production was analysed by linear regression. A principal component analysis (PCA), in the CANOCO 4.5 program, was applied to all collected data together (concentrations of elements in the soil and biomass, pH, and biomass of organs). We used standardised ‘species data’ because data of different character and units were analysed together. The PCA was used to make visible correlations between all analysed data and similarity of different treatments. The results were visualised in the form of a bi-plot ordination diagram in the CanoDraw program.
Results
Biomass production
The effect of treatment on the total biomass of R. obtusifolius was significant (Fig. 1), and the total biomass weight ranged from 1.3 to 43.3 g plant−1 in the LP and the McC treatments, respectively. Belowground biomass was also significantly affected by the treatment, and ranged from 0.4 to 29.0 g plant−1 in the LC and the McC treatments, respectively (Table 3). Biomass production of all aboveground organs together was also significantly influenced by treatment. No stems or seeds were produced in the LC and LP treatments. Leaf biomass ranged from 0.1 to 5.8 g plant−1 in the LC and the McC treatments. In treatments with stems and seed production, stem biomass ranged from 1.1 to 4.1 g plant−1 in the MC and the LCa treatments, and seed biomass ranged from 1.9 to 7.9 g plant−1 in the MC and the LCa treatments, respectively. Significant negative relationships were recorded between concentrations of micro- and risk elements and total biomass of all organs in the case of Cd (r = −0.606; p < 0.01), Ni (r = −0.373; p = 0.033), Pb (r = −0.356; p = 0.042), and of Zn (r = −0.552; p < 0.01).
Effect of treatment on the total biomass of R. obtusifolius (below- and aboveground biomass together) at the end of the experiment. Treatment abbreviations: LC Litavka control soil without any additive, LCa Litavka soil with lime, LP Litavka soil with superphosphate, MC Malín control soil without any additive, MCa Malín soil with lime, MP Malín soil with superphosphate, and McC Mšec, non-contaminated control soil. Error bars represent SE. Based on Kruskal–Wallis test, differences between treatments were significant based on a 0.01 (**) probability level. Using the multiple comparisons of mean ranks, treatments with the same letter were not significantly different
Concentration of macroelements in the organs
The concentrations of N, P, and Ca were significantly affected by treatments, and the concentrations of N, P, K, Ca, and Mg differed between individual organs (see Table 3 for details). The concentration of N ranged from 3.8 g kg−1 in stems in the MCa treatment, to 29.9 g kg−1 in leaves in the LP treatment. The concentration of P ranged from 0.3 g kg−1 in stems in the LCa treatment to 3.5 g kg−1 in leaves in the MC treatment; the concentration of K ranged from 5.6 g kg−1 in belowground organs to 38.9 g kg−1 in leaves in the LC and McC treatments, respectively, and the concentration of Ca ranged from 2.8 g kg−1 in stems to 15.4 g kg−1 in leaves both in the McC treatment. The concentration of Mg lay between 0.8 g kg−1 in stems in the McC treatment and 5.7 g kg−1 in leaves in the McC treatment.
Concentration of microelements in the organs
The concentrations of Cu, Fe, Mn, and Ni were significantly affected by treatments, and the concentrations of Cu, Fe, Mn, Ni, and Zn differed between the individual organs (see Table 4 for details). The concentration of Cu ranged from 2.7 mg kg−1 in seeds in the LCa to 91 mg kg−1 in belowground organs in the LP treatment; the concentration of Fe ranged from 51.5 mg kg−1 in stems in the LCa treatment, to 5357 mg kg−1 in leaves in the MP treatment; the concentration of Mn ranged from 3.5 mg kg−1 in stems in the LCa treatment, to 228 mg kg−1 in belowground organs in the LC treatment; the concentration of Ni ranged from 0.5 mg kg−1 in stems in the LCa treatment, to 5.9 mg kg−1 in leaves in the MP treatment; and finally, the concentration of Zn ranged from 24 mg kg−1 in stems, to 83 mg kg−1 in belowground organs in the non-contaminated McC treatment.
Concentration of risk elements in the organs
The concentrations of As, Cd, Cr, Pb, and Zn were significantly affected by treatments and analysed plant organs (see Table 4 for details). The concentration of As ranged from 0.22 mg kg−1 in stems in the McC treatment to 189 mg kg−1 in leaves in the MCa treatment; the concentration of Cd ranged from 0.2 mg kg−1 in stems in the MCa treatment, to 29 mg kg−1 in belowground organs in the LC treatment; the concentration of Cr ranged from 0.07 mg kg−1 in seeds in the McC treatment, to 6.8 mg kg−1 in leaves in the MP treatment; the concentration of Pb ranged from 0.1 mg kg−1 in stems in the McC treatment to 235 mg kg−1 in belowground organs in the LC treatment; and finally, the concentration of Zn, in plants grown on contaminated soil, ranged from 30 mg kg−1 in stems in the MC and MCa treatments, to 1479 mg kg−1 in belowground organs in the LC treatment.
Bioaccumulation and translocation factors
Bioaccumulation factors for all elements were significantly affected by treatments (Table 5). In the non-contaminated McC treatment, the BF was above one only for Cd and Ni and in contaminated soils of LC and MC treatments, the BF was below one for all elements. Bioaccumulation factors for As, Cd, Cu, Mn, Ni, Pb, and Zn were affected by their level of soil contamination (As: r = 0.541, p = 0.001; Cd: r = −0.360, p = 0.040; Cu: r = −0.377, p = 0.031; Mn: r = −0.587, p < 0.01; Ni: r = −0.368, p = 0.035; Pb: r = −0.457, p < 0.01 and Zn: r = −0.376, p = 0.031). Liming (MCa treatment) and application of superphosphate (LP and MP treatments) did not affect the BF in contaminated soils.
Translocation factors for As, Cu, and Ni were significantly affected by treatments (Table 5). Liming (LCa and MCa treatments) and application of superphosphate (MP treatment) affected the TF in contaminated soils.
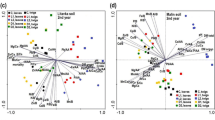
Result of PCA analysis
The first axis of the PCA analysis explained 35 %, the first two axes 56 % and the first four axes together, 82 % of the variability of all analysed data (Fig. 2). The first ordination axis divided individual pots into the Litavka group on the right side and Malín and Mšec groups on the left side of the diagram. This indicates an effect of soil properties on the availability of elements in soil and biomass production as well as on element accumulation. In Litavka, in contrast to Malín soil, data for lime treatment (LCa) were clearly separated from all marks for control (LC) and superphosphate treatments (LP). This indicates a large effect of lime application on all the recorded data in Litavka soil and a minimal effect in Malín soil. In the majority of treatments, data for stems and seeds were grouped into the upper part of the diagram, indicating the lowest concentrations of elements in these organs, since the vectors for the majority of elements in the biomass grouped on the opposite site of the diagram.
Ordination diagram showing the results of PCA analysis with element concentrations in organs of R. obtusifolius plants grown on contaminated and non-contaminated soils. Soil abbreviations: Litavka slightly acidic contaminated soil, Malín alkaline contaminated soil, Mšec acidic non-contaminated soil. Treatment abbreviations: C control, Ca lime application, P superphosphate application; C, belowground organs concentrations of elements in belowground organs in control and etc. Element abbreviations:/B total concentrations of elements in plant biomass,/Ca plant-available concentrations of elements in soil (extracted by 0.01 mol L−1 CaCl2) and/AA acid-extractable concentrations of elements in soil (extracted by 0.11 mol L−1 CH3COOH). Other abbreviations: DM dry matter biomass per organ and pH mean soil pH
The length and direction of the vectors relating to the individual elements indicate the association of elements with their respective treatments. For example, Zn concentration was the highest in belowground organs in the LC treatment, but the lowest in stems in the McC treatment. The concentration of Zn in plant biomass (Zn/B) was positively correlated with plant-available Zn (Zn/Ca) and also with acid-extractable Zn (Zn/AA) concentrations in the soil as indicated by an angle between the vectors for Zn/B and Zn/Ca or Zn/AA of less than 90°. The concentration of Zn in plant biomass was negatively correlated with biomass of organs (DM) as the angle between vectors for Zn/B and DM was greater than 90°. Two vectors did not positively correlate, if the angle between them is larger than 90°. A long vector for a particular variable indicates that it greatly affected the results of the analysis and the opposite was the case for a short vector. For example, there was no effect of soil and treatment on the concentrations of K and Mg in plant biomass, as vectors for these elements (K/B and Mg/B) were very short.
Discussion
Biomass production
Biomass production (i.e. total biomass of all organs) of R. obtusifolius was clearly negatively related to the concentrations of Cd, Ni, Pb, and Zn in its biomass, indicating their toxicity to plants. Very high concentrations of micro- and risk elements in plants (i.e. depending on the plant species, >5 mg As kg−1, >5 mg Cd kg−1, >5 mg Cr kg−1, >5 mg Ni kg−1, >30 mg Pb kg−1 or >100 mg Zn kg−1) can reduce biomass production, because micro- and risk elements can cause inhibition of cell elongation and division (Anton and Mathe-Gaspar 2005; Chen and Wong 2006; Barrutia et al. 2009). In LC and LP treatments, the toxicity of risk elements was high enough to inhibit the development of stems and generative organs. R. obtusifolius is thus, a species with a high sensitivity to metal(loid) toxicity.
The highest biomass production of R. obtusifolius in the McC treatment was probably connected with the lower micro- and risk elements concentrations (i.e. depending on the plant species, in range of 1–1.7 mg kg−1 for As, 0.05–2 mg kg−1 for Cd, 0.1–0.5 mg kg−1 for Cr, 0.5–5 mg kg−1 for Ni, 0.5–10 mg kg−1 for Pb or 10–150 mg kg−1 for Zn), but also to better N and K nutrition as shown by the N and K concentrations in leaves. In the Litavka soil, a greater biomass production in the LCa than the LC treatment was probably connected with the obvious trend that lime substantially reduced the mobility of micro- and risk elements, in particular of Cd, Mn, and Zn (Table 2). Similar results, i.e. an increased growth of several crops and weedy species on acid soils contaminated by Cd, Cu, Ni, Pb, and Zn after lime application has also been recorded by other authors (Chen and Wong 2006; Tlustoš et al. 2006; Alvarenga et al. 2008).
Concentrations of macroelements in plant organs
In the McC treatment, the highest concentrations of N, K, Ca, and Mg were recorded as expected, in leaves, because leaves are the most metabolically active organs, with high nutrient requirements (López-Lefebre et al. 2001). The most surprising result was the highest Ca concentration in belowground organs, leaves and seeds in the McC treatment, despite the lowest soil Ca availability (Tables 1 and 2). Similarly, the Ca concentration in belowground organs and leaves in LC, LCa, and LP treatments was higher than in the MC, MCa, and MP treatments, despite a higher Ca availability in the MC, MCa, and MP treatments. The lowest availability of Ca in the McC treatment was reflected only by the lowest concentration of Ca in stems. The explanation for these discrepancies between Ca availability and Ca concentrations in belowground organs and leaves appears to be at least partly due to competition between Ca and Mg ions (Appenroth and Gabrys 2003). This is because of obvious trend that the highest concentrations of Mg in belowground organs were generally in the treatments where low concentrations of Ca were recorded. The highest concentration of P was recorded in seeds, due to the high P requirements for generative reproduction (Jiang et al. 2007; White and Veneklaas 2012). With the exception of the MP treatment, the P concentration in seeds was below the critical value of 3 g kg−1, below which there is a decrease in the germination ability of R. obtusifolius or R. crispus (Hrdličková et al. 2011; Hejcman et al. 2012a). The lowest concentrations of N and P were recorded in stems and belowground organs, of Ca and Mg in stems and of K in belowground organs. A low concentration of macroelements in stems is connected with their low metabolic activity and with a high mobility of N, P, K, and Mg in plants and therefore, considerable translocation into plant apices (Anton and Mathe-Gaspar 2005; Gaweda 2009).
In the LCa treatment, a significant decrease in the concentrations of N and Ca in belowground organs after liming might be associated with a dilution effect caused by greater biomass production (Chen and Wong 2006; Tlustoš et al. 2006).
No effect of liming or superphosphate application on the distribution of K, Ca, and Mg in plant biomass was recorded in either acid- or alkaline-contaminated soils. Similarly, no effect of liming on the Mg concentration in plant tissues of other weedy species was found by Alvarenga et al. (2008).
Concentrations of microelements in plant organs
In the McC treatment, the highest concentrations of Fe and Zn were recorded in belowground organs; that of Cu in belowground organs and leaves; of Mn in leaves; the lowest concentrations of Cu, Mn, and Zn in stems; and of Fe in seeds. Similar concentrations of Ni were recorded in all organs. Variability of all microelements in different organs might be due to compartmentalisation and translocation in the vascular system (Bose and Bhattacharyya 2008; Hansch and Mendel 2009). Similar results, i.e., concentrations of Cu, Fe, and Ni in the order belowground organs > leaves > stems is consistent with results for R. acetosa, but inconsistent for those for Cu, Fe, and Zn in R. dentatus when both were grown in non-contaminated soils (Barman et al. 2000; Gaweda 2009). A different distribution of Mn was recorded, with the order leaves > seeds > belowground organs > stems, which clearly separated the distribution of Mn in R. obtusifolius from that of other microelements. This result is inconsistent with the distribution of Mn in R. acetosa (belowground organs > leaves > stems, Gaweda 2009), which shows a difference in Mn distribution within Rumex species.
In LC and MC treatments, a tendency for a restricted transport of microelements from belowground organs into leaves in comparison to the McC treatment was recorded. This was probably connected with protection against excessive concentrations of microelements in aboveground organs (i.e. depending on the plant species as well as organ, >20 mg Cu kg−1, >500 mg Fe kg−1, >356 mg Mn kg−1, >5 mg Ni kg−1 or >100 mg Zn kg−1, Hansch and Mendel 2009).
In LCa and MCa treatments, a tendency for increased transport of Cu, Fe, Mn, and Ni from belowground organs into leaves in comparison to LC and MC treatments was recorded, as also demonstrated by higher TFs. We speculate that changes in the distribution pattern of microelements are connected to the presence of organic acids (mainly oxalate) for the formation of stable complexes, similar to the internal defence mechanism of oxalate plants against excess Ca (Tolra et al. 2005; Miyagi et al. 2013). Therefore, we can speculate that micro- and risk elements are precipitated with oxalate in roots in contaminated control soils. On the other hand, in contaminated soils with lime, oxalate is precipitated with Ca and thus is not available for micro- and risk elements that can easy transport to leaves. In the MP treatment, an increased transport of all microelements from belowground organs into leaves in comparison to the MC treatment was recorded probably because of the sufficient amount of Ca available from superphosphate as well as from soil solution precipitated oxalates as Ca-oxalate and thus available microelements can be easily transported to leaves. Changes in the translocation of microelements in plants after liming and superphosphate application require further research that focuses on differences between oxalate and non-oxalate plants.
Concentrations of risk elements in plant organs
In the McC treatment, the highest concentrations of risk elements (As, Cd, Cr, and Pb) were recorded in belowground organs or leaves and the lowest were recorded in stems and seeds. This was connected with low concentrations of risk elements in reproductive organs and with their lower metabolic activity in stems (Anton and Mathe-Gaspar 2005; Bose et al. 2008; Gaweda 2009).
In the LC and MC treatments, there was a higher transport of risk elements from stems into seeds and restricted transport from belowground organs into leaves in comparison to the McC treatment. The clear tendency for the accumulation of risk elements in belowground organs was connected with the exclusion strategy of R. obtusifolius and the function of roots as a barrier that limits the translocation of risk elements from the soil to the aboveground organs in soils contaminated by risk elements (Gaweda 2009; Zhang et al. 2010).
In the LCa and MCa treatments, there was a tendency for a greater transport of As, Cd, Cr, Pb, and Zn from belowground organs into leaves in comparison to the LC and MC treatments, in the most cases demonstrated also by higher TFs.
The results for Cd, Pb, and Zn transport were inconsistent with those for Triticum aestivum published by Tlustoš et al. (2006)—i.e. decreasing shoots/roots ratio after lime application in comparison to R. obtusifolius. As described above, we presume that oxalate plants possess an internal defence mechanism against risk elements, such as forming Ca-oxalate (Miyagi et al. 2013), because risk elements (mainly divalent Cd, Pb, and Zn) compete with divalent Ca for sites to form complexes with oxalate. There was a tendency for a higher transfer of Cd and Zn from belowground organs into leaves in the LP and MP treatments, in comparison to the LC and MC treatments, partly also demonstrated by higher TFs. Similar results were obtained for As and Pb, but only in alkaline-contaminated soil. This was demonstrated also by higher TFs. The results for Cd and Zn transport are inconsistent with observations for Zea mays and Brassica parachinensis (Jiang et al. 2007; Qiu et al. 2011). Therefore, we speculate that differences in distribution can be connected to the presence of oxalate (available for the formation of less toxic complexes as well as internal defence mechanism) in R. obtusifolius. Using lime and superphosphate application increased the in vivo mobility of As, Cd, Cr, Pb, and Zn into leaves of R. obtusifolius. For this reason, differences in the translocation of risk elements after liming and superphosphate application deserve closer examination, with a focus on the differences between oxalate and non-oxalate plants.
Bioaccumulation and translocation factors
In the non-contaminated McC treatment, the BF of micro- and risk elements for R. obtusifolius ranged from 0.01 to 2.9 and in the LC and MC treatments, ranged from 0.01 to 0.4. A decrease in the BF with increasing pseudo-total concentrations of elements (Cd, Zn, Pb, Ni, Mn, and Cu) in soils was consistent with results of Cd and Zn published by Zhao et al. (2003). Therefore, we do not recommend the use of R. obtusifolius for phytoextraction in heavily contaminated soils, but it might be suitable for moderately contaminated soils, as is R. acetosa (Gaweda 2009). In addition, because of the sensitivity of R. obtusifolius to risk elements, it can be used for the identification of contaminated soils by field vegetation mapping, i.e. according to the symptoms of risk elements toxicity visible on aboveground organs in different phenological stages (Hejcman et al. 2012b). Liming tended to decrease the BF of micro- and risk elements in the contaminated Litavka soil, due to the reduction in element availability in soil and subsequently in plants. In the LP, MCa and MP treatments, no effect on the BF of micro- and risk elements was observed, because there was no reduction of element availability in the soil.
In the LC treatment, the TFs for risk elements (As, Cd, Pb, and Zn) ranged from 0.5 to 1.0, indicating an exclusion strategy by R. obtusifolius. In the MC treatment, the TFs for risk elements were higher, confirming that the ability to exclude risk elements was affected by their availability in the soil. The classification of R. obtusifolius as a metal-excluder with restricted risk element transfer to aboveground organs is consistent with observations for R. acetosa (Barrutia et al. 2009; Gaweda 2009). It appears that there is consistency in the physiological responses of different Rumex species to the availability of risk elements in the soil, but this requires further research. Plants with the ability to accumulate risk elements can be used to phytoremediate contaminated soils (Baker 1981). Therefore, the TF is only relevant for elements that exceed background concentrations in comparison to those of non-contaminated soils (see Table 1). In the LCa and MCa treatments, the TFs for As, Cd, Pb, and Zn ranged from 0.9 to 4.5, which is characteristic for indicators or accumulators. Liming substantially increased the translocation of risk elements from belowground organs into leaves. A similar result was observed for superphosphate application, but only in alkaline-contaminated soil. We conclude that the identification of plants for the phytoremediation of contaminated soils must proceed with caution, because TF values depend on the chemical properties of the soil.
Conclusions
The ionome, i.e., the elemental composition of different organs is greatly affected by soil chemical properties. Soil chemical properties affect not only the concentrations of individual elements in individual organs, but also their distribution between plant organs. Variability in the concentrations of micro- and risk elements is much greater than variability in the concentrations of macroelements, especially on metal(loid)-contaminated soils. Liming of contaminated soils as well as superphosphate application can modify the distribution pattern of elements and can increase the translocation of micro- and risk elements from belowground organs to leaves.
The oxalate plant, R. obtusifolius, is sensitive to Cd, Ni, Pb, and Zn toxicity, as its biomass production is reduced due to their availability in the soil and consequently due to reduction of their high concentrations in plant organs. The restricted translocation of micro- and risk elements from belowground organs to leaves in R. obtusifolius is consistent with this species being an As-, Cd-, Pb-, and Zn-excluder and not suitable for phytoremediation of heavily contaminated soils. However, sensitivity to risk elements can be used for identification of metal(loid)-contaminated soils by field vegetation mapping.
References
Alkorta I, Hernández-Allica J, Becerril JM, Amezaga I, Albizu I, Garbisu C (2004) Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead and arsenic. Rev Environ Sci Biotechnol 3:71–90
Allen SE (1989) Analysis of ecological materials, 2nd edn. Blackwell, Oxford
Alvarenga P, Goncalves AP, Fernandes RM, de Varennes A, Vallini G, Duarte E, Cunha-Queda AC (2008) Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci Total Environ 406:43–56
Anonymous (1994) Public notice No. 13/1994 for the managemenet of soil protection. Czech Ministry of the Environment, Prague, In Czech
Anton A, Mathe-Gaspar G (2005) Factors affecting heavy metal uptake in plant selection for phytoremediation. Z Naturforsch C 60:244–246
Appenroth KJ, Gabrys H (2003) Ion antagonism between calcium and magnesium in phytochrome-mediated degradation of storage starch in Spirodela polyrhiza. Plant Sci 165:1261–1265
Baker AJM (1981) Accumulators and excluders—strategies in the response of plants to heavy-metals. J Plant Nutr 3:643–654
Barman SC, Sahu RK, Bhargava SK, Chaterjee C (2000) Distribution of heavy metals in wheat, mustard, and weed grown in field irrigated with industrial effluents. Bull Environ Contam Toxicol 64:489–496
Barrutia O, Epelde L, Garcia-Plazaola JI, Garbisu C, Becerril JM (2009) Phytoextraction potential of two Rumex acetosa L. accessions collected from metalliferous and non-metalliferous sites: effect of fertilization. Chemosphere 74:259–264
Borůvka L, HuanWei C, Kozák J, Krištoufková S (1996) Heavy contamination of soil with cadmium, lead and zinc in the alluvium of the Litavka river. Rostl Výr 42:543–550
Bose S, Bhattacharyya AK (2008) Heavy metal accumulation in wheat plant grown in soil amended with industrial sludge. Chemosphere 70:1264–1272
Bose S, Chandrayan S, Rai V, Bhattacharyya AK, Ramanathan A (2008) Translocation of metals in pea plants grown on various amendment of electroplating industrial sludge. Bioresour Technol 99:4467–4475
Chen Q, Wong JWC (2006) Growth of Agropyron elongatum in a simulated nickel contaminated soil with lime stabilization. Sci Total Environ 366:448–455
Garcia-Salgado S, Garcia-Casillas D, Quijano-Nieto MA, Bonilla-Simon MM (2012) Arsenic and heavy metal uptake and accumulation in native plant species from soils polluted by mining activities. Water Air Soil Pollut 223:559–572
Gaweda M (2009) Heavy metal content in common sorrel plants (Rumex Acetosa L.) obtained from natural sites in Malopolska province. Pol J Environ Stud 18:213–218
Guleryuez G, Arslan H, Celik C, Gucer S, Kendall M (2008) Heavy metal content of plant species along Nilufer stream in industrialized Bursa City, Turkey. Water Air Soil Pollut 195:275–284
Gupta S, Nayek S, Saha RN, Satpati S (2008) Assessment of heavy metal accumulation in macrophyte, agricultural soil, and crop plants adjacent to discharge zone of sponge iron factory. Environ Geol 55:731–739
Hann P, Trska C, Kromp B (2012) Effects of management intensity and soil chemical properties on Rumex obtusifolius in cut grasslands in Lower Austria. J Pest Sci 85:5–15
Hansch R, Mendel RR (2009) Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr Opin Plant Biol 12:259–266
Hejcman M, Strnad L, Hejcmanová P, Pavlů V (2012a) Response of plant species composition, biomass production and biomass chemical properties to high N, P and K application rates in Dactylis glomerata- and Festuca arundinacea-dominated grassland. Grass Forage Sci 67:488–506
Hejcman M, Vondráčková S, Müllerová V, Červená K, Száková J, Tlustoš P (2012b) Effect of quick lime and superphosphate additives on emergence and survival of Rumex obtusifolius seedlings in acid and alkaline soils contaminated by As, Cd, Pb, and Zn. Plant Soil Environ 58:561–567
Horák J, Hejcman M (2013) Use of trace elements from historical mining for alluvial sediment dating. Soil Water Res 8:77–86
Hrdličková J, Hejcman M, Křišťálová V, Pavlů V (2011) Production, size, and germination of broad-leaved dock seeds collected from mother plants grown under different nitrogen, phosphorus, and potassium supplies. Weed Biol Manag 11:190–201
Hujerová R, Pavlů V, Hejcman M, Pavlů L, Gaisler J (2013) Effect of cutting frequency on above- and below-ground biomass production of Rumex alpinus, R. crispus, R. obtusifolius, and the Rumex hybrid (R. patienta x R. tianschanicus) in the seeding year. Weed Res 53:378–386
Jiang HM, Yang JC, Zhang JF (2007) Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ Pollut 147:750–756
Kabata-Pendias A (2001) Trace elements in soils and plants, 3rd edn. CRC Press, Boca Raton
Levy DB, Redente EF, Uphoff GD (1999) Evaluating the phytotoxicity of Pb-Zn tailings to big bluestem (Andropogon gerardii Vitman) and switchgrass (Panicum virgatum L.). Soil Sci 164:363–375
Lindström BEM, Frankow-Lindberg BE, Dahlin AS, Wivstad M, Watson CA (2013) Micronutrient concentrations in common and novel forage species and varieties grown on two contrasting soils. Grass Forage Sci 68:427–436
López-Lefebre LR, Rivero RM, Garcia PC, Sanches E, Ruiz JM, Romero L (2001) Effect of calcium on mineral nutrient uptake and growth of tobacco. J Sci Food Agric 81:1334–71338
Lorestani B, Cheraghi M, Yousefi N (2011) Accumulation of Pb, Fe, Mn, Cu and Zn in plants and choice of hyperaccumulator plant in the industrial town of Vian, Iran. Arch Biol Sci 63:739–745
Mahler RL (2004) Nutrients plants require for growth. CIS1124 Publishing University of Idaho College of Agricultural and Life Sciences. http://www.cals.uidaho.edu/edcomm/pdf/CIS/CIS1124.pdf. Accessed 16 Feb 2014
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Mehlich A (1984) Mehlich-3 soil test extractant—a modification of Mehlich-2 extractant. Commun Soil Sci Plant Anal 15:1409–1416
Miyagi A, Uchimiya M, Kawai-Yamada M, Uchimiya H (2013) Impact of aluminium stress on oxalate and other metabolites in Rumex obtusifolius. Weed Res 53:30–41
Pugh RE, Dick DG, Fredeen AL (2002) Heavy metal (Pb, Zn, Cd, Fe, and Cu) contents of plant foliage near the anvil range lead/zinc mine, Faro, Yukon Territory. Ecotoxicol Environ Saf 52:273–279
Qiu Q, Wang YT, Yang ZY, Yuan JG (2011) Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem Toxicol 49:2260–2267
Quevauviller P (1998) Operationally defined extraction procedures for soil and sediment analysis—I. standardization. TrAC Trends Anal Chem 17:289–298
Salt DE, Baxter I, Lahner B (2008) Ionomics and the study of the plant ionome. Annu Rev Plant Biol 59:709–733
Schwertfeger DM, Hendershot WH (2009) Determination of effective cation exchange capacity and exchange acidity by a one-step BaCl2 method. Soil Sci Soc Am J 73:737–743
Šrek P, Hejcman M, Kunzová E (2012) Effect of long-term cattle slurry and mineral N, P and K application on concentration of N, P, K, Ca, Mg, As, Cd, Cr, Cu, Mn, Ni, Pb and Zn in peeled potato tubers and peels. Plant Soil Environ 58:167–173
Strnad L, Hejcman M, Hejcmanová P, Křišťálová V, Pavlů V (2012) Performance and mortality of Rumex obtusifolius and R. crispus in managed grasslands are affected by nutrient availability. Folia Geobotanica 47:293–304
Sytar O, Kumar A, Latowski D, Kuczynska P, Strzalka K, Prasad MNV (2013) Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol Plant 35:985–999
ter Braak CJF, Smilauer P (2002) CANOCO reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca
Thompson K, Parkinson JA, Band SR, Spencer RE (1997) A comparative study of leaf nutrient concentrations in a regional herbaceous flora. New Phytol 136:679–689
Tlustoš P, van Dijk D, Száková J, Pavlíková D (1994) Cd and Zn release through the selected extractants. Rostl Výr 40:1107–1121
Tlustoš P, Száková J, Kořínek K, Pavlíková D, Hanč A, Balík J (2006) The effect of liming on cadmium, lead, and zinc uptake reduction by spring wheat grown in contaminated soil. Plant Soil Environ 53:16–24
Tolra RP, Poschenrieder C, Luppi B, Barcelo J (2005) Aluminium-induced changes in the profiles of both organic acids and phenolic substances underlie Al tolerance in Rumex acetosa L. Environ Exp Bot 54:231–238
Tyler G, Ström L (1995) Differing organic-acid exudation pattern explains calcifuge and acidifuge behaviour of plants. Ann Bot 75:75–78
Vondráčková S, Hejcman M, Tlustoš P, Száková J (2013) Effect of quick lime and dolomite application on mobility of elements (Cd, Zn, Pb, As, Fe, and Mn) in contaminated soils. Pol J Environ Stud 22:577–589
Vondráčková S, Hejcman M, Tlustoš P, Száková J (2014) Effect of rock phosphate and superphosphate application on mobility of elements (Cd, Zn, Pb, As, Fe, Mn) in contaminated soils. Environ Eng Manag J. In press.
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
White PJ, Veneklaas EJ (2012) Nature and nurture: the importance of seed phosphorus content. Plant Soil 357:1–8
White PJ, Broadley MR, Thompson JA, McNicol JW, Crawley MJ, Poulton PR, Johnston AE (2012) Testing the distinctness of shoot ionomes of angiosperm families using the Rothamsted Park grass continuous hay experiment. New Phytol 196:101–109
Zhang XH, Liu J, Huang HT, Chen J, Zhu YN, Wang DQ (2007) Chromium accumulation by the hyperaccumulator plant Leersia hexandra Swartz. Chemosphere 67:1138–1143
Zhang XF, Xia HP, Lia ZA, Zhuang P, Gao B (2010) Potential of four forage grasses in remediation of Cd and Zn contaminated soils. Bioresour Technol 101:2063–2066
Zhao FJ, Lombi E, McGrath SP (2003) Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant Soil 249:37–43
Zhao H, Guo B, Wei Y, Zhang B (2013) Multi-element composition of wheat grain and provenance soil and their potentialities as fingerprints of geographical origin. J Cereal Sci 57:391–397
Zhuang P, Yang QW, Wang HB, Shu WS (2007) Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut 184:235–242
Acknowledgments
The finalisation of this manuscript was supported by projects NAZV QJ 1210211 and by the project CIGA 20124205.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philip John White.
Rights and permissions
About this article
Cite this article
Vondráčková, S., Hejcman, M., Száková, J. et al. Soil chemical properties affect the concentration of elements (N, P, K, Ca, Mg, As, Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn) and their distribution between organs of Rumex obtusifolius . Plant Soil 379, 231–245 (2014). https://doi.org/10.1007/s11104-014-2058-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2058-0