Abstract
In this study, heavy metal content (Cr, Cu, Mn, Ni, Zn) was determined in sediments and different organs of Rumex obtusifolius L. and Polygonum lapathifolium L. (Polygonaceae), Urtica dioica L. (Urticaceae) and Xanthium strumarium L. (Asteraceae) species. These species grow ubiquitously and vigorously on the periodic flooding areas of Nilüfer stream which have been polluted by different local industrial activities. Below and above-ground parts of plant samples and their sediments were analyzed by ICP-MS for their elemental contents. In general, the variations in the heavy metal content in sediments are reflected in heavy metal status of plant organs. However, this variation depends on plant species and heavy metals. R. obtusifolius, U. dioica and X. strumarium species have elevated levels of Cr, Cu, Ni, and Zn whereas Mn was observed only in P. lapathifolium. The contribution of different organs to the accumulation capacity of the total phytomass is specific to species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The soil nutrients include essential and non-essential heavy metals such as copper, iron, manganese or nickel and, they have important roles in plant growth (Marschner 1995; Hagemeyer 2004). Heavy metal content of soil is dependent on both natural and anthropogenic sources in the local ecosystems. While natural forms are usually found in relatively low concentrations, in recent decades the number and intensity of anthropogenic sources such as rubbish tips, smelter stacks, waste incineration fertilizers, vehicle emissions, agricultural waste and sewage sludge have increased the local environmental heavy metal concentrations (Bargagli 1998; Koch and Rotard 2001). Historical pollution of unchecked emissions of heavy metals may persist for decades.
The responses of plants to high heavy metal content of soil, sediment and water vary with species. For instance, some plant species can be injured by the increased heavy metal content in their environment. On the other hand, some plant species called indicators can tolerate heavy metals, reflecting the external heavy metal (Baker 1981), and they can be used as bio-indicators or biomonitors for quality assessment in aquatic and terrestrial ecosystems (Pugh et al. 2002). Also, some plants have the capability to safely accumulate of heavy metals in different ways (Baker 1981; McIntyre 2003; Kim et al. 2003). Many studies have focused on the heavy metal accumulation capacities of terrestrial plants (e.g. Ernst 1996; Aksoy and Öztürk 1997; Aksoy et al. 1999; Freitas et al. 2004; Güleryüz et al. 2002, 2006; Swaileh et al. 2004; Zeidler 2005; González and González-Chávez 2006) and aquatic merged or submerged macrophytes (e.g. Lewander et al. 1996; Biernacki and Lovett-Doust 1997; Ghate and Chaphekar 2000; Samecka-Cymerman and Kempers 2001, 2002; Scott et al. 2002; Kim et al. 2003; Demirezen and Aksoy 2004, 2006) in contaminated sites.
Anthropogenic activities as sources of increased heavy metal content in both terrestrial and aquatic systems are common in Turkey. Bursa is one of the most important industrialized and urbanized cities in Turkey, which is experiencing rapid industrial development. Industrial activities together with agricultural activities, a rapidly increasing population and non-planned urbanization processes have challenged the ecological balance in Bursa City (Karaer and Küçükballı 2006). Nilüfer stream is used for the agricultural irrigation in Bursa plain. However, domestic, industrial and agricultural wastes are discharged into the Nilüfer stream as reported in several studies. For instance, Yılmaz et al. (1998) found that chromium and lead concentrations were above the standard limits given for the heavily polluted class of water. Dere et al. (2006) showed the extent of the pollution by some other environmental pollution parameters (epipelic diatom taxa, fecal coliforms, total coliforms and total bacteria, and also dissolved oxygen, biochemical oxygen demand—BOD5, electrical conductivity, total dissolved substance).
The aim of this study was to determine the heavy metal (Cr, Cu, Mn, Ni and Zn) content of organs in four plant species [Rumex obtusifolius L. subsp subalpinus (Schur.) Célak and Polygonum lapathifolium L. (Polygonaceae); Urtica dioica L. (Urticaceae) and Xanthium strumarium L. (Asteraceae)] which naturally grow along the Nilüfer stream. We further relate the plant concentrations to their corresponding sediments. Heavy metal loading of plant organs is compared with the corresponding sediment heavy metal contents.
2 Material and Methods
2.1 Study Area
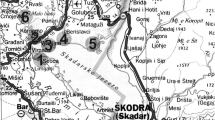
The Nilüfer basin is located in northwest Anatolia. It includes the Nilüfer Stream (168 km) and the industrialized city of Bursa which lies at the intersection of 40°11′ N latitude and 29°04′ E longitude. The average total precipitation of Bursa city is 696.5 mm and the average temperature is 14.6°C (Güleryüz 1992). The precipitation period is between September and April. The Nilüfer stream passes through the city and supplies drinking water to Bursa city via Doğancı dam which was built on upstream of the city (Fig. 1). Not only does it supply drinking water to Bursa, but Nilüfer stream also it supplies irrigation water for agricultural sites around the city via its tributaries such as Ayvalı, Kaplıkaya, Gökdere, Cilimboz and Deliçay creeks. The Nilüfer stream and its tributaries have been polluted by organic and inorganic pollutants from the industrial and domestic wastes resulting from industrialization and urbanization activities in Bursa. Because there are many industrial zones around Bursa, wastes are discharged into this stream and it’s tributaries at seven main points. Industrial activities occur in both main industrial zones (Bursa Organized Industrial Zone, Demirtaş Organized Industrial Zone, and Nilüfer Organized Industrial Zone) and in small industrial zones such as Gürsu, Hasanağa, Kestel Organized Industrial Zones and Yıldırım Industrial Building. Although, there are many industrial zones around Bursa, only two public wastewater facilities were built on this area (Fig. 1), and significant amounts of industrial waste is directly dumped to the Nilüfer stream, without treatment.
2.2 Sample Sites and Sampling
Four sampling sites were selected (Buski, Cilimboz, Buttim, B. Balıklı) along the Nilüfer stream. The Buski sampling site is upstream of industrial activities on the upper part of Nilüfer stream which has no industrial activities. Since there is no industrial activity between Buski and the spring points of Nilüfer stream, we propose Buski as unpolluted reference point. The Cilimboz sampling site is the point where the Cilimboz creek mix with the Nilüfer stream. Climboz creek is carries the wastes of the leather tanning industries, and this sampling site is the first polluted area. Buttim is the next site downstream and is affected by the wastes of the automotive and textile industries. B. Balıklı sampling site is located in the downtown of Bursa city. Many creeks transport the wastes of industrial, agricultural and urban activities are mixed to the Nilüfer stream before this site (Fig. 1).
Polygonum lapathifolium L. “Willow Weed, Curlytop Knotweed”, Rumex obtusifolius L. subsp subalpinus (Schur.) Célak. “Sorrel”, (Polygonaceae), Urtica dioica L. “Stinging Nettle, Common Nettle” (Urticacea) and Xanthium strumarium L. “Coklebur, Rough Cocklebur” (Asteracea) are common species on these sites. They grow ubiquitously and vigorously on the periodic flooding areas of the Nilüfer stream. Their basic properties are given in Table 1; the text on the Flora of Turkey and the East Aegean Islands is referred to for the names of taxa cited here (Davis 1965–1985).
Plant and sediment samples were taken from four different places at each sampling site. Sampling was conducted from March to August 2002, and all plant samples were in the flowering phase. After the plant samples were carefully uprooted using a plastic shovel, a sample of sediment was taken from each plant’s habitat. Soil cores, which have 10 × 15 cm dimensions, were taken using a plastic pipe. They were sifted using with a standard 4-mm stainless steel sieve and then transferred to the laboratory in plastic bags. They were dried in laboratory conditions. Uprooted plant samples were also transferred to the laboratory in plastic bags and they were carefully separated below-ground (roots and below-ground stems) and above-ground (stems and leaves) parts. Plant materials were washed with tap water and then de-ionized water. After they were dried in an oven (105°C) until their weight became constant, samples were ground with a mortar and pestle. Homogenized plant material and soil samples were stored in clear paper bags for analyses.
2.3 Analysis of Sediments and Plant Samples
The pH of air-dried soil samples was measured with a soil/water ratio of 1:2.5 (in saturated mud; Steubing 1965). Exchangeable cations of sediments were analysed in ammonium acetate which was prepared by mixing of 57.5 ml of glacial acetic acid and 60 ml of NH3 (d = 0.91) in water and diluting the resultant mixture to exactly 1 l. The diluting solution was buffered to the mean soil pH value of sediments (pH = 7.3). Air dried sediment samples (10 g) were mechanically shaken in ammonium acetate solution with a vertical shaker for 30 min and then filtered through Whatman blue-band (no. 3) filter paper.
Sediment samples (0.5 g dry weight) were digested with 6 ml HNO3 and 4 ml HCl in a microwave oven (160°C, 2 h) and digestion samples were diluted to 100 ml volume with de-ionized water for acid-soluble cation analyses. Homogenized plant samples (0.5 g dry weight) were also prepared using the same procedure for metal analyses. All sediments and plant material solutions were anlaysed for Cr, Cu, Mn, Ni and Zn by ICP-MS Agilent® 7500 in Tubitak, Bursa Test and Analysis Laboratory (Tubitak-Butal).
2.4 Statistical Analysis
The differences between the sample sites regarding to heavy metal contents of plant organs and sediments were tested by one-way ANOVA. The difference groups among sample sites were determined by Tukey HSD post hoc test (HSD, honestly significant difference). All statistical analyses were based on significance level of α = 0.05 using Statistica Ver 5.0 (StaSoft Inc., 1984–1995, Tulsa, OK, USA) program.
3 Results and Discussion
3.1 Soil pH and Heavy Metal Levels in Sediments
Soil pH and heavy metal contents (exchangeable and acid-soluble) of sediments are given in Table 2. There was no significant difference among sample sites regarding pH levels (P > 0.05). The soil pH varied from 7.4 to 7.8 at sample sites.
Heavy metal contents in sediments of all sample sites in were above detectable levels. Significant differences in all examined exchangeable heavy metals were found between samples sites (P < 0.05). In general, the exchangeable element concentration of sediment increased at the Buttim and B. Balıklı sites (Table 2). For example, the exchangeable Cu content of sediment was 3.6 mg kg−1 dry weight at the B. Balıklı sample site where industrial activities are performed heavily. The 0.7 mg kg−1 dry weight at the Buski sample site was assumed to have no contamination by industrial activities. Similar variation in element concentration according to sample sites was observed for exchangeable Cr. Cr contents were high at the Buttim and B. Balıklı sites (2.3 and 1.7 mg kg−1 dry weight, respectively). Highest Mn, Ni and Zn contents were also measured at the Buttim site which is close to industrial activities (Table 2). Our results indicate that there was an increase in sediment heavy metal content along the Nilüfer stream depending on industrial activities. These results are supported by other authors who previously studied this area (Aydınalp et al. 2005; Dere et al. 2006; Karaer and Küçükballı 2006). Significant difference was found among sample sites regarding to the acid-soluble elements of sediments (P < 0.05). The acid-soluble Cr, Cu, Mn, Ni and Zn contents increased at the B. Balıklı sample site (Table 2).
3.2 Heavy Metal Levels in Plants
Mean heavy metal contents in different organs of four plant species are presented in Tables 3, 4, 5, 6. According to Markert (1994), the chromium (Cr) level in a plant is 1.5 mg kg−1 dry weight but the chromium level above 0.5 mg kg−1 dry weight is considered as toxic to plants (Allen 1989). Therefore, it can be accepted that all examined species have Cr accumulation capacity. In this study, the mean Cr content in total phytomass of all species is much higher than the poisonous level (Tables 3, 4, 5, 6). Even the lowest Cr content in total phytomass of U. dioica sampled from Buski site was fifty-two fold higher than the 0.5 mg kg−1 dry weight level. Also, the high Cr contents in below-ground parts, stems and leaves indicate the Cr accumulation and detoxifying capability of these organs were determined in all species. While the distribution model for Cr was not observed in P. lapathifolium, U. dioica and X. strumarium (Tables 3, 5 and 6), it was accumulated in above-ground parts, especially in stems of R. obtusifolius (Table 4). A significant difference was found between the mean Cr content of total phytomass of all species at the different sampling sites (P < 0.05; Tables 3, 4, 5, 6). The mean Cr contents in total phytomass of U. dioica and X. strumarium reflect the exchangeable Cr content in their sediments. The highest Cr content was measured in total phytomass of U. dioica (112 mg kg−1 dry weight; Table 5) and X. strumarium (193 mg kg−1 dry weight; Table 6) taken from the Buttim site which had the highest Cr content in sediment (Table 2).
The mean Cu content in total phytomass of all species was higher than the copper content in normal plants (4–15 mg kg−1 dry weight; Shaw et al. 2004). The mean Cu content ranged between 34–89 mg kg−1 dry weight (Table 4) in total phytomass of R. obtusifolius L. and this reflects the exchangeable Cu content in sediment (Table 2). The exchangeable Cu content of R. obtusifolius (89 mg kg−1 dry weight) increased towards the B. Balıklı sampling site which has the highest sediment Cu content (3.6 mg kg−1 dry weight). In other examined species, the highest Cu content of total phytomass was measured in plant samples taken from the Buttim site (Tables 3, 4, 5, 6). Although Cu content of above-ground parts of sorrel plants were higher than the Cu content in normal plants, the Cu contents of below-ground parts were at normal levels (8–17 mg kg−1 dry weight). Leaves seem to be the main Cu storage organs in above-ground phytomass of R. obtusifolius species (Table 4). The high Cu contents in leaves can be attributed to the function of this element in the basic metabolic activities of these organs. Copper is a co-factor in enzymes, e. g. plastocyanin, superoxide dismutase an amine oxidases (Hagemeyer 2004). Although a significant difference between in mean Cu content of total phytomass of all species (Tables 3, 4, 5, 6; P < 0.05) between sites, the mean Cu contents of these species do not reflects the exchangeable Cu content in their sediments. These results indicate the Cu accumulation capability of all examined species and agree with previous studies. For instance, another Polygonum species, P. thunbergii, P. mite, accumulated Cu in studies by Samecka-Cymerman and Kempers (2002) and Kim et al. (2003). But the distribution model of Cu in P. lapathifolium and X. strumarium is not clear and is not similar to Cu distribution model in other examined species.
Among all species, the highest Mn content of total phytomass was found in P. lapathifolium and it varied between 234 and 566 mg kg−1 dry weight (Table 3). Mn values of this species were higher than that of a normal plant (200 mg kg−1 dry weight; Markert 1994) and these values indicate the Mn accumulation capacity of P. lapathifolium. But there was no a correlation between mean Mn content of total phytomass in this species and Mn content in sediment. For example: high Mn contents were measured in P. lapathifolium plants collected at the Buski and Cilimboz sample sites (566 and 513 mg kg−1 dry weight, respectively) which have lower sediment Mn contents than B. Balıklı (Table 2). This may reflect the high Mn accumulation capacity of P. lapathifolium, because it was reported that Polygonum species are good bioaccumulators (Qian et al. 1999; Samecka-Cymerman and Kempers 2001, 2002). In general, the mean Mn content in total phytomass of R. obtusifolius, U. dioica and X. strumarium was lower than that of normal plants (200 mg kg−1 dry weight; Markert 1994) and reflected Mn content in sediments. The mean Mn content of these species was highest at site which had the highest exchangeable Mn content. There was significant difference between sample sites regarding to the Mn content in total phytomass of all species (P < 0.05; Tables 3, 4, 5, 6). With the exception of cocklebur plants taken from the Buttim sample site, the mean Mn contents in above-ground parts of plants were lower than that of below-ground parts. The mean Mn contents of stems were lower than the mean Mn content of leaves in all species and all cases. For example, the mean Mn contents in leaves of P. lapathifolium plants taken from Buski are 3.5 fold higher than the Mn content of stems (Table 3). The contribution of leaves in Mn accumulation of plants reflects its function in plant metabolism, particularly its important role in the Hill reaction, where H2O splits and O2 releasing process (Hagemeyer 2004).
Ni concentrations in normal plants range from 0.5 to 5 mg kg−1 dry weight and the values exceeding these limits are reported as poisonous (Allen 1989). The mean Ni contents in all plant organs and in total phytomass of all examined species in this study were higher than this poisonous Ni level in normal plants (Tables 3 and 6). The mean Ni content was increased in the samples of P. lapathifolium, U. dioica and X. strumarium taken from the Buttim site (Tables 3, 5 and 6). The exchangeable Ni content of sediment was highest at Buttim (5.6 mg kg−1 dry weight) and this indicates that the Ni accumulation in these species increases depending on the Ni status of their habitats. Although high Ni contents of these species indicate a Ni detoxifying mechanism, there was no significant Ni distribution between plant parts in all species. Except for R. obtusifolius, significant differences were found between sample sites regarding to the mean Ni content in total phytomass of other species (Tables 3, 4, 5, 6).
Normal Zn concentration in plants is 50 mg kg−1 dry weight (Markert 1994). Allen (1989) reported that Zn concentrations in plants above 5–20 mg kg−1 dry weight are regarded as poisonous. The mean Zn content in total phytomass of all examined species was higher than these Zn levels (Tables 3, 4, 5, 6). This indicates that all species have Zn accumulation capacity in their organs. Our findings on Zn accumulation capacities of P. lapathifolium and U. dioca agree with the results of previous studies; Kim et al. (2003) concluded that P. thunbergii can effectively accumulate Zn. The mean Zn content in total phytomass of all species differed with site (P < 0.05; Tables 3, 4, 5, 6), and the Zn plant content increased with increasing Zn content (Table 2).
Our findings suggest that R. obtusifolius, P. lapathifolium, U. dioica and X. strumarium may be considered bioaccumulation species for Cr, Cu, Ni, and Zn. Therefore, it seems that they can play a substantial role in remediation of polluted sites. But the contribution of different organs to accumulation capacity of total phytomass is specific to species. While P. lapathifolium have also Mn accumulation capacity, it was not observed in R. obtusifolius, U. dioica and X. strumarium. Our results demonstrate that the species studied can tolerate high heavy metal sediments and phytomass loadings.
References
Aksoy, A., Hale, W. H. G., & Dixon, J. M. (1999). Capsella bursa-pastoris L. Medic. as a biomonitor of heavy metals. The Science of the Total Environment, 226, 177–186. Medline. DOI 10.1016/S0048-9697(98)00391-X.
Aksoy, A., & Öztürk, M. A. (1997). Nerium oleander L. as a biomonitor of lead and other heavy metal pollution in Mediterranean environments. The Science of the Total Environment, 205, 145–150. DOI 10.1016/S0048-9697(97)00195-2.
Allen, S. E. (1989). Analysis of ecological materials (2nd ed.). Oxford: Blackwell.
Aydinalp, C., FitzPatrick, E. A., & Cresser, M. S. (2005). Heavy metal pollution in some soil and water resources of Bursa Province, Turkey. Communications in Soil Science and Plant Analysis, 36, 1691–1716. DOI 10.1081/CSS-200062425.
Baker, A. J. M. (1981). Accumulators and excluders strategies in the response of plants to heavy metals. Journal of Plant Nutrition, 3, 643–654.
Bargagli, R. (1998). Trace elements in terrestrial plants. An ecophysiological approach to biomonitoring and biorecovery. Berlin: Springer.
Biernacki, M., & Lovett-Doust, J. (1997). Vallisneria americana (Hydrocharitaceae) as a biomonitor of aquatic ecosystems: Comparison of cloned genotypes. American Journal of Botany, 84, 1743–1751. DOI 10.2307/2446474.
Davis, P. H. (1965–1985). Flora of Turkey and the East Aegean Islands. Edinburg: Edinburg University Press.
Demirezen, D., & Aksoy, A. (2004). Accumulation of heavy metals in Typha angustifolia (L.) and Potamogeton pectinatus (L.) living in Sultan Marsh (Kayseri, Turkey). Chemosphere, 56, 685–696. Medline. DOI 10.1016/j.chemosphere.2004.04.011.
Demirezen, D., & Aksoy, A. (2006). Common hydrophytes as bioindicators of iron and manganese pollutions. Ecological Indicators, 6, 388–393. DOI 10.1016/j.ecolind.2005.04.004.
Dere, Ş., Dalkıran, N., Karacaoğlu, D., Elmacı, A., Dülger, B., & Şentürk, E. (2006). Relationships among epıpelıc diatom taxa, bacterial abundances and water quality in a highly polluted stream catchment, Bursa-Turkey. Environmental Monitoring and Assessment, 112, 1–22. Medline. DOI 10.1007/s10661-006-0213-7.
Ernst, W. H. O. (1996). Bioavailibility of heavy metals and decontamination of soils by plants. Applied Geochemistry, 11, 163–167. DOI 10.1016/0883-2927(95)00040-2.
Freitas, H., Prasad, M. N. V., & Pratas, J. (2004). Analysis of serpentinophytes from north-east of Portugal for trace metal accumulation-relevance to the management of mine environment. Chemosphere, 54, 1625–1642. Medline. DOI 10.1016/j.chemosphere.2003.09.045.
Ghate, S., & Chaphekar, S. B. (2000). Plagiochasma appendiculatum as a biotest for water quality assessment. Environmental Pollution, 108, 173–181. Medline. DOI 10.1016/S0269-7491(99)00243-2.
González, R. C., & González-Chávez, M. C. A. (2006). Metal accumulation in wild plants surrounding mining wastes. Environmental Pollution, 144, 84–92. Medline. DOI 10.1016/j.envpol.2006.01.006.
Güleryüz, G. (1992). The studies on the nutrient turnover and productivity at some plant communities of the Uludağ Alpine Region. PhD dissertation, University of Uludağ, Bursa.
Güleryüz, G., Arslan, H., İzgi, B., & Güçer, Ş. (2006). Element content (Cu, Fe, Mn, Ni, Pb and Zn) of a ruderal plant species (Verbascum olympicum Boiss.) from East Mediterranean. Zeitschrift für Naturforschung C, 61, 357–362.
Güleryüz, G., Arslan, H., Kırmızı, S., & Güçer, Ş. (2002). Investigation of influence of tungsten mine wastes on the elemental composition of some alpine and subalpine plants on Mount Uludağ, Bursa, Turkey. Environmental Pollution, 120, 707–716. Medline.
Hagemeyer, J. (2004). Ecophysiology of plant growth under heavy metal stress. In M. N. V. Prasad (Ed.), Heavy metal stress in plants: From molecules to ecosystems (pp. 201–222, 2nd ed.). Berlin: Springer.
Karaer, F., & Küçükballı, A. (2006). Monitoring of water quality and assessment of organic pollution load in the Nilüfer Stream, Turkey. Environmental Monitoring and Assessment, 114, 391–417. Medline. DOI 10.1007/s10661-006-5029-y.
Kim, I. S., Kang, K. H., Johnson-Green, P., & Lee, E. J. (2003). Investigation of heavy metal accumulation in Polygonum thunbergii for phytoextraction. Environmental Pollution, 126, 235–243. Medline. DOI 10.1016/S0269-7491(03)00190-8.
Koch, M., & Rotard, W. (2001). On the contribution of back-ground sources to heavy metal content of municipal sewage-sludge. Water Science and Technology, 43, 67–74. Medline.
Lewander, M., Greger, M., Kautsky, L., & Szarek, E. (1996). Macrophytes as indicators of bioavailable Cd, Pb and Zn flow in the river Przemsza, Katowice region. Applied Geochemistry, 11, 169–173. DOI 10.1016/0883-2927(95)00074-7.
Markert, B. (1994). Plants as biomonitors-potential advantages and problems. In D. C. Adriano, Z. S. Chen, & S. S. Yang (Eds.), Biogeochemistry of trace elements (pp. 601–613). Northwood, NY: Science and Technology Letters.
Marschner, H. (1995). Mineral nutrition of higher plants (2nd ed.). London: Academic.
McIntyre, T. (2003). Phytoremediation of heavy metals from soils. In T. Scheper (Ed.), Advances in biochemical engineering/biotechnology (pp. 97–123). Berlin: Springer.
Pugh, R. E., Dick, D. G., & Fredeen, A. L. (2002). Heavy metal (Pb, Zn, Cd, Fe and Cu) contents of plant foliage near the Anvil range leaf/zinc mine, Faro, Yukon territory. Ecotoxicology and Environmental Safety, 52, 273–279. Medline. DOI 10.1006/eesa.2002.2201.
Qian, J. H., Zayed, A., Zhu, Y. L., Yu, M., & Terry, N. (1999). Phytoaccumulation of trace elements by wetland plants: III Uptake and accumulation of ten trace elements by twelve plant species. Journal of Environmental Quality, 28, 1448–1455.
Samecka-Cymerman, A., & Kempers, A. J. (2001). Concentrations of heavy metals and plant nutrients in water, sediments and aquatic macrophytes of anthropogenic lakes (former open cut brown coal mines) differing in stage of acidification. The Science of the Total Environment, 28, 87–98. DOI 10.1016/S0048-9697(01)00838-5.
Samecka-Cymerman, A., & Kempers, A. J. (2002). Aquatic macrophytes as biomonitors of pollution by textile industry. Bulletin of Environmental Contamination and Toxicology, 69, 82–96. Medline. DOI 10.1007/s00128-002-0013-x.
Scott, W. A., Adamson, J. K., Rollınson, J., & Parr, T. W. (2002). Monitoring of aquatic macrophytes for detection of long-term change in river systems. Environmental Monitoring and Assessment, 73, 131–153. Medline. DOI 10.1023/A:1013023501051.
Shaw, B. P., Sahu, S. K., & Mishra, R. K. (2004). Heavy metal induced oxidative damage in terrestrial plants. In M. N. V. Prasad (Ed.), Heavy metal stress in plants: from molecules to ecosystems (pp. 84–126, 2nd ed.). Berlin: Springer.
Steubing, L. (1965). Pflanzenökplogisches Praktikum. Berlin: Parey.
Swaileh, K. M., Hussein, R. M., & Abu-Elhaj, S. (2004). Assessment of heavy metal contamination in roadside surface soil and vegetation from the West Bank. Archives of Environmental Contamination and Toxicology, 47, 23–30. Medline. DOI 10.1007/s00244-003-3045-2.
Yılmaz, A., Özer, U., Torunoğlu, T., & Aksoy, S. (1998). Evaluation of heavy metal contents, polyaromatic petroleum hydrocarbons and some inorganic pollutants in the South Marmara Rivers, Turkey. Fresenius Environmental Bulletin, 7, 269–276.
Zeidler, M. (2005). Heavy metals in two herb species (river Morava, Czech Republic). Polish Journal of Ecology, 53, 185–195.
Acknowledgements
This study was supported by the Scientific Research Commission of Uludağ University (to G.Güleryüz, Project No: F-2003/4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Güleryüz, G., Arslan, H., Çelik, C. et al. Heavy Metal Content of Plant Species along Nilüfer Stream in Industrialized Bursa City, Turkey. Water Air Soil Pollut 195, 275–284 (2008). https://doi.org/10.1007/s11270-008-9745-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9745-5