Abstract
Background and aims
In Mediterranean steppes, Stipa tenacissima tussocks facilitate the establishment of vascular plants. We hypothesized that this effect may partially reflect the indirect interaction between Stipa tenacissima, biological soil crusts (BSC), and seeds.
Methods
We explored the relationship between BSC composition and soil surface conditions (surface roughness and hydrophobicity by using the water drop penetration time test), and seed germination and seedling rooting in a S. tenacissima steppe in southeastern Spain. We explored the causal factors of seed germination at two spatial scales and used SADIE index to represents the soil surface heterogeneity.
Results
Microsites strongly differed in BSC composition and soil surface conditions. Germination of two key species, Pistacia lentiscus and Brachypodium retusum, was not affected by BSC type. In contrast, rooting was lower on soil from open areas covered by BSC than on soil from open areas dominated by bare soil and soil collected under the tussocks. The effect was similar in both species. Lichens were probably responsible for the decrease in rooting.
Conclusions
Our results suggest that lichen cover and the cover of bare soil and mosses may hamper and facilitate rooting, respectively. By affecting seedling rooting, BSC may contribute to the facilitative effect of Stipa tenacissima.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological soil crusts (BSC) cover open spaces between vascular plants in arid and semiarid regions throughout the world. BSC are integrated by associations of cyanobacteria, mosses, lichens and green algae widely distributed in the surface soil layer (Belnap and Gillette 1998; Briggs and Morgan 2011). BSC may control soil erosion (Belnap 2001; Zhang et al. 2010), increase soil fertility through nitrogen and carbon fixation (Belnap 1994, 2002), and help retaining plant available nutrients (Kimball and Belnap 2001; Belnap et al. 2004; Delgado-Baquerizo et al. 2013). Likewise, abundance and type of BSC affect water infiltration rate and other soil functions, even at small spatial scales (Maestre et al. 2005).
The effect of BSC upon seed germination can be positive, negative or neutral (Zaady et al. 1997; Boeken et al. 1998; Prasse and Bornkamm 2000; Rivera-Aguilar et al. 2005; Deines et al. 2007; Escudero et al. 2007; Godínez-Alvarez et al. 2011). BSC may affect seed performance and influence the dynamics of vascular plants by hampering seed transport and modifying seed microenvironment. BSC tortuosity or roughness may also influence water infiltration, runoff, and other ecosystem functions affecting seed performance (Belnap 2001). In addition, hydrophobicity associated with some BSC types affects water redistribution and rainwater infiltration (Maestre and Cortina 2002), and may also affect seed germination and seedling establishment (Prasse and Bornkamm 2000). BSC effects on the early stages of plant life cycle may also be related to plant morpho-functional traits (Prasse and Bornkamm 2000; Quintana-Ascencio and Menges 2000; Su et al. 2009), and seed morphology (Briggs and Morgan 2011). However, to our knowledge, studies assessing the interactive effects of BSC traits and seed morphology on seed germination and seedling establishment are lacking.
In semiarid steppes of southeastern Spain, vascular plant germination and establishment on open spaces may be lower than in the vicinity of the dominant Stipa tenacissima L. tussocks (Maestre et al. 2001; Barberá et al. 2006). We think that coupled with S. tenacissima facilitation, the microenvironment created by biological crusts under S. tenacissima tussocks promotes germination and root penetration, whereas open areas dominated by bare soil may have the opposite effect. Likewise, BSC hydrophobicity and soil surface roughness may hamper and promote seed germination, respectively, by affecting moisture availability. We investigated the effects of different biological and physical crusts on seed germination and early rooting of two key vascular plant species of S. tenacissima steppes in southeastern Spain. We explored the causal factors at two spatial scales. At a larger scale, we tested the hypothesis that composition and cover of BSC in different locations within S. tenacissima steppes influence germination rate and root penetration. At a lower scale, our hypothesis is that physical properties as hydrophobicity and soil surface roughness associated to particular BSC morphotypes influence germination and early rooting.
Material and methods
Study area
The study was performed in a S. tenacissima steppe in Aigües (Alicante, southeastern Spain; 460 m asl; 38°31′ N, 0°21′ W). The climate is semiarid, with 388 mm mean annual rainfall and mean temperature of 16 °C (Maestre and Cortina 2002). The soil is alkaline Lithic calciorthid and the soil texture is silty-clay (Maestre and Cortina 2002). The soil surface, when exposed, is highly prone to form structural crusts that can scarcely retain seeds, sediments, water or litter (See Belnap 2001). Vegetation cover is dominated by the perennial grass S. tenacissima and Rosmarinus officinalis L., with patches of Brachypodium retusum (Pers.) P. Beauv. and other, mostly nanophanerophyte species.
Experimental approach
Biological crusts under vascular plants and in open spaces differ in their composition (Maestre and Cortina 2002; Maestre et al. 2007). Based on this, we performed a stratified sampling at the first level of heterogeneity. We sampled three microenvironments: areas underneath S. tenacissima tussocks (TC), and open areas where BSC or bare soil dominated (OC and OU, respectively). Samples were collected within a 50 m × 50 m experimental plot.
We collected twenty samples per microenvironment in late October 2007. Before sampling, we sprayed the soil surface with distilled water to avoid crust breaking, and a sample of the topsoil layer (8.4 cm diameter, 1 cm depth) was collected with a Petri dish so that the soil surface remained almost unaltered (Appendix I). Samples were divided into two groups of ten Petri dishes, and each group was then sown with either B. retusum or P. lentiscus seeds. However, to characterize BSC microsites in the Petri dishes, 63 samples were employed.
BSC and soil measurements
Soil surface in the Petri dishes was characterized according to their components of BSC (lichens, mosses and cyanobacteria) and bare soil, at the same points where surface roughness was measured (see below).
The lichen community was dominated by Psora crenata in OC and OU microenvironments (15 % and 1 % surface cover, respectively), and Squamarina sp. in TC microenvironments (7 % surface cover). Psora crenata forms small squamules that may be disperse to imbricate. Squamarina sp. forms a squamulose to sub-folious thallus. Fulgensia sp. covered 6 % of the area in OC microenvironments, and less than 2 % of the area in OU and TC microenvironments. Their tallus is squamulose.
Mosses and cyanobacteria were not determined in this study. Most abundant mosses in S. tenacissima steppes in southeastern Spain are small and sparse. They include Dydimodon acutus and Weissia sp. Other less abundant mosses are Gymnostomum lusieri, Aloina rigida and Crossidium sp. (Vicent Calatayud, Fundación CEAM, pers. comm.). Cyanobacteria in these steppes develop into thin films covering the soil surface. Species common in the cyanobacteria community include Microcoleus steentrupii, Leptolyngbya boryanum, L. foveolarum, Oscillatoria sp., Phormidium sp., and Chroococcidiopsis sp. (Maestre et al. 2006).
Soil surface roughness was estimated by measuring relative soil surface height with a pin profilometer at 45 points distributed evenly in a 1 × 1 cm grid throughout the Petri dishes. We considered depth heterogeneity as a measure of topsoil roughness and it was represented by using spatial aggregation index. The aggregation index Ia, measures the relative distances to regularity, where Ia quantifies the general spatial pattern of the analyzed variable indicating whether the sample is randomly distributed (Ia close to unity), aggregated (Ia > 1) or regularly distributed (Ia < 1) (Perry 1998). We used the Spatial Analysis by Distance Indices (SADIE) methodology to calculate Ia index with the software described by Perry et al. (1999). Hence, Ia represents the soil surface heterogeneity for each experimental unit (each microenvironment and Petri dish).
The water drop penetration time test (WDPTT) was used to assess water repellency or hydrophobicity. WDPTT allows making categories as hydrophilic or wettable soil (Letey et al. 2000; Jaramillo-Jaramillo 2006; Bachmann et al. 2007). The test consists of applying a distilled water drop on the soil surface and recording the time (in seconds) required for water to infiltrate. The test was carried out on 24 regularly distributed points over the soil surface in each Petri dish, and the 24 measurements were later averaged. The breakthrough time of water drop was reported into two groups: from the immediate penetration of the water drop to 20 s, the soil was considered wettable; above 20 s, the soil was considered hydrophobic (King 1981; Doerr et al. 2000). Despite its limitations, including the use of distilled water and flat surfaces, the WDPT test has been widely used to evaluate soil hidrophobicity (Jaramillo-Jaramillo 2006; Bachmann et al. 2007).
Seed germination and rooting
We seeded Brachypodium retusum and Pistacia lentiscus on the Petri dishes. These species were selected since they are native key species, which have been considered for the restoration of arid areas in southeastern Spain (Vilagrosa et al. 2003; Cortina et al. 2004; De Baets et al. 2007). Seeds came from Intersemillas, Inc. and the Forest Seed Bank of the Regional Government of Valencia, respectively. Each experimental unit was randomly placed on trays in a growth chamber and was replicated ten times: three microsites, two seeded species, ten replicates (60 Petri dishes in total). We sowed 45 seeds per experimental unit on each point of the grid where roughness was measured. The dishes were placed in a germination chamber with 12 h of photosynthetic active radiation of 600 μmol m−2 s−1 and temperature of 20 °C. Every other day, we watered the trays to keep gravimetric moisture content between 10 % and 30 %. Biological crusts may function at very low soil moisture availability (Delgado-Baquerizo et al. 2013). Seeds were considered as germinated once radicle elongation was detected. For rooting evaluation, we let the samples dry for a week and recorded the number of seedlings whose roots penetrated the soil surface and did not fall on the floor after turning the Petri dish upside down (Appendix I).
Statistical analyses
Data were analyzed at two spatial scales. At the larger scale, we conducted univariate ANOVA to evaluate the effect of microsite (fixed factor with three levels: TC, OC and OU) on BSC cover, hydrophobicity and soil surface roughness. We used bivariate ANOVA and Tukey-HSD post-hoc tests to determine the effect of species and microsite (fixed factors with 2 and 3 levels, respectively) on germination, rooting and the ratio rooting:germination. Percentage germination was transformed to arc sin (germination/100)0.5 to comply with assumptions of normality and homoscedasticity.
At a medium scale, we used nonmetric multidimensional scaling analysis (NMDS) to ordinate Petri dishes in terms of their soil surface cover. This method is well suited to non-normal data, arbitrary, discontinuous or questionable scales (McCune and Grace 2002). The NMDS analyses were performed with R version 2.13.0 (http://www.r-project.org) using isoMDS MASS package (Oksanen et al. 2011), and Bray-Curtis similarity index as distance measure (set.seed(0)). Then, we used Pearson correlation analyses to examine the relationship between NMDS axes 1 and 2 with soil properties (hydrophobicity, roughness and BSC). Seed germination, rooting and the ratio rooting:germination were analyzed separately for each species (B. retusum and P. lentiscus), using stepwise linear regression analyses to identify which components of the BSC, represented by NMDS axes and soil surface features, explained the larger amount of variability of the response variables. All statistical analyses but NMDS and Ia were performed using software SPSS 18.0 for Windows (SPSS Inc. Chicago, IL, USA.).
Results
Soil surface cover and physical properties
BSC cover was highly dependent on microsite (Table 1). Open areas with BSC (OC microsites) had higher cyanobacteria and lichen cover, and lower moss cover than OU and TC microsites. Bare soil was higher in OU microsites and lower in OC microsites. Cyanobacteria and lichen cover in TC and OU microsites were not significantly different. Gravel cover was low and similar in all microsites. OU microsites showed lower hydrophobicity and higher Ia index (i.e., relative height of the soil surface showed an aggregated pattern rather than a random or regular distribution). OC and TC areas did not differ in roughness or hydrophobicity.
Lichen cover was positively correlated with hydrophobicity (r = 0.413, n = 60, p = 0.001) and negatively correlated with bare soil cover (r = 0.608, n = 63, p = 0.001). Bare soil cover was negatively correlated with hydrophobicity (r = 0.449, n = 60, p < 0.001) and positively correlated with surface roughness (Ia; r = 0.311, n = 63, p = 0.013).
Soil surface features, seed germination and rooting
The two species differed in the amount of germination and rooting. Thus, post hoc tests were performed separately for each species. Seed germination was higher in B. retusum than in P. lentiscus (Fig. 1). Microsite had only a marginal effect on seed germination. In contrast, microsites and species had a significant effect on rooting. In B. retusum rooting was higher than in P. lentiscus, and lower in OC than in OU and TC microsites. The proportion of germinated seeds that rooted was also affected by species and microsite. Rooting efficiency was higher in B. retusum than in P. lentiscus, and was lower in OC microsites than in TC microsites. Species by microsites interactions were not significant for any variable measured.
Germination and rooting of two key species of Stipa tenacissima steppes of southeastern Spain seeded on soils from three microsites (OC open areas with BSC, OU open areas bare soil dominated, TC crusted areas underneath Stipa tenacissima tussocks). Means (±SE) for n = 30 and 20 experimental units for species and microsite, respectively. Results of a bivariate ANOVA are shown. Different letters correspond to significant differences by species (Tukey-HSD test, p ≤ 0.05)
NMDS analysis clearly separated the three types of BSC (Fig. 2; stress = 16.15). Axis 1 was negatively correlated with cyanobacteria and lichen cover, and positively correlated with the cover of mosses and bare soil. Axis 2 was negatively correlated with lichen cover and hydrophobicity, and positively correlated with gravel cover. In addition, hydrophobicity and soil surface roughness were marginally correlated with Axis 1 (r = −0.249, n = 60, p = 0.055 and r = 0.245, n = 60, p = 0.053; Appendix II).
Germination was not related to soil surface features in B. retusum (Table 2). In contrast, germination of P. lentiscus seeds was significantly related to NMDS Axis 2. Rooting was dependent on soil surface features, which explained one fourth of the observed variability of the dependent variable in both species. We could not fit a regression model to relate rooting efficiency for any of the two species tested.
Discussion
BSC are key components of dryland ecosystems, as they affect ecosystem processes and community composition. By modifying soil surface properties, BSC may affect seed germination and seedling establishment. However, the complexity of the processes involved prevents generalizations on the outcomes of the interactions between BSC and vascular plants. This complexity is partly the result of heterogeneity in BSC cover and composition.
We have found that soil in open areas covered by BSC (and thus, relatively stable) differ in composition from soil in open areas and soil under S. tenacissima tussocks, where BSC are not dominant. Differences in the relative abundance of mosses under the tussocks compared to open areas had been previously described (Maestre et al. 2001, 2002). In this respect, as moss cover was relatively high in exposed OU microsites, factors other than shade may affect the abundance of mosses. Their presence in OU microsites, and the relatively high cover of bare soil under the tussocks (close to 50 %), suggest that mosses may be early colonizers in these soils, and lichens are unable to establish in such disturbed microsites. Moss dominance under S. tenacissima tussocks could be promoted by the disturbance created by anecic earthworms, whose activity is higher in these microsites (Maestre and Cortina 2002). The successional role of mosses in drylands is controversial (Esposito et al. 1999; Martínez et al. 2006), and probably depends on the intensity and type of disturbance. On the other hand, OC microsites were dominated by cyanobacteria, as described elsewhere (Maestre and Cortina 2002). Lichens were more abundant in OC than in TC microsites, which is in disagreement with previous observations (Maestre and Cortina 2002). Temporal changes in earthworm activity under the tussocks may explain these differences, but information on BSC colonization dynamics in S. tenacissima steppes is lacking. NMDS analysis emphasized the contrast between the three types of microsites and the importance of lichen cover in determining these differences.
As a result of differences in surface features, microsites also differed in physical soil properties. Thus, hydrophobicity was lower and roughness higher in OU than in OC and TC microsites. OC and TC areas did not differ in roughness or hydrophobicity, suggesting that these differences were associated with bare soil cover rather than BSC cover. Low hydrophobicity was probably the result of low cover of lichens and high cover of mosses and bare soil, as suggested by the significant relationships found between hydrophobicity and NMDS Axes. The spatial pattern showed by soil surface roughness may reflect the relatively recent disturbance caused by earthworms in this microsite. It is worth noting the strong tendency of exposed silty soils, as those common in the area, to form smooth structural crusts (Valentin and Bresson 1992; Cortina et al. 2010).
Germination was weakly affected by microsite. This was probably due to the relative high water content of the soil surface in the experimental units, which probably buffered the effect of soil cover on seed performance. Despite that wet conditions in the soil surface may occasionally occur (De Luís et al. 2001), in our experiment, which prioritized seed germination, soil surface was probably too wet, compared to what is common in the field. However, it is important to note that recruitment of P. lentiscus concentrates in these rare events when soil moisture is high during a prolonged period of time (García-Fayos and Verdú 1998), and thus, the effect of BSC on germination of this species would be reduced under field conditions as well. Studies on the effect of BSC on germination of vascular plants show contrasting results (Zaady et al. 1997; Prasse and Bornkamm 2000; Rivera-Aguilar et al. 2005; Deines et al. 2007; Godínez-Alvarez et al. 2011) which may reflect differences in BSC composition and morphology, soil properties and the ecological strategy of colonizing plants (Belnap 2001).
Microsite had a strong effect on seedling rooting. OC microsites decreased rooting in both species, compared to OU and TC microsites. Similar results were obtained by Deines et al. (2007). The decrease in rooting was related to the cover of the surface soil as shown by regression models (NMDS Axes 1 and 2). Direct observations suggest that lichens represent a physical barrier for rooting, and thus, they may hamper establishment. Following our observations, both lichen cover and spatial distribution (e.g., distance to discontinuities between adjacent lichens) may be relevant for rooting. Similarly, lichen disturbance may facilitate rooting. Rooting efficiency, that is the ratio between rooting and germination, was also sensitive to microsite. In this case, OC and TC microsites differed significantly, whereas OC and OU showed no statistical differences. Soil surface composition in TC microsites suggests that mosses and bare soil may have facilitated seedling rooting, although we could not fit any simple model to these data.
The effect of microsite on seed germination and seedling rooting was similar in both species. Indeed species x microsite interactions were not statistically significant for any of the three response variables studied, suggesting that the effect of microsite was strong enough to override differences in seed morphology under the experimental conditions of our study.
Finally, comparisons between B. retusum and P. lentiscus may be confounded by uncontrolled differences in seed quality and storage conditions. However, it is worth noting that in both germination and rooting, B. retusum performed better than P. lentiscus. Differences in germination and rooting may be related to differences in seed morphology. Brachypodium retusum seeds are long and narrow, compared to bean-shaped P. lentiscus seeds, and may favor contact with the underlying soil (Briggs and Morgan 2011). On the other hand, P. lentiscus recruitment is promoted by facilitative interactions with other vascular plants, including S. tenacissima (Maestre et al. 2001). The main driver of this interaction is concentrated seed dispersion and shade (Verdú and García-Fayos 1996; Maestre et al. 2003). Our results suggest that soil surface features may further hamper establishment by creating a physical filter to seedling rooting, and conversely, soil surface under S. tenacissima tussocks may facilitate P. lentiscus recruitment.
In conclusion, the soil surface in a S. tenacissima steppe showed sharp contrasts in composition and function between different microsites. These differences had a weak effect on the germination of two key species, but a large effect on seedling rooting. Open crusted areas, particularly where lichens dominate, represent a barrier for rooting. Recruitment may be promoted by the tussocks. This mechanism may contribute to the facilitative effect of S. tenacissima on the establishment of key vascular plants.
References
Bachmann J, Deurer M, Arye G (2007) Modeling water movement in heterogeneous water-repellent soil: 1. Development of a contact angle-dependent water-retention model. Vadose Zone J 6:36–445
Barberá GG, Navarro-Cano JA, Castillo VM (2006) Seedling recruitment in a semi-arid steppe: the role of microsite and post-dispersal seed predation. J Arid Environ 67:701–714
Belnap J (1994) Potential of cryptobiotic soil crust in semiarid rangeland. In: Monsen SB, Ketchumn SG (eds) Proceedings-ecology and management of annual rangelands. US Department of Agriculture, Forest Service, General Technical Report INT-GTR-313. Ogden, Utah, pp 179–185
Belnap J (2001) Comparative structure of physical and biological soil crust. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Springer, Berlin, pp 177–191
Belnap J (2002) Nitrogen fixation in biological soil crusts from southeast Utah, USA. Biol Fertil Soils 35:128–135
Belnap J, Gillette DA (1998) Vulnerability of desert biological soil crusts to wind erosion: the influences of crust development, soil texture, and disturbance. J Arid Environ 39(2):133–142
Belnap J, Phillips SL, Miller ME (2004) Response of desert biological soil crust to alteration in precipitation frecuency. Oecologia 141:306–316
Boeken B, Lipchin C, Gutterman Y, Van Rooyen N (1998) Annual plant community responses to density of small-scale soil disturbances in the Negev desert of Israel. Oecologia 114:106–117
Briggs AL, Morgan JW (2011) Seed characteristics and soil surface patch type interact to affect germination of semi-arid woodland species. Plant Ecol 212:91–103
Cortina J, Bellot J, Vilagrosa A, Caturla RN, Maestre FT, Rubio E, Ortíz De Urbina JM, Bonet A (2004) Restauración en semiárido. In: Vallejo VR, Alloza JA (eds) Avances en el estudio de la gestión del monte Mediterráneo. Fundación Centro de Estudios Ambientales del Mediterráneo-CEAM, Spain, pp 345–406
Cortina J, Martín N, Maestre FT, Bautista S (2010) Disturbance of the biological soil crusts and performance of Stipa tenacissima in a semi-arid Mediterranean steppe. Plant Soil 334:311–322
De Baets S, Poesen J, Knapen A, Barberá GG, Navarro JA (2007) Root characteristics of representative Mediterranean plant species and their erosion-reducing potential during concentrated runoff. Plant Soil 294:169–183
De Luís M, García-Cano MF, Cortina J, Raventós J, González-Hidalgo JC, Sánchez JR (2001) Climatic trends, disturbances and short-term vegetation dynamics in a Mediterranean shrubland. For Ecol Manage 147:25–37
Deines L, Rosentreter R, Eldridge DJ, Serpe MD (2007) Germination and seedling establishment of two annual grasses on lichen-dominated biological soil crusts. Plant Soil 295:23–35
Delgado-Baquerizo M, Maestre FT, Rodríguez JGP, Gallardo A (2013) Biological soil crusts promote N accumulation in response to dew events in dryland soils. Soil Biol Biochem 62:22–27
Doerr SH, Shakesby RA, Walsh RPD (2000) Soil water repellency: its causes, characteristics and hydro-geomorphological significance. Earth-Sci Rev 51:33–65
Escudero A, Martínez I, de la Cruz A, Otálora MG, Maestre FT (2007) Soil lichens have species-specific effects on the seedling emergence of three gypsophile plant species. J Arid Environ 70:18–28
Esposito A, Mazzoleni S, Strumia S (1999) Post-fire bryophyte dynamics in Mediterranean vegetation. J Veg Sci 10:261–268
García-Fayos P, Verdú M (1998) Soil seed bank, factors controlling germination and establishment of a Mediterranean shrub: Pistacia lentiscus L. Acta Oecol 19:357–366
Godínez-Alvarez H, Morín C, Rivera-Aguilar V (2011) Germination, survival and growth of three vascular plants on biological soil crust from a Mexican tropical desert. Plant Biol 14:157–162
Jaramillo-Jaramillo DF (2006) Repelencia al agua en suelos: Una síntesis. Rev Acad Colomb Cienc 30(115):215–232
Kimball T, Belnap J (2001) The influence of biological soil crusts on mineral uptake by associated vascular plants. J Arid Environ 47:347–357
King PM (1981) Comparison of methods for measuring severity of water repellence of sandy soils and assessment of some factors that affect its measurement. Aust J Soil Res 19:275–285
Letey J, Carrillo MLK, Pang XP (2000) Approaches to characterize the degree of water repellency. J Hydrol 231–232:61–65
Maestre FT, Cortina J (2002) Spatial patterns of surface soil properties and vegetation in a Mediterranean semi-arid steppe. Plant Soil 241:279–291
Maestre FT, Bautista S, Cortina J, Bellot J (2001) Potential for using facilitation by grasses to establish shrubs on a semiarid degraded steppe. Ecol Appl 11(6):1641–1655
Maestre FT, Huesca MT, Zaady E, Bautista S, Cortina J (2002) Infiltration, penetration resistance and microphytic crust composition in contrasted microsites within a Mediterranean semi-arid steppe. Soil Biol Biochem 34:895–898
Maestre FT, Bautista S, Cortina J (2003) Positive, negative and net effects in grass-shrub interactions in Mediterranean semiarid grasslands. Ecology 84(12):3186–3197
Maestre FT, Escudero A, Martinez I, Guerrero C, Rubio A (2005) Does spatial pattern matter to ecosystem functioning? Insights from biological soil crusts. Funct Ecol 19:566–573
Maestre FT, Martín N, Díez B, López-Poma R, Santos F, Luque I, Cortina J (2006) Watering, fertilization, and slurry inoculation promote recovery of biological crust function in degraded soils. Microb Ecol 52:365–377
Maestre FT, Ramírez DA, Cortina J (2007) Ecología del esparto (Stipa tenacissima L) y los espartales de la Peninsula Ibérica. Ecosistemas 16:111–130, URL http://www.revistaecosistemas.net/index.php/ecosistemas/article/view/458
Martínez I, Escudero A, Maestre FT, De la Cruz A, Guerrero C, Rubio A (2006) Small-scale of abundance of moss and lichens forming biological soil crust in two semi-arid gypsum environments. Aust J Bot 54:339–348
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Oksanen J, Blanchet FG, Kindt R, Legendre P, O’hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2011) VEGAN: community ecology package. R package version 1.17-10. URL http://CRAN.R-project.org/package=vegan
Perry JN (1998) Measures of spatial pattern for counts. Ecology 79:1008–1017
Perry JN, Winder L, Holland JM, Alston RD (1999) Red-blue plots for detecting clusters in count data. Ecol Lett 2:106–113
Prasse R, Bornkamm R (2000) Effect of microbiotic soil surface crusts on emergence of vascular plants. Plant Ecol 150:65–75
Quintana-Ascencio PF, Menges ES (2000) Competitive abilities of three narrowly endemic plant species in experimental neighborhoods along a fire gradient. Am J Bot 87:690–699
Rivera-Aguilar V, Godínez-Alvarez H, Manuell-Cacheux I, Rodríguez-Zaragoza S (2005) Physical effects of biological soil crusts on seed germination of two desert plants under laboratory conditions. J Arid Environ 63:344–352
Su YG, Li XR, Zheng JG, Huang G (2009) The effect of biological soil crusts of different successional stages and conditions on the germination of seeds of three desert plants. J Arid Environ 73:931–936
Valentin C, Bresson LM (1992) Morphology, genesis and classification of surface crusts in loamy and sandy soils. Geoderma 55:225–245
Verdú M, García-Fayos P (1996) Nucleation processes in a Mediterranean bird-dispersed plant. Funct Ecol 10:275–280
Vilagrosa A, Bellot J, Vallejo VR, Gil-Pelegrin E (2003) Cavitation, stomatal conductance, and leaf dieback in seedlings of two co-occurring Mediterranean shrubs during an intense drought. J Exp Bot 54:2015–2024
Zaady E, Gutterman Y, Boeken B (1997) The germination effects of cyanobacterial soil crust on mucilaginous seeds of three desert plants: Plantago coronopus, Reboudia pinnata and Carrichtera annua. Plant Soil 190:247–252
Zhang YM, Wu N, Zhang BC, Zhang J (2010) Species composition, distribution patterns and ecological functions of biological soil crusts in the Gurbantunggut Desert. J Arid Land 2(3):180–189
Acknowledgments
This research was funded by Consejo Nacional de Ciencia y Tecnología, CONACYT, European Union DGXII (Support Action PRACTICE), and the Spanish Ministries of Science and Innovation (Project GRACCIE-CSD2007-00067, CONSOLIDER-INGENIO 2010 Program), and Economy and Competitivity (Project UNCROACH-CGL2011-30581-C02-01). We thank the Banc de Llavors Forestals de la Comunitat Valenciana for providing P. lentiscus seeds. Useful suggestions from Dr. Humberto González helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jeffrey Walck.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix I
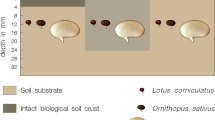
Representative examples of experimental units used in this study (8.4 cm diameter Petri dishes). Microsites are identified as OC (open areas with BSC), OU (open areas bare soil dominated), and TC (crusted areas underneath Stipa tenacissima tussocks). Images show the experimental units before seeding (A) and units at the end of the experiment (B), after turning Petri dishes upside down. We may note tortuosity of Pistacia lentiscus radicles. (JPEG 76 kb)
Appendix II
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Mendoza-Aguilar, D.O., Cortina, J. & Pando-Moreno, M. Biological soil crust influence on germination and rooting of two key species in a Stipa tenacissima steppe. Plant Soil 375, 267–274 (2014). https://doi.org/10.1007/s11104-013-1958-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1958-8