Abstract
Aims
The main objective of this study was to test the hypothesis that isolating and characterizing bacterial suspensions (undefined consortia) from the root/rhizosphere of rice will contribute to the selection of mixtures of rhizobacteria with better growth-promoting activity of rice plants.
Methods
Bacterial consortia were obtained from roots/rhizosphere soil samples of rice plants grown under upland and irrigated production systems. Those undefined consortia were subjected to five consecutive passes every 7 days in NFb (N-free broth) semisolid medium. Thereafter, strains of each growth-promoting consortia were isolated by plating on three different culture media. Then, undefined consortia, as well as mix and single bacterial strains, were characterized in terms of indoleacetic acid production, nitrogen fixation capacity, and growth promotion of rice plants.
Results
Of the 72 consortia analyzed, 41.7 % and 50.0 % increased nitrogenase activity and the production of indolic compounds, respectively, after 5 continuous passes in NFB medium. Three undefined consortia, 11 single strains and 5 strain mixtures, exhibited plant growth promotion in rice plants under greenhouse conditions.
Conclusions
Continuous enrichment in Nfb medium of undefined consortia from root/rhizosphere soil is a good strategy for the selection of plant growth-promoting bacteria for rice plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant growth-promoting rhizobacteria (PGPR), such as Azotobacter, Clostridium, Azospirillum, Herbaspirillum, Burkholderia, Rhizobium (Choudhury and Kennedy 2004), and a variety of Enterobacteriaceas (Kennedy et al. 2004), have been previously isolated from the rhizosphere of rice based on their capacity to promote plant growth by N fixation, increase root surface area by the secretion of plant growth regulators such as indoleacetic acid (IAA), increase the availability of nutrients, or promote growth by combined modes of action (Vessey 2003; Rodrigues et al. 2008). The activities of individual PGPR strains have been demonstrated through the development of mixed inoculants, thereby allowing fertilizer applications to be decreased by up to 25 and 50 % for N and P, respectively, compared to uninoculated controls (Bashan et al. 2004).
Mixed inoculants,defined as mixtures of two or more strains, have been produced with endophytic (Govindarajan et al. 2008) or rhizospheric PGPR with high nitrogenase activity (Alam et al. 2001), phosphate solubilization capacity, production of growth-regulating substances (Rodriguez and Fraga 1999; Rojas et al. 2001; Raja et al. 2006), or even with arbuscular mycorhizal fungi able to promote growth in rice plants under well-watered or drought conditions (Ruíz-Sánchez et al. 2011). These mixed inoculants respond flexibly to environmental changes by using available energy flows and substrates more efficiently than pure cultures (Zlotnikova et al. 2007). In addition, they can stimulate an increase in the amounts of sugars and amino acids exuded by the root and thus stimulate N fixation and the production of growth regulators (Raja et al. 2006), thereby generating an improved plant growth (Nandakumar et al. 2001; Piao et al. 2005; Ruíz-Sánchez et al. 2011). However, the promotion of plant growth by mixed inoculants has been inconsistent (de Freitas 2000; Oliveira et al. 2002; Mehnaz et al. 2010). Some PGPR mixtures can even result in plant growth inhibition, although their component members are growth promoters (Felici et al. 2008). Among other reasons, this phenomenon might be due to the interruption of plant-microbial interactions that are established in the rhizosphere (Newton and Fray 2004). The development of bacterial mixtures between PGPR does not take in account the role of organisms without a direct growth-promoting activity into establishing synergistic relationships. They participate in increasing the availability of nutrients (Shrestha et al. 2007), removal of inhibitory products (Minamisawa et al. 2004), stimulation of mechanisms to promote plant growth (Holguin and Bashan 1996) and interactions still unknown.
The isolation and selection of diazotrophs by enrichment in nitrogen-free medium (NFb) is a common practice (Döbereiner 1995; Jha et al. 2009). However, the nitrogenase activity of strains obtained by enrichment is often less than the original activity (Holguin et al. 1992), most likely due to the disturbance of synergistic relations that promote N-fixation in the rhizosphere environment (Holguin and Bashan 1996; Minamisawa et al. 2004). On the other hand, microbial ecology studies claim that highly diverse communities give rise to highly competitive populations after several generations by making better use of the nutrient sources and growth conditions. This rise in highly competitive populations results from changes in the structure of the population due to constant replacement of the organisms (Kassen and Rainey 2004). The main objective of this study was to test the hypothesis that isolating bacterial suspensions (undefined consortia) from the root/rhizosphere of rice will select mixtures of rhizobacteria with better growth-promoting activity of rice plants.
The methodology of continuous enrichment has permitted the achievement of undefined consortia with high capacities to degrade various xenobiotic agents (Marron-Montiel et al. 2006; Li et al. 2008). However, to the best of our knowledge, this method has not been used to obtain mixed inoculants with plant growth-promoting activity. In this regard, the objectives of this study were to i) evaluate a strategy to obtain bacterial undefined consortia with growth-promoting activity in rice plants using continuous enrichment in NFb culture medium and ii) determine the plant growth-promoting activities of isolates obtained from the undefined consortia in specific mixtures and individually.
Materials and methods
Study site and sampling
Twelve rice farms were sampled in the departments of Tolima (4 farms) and Meta (8 farms) (Colombia) under irrigated and upland production systems during July and August of 2008. In the upland rice production system, the water supply is rain-fed, whereas in the irrigated rice production systems, water is supplied by the district water system. In Meta, four irrigated (irrigated Meta, IM) and four upland farms (upland Meta, UM) were sampled. Meta experiences an annual average temperature of 24 °C and annual rainfall of 3,500 mm. The predominant soils are Oxisols with low fertility and high contents of Fe and Mn (Fedearroz 2000). In the southern zone of Tolima (in the municipality of Saldaña), four farms were sampled under irrigated rice production systems (irrigated Tolima, IT). In this zone, the annual average temperature is 23 °C and annual rainfall is 1,122 mm. The soils are mainly Inceptisols and Entisols (Fedearroz 2000). Fifteen plants distributed every 15–25 m were taken from each cropland. Each plant was shaken vigorously and divided into three approximately equal parts to form three integrated samples, with 15 subsamples each, of roots with rhizosphere soil. The samples were deposited in propylene bags, transported at 4 °C, and processed after arrival at the laboratory. For each farm, a composite soil sample was taken to determine the physicochemical parameters according to standard techniques (Horwitz 2000).
Obtaining root-rhizosphere bacterial suspensions (undefined consortia)
One gram (fresh weight) of root segments and rhizosphere soil from each composite sample was macerated and diluted in 9 mL of saline solution (0.85 % NaCl) to develop serial dilutions until 10−3 dilution. Then, 1 mL of the 10−3 dilution was inoculated into 50-mL tubes that contained 22 mL of modified NFb semisolid (1.28 % bacteriological agar, Merck) medium (Rennie 1981). This medium was composed of malic acid (5 g L−1), K2HPO4 (0.5 g L−1), NaCl (0.1 g L−1), MgSO4.7H2O (0.2 g L−1), CaCl2.2H2O (0.02 g L−1), KOH (2 g L−1), glucose (8 g L−1), mannitol (8 g L−1), bromothymol blue (0.05 g L−1), 2 mL of a micronutrient solution (for 200 mL: 0.02 g Na2MoO4.2H2O, 0.235 g MnSO4, 0.280 g H3BO3, 0.008 g CuSO4.5H2O and 0.0024 g ZnSO4.7H2O), 4 mL of EDTA-Fe (0.8258 g FeSO4.7H2O and 0.556 g Na2EDTA.2H2O), and 1 mL of vitamins biotin (0.1 g L−1) and pyridoxine (0.2 g L−1). The medium was adjusted to pH 7. After incubation for 7 days at 30 °C, 1 mL of bacterial suspension was inoculated into 22 mL of semisolid NFb (first generation). Given the impossibility of centrifuging the semisolid medium, 0.5 mL was taken from the surface of the tube to retrieve the aerobic diazotrophs and 0.5 mL was taken after stirring for 30 s in a vortex to obtain an evenly distributed mixture of microorganisms throughout the tube. Every 7 days, the procedure was repeated until completion of the fifth generation. This procedure was carried out in triplicate, and an aliquot of each undefined bacterial suspension per generation was stored in the presence of 15 % glycerol at −20 °C until further testing to select and identify the cultivable bacteria present in each bacterial suspension that exhibited growth promotion capacity.
Nitrogenase activity
To evaluate the effect of continuous enrichment on the nitrogenase activity of undefined consortia obtained from sampled farms, an acetylene reduction assay was performed (Holguin et al. 1992) for the first and fifth generations (subcultured in NFB medium). Briefly, 50 μl of a bacterial suspension (undefined consortia), which had been stored at −20 °C, were used to inoculate 5 mL of NFb supplemented with 1 g L−1 of yeast extract (NFb + YE). The bacterial suspension was adjusted to 0.2 absorbance units at 600 nm, with a solution of NaCl (0.85 %) after being incubated for 24 h at 30 °C and 150 rev min−1 (rpm). Then, 0.1 mL of adjusted suspension was inoculated into 10 mL of semisolid NFb. After 24 h of incubation, 10 % of the atmosphere was replaced by acetylene, and the suspension was incubated for 24 h. Finally, 1 mL of air was removed from the bottles and tested for acetylene and ethylene using a gas chromatograph (Varian 6000 Instrument Group, USA). Three undefined consortia (one for each subsample) were evaluated from each farm using four replicates per sample. The strains obtained from each undefined consortia were then evaluated similarly, except that the strains were reactivated in NFb + YE solid medium.
Production of indolic compounds
To evaluate the effect of continuous enrichment on the production of indolic compounds, a Salkowsky colorimetric test was performed (Glickmann and Dessaux 1995) for the first and fifth generations. A 0.1-mL aliquot of each undefined consortia, which was obtained in the same way as outlined previously for nitrogenase activity detection, was inoculated into 10 mL of LB medium (10 g L−1 tryptone, 5 g L−1 of yeast extract and 10 g L−1 NaCl) supplemented with tryptophan (0.3 mM). After 72 h of incubation at 150 rpm and 30 °C, 1 mL of supernatant was mixed with 1 mL of Salkowsky reagent (Glickmann and Dessaux 1995). The reaction was kept in the dark for 30 min, after which, spectroscopy readings were taken at 540 nm. Three undefined consortia were evaluated per farm, and each sample was analyzed in triplicate. Similarly, the strains isolated from each undefined consortia exhibiting growth- promoting activity were evaluated for IAA production.
Evaluation of growth promotion of undefined consortia under greenhouse conditions
To determine whether the rhizosphere-root bacterial suspensions (undefined consortia), obtained from continuous enrichment, promoted growth in rice plants, three undefined consortia of the first and fifth generation per each of the 12 farms (72 treatments) were evaluated under greenhouse conditions in a completely randomized design. Bacterial suspensions were grown in NFb + YE broth for greenhouse testing. After 24 h at 30 °C and 150 rpm, the bacterial suspensions of each undefined consortia were adjusted with sterile distilled water to 0.2 absorbance units (600 nm). Five seeds per consortium were disinfected first with 70 % ethanol for 6 min and then with 2 % NaOCl for 6 min. They were finally rinsed with abundant sterile distilled water to remove residual disinfectants. Then disinfected seeds were inoculated with 1 mL of bacterial suspension after being soaked in sterile distilled water for 48 h. Three replicates of each undefined consortia were used to inoculate 5 seeds per replicate that were then sown in pots with 200 g of soil from the sampled areas, depending on the origin of the rhizosphere soil-root sample (Table 1). Each replicate consisted of one pot with three plants inoculated since then after a week of growth, only three plants were left. A negative control of uninoculated seeds, which were soaked in 5 mL of sterile distilled water, and positive control pots inoculated with the PGPR Enterobacter hormaechei G10 (Vanegas 2007), which was also adjusted to 0.2 absorbance units at 600 nm, were used. After a week of growth, only three plants per pot were left. The undefined consortia from soils obtained from Meta and Tolima were used to inoculate rice seeds of Fedearroz 369 and Fedearroz 60 cultivars, respectively, because those bacterial suspensions were obtained from rhizosphere soils of those cultivars (Vanegas et al. 2013). The pots were fertilized with K2HPO4 (18 kg P ha−1), K2SO4 (102 kg K ha−1), and a solution of 20 mL of Hoagland’s micronutrients at 20 % (Hoagland and Arnon 1950) at 7 days after seed sowing. At 20 days after seed sowing, each pot was fertilized with 25 mL of a solution of 20 % Hoagland’s micronutrients without N. Finally, 40 days after planting, the dry weight of the stem and roots was determined for the treated plants.
Characterization of the bacterial members of each undefined consortium
To determine the growth-promoting capacity of the members of each bacterial suspension in rice plants, all bacterial morphotypes that grew on three different media were selected. Briefly, the bacterial suspensions that exhibited growth-promoting capacity were grown in NFb + YE broth for 24 h. Serial dilutions were then prepared (10-4–10-8) and plated on three culture media LB, NFb, and NFb + YE. The morphotypes observed on the three media were purified and stored at −20 °C. Nitrogenase activity and indolic compound production were evaluated for each morphotype as previously described. To evaluate growth promotion in rice plants, the methodology described previously was followed, with the exception that the seeds were sown in peat and fertilized with a solution of 50 % Hoagland’s micronutrients (Hoagland and Arnon 1950) without N. Bacterial suspensions prepared at 0.2 optical densities at 600 nm of each isolate (approximately 108 CFU. mL−1), obtained from each culture medium, were evaluated individually and by mixing equal volumes of all isolates that belong to the same culture medium. For each treatment, four replicates were used. Each undefined consortium with growth-promoting activity and their respective morphotypes were evaluated under a completely randomized design in a single test. Bacteria with growth-promoting capacity were selected and identified by the amplification of 16S rDNA using the universal primers 27f and 1492r under standard conditions (Martin-Laurent et al. 2001). The PCR products were sequenced by Macrogen Inc., and the taxonomic assignment was conducted using the SeqMatch tool from the Ribosomal Database Project (Cole et al. 2009).
Statistical analysis
Significant differences between treatments were evaluated using ANOVA (P ≤ 0.05). The treatment means were compared using the Tukey test, with the exception of the greenhouse experiments, where a least significant difference test (LSD) was used. The trials that did not meet the assumptions of ANOVA were differentiated using the Kruskal-Wallis test (Kruskal and Wallis 1952) in the statistical program Minitab 14.1.
Results
Physicochemical analysis
The soil samples from the three zones of study were differentiated by various chemical parameters (Table 1). However, it is worth mentioning that soils did not differ significantly in % TN, % OC, % clay, % sand, or % silt. Furthermore, they did not differ in the concentrations of K, P, and micronutrients such as Mn and B.
In order to have a general view of the effect of enrichment on the nitrogenase activity and indolic compound production from a whole set of undefined consortia obtained per farm, the average of replicates of every integrated sample from each cropland was summarized in Table 2. In this context the tendency of undefined consortia obtained from rhizosphere/root samples from Tolima was to increase nitrogenase activity, whereas undefined consortia from Meta did not show any increase in the nitrogenase activity by continuous enrichment (Table 2). On the other hand, the production of indolic compounds increased in 50 % of the undefined consortia in no particular pattern. Two of the averaged samples were from upland areas and two were from each of the irrigated crop zones. The production of indolic compounds decreased in 33.33 % of the analyzed undefined consortia (two were from upland crops and two were from irrigated crops) (Table 2).
Promotion of growth by each undefined consortia
For assays under greenhouse conditions, three replicates of each undefined consortium from the 12 farms were evaluated as independent treatments for the first and fifth generations. Of 72 bacterial suspensions evaluated, 3 showed significant increases in the promotion of rice plant growth compared to uninoculated plants. An undefined consortium of the first generation from cropland 10, irrigated Tolima (G1.F10.1.IT), increased the dry weight of stems by 43.52 % compared to the control (Table 3). Undefined consortia of the fifth generation, G5.F9.2.IT (Table 4) and G5.F1.2.UM (Table 5), increased the dry weight of stems by 193.17 %, and 65.63 % respectively, compared with uninoculated controls.
Plant growth promotion assays for undefined consortia and their members in rice plants under greenhouse conditions
As previously mentioned, three undefined consortia promoted plant growth compared to uninoculated controls (Tables 3, 4 and 5). Single isolates and mixed inoculants developed by mixing strains according to the selection medium (LB, NFb or NFb + YE) were used to inoculate rice seeds. Inoculation of rice plants with the undefined consortium G1.F10.1.IT and 6 of its isolates and 2 mixtures showed significant increases in root dry weight by at least 70.60 % (Table 3) (All percent increases are in comparison to control). The mixture of strains S8 and S9 showed the largest increase in foliar dry weight (108.49 %) and root dry weight (44.03 %). From the undefined consortium G5.F9.2.IT, 5 isolates and one bacterial mixture (S5/S6/S7/S8) promoted increases in root dry weight by up to 88.87 % (Table 4). With respect to the undefined consortium G5.F1.2.UM, the bacterial mixture (S3/S4/S6) increased root dry weight by 57.10 % and foliar dry weight by 92.57 % (Table 5). The strains that showed the greatest increases in foliar dry weight were S8, S10, and S6 (Table 5).
Indolic compound production and nitrogenase activity of isolated strains
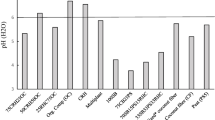
The three bacterial suspensions from undefined consortia with plant growth-promoting activity presented lower indolic compound production than those recorded for the isolates (Fig. 1). Isolates of the undefined consortium G5.F9.2.IT exhibited indolic compound production of between 84.99 and 160.16 μg mL−1. Isolates of the undefined consortium G5.F1.2.UM exhibited indolic compound production of between 66.83 and 144.62 μg mL−1, and isolates of the undefined consortium G1.F10.1.IT exhibited indolic compound production of between 37.55 and 181.48 μg mL−1 (Fig. 1). Despite the fact that the bacterial suspensions from undefined consortia were selected in NFb culture medium, theoretically favoring obtaining diazotrophs, they exhibited low nitrogenase activity: 6.97, 4.56, and 0.46 nmol of ethylene h−1 for G5.F1.2.UM, G5.F9.2.IT, and G1.F10.1.IT, respectively. None of the isolates obtained from the three undefined consortia exhibited nitrogenase activity greater than 0.1 nmol ethylene h−1.
Indolic compound production (μg mL−1 of indolic acetic acid, AIA) of strains (S) isolated from three undefined consortia (C), G5.F9.2.IT, G1.F10.1.IT, and G5.F1.2.UM, exhibiting growth promotion activity in rice plants. The bars represent the means of three replicates with their respective standard errors. Different letters represent significantly different results at P ≤ 0.05
Molecular identification
The strains of the undefined consortium G1.F10.1.IT were identified as: Enterobacter sp. strains S2, S3, S5, S6, S8, and S9 (accession numbers JQ779327, JQ779328, JQ779330, JQ779331, JQ779333, and JQ779334 respectively); Chryseobacterium sp. strains S4 and S7 (accession numbers JQ779329 and JQ779332 respectively); and Lactococcus sp. strain S6 (JQ779331). The strains of the bacterial suspension G5.F9.2.IT (S5/S6/S7/S8), which exhibited the highest increase of root dry weight (88.87 %) compared to control, belong to the genus Enterobacter sp (accession numbers JQ779335, JQ779336, and JQ779337 for S5, S6, and S7, respectively) with the exception of strain S8, which belongs to the genus Acinetobacter sp (JQ779338).
Discussion
The selection of strains for the development of a mixed inoculant is a critical step (Bhattacharjee et al. 2008). No standard protocol is available to create a successful mixed inoculum; however, the combination of different PGPR with potentially complementary mode of actions is most likely the most commonly used criterion (Belimov et al. 1995; Wani et al. 2007; Adesemoye and Kloepper 2009). Despite the obvious convenience of such an approach, it does not consider compatibility in terms of the microbial interactions between strains at the niche level (de Freitas 2000; Rojas et al. 2001; Oliveira et al. 2002; Felici et al. 2008; Xu et al. 2011). This highlights the importance of implementing methodologies that promote the selection of bacterial mixtures that exhibit good growth-promotion capacity and already interact within the rhizosphere.
The methodology proposed in this study allows researchers to obtain undefined consortia and mixed inoculants with high plant growth-promoting capacity using an enrichment strategy through five consecutive subcultures in a nitrogen-free medium from different root/rhizosphere bacterial suspensions. This strategy is a valuable tool for the development and establishment of mixed inoculants with plant growth-promoting activity without the need to further evaluate the compatibility between the members of the mixed inoculants. In addition, single strains with plant growth-promoting activity were also obtained (Tables 3, 4 and 5).
The enrichment through five consecutive passes evaluated in this study allowed the selection of more efficient nitrogen-fixing undefined consortia from irrigated rice fields at Tolima (Table 2). In contrast, undefined consortia from upland and irrigated fields at Meta did not show any increase in nitrogen-fixation capacity. The crops at Tolima are fertilized with twice as much applied nitrogen than those at Meta, where, on average, 100 kg N ha−1 is used. The large inputs of N applied to the fields of Tolima and even Meta may inhibit the nitrogenase activity of most diazotrophic bacteria (Vanegas et al. 2013), as it has been shown that applications of between 20 and 100 kg of N ha−1 reduce N2 fixing activity by up to 85 % (Shrestha and Maskey 2005). In addition, the crops of Tolima presented better fertility parameters (i.e., CEC and nutrient availability) than crops of Meta (Table 1), which may have allowed the establishment of a greater diversity of diazotrophs (Ueda et al. 1995; Rao et al. 1998). Consequently, Tolima samples may provide conditions that induce a faster response to the selection pressure associated with subculturing in a N-free medium (Döbereiner 1995).
After obtaining these results, the effect of subculturing up to the tenth generation was evaluated (data not shown). The results showed that only one undefined consortia from the upland crops (4 UM) of Meta exhibited increased nitrogen-fixing capacity (22.38 nmol of ethylene h−1). However, the four undefined consortia from irrigated crops at Meta either maintained (IM6) a relatively high value (22.68 nmol of ethylene h−1) or increased the nitrogenase activity to between 9.13 nmol of ethylene h−1 (IM5) and 22.33 nmol of ethylene h−1 (IM7) after the tenth generation (data not shown). These results support the hypothesis that continuous subculturing of an undefined consortium obtained from rhizosphere/root soil samples of rice on an N-free medium enriches the diazotrophic microbial community that is able to fix nitrogen. However, the number of generations needed to select a microbial community with improved N-fixing capacity is sample specific and depends not only on the origin of the samples, but also on the original physico-chemical conditions of the crop from where the samples are taken, most likely due to the original structure of the diazotrophic microbial community. In fact, it has been reported that under flooded conditions, N fixation is increased due to an increase in the availability of nutrients and microhabitats with different oxygen tensions that allow the establishment of a wider variety of diazotrophs (Ueda et al. 1995; Roger 1995; Liesack et al. 2000). This finding may explain why the selection response in terms of nitrogen fixation capacity was better for the irrigated than the upland croplands.
Enrichment in NFb culture medium has been widely used to obtain diazotrophic PGPR (Döbereiner 1995; Jha et al. 2009). However, the bacterial suspensions obtained through such enrichment have not been used directly as inocula to promote plant growth. We do not know whether the strains that were isolated from each undefined consortia were representative of the complete bacterial community that promoted the growth of rice plants because we are limited to evaluating only the culturable bacteria favored by the nutritional conditions of each medium. In addition, it is unknown whether these isolates were present at different abundance levels within the undefined consortia. It is worth mentioning that in each culture medium different strains predominated, which indicates the variability of the culturable microbial community within each undefined consortia.
The continuous enrichment strategy permitted us to obtain mixtures of rhizobacteria and individual rhizobacterial strains with growth-promoting activity in rice plants (Tables 3, 4 and 5). For the undefined consortium G1.F10.1.IT, the strain mixture S5/S6/S7 promoted increased root and stem dry weights; however, when these strains were evaluated individually, each by itself did not have a significant effect on plant growth (Table 3). Similar results were shown for the strain mixture S3/S4/S6 of the bacterial consortia G5.F1.2.UM (Table 5). Synergistic effects on growth promotion with significant increases in total N uptake and rice yield have been previously reported when using Azotobacter armeniacus and A. nigricans together, but no effect was observed when either of these strains was used individually (Piao et al. 2005). A similar result was reported by Nandakumar et al. (2001).
Despite the relevance of these synergistic relationships, the mechanisms that mediate these interactions have not yet been established. It has been suggested that low molecular weight molecules associated with cellular communication such as N-acyl homoserine lactones (AHL), may contribute with the establishment of such interactions. Those molecules not only regulate diverse behaviors in rhizosphere inhabiting bacteria (Holguin and Bashan 1996; Ortiz-Castro et al. 2009), but can also trigger an extensive range of functional responses in plants (Mathesius et al. 2003). A preliminary characterization of the presence of this kind of molecules, using the biosensor Chromobacterium violaceum CV026 in cross-streak experiments was done as suggested by McClean et al. (1997) for the members of consortium G1.F10.1.IT. Strains S5, S8 and S9 were positive for the presence of AHL (results not shown), suggesting a possible communication at least between strains S8 and S9 that were tested together as plant growth-promoting mix of bacteria (Table 3). We may speculate that the presence of such molecules explains the effect on growth promotion of the mix of those strains, however even if such communication exists it did not improve the growth promotion capacity of S9 strain that is the one that presented growth promotion activity when tested alone. Further experiments, out of the scope of this study, should be done to demonstrate the role of AHL in the growth promotion capacity of S8-S9 mix.
It is considered that the success of mixed inoculants is due to synergism between promotion mechanisms (Rojas et al. 2001; Raja et al. 2006), the ability to occupy different niches within the plant, which produces a cooperative effect in terms of growth promotion and a more competitive bacterial mix (Govindarajan et al. 2007), metabolic synergy that increases the availability of nutrients (Shrestha et al. 2007), removal of inhibitory products such as oxygen (Bashan 1998; Minamisawa et al. 2004), and stimulation of plant promotion mechanisms (Holguin and Bashan 1996). Further studies are needed to determine which of these mechanisms, or possibly other mechanisms, may contribute to the plant growth promotion effect of the bacterial mixes obtained in this study.
Forty-five percent of single strains that were obtained from the three undefined consortia had the capacity to promote plant growth in terms of either root and/or stem dry weight. However, there was no correlation between the growth promotion and nitrogen fixation capacity, as the strains isolated from the different undefined consortia exhibited negligible nitrogenase activity compared to the original suspensions. Similar results were found by Holguin et al. (1992) and Minamisawa et al. (2004) when they isolated and evaluated the nitrogenase activity of strains obtained from enrichment in N-free media. Of the undefined consortia, 50.00 % increased the production of indolic compounds (Table 2). Again, no correlation was observed between the production of indolic compounds and growth promotion activity.
The undefined consortia that promoted growth under greenhouse conditions presented neither high nitrogenase activity nor high indolic compound production (Fig. 1). However, the strains obtained from each of the three undefined consortia presented low or undetectable nitrogenase activity, whereas the production of indolic compounds was present and relatively high for most strains (Fig. 1). This result is consistent with the mode of action of other diazotrophic PGPR such as Azospirillum brasilense, the main mode of action of which, despite its status as a nitrogen-fixing bacterium, is related to the production of plant growth-regulating substances such as auxins (Spaepen et al. 2008). The results suggest that besides the auxin production, other modes of action might be involved in the plant growth promotion activity of the isolated strains. These modes include the production of other plant growth regulators such as gibberellins and/or cytokinins (Azcon and Barea 1975), phosphate solubilization (Yu et al. 2012), siderophores production (Sharma and Johri 2003), regulation of the endogenous content of growth regulator substances through mechanism such as ACC deaminase (Glick 2013) and colonization (Verma et al. 2001; Steenhoudt and Vanderleyden 2000), mechanisms that will be studied in the future for the growth promoting consortia and strains described in this study to understand their mode of action as plant growth promoters.
The bacterial consortium G1.F10.1.IT included 5 morphotypes belonging to the family Enterobacteriaceae. Similarly, the mixture S5/S6/S7/S8 from the bacterial consortium G5.F9.2.IT included 3 morphotypes of the genus Enterobacter. Several genera of Enterobacteriaceae have been found in the rhizosphere of rice plants and were reported as PGPR (Barraquio et al. 2000; Mehnaz et al. 2001; Verma et al. 2001; Kennedy et al. 2004; Shankar et al. 2011), suggesting an important role of this taxonomic group in the rhizosphere of rice (Ladha and Reddy 2000). The ubiquity of this genus in the rhizosphere has been attributed to its rapid growth, strong seed adherence, root colonization capacity, metabolic diversity, and use of multiple mechanisms to promote plant growth (Barraquio et al. 2000; Shankar et al. 2011). This may explain why, despite their putative constraints on human health, several reports exist of the use of mixed inoculants using rhizobacteria, including some members of the Enterobacteriaceae (Holguin and Bashan 1996; Shrestha et al. 2007; Mehnaz et al. 2010). The other rhizobacteria found in the undefined consortia belong to the genus Chryseobacterium, which has been reported to act as a biocontrol for rice blast through the induction of systemic resistance and to increase the productivity of rice (Lucas et al. 2009). The use of Lactococcus as PGPR in rice has not been reported. However, this genus is widely distributed in rice silage (Ennahar et al. 2003) and has been recognized as a producer of bacteriocins (Delves-Broughton et al. 1996) and of lactic acid from rice straw (Fukushima et al. 2004).
Conclusions
The continuous enrichment strategy using up to five passages proved to be a good methodology for the selection of undefined consortia with increased nitrogenase activity from rhizosphere/root samples of irrigated crops at Tolima. More importantly, this relatively simple strategy resulted in three bacterial suspensions of undefined consortia, five mixtures of rhizobacteria, and 11 individual rhizobacterial strains that exhibited growth-promoting capacity in rice plants. Although the continuous enrichment strategy increased nitrogenase activity and the production of auxins in some undefined consortia, the mechanisms of action of the consortia and even of strains or mixtures of strains that promoted growth were not identified. Currently, new studies are being carried out in our laboratory to identify the mode of action and the potential of the selected strains to be developed as inoculants.
References
Adesemoye AO, Kloepper JW (2009) Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol 85:1–12
Alam MS, Cui Z, Yamagishi T, Ishii R (2001) Grain yield and related physiological characteristics of rice plants (Oryza sativa L.) inoculated with free-living rhizobacteria. Plant Prod Sci 4:126–130
Azcon R, Barea JM (1975) Synthesis of auxins, gibberellins and cytokinins by Azotobacter vinelandii and Azotobacter beijerinckii related to effects produced on tomato plants. Plant Soil 43:609–619
Barraquio WL, Segubre EM, Gonzalez MAS, Verma SC, James EK, Ladha JK, Tripathi AK (2000) Diazotrophic Enterobacteria: what is their role in the rhizosphere of rice. In: Ladha JK, Reddy PM (eds) The quest for nitrogen fixation in rice. International Rice Research Institute, Makati, pp 93–118
Bashan Y (1998) Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol Adv 16:729–770
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003). Can J Microbiol 50:521–577
Belimov AA, Kojemiakov AP, Chuvarliyeva CV (1995) Interaction between barley and mixed cultures of nitrogen fixing and phosphate-solubilising bacteria. Plant Soil 173:29–37
Bhattacharjee RB, Singh A, Mukhopadhyay SN (2008) Use of nitrogen-fixing bacteria as biofertiliser for non-legumes: prospects and challenges. Appl Microbiol Biotechnol 80:199–209
Choudhury A, Kennedy IR (2004) Prospects and potentials for systems of biological nitrogen fixation in sustainable rice production. Biol Fertil Soils 39:219–227
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:141–145
de Freitas JR (2000) Yield and N assimilation of winter wheat (Triticum aestivum L., var. Norstar) inoculated with rhizobacteria. Pedobiologia 44:97–104
Delves-Broughton J, Blackburn P, Evans RJ, Hugenholtz J (1996) Applications of the bacteriocin, nisin. Antonie Van Leeuwenhoek 69:193–202
Döbereiner J (1995) Isolation and identification of aerobic nitrogen-fixing bacteria from soil and plants. In: Alef K, Nannipien P (eds) Methods in applied soil microbiology and biochemistry. Academic, London, pp 134–141
Ennahar S, Cai Y, Fujita Y (2003) Phylogenetic diversity of lactic acid bacteria associated with paddy rice silage as determined by 16S ribosomal DNA analysis. Appl Environ Microbiol 69:444–451
Fedearroz (2000) Manejo y conservación de suelos para la producción de arroz en Colombia. Fedearroz - Fondo Nacional del Arroz, Bogotá
Felici C, Vettori L, Giraldi E, Forino LMC, Toffanin A, Tagliasacchi AM, Nuti M (2008) Single and co-inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicum esculentum: Effects on plant growth and rhizosphere microbial community. Appl Soil Ecol 40:260–270
Fukushima K, Sogo K, Miura S, Kimura Y (2004) Production of D lactic acid by bacterial fermentation of rice starch. Macromol Biosci 4:1021–1027
Glick BR (2013) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. doi:10.1016/j.micres.2013.09.009
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
Govindarajan M, Kwon SW, Weon HY (2007) Isolation, molecular characterization and growth-promoting activities of endophytic sugarcane diazotroph Klebsiella sp. GR9. World J Microbiol Biotechnol 23:997–1006
Govindarajan M, Balandreau J, Kwon SW, Weon HY, Lakshminarasimhan C (2008) Effects of the inoculation of Burkholderia vietnamensis and related endophytic diazotrophic bacteria on grain yield of rice. Microb Ecol 55:21–37
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular, Berkeley
Holguin G, Bashan Y (1996) Nitrogen-fixation by Azospirillum brasilense Cd is promoted when co-cultured with a mangrove rhizosphere bacterium (Staphylococcus sp.). Soil Biol Biochem 28:1651–1660
Holguin G, Guzman MA, Bashan Y (1992) Two new nitrogen-fixing bacteria from the rhizosphere of mangrove trees: their isolation, identification and in vitro interaction with rhizosphere Staphylococcus sp. FEMS Microbiol Lett 101:207–216
Horwitz W (ed) (2000) Official methods of analysis of AOAC international. AOAC International, Gaithersburg
Jha B, Thakur MC, Gontia I, Albrecht V, Stoffels M, Schmid M, Hartmann A (2009) Isolation, partial identification and application of diazotrophic rhizobacteria from traditional Indian rice cultivars. Eur J Soil Biol 45:62–72
Kassen R, Rainey PB (2004) The ecology and genetics of microbial diversity. Annu Rev Microbiol 58:207–231
Kennedy IR, Choudhury A, Kecskés ML (2004) Non-symbiotic bacterial diazotrophs in crop-farming systems: can their potential for plant growth promotion be better exploited? Soil Biol Biochem 36:1229–1244
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47:583–621
Ladha JK, Reddy PM (2000) The quest for nitrogen fixation in rice. International Rice Research Institute, Makati
Li X, Li P, Lin X, Zhang C, Li Q, Gong Z (2008) Biodegradation of aged polycyclic aromatic hydrocarbons (PAHs) by microbial consortia in soil and slurry phases. J Hazard Mater 150:21–26
Liesack W, Schnell S, Revsbech NP (2000) Microbiology of flooded rice paddies. FEMS Microbiol Rev 24:625–645
Lucas JA, Ramos Solano B, Montes F, Ojeda J, Megias M, Gutierrez Mañero FJ (2009) Use of two PGPR strains in the integrated management of blast disease in rice (Oryza sativa) in Southern Spain. Field Crops Res 114:404–410
Marron-Montiel E, Ruiz-Ordaz N, Rubio-Granados C, Juárez-Ramírez C, Galíndez-Mayer CJ (2006) 2,4-D-degrading bacterial consortium isolation, kinetic characterization in batch and continuous culture and application for bioaugmenting an activated sludge microbial community. Process Biochem 41:1521–1528
Martin-Laurent F, Philippot L, Hallet S, Chaussod R, Germon JC, Soulas G, Catroux G (2001) DNA extraction from soils: old bias for new microbial diversity analysis methods. Appl Environ Microbiol 67:2354–2359
Mathesius U, Mulders S, Mengsheng G, Teplikski M, Caetano-Agolles G, Rolfe BG, Bauer WD (2003) Extensive and specific responses of a eukaryote bacterial quorum-sensing signals. Proc Natl Acad Sci U S A 100:1444–1449
McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P (1997) Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703–3711
Mehnaz S, Mirza MS, Haurat J, Bally R, Normand P, Bano A, Malik KA (2001) Isolation and 16S rRNA sequence analysis of the beneficial bacteria from the rhizosphere of rice. Can J Microbiol 47:110–117
Mehnaz S, Kowalik T, Reynolds B, Lazarovits G (2010) Growth promoting effects of corn (Zea mays) bacterial isolates under greenhouse and field conditions. Soil Biol Biochem 42:1848–1856
Minamisawa K, Nishioka K, Miyaki T, Ye B, Miyamoto T, You M, Saito A, Saito M, Barraquio WL, Teaumroong N (2004) Anaerobic nitrogen-fixing consortia consisting of clostridia isolated from gramineous plants. Appl Environ Microbiol 70:3096–3102
Nandakumar R, Babu S, Viswanathan R, Sheela J, Raguchander T, Samiyappan R (2001) A new bio-formulation containing plant growth promoting rhizobacterial mixture for the management of sheath blight and enhanced grain yield in rice. Biocontrol 46:493–510
Newton JA, Fray RG (2004) Integration of environmental and host derived signals with quorum sensing during plant-microbe interactions. Cell Microbiol 6:213–224
Oliveira ALM, Urquiaga S, Döbereiner J, Baldani JI (2002) The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 242:205–215
Ortiz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bocia J (2009) The role of microbial signals in plant growth and development. Plant Signal Behav 4:701–712
Piao Z, Cui Z, Yin B, Hu J, Zhou C, Xie G, Su B, Yin S (2005) Changes in acetylene reduction activities and effects of inoculated rhizosphere nitrogen-fixing bacteria on rice. Biol Fertil Soils 41:371–378
Raja P, Uma S, Gopal H, Govindarajan K (2006) Impact of bio inoculants consortium on rice root exudates, biological nitrogen fixation and plant growth. J Biol Sci 6:815–823
Rao VR, Ramakrishnan B, Adhya TK, Kanungo PK, Nayak DN (1998) Review: Current status and future prospects of associative nitrogen fixation in rice. World J Microbiol Biotechnol 14:621–633
Rennie RJ (1981) A single medium for the isolation of acetylene-reducing (dinitrogen-fixing) bacteria from soils. Can J Microbiol 27:8–14
Rodrigues EP, Santos L, Martinez AL, Baldani VLD, dos Santos KR, Urquiaga S, Massena (2008) Azospirillum amazonense inoculation: effects on growth, yield and N2 fixation of rice (Oryza sativa L.). Plant Soil 302:249–261
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Roger PA (1995) Biological N2-fixation and its management in wetland rice cultivation. Nutr Cycl Agroecosyst 42:261–276
Rojas A, Holguin G, Glick BR, Bashan Y (2001) Synergism between Phyllobacterium sp (N2 fixer) and Bacillus licheniformis (P solubilizer), both from a semiarid mangrove rhizosphere. FEMS Microbiol Ecol 35:181–187
Ruíz-Sánchez A, Armada E, Muñoz Y, García IE, Aroca R, Ruíz-Lozano JM, Azcon R (2011) Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J Plant Physiol 168:1031–1037
Shankar M, Ponraj P, Ilakiam D, Gunasekaran P (2011) Root colonization of a rice growth promoting strain of Enterobacter cloacae. J Basic Microbiol 51:523–530
Sharma A, Johri BN (2003) Combat of iron-deprivation through a plant growth promoting fluorescent Pseudomonas strain GRP3A in mung bean (Vigna radiata L Wilzech). Microbiol Res 158:77–81
Shrestha RK, Maskey SL (2005) Associative nitrogen fixation in lowland rice. Nepal Agric Res J 6:112–121
Shrestha A, Toyota K, Okazaki M, Suga Y, Quevedo MA, Loreto AB, Mariscal AA (2007) Enhancement of nitrogen-fixing activity of Enterobacteriaceae strains isolated from sago palm (Metroxylon sagu) by microbial interaction with non-nitrogen fixers. Microbes Environ 22:59–70
Spaepen S, Dobbelaere S, Croonenborghs A, Vanderleyden J (2008) Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant Soil 312:15–23
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free living nitrogen fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Ueda T, Suga Y, Yahiro N, Matsuguchi T (1995) Remarkable N2-fixing bacterial diversity detected in rice roots by molecular evolutionary analysis of nifH gene sequences. J Bacteriol 177:1414–1417
Vanegas J (2007) Mitigación del estrés salino en plántulas de Avicennia germinans y Capsicum annuum por bacterias promotoras de crecimiento vegetal. Dissertation, Universidad Nacional de Colombia
Vanegas J, Landazabal G, Melgarejo LM, Beltran M, Uribe-Vélez D (2013) Structural and functional characterization of the microbial communities associated with the upland and irrigated rice rhizospheres in a neotropical Colombian savannah. Eur J Soil Biol 55:1–8
Verma SC, Ladha JK, Tripathi AK (2001) Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol 91:127–141
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Wani PA, Khan MS, Zaidi A (2007) Synergistic effect of the inoculation with nitrogen-fixing and phosphate–solubilizing rhizobacteria on performance of field-grown chickpea. J Plant Nutr Soil Sci 170:283–287
Xu XM, Jeffries P, Pautasso M, Jeger MJ (2011) Combine use of biocontrol agents to manage plant disease in theory and practice. Phytophatology 101:1024–1031
Yu X, Liu X, Zhu TH, Liu GH, Mao C (2012) Co-inoculation with phosphate-solubilizing and nitrogen-fixing bacteria on solubilization of rock phosphate and their effect on growth promotion and nutrient uptake by walnut. Eur J Soil Biol 50:112–117
Zlotnikova AK, Kazakova ML, Zlotnikov KM, Kazakov AV, Umarov MM (2007) Physiological and biochemical properties of the bacterial association of Klebsiella terrigena E6 and Bacillus firmus E3. Appl Biochem Microbiol 43:304–312
Acknowledgments
This work was funded by the Ministry of Agriculture and Rural Development of Colombia, Universidad Nacional de Colombia and the Colombian Federation of Rice (Fedearroz). JV was the beneficiary of a doctoral fellowship awarded by Colciencias-Icetex-Sena. Authors want to acknowledge the critical review of Dr Vera Baldani and Kristina Lyons to the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jesus Mercado-Blanco.
Rights and permissions
About this article
Cite this article
Vanegas, J., Uribe-Vélez, D. Selection of mixed inoculants exhibiting growth-promoting activity in rice plants from undefined consortia obtained by continuous enrichment. Plant Soil 375, 215–227 (2014). https://doi.org/10.1007/s11104-013-1960-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1960-1