Abstract
Aims
Plantation forests are often assumed to have reduced biodiversity relative to unmanaged forests. However, existing knowledge is based on studies of rotation-aged tree crops. We investigated how Eucalyptus afforestation of agricultural land affected plant species composition and biodiversity across a range of plantation ages (1–10 years). We also studied whether the soil seed bank could contribute to regeneration of existing vegetation in such plantations.
Methods
We used a chronosequence approach to evaluate plant and seed species composition and diversity in forests and soil seed banks. We also quantified the similarity of seed banks and aboveground vegetation within plantation sites of a given age. Plantation sites were also compared to a nearby, mature pine forest.
Results
Total plant species number, density and diversity in Eucalyptus grandis plantations increased for the first 3 years plantation establishment, then stabilized or decreased for the next 1–2 years and then increase significantly over the following years. Species number and density in soil seed bank increased significantly with plantation age only after an initial 6-year decrease. Shannon–Wiener index of total species diversity did not significantly differ with plantation age. The understory vegetation and soil seed bank were dominated by pioneer species in the first 3 years, but intermediate-successional and shade-tolerant species gradually invaded as plantations developed further. After 7 years, E. grandis plantation understories were composed of mainly shade-tolerant species. Nevertheless, the diversity of the diversity of intermediate-successional in soil seed banks were higher than that of shade-tolerant species in soil seed banks at this age range (7–10 year). Among species successfully germinated from soil seed banks, 48 % were not found in the aboveground plant community. Similarities between the species in the soil seed bank and the aboveground vegetation were low for both plantation and control forests and did not significantly change with plantation ages.
Conclusions
E. grandis likely produces a changing microclimate during plantation development, which in turn drives composition and diversity dynamics in understory vegetation and soil seed banks after the afforestation of agricultural land. The first 4 years after plantation establishment is associated with lower plant and soil seed bank diversity, meriting a greater focus on biodiversity stabilization and possibly longer rotation periods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forest plantations, which occupy approximately 0.20 billion ha worldwide, support increasing local and global demands for wood (FAO 2007) and are considered rapid-response carbon sinks that may help mitigate increasing atmospheric CO2. However, scientists increasingly recognise that the large-scale development of commercial plantations can have ecological consequences. Fast-growing trees can affect the establishment of natural vegetation and understorey ecology via resource competition (Smith et al. 2000; Bremer and Farley 2010), allelopathy (Ahmed et al. 2008; Zhang et al. 2010b) or degradation soil fertility (Wall and Heiskanen 2003; Sicardi et al. 2004; Zhang et al. 2010a, b). Therefore, understanding how plantations afforestation would affect the biodiversity is critical to understanding the ecological functions of plantations and improving their management.
Land use change has been recognised as the most important driver of changes in biodiversity in the current century (Brokerhoff et al. 2008). In recent decades, the afforestation of agricultural land has represented a major change in land use in some developing countries (e.g. Brazil, India and China). In particular, China, which has the largest area of plantation forests in the world, is engaging in massive ongoing afforestation programs, such as the project to return croplands to forests (FAO 2006), to mitigate environmental problems resulting from previous substantial losses of forests and forest biodiversity. Most previous studies have found that converting natural and semi-natural grasslands and shrublands or primary forest into plantations is likely to be detrimental for biodiversity (Barlow et al. 2007; Gibson et al. 2011). Furthermore, an expanding body of literature has found lower levels of biodiversity in plantations, especially when used for commercial production (Humphrey et al. 2000; Cao et al. 2010; Sang et al. 2013). Nevertheless, a few studies have shown that other types of plantations (restoration, wildlife conservation, etc.) can play important roles in biodiversity conservation and species restoration (Hartley 2002; Carnus et al. 2006; Brokerhoff et al. 2008; Cao et al 2008). This discrepancy has led to a lack of consensus as to whether or not plantations might be detrimental to biodiversity conservation. Numerous factors ultimately determine the likely effects of plantations on biodiversity, such as stand age, species grown, land use preceding the establishment of a plantation, the plantation species and whether plantation afforestation is for commercial, restoration, wildlife conservation or other purposes (Brokerhoff et al. 2008; Bremer and Farley 2010). We suggest that these factors known to influence biodiversity, along with others such as changes in understory microclimate after plantation establishment, position within the landscape matrix and whether the area is managed for conservation goals, should all be considered when determining the effects of a plantation forest on biodiversity conservation.
Plant diversity in the plantation understory plays important roles in improving soil fertility, stimulating the soil nutrient cycle and maintaining soil quality (Halpern 1995; Carnus et al. 2006; Wang et al. 2011). The maintenance of plant diversity in plantations depends on the soil seed bank and other propagules. Soil seed banks reflect past ecological conditions and offer important potential for regenerating current and past vegetation (Aparicio and Guisande 1997; Fenner 2000; Díaz-Villa et al. 2003; Macdonald and Fenniak 2007). However, little information regarding soil seed bank characteristics is available from plantations established on formerly arable lands. Eucalyptus is a fast-growing, commercially available tree that has been introduced in developing countries such as Brazil, India and China. In such countries, Eucalyptus plantations are generally managed with a short rotation period (5–7 years) for wood production (Zhang et al. 2010a, 2012). These plantations cover more than 200,000 ha in southwestern China (Zhang et al. 2010a), where Eucalyptus grandis Hill ex Maiden is one of the primary tree species used for afforestation of arable land. Previous studies have indicated that Eucalyptus plantations have impoverished floral, faunal and soil biodiversity (Pellen and Garay 1999; Behera and Sahani 2003; Cao et al. 2010; Calvino-Cancela et al. 2012). However, most of these studies were conducted in plantations of a particular age prior to the rotation period, providing little knowledge of biodiversity in well-established Eucalyptus plantations.
In order to help improve our current understanding about the effects of Eucalyptus plantations on biodiversity conservation, we measured changes in plant and soil seed bank diversity in E. grandis plantations converted from agricultural land. Using a chronosequence approach, we tested the hypotheses that (a) plant and soil seed bank diversity would increase with the development of E. grandis plantations and (b) plantation soil seed banks could offer the potential for the regenerating understory vegetation. The findings can help support the development of biodiversity management strategies for Eucalyptus stands in southwestern China.
Materials and methods
Study region and site locations
The study was conducted in the Danling region (102°57′–103°04′E, 29°55′–29°59′N, 570–592 m a.s.l) located to the western Sichuan Province of southwestern China. The study site has a subtropical climate, with a mean annual temperature, precipitation and relative humidity of the site are 17.5 °C, 1,397 mm and 82 %, respectively. The soil is classified as ferralsol derived from Pleistocene alluvium and has a yellow colour, loam texture and granular structure (Zhang et al. 2010a, b).Within the Danling region, Eucalyptus plantations, cultivated land and unmanaged forests formed a landscape mosaic in this region. The large, recently afforested plantations are located on former agricultural land and contain E. grandis ranging from 1 to 10 years old. Pinus massoniana lamb. trees dominate the unmanaged stands, along with a very few Cyclobalanopsis glauca (Thunb.) Oerst. trees. Although P. massoniana forests cannot be regarded as native or natural forests, these unmanaged forest areas represent stable, mature forest ecosystems and were treated as ecological controls for afforested sites.
Experimental site selection was based in part on consultations with forest survey personnel and landowners to confirm site suitability. The soils of all the sites were developed from the same parent material, and all sites had similar soil type, slope and land use history. In particular, all sites had been ploughed prior to afforestation, used agriculturally for at least 100 years prior, and previously managed with an intensity and cropping system typical for the region and similar to present agricultural sites. Management practices after afforestation had been similar across plantation sites, with no fertilization or weed control treatments applied. E. grandis trees had been planted no more than 2 years after tillage termination, and trees in the young stands had not been thinned. In summary, all study sites were larger than 10 ha, located on relatively flat terrain (<5°) and had been afforested from 1 to 10 years (Table 1, Fig. 1).
The above site selection criteria are consistent with the assumption that the study sites had similar soil characteristics prior to afforestation and that an adjacent agricultural site could be considered as another control. At all agricultural sites selected, crop rotations were similar and had remained the same for several decades. Rice, potatoes, white gourd and broccoli were grown during the sampling year (2008), although oat and sweet potato had also been planted in the past 5 years. Fertilisation rates were generally low and comparable at all sites, with yard manure applied every year at a rate of 15 t ha−1 and occasional applications of mineral fertilizers. None of these sites had been limed during the past 6 years. Seeds in previous agricultural use were always harvested with the crop.
Plant survey and soil sampling
A chronosequence approach was used to evaluate changes in plant diversity and soil seed banks following afforestation. We divided plantation sites into three age categories: (1) prior to rotation period (1–4 years), (2) during rotation period (5–7 years) and (3) after rotation period (8–10 years). Three sites of each age were selected for sampling. Soil physicochemical properties for each plantation age were shown in Table 2. One agricultural site and three P. massoniana forests within approximately 1 km of the plantation sites were chosen as control sites (Fig. 1). At each site, one 20 × 20 m plot was established in October 2008, and vegetation surveys were conducted in these plots. Within each plot, five quadrats of 5 × 5 m, each containing a 1 × 1 m sub-quadrat at its centre, were designated to quantify the shrub layer and herbaceous layer (Fig. 1). For both shrubs (tree seedlings and woody climbing plants with <2.5 cm stem diameter) and herbaceous plants (perennial and annual herbs, herbaceous climbing plants and ferns), the number of stems and plant heights were tallied for each species. The coverage of each seed plant species was estimated visually, and species were classified into pioneer species, intermediate-successional and shade-tolerant species (Flora Republicae Popularis 2004)
After manually removing stones, roots and plant fragments, six soil samples of 10 × 10 × 10 cm were randomly excavated from each 20 × 20 m plot with enough care to keep the litter intact. The soil samples were split into three depth classes (litter layer, 0–5 cm and 5–10 cm), then these subsamples were placed in plastic bags, mixed thoroughly, and taken to the laboratory for seed bank analysis.
Laboratory analyses
To assess seed abundance (density) and species composition in the seed banks at each site, we conducted seed germination trials. Each soil sample was first passed through a 2-mm sieve to remove coarse debris; then seeds with a diameter larger than 2 mm were retrieved and returned to their respective sieved samples. Each sieved soil sample was spread on a layer of heat-sterilised (100 °C, 10 h) sand in a seed germination tray. All the germination trays were covered with nylon nets to prevent seed intrusion from or within the glasshouse and were placed in an experimental greenhouse, watered daily to keep the soil moist. Newly germinated seedlings were identified at the species level, counted and then removed from the seed trays every 2–5 days. Unidentified seedlings were transplanted into additional germination trays for further growth until the species could be identified. The germination assay continued until no new seedlings emerged for 4 weeks (Wang et al. 2009). The greenhouse had a mean temperature of 25 °C and mean relative humidity of 65 %.
Data analysis
Seed density was calculated from the number of emerged seedlings per square metre. Shannon–Wiener (H′) values were selected to determine the diversity of plants and of the soil seed bank (Halpern 1995). Similarities in species composition between the soil seed banks and the aboveground vegetation in E. grandis plantation and control forests were analysed using the Sorensen index (SI), SI = c / (a + b − c), where a is the number of species present in site A, b is the number of species present in site B and c is the number of species that site A and site B have in common (Du et al. 2007).
All data were statistically analysed using SPSS v.21.0. After the pairwise comparisons tests made upon the MANOVA, the responses of the abundance and diversity of the vegetation and the soil seed bank properties to age were evaluated by liner and non-parametric LOESS regression with the 95 % confidence intervals (95 % CI). After verifying the general ANOVA hypothesis, detailed post hoc mean comparisons for the study stands and the control sites were performed using Tukey's HSD. The homogeneity of the variances was tested by the Levene's test. Any data sets failing this test were log-transformed before further analysis to help satisfy the requirement of variance homogeneity. Differences among means were considered statistically significant if p < 0.05, with levels of significance denoted as follows: ***p < 0.001, significant at **p <0.01, significant at *p < 0.05, ns = not significant.
Results
Composition and diversity of understorey plant species
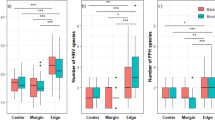
Seventy-eight species in 44 vascular plant families were observed in 1- to 10-year-old E. grandis plantation sites (Table 5 in Appendix). Herbs, followed by woody plant, dominated the composition of the understorey vegetation in terms of species richness. Among the total herbaceous species across all sampled plantation ages, perennial herbs were dominant (55–73 %), followed by annual herbs (15–27 %) and climbing herbaceous plants (7–21 %) plants. For total species (T), plant species number, density and Shannon–Wiener index, all increased significantly in the first 3 years exhibited a decrease or no change during the fourth and fifth years, and increased again with additional years (Fig. 2). The pioneer species (P) remained at low richness and density through the chronosequence (Fig. 2a, b). The Shannon–Wiener index of pioneer species decreased during the first 5 years, then increased during following years (Fig. 2c). The number and density of intermediate-successional species (I) also tracked the corresponding trends for total species, while the Shannon–Wiener index for this group increased significantly with plantation age (Fig. 2c). Finally, while the number of shade-tolerant species (S) followed the same trend as seen for total species (Fig. 2a), species density and Shannon–Wiener index for this group both increased significantly with plantation age (Fig. 2b, c).
Composition, size and diversity of the soil seed bank
The seeds of 58 species in 38 vascular plant families were observed in the soil seed banks of 1- to 10-year-old E. grandis plantations sites (Table 6 in the Appendix). Herbaceous species again dominated, both in terms of species number and seed density. Most seeds were found in 0–5 cm soil subsample, with fewer in the leaf litter and the fewest in the 5–10-cm soil layer (F = 561.165, p < 0.001). The species number of total species increased significantly with plantation age (Fig. 3a), while the total seed density decreased significantly for the first 6 years before increasing with additional plantation age (Fig. 3b). No significant responses to age were found for the Shannon–Wiener index of total species of seeds (Fig.3c). While the number of pioneer species decreased during the first 4 years before beginning to increase with age (Fig. 3a), the density and Shannon–Wiener index of this group decreased during the first 6 years before increasing (Fig. 3b, c). The species number, density and Shannon–Wiener index of intermediate-successional species increased during the first 3 years, then stabilized during years 4 through 6 before increasing with age (Fig. 3).This pattern was also seen for the number of shade-tolerant species' seed, while the density and Shannon–Wiener index for this type of seed showed a continuous, significant increase with plantation age (Fig. 3b, c).
Across 10-year range of plantation ages, 75 % of species in the extant vegetation were present in the soil seed bank and 62 % of the species in the soil seed bank were present in the extant vegetation. The species composition similarity of herb (0.08–0.14) and total (0.06–0.32) between the soil seed bank and the aboveground vegetation in the study sites was low. The species composition similarity of shrub species in 2–10-year-old stands was low (0.18–0.47), with the exception of the shrub species in 1-year-old plantation (0.80). Despite this observation, plantation age did not significantly influence species composition similarity (Table 3).
Comparison with the control sites
Eight weed species, belonging to 6 families, were found in the agricultural sites; 16 weed species, belonging to 10 families, germinated from the soil samples of these sites (data not shown). Multiple comparisons test indicated that species richness, density and diversity for both the vegetation and soil seed bank of the E. grandis study sites were significantly higher (p < 0.001) than the corresponding measurements in the agricultural land (data not shown).
One hundred and sixty-four understorey plant species, belonging to 75 families, were found in the forest sites (P. massoniana) (Table 7 in the Appendix), while 95 plant species, belonging to 74 families, germinated from the soil samples of these sites (Table 8 in the Appendix). Herbaceous species dominated the composition of both the understory plants and soil seed bank. As seen for the plantations, the species composition similarity (0.32) of the species between the soil seed bank and aboveground vegetation were relatively low (Table 3). Species found in aboveground vegetation at these control sites accounted for 87 % of those found in the soil seed banks of plantation sites overall. Multiple comparisons test (Table 4) indicated that species richness and density for both the vegetation and soil seed bank of the E. grandis study sites were significantly lower than the corresponding measurements in the control forests. The Shannon–Wiener indices for all understorey vegetation in the 1-, 2- and 5-year-old E. grandis plantations were significantly lower than that found for the control forests; however, the indices for 3-, 4-, and 6–10-year-old E. grandis plantation were similar to those in the control forests. The Shannon–Wiener indices for herbaceous species in the soil seed bank of E. grandis plantations 1–8 years old were significantly lower than that of the control forests; in the 9- and 10-year-old plantations, they were similar to that for the control forests. In plantation sites of 1–2 years old, the Shannon–Wiener indices for all plant seeds and for woody species' seeds in the soil seed bank were significantly lower than that of the control forests. However, E. grandis plantations in the mid- to post-rotation phase (6–10 years) showed no significant differences in Shannon–Wiener indices compared to control forests.
Discussion
Understorey vegetation
Our hypotheses that plant would increase with the development of E. grandis plantations were supported by the findings in this study. However, our results showed that plant abundance and diversity in E. grandis plantations increased for the first 3 years plantation establishment, then stabilized or decreased for the next 1–2 years and then increase significantly over the following years. A few weed species were found in previous agricultural site because the tillage weeding was applied and seeds were always harvested with the crop. Although the plantations in this study were afforested on the former cropland many, plant propagates (e.g. root stumps, soil seed banks in deeper soil layers) remained (Egler 1954). Furthermore, plant regeneration during the initial stage of E. grandis afforestation (the first 3 years) most likely resulted from the cessation of agricultural management and the closure of the lower canopy, which facilitated the germination and dispersal of seeds from the pioneer, shade-intolerant herbaceous species, a few intermediate-successional species and the growth of younger seedlings (Sem and Enright 1995; Garay et al. 2004; Wang et al. 2009). A previous study of the sites investigated here indicated that the growth rate of E. grandis increased and reached a maximum rate before the rotation period began, approximately 4 years after initial afforestation, and then decreased with plantation age (Zhang et al. 2012). At a plantation age of about 4–5 years, fast-growing E. grandis could likely still compete for soil resources with other plants. Soil organic matter has previously been shown to exhibit an initial decline over the first 4 years of E. grandis plantation establishment, but it increases significantly over time in the upper soil layers thereafter (Zhang et al. 2012). At this stage, higher concentrations of allelochemicals and more severe allelopathic effects from roots and soil in E. grandis plantations are known to inhibit seed germination and growth of younger seedlings, reducing the abundance of other species (Zhang et al. 2010a, b). Canopy cover has been found to support the occurrence and growth of shade-tolerant and reduce intolerant species (Parrotta et al. 1997; Plue et al 2010). The rapid growth rate of the Eucalyptus trees in plantations creates a closed canopy prior to the rotation period, and subsequent limited light conditions did not facilitate the input of shade-intolerant seeds or the regeneration of younger seedlings. Microclimatic changes of limited light availability, severe competition for water and nutrients, and allelopathy could help account for the relative stability or decrease in understory plant biodiversity during the fourth and fifth years after plantation establishment. As E. grandis stands aged (6–10 years), the understory vegetation was gradually replaced by shade-adapted species. This may be due to a gradual increase in facilitative effects of the planted trees for shade-adapted species based on increased lateral and vertical heterogeneity, better development of soil organic layers and associated fungal flora, increased deadfall and a better light environment over time (Allen et al. 1995; Brockerhoff et al. 2003; Bremer and Farley 2010; Zhang et al 2012). Furthermore, the abundant availability of precipitation in this region could weaken any allelopathic effects associated with greater plantation ages (Moline 1991; Zhang et al. 2010a). As a whole, changing microclimatic conditions across the E. grandis chronosequence was a likely driving factor in plant species composition and diversity dynamics after afforestation.
Seed banks
We did not find support for our hypothesis that soil seed bank diversity would increase with the development of E. grandis plantations. The present results showed that Shannon–Wiener index of total species diversity of soil seed bank did not significantly differ with plantation age. However, species number and density in soil seed bank increased significantly with plantation age only after an initial 6-year decrease. Seed bank density at all study sites were relatively higher compared with those found in some previous studies of the effects of plantations afforestation (Wang et al. 2009; Li et al. 2012). Seed bank density in plantations afforested on grasslands or shrublands has often been found to peak early (initial 1–2 years) after afforestation, then slowly decline (Sem and Enright 1995; Huang et al. 1996; Thompson 2000; Plue et al. 2010). In the present study, species abundance and diversity was greatest at the initial stage of E. grandis afforestation of former agricultural land. This was associated with high number of pioneer annual and perennial herbs, suggesting a high seed input for these species upon cessation of agricultural management. Seeds in previous agricultural use were always harvested with the crop. Later in the chronosequence, pioneer species' seed densities and diversity decreased, while abundance and diversity of intermediate-successional and shade-tolerant species became relatively stable. This might be attributable to microclimatic changes associated with the development of the plantations, such as canopy closure, reduced light availability, greater allelopathy, germination of the pioneer seeds without survival to the reproductive phase and seed death caused by factors such as higher soil moisture or invertebrate predation. After 7 years, the composition of soil seed bank was increasingly composed of intermediate-successional and shade-tolerant species, which might be attributed to continuing changes in microclimatic conditions resulting from the plantations' succession. The aboveground structural complexity developed after the rotation period (7–10 years) is known to be more conducive to seed input and dispersal for intermediate-successional and shade-tolerant species (Li et al. 2012; Bremer and Farley 2010). The diversity of intermediate-successional species in soil seed banks was higher than that of shade-tolerant species. Furthermore, among the 58 plant species observed in plantation the soil seed banks, herbaceous species accounted for 40.5–83.3 %, with annuals comprising 24.3–50 % and perennials comprising herbs comprising 16.2–33.3 %, suggesting that the plant community was at a comparatively early successional stage (Thompson 2000). We also found that soil seed bank density was significantly higher in 0–5-cm soil layer than in leaf litter or 5–10 cm layer, inconsistent with previous results in which seeds in the litter layer accounted for the highest proportion of the total number of seeds (Du et al. 2007). Such a result might be attributable to a looser litter layer, greater turnover of leaf litter or greater seed dispersal by birds, vertebrates and soil fauna in E. grandis plantations (Appleby 1991; Yan et al. 2010; Zhang et al. 2010a, b). Despite observed changes in species number and abundance, species diversity (Shannon–Wiener index) of the soil seed bank did not change across the chronosequence, possibly indicating changes in species evenness related to the dominance of species such as Polygonum hydropiper, Digitaria sanguinalis or Oplismenus undulatifolius under E. grandis plantations.
Under natural conditions, seeds in the soil seed bank are both derived from the local aboveground vegetation and contribute to the regeneration of that aboveground vegetation through germination and seedling growth. Significant differences between the composition of the seed bank and the actual vegetation are not uncommon (Thompson 2000; Díaz-Villa et al. 2003; Vilà and Gimeno 2007) and may be attributed to the environmental variability that affects seed dispersal and the effect of dormancy, which buffers against rapid changes in species composition (Thompson and Grime 1979; Thompson 1992; Yan et al. 2010; Plue et al. 2010). A few studies have indicated that the species number in common between soil seed bank and aboveground vegetation decreases with forest succession (Bremer and Farley 2010; Hu et al. 2013). Across a range of E. grandis plantation ages (1–10 years), 75 % of species in the extant vegetation were present in the soil seed bank, indicating that the succession of E. grandis plantations facilitated seed input and seedling regeneration, likely in conjunction with changes in understory microclimate. Our hypothesis that plantation soil seed banks could offer the potential for the regenerating understory vegetation was supported by the findings in this study. At the same time, 48 % of the species germinated in soil seed bank samples were not found in the aboveground plant community. Meanwhile, 87 % of the species in the soil seed bank of E. grandis plantations were identified located in the aboveground vegetation of the control sites (P. massoniana). The planted trees therefore affected the makeup of understory vegetation both during plantation development and relative to the control site. Such observations may be related to changing understory microclimates or the greater development of litter and humus layers in Eucalyptus plantations over time. These changes may have led to increased seed inputs from the neighbour forests by seed-dispersing wildlife attracted to the plantations (Thompson 2000; Brockerhoff et al. 2008). However, factors such as the closed canopy, more severe allelopathy and relatively thicker litter layer during or prior to the rotation period (starting about 4–6 years after establishment) may account for a stabilization in seed species number, density or diversity index in soil seed banks of the E. grandis plantations.
Conservation implications
Most of the studies on plantations biodiversity have compared species measures to those in natural forests without considering whether such comparisons are appropriate (Brokerhoff et al. 2008). Eucalyptus plantation forests are often assumed to support lower species abundance and diversity compared to natural forests (Nsabimana et al. 2004; Stephen and Wagner 2007; Bremer and Farley 2010). However, most of these studies focused on young Eucalyptus plantations prior to the rotation period. The short rotation period of 5–7 years generally used for wood production in developing countries can inhibit a clear, comprehensive understanding of the effects of Eucalyptus plantations on understory plant diversity. In the present study, the species abundance and diversity of plants and soil seed banks exhibited dynamic changes after the afforestation of agricultural land with E. grandis. Plant and soil seed bank diversity in E. grandis plantations were not significantly different from those measures in control forests once the plantations have surpassed the rotation period. Although the control forests, consisting mainly of P. massoniana, do represent stable, mature forest ecosystems common in this region, they were not native forests, but forests dominated by an exotic species. Furthermore, similarity indices between seed banks and vegetation at these sites were relatively low, and the indices for plantation sites did not significantly change with succession. Therefore, the recovery of plant diversity in the study plantations is likely to last a longer period of time than that before which E. grandis plantations are typically harvested (<5–7 years).
Such findings overall support the use of scientific management measures to support biodiversity conservation in E. grandis plantations, especially for the relatively lower plant diversity found in E. grandis plantations prior to the rotation period (∼4 years). Although Eucalyptus plantations of different ages are found in study region, they are typically composed of the single tree species. The establishment of plantations with diverse tree species would provide more habitat types for native species (Lamb 1998; Norton 1998; Hartley 2002; Hu et al. 2013). Furthermore, avoiding intensive site preparation of plantations may help limit the destruction of existing herbaceous vegetation and woody debris (Hartley 2002; Lindenmayer and Franklin 2002; Carnus et al. 2006). Wider tree spacing during plantation establishment support better maintenance of understorey vegetation (Brokerhoff et al. 2008). Although the afforestation of agricultural land in China aims to mitigate environmental problems, the majority of plantation forests are managed primarily for production purposes and have a short rotation period. An increase in rotation length has been advocated as a means to enhance the native biodiversity in plantations (Rosoman 1994; Humphrey et al. 2006; Bremer and Farley 2010). This approach is also supported by the results of our chronosequence study of plant and seed bank biodiversity. Scientific thinning, fertilising, watering and soil burrowing for the first 4 years after establishment while forbidding litter collection may lessen competition for resources and increase the environmental heterogeneity, by facilitating the regeneration of understorey vegetation.
Conclusions
In conclusion, the present chronosequence study of biodiversity in E. grandis plantations partly supports the hypothesis that plant's abundance and diversity in such plantations can increase given a more comprehensive time frame. In particular, the plant species abundance and diversity increased with plantation age for the first 3 years, then stabilized or slightly decreased during years 4 and 5. Our hypothesis that plantation soil seed banks could offer the potential for the regenerating understory vegetation was supported by the findings in this study. Seed species number and density of plantation soil seed banks increased significantly with plantation age, only after an initial 6-year decline in total seed species density. Contrary to our initial hypothesis, the diversity index of total species of soil seed bank did not respond significantly to plantation age. Some evidence of succession was seen in this study, as the understory vegetation and soil seed bank were dominated by pioneer species in the first 3 years, with intermediate-successional and shade-tolerant species gradually invading as plantations aged. After 7 years, the understory vegetation in E. grandis plantations was primarily made up of shade-tolerant species; however, the diversity of intermediate-successional seeds in soil seed banks was higher than that of shade-tolerant species. Overall, our results suggest the use of scientific management measures may help support greater biodiversity in E. grandis plantations, especially for plantations prior to the rotation period (∼4 years), which exhibited relatively low plant diversity.
References
Ahmed R, Hoque ATM, Hossain MK (2008) Allelopathic effects of leaf litters of Eucalyptus camaldulensis on some forest and agricultural crops. J For Res 19:19–24
Allen RB, Platt KH, Coker REJ (1995) Understorey species composition patterns in a Pinus radiata D. Don plantation on the central North Island volcanic plateau, New Zealand. N Z J Ecol 25:301–317
Aparicio A, Guisande R (1997) Replenishment of the endangered Echinospartum algibicum (Genisteae, Fabaceae) from the soil seed bank. Biol Conserv 81:267–273
Appleby M (1991) The invasion of Eucalyptus regnant forests by weed species. BSc (Hons) thesis, University of Melbourne, Australia
Barlow J, Gardner TA, Araujo IS, Ávila-Pires TCA, Bonaldo AB et al (2007) Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. PNAS 104:18555–18560
Behera N, Sahani U (2003) Soil microbial biomass and activity in response to Eucalyptus plantation and natural regeneration on tropical soil. For Ecol Manag 174:1–11
Bremer LL, Farley KA (2010) Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv 19:3893–3915
Brockerhoff EG, Ecroyd CE, Leckie AC, Kimberley MO (2003) Diversity and succession of adventive and indigenous vascular understorey plants in Pinus radiata plantation forests in New Zealand. For Ecol Manag 185:307–326
Brokerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J (2008) Plantation forests and biodiversity: oxymoron or opportunity? Biodivers Conserv 17:925–951
Calvino-Cancela M, Kasel S, Bennett LT, Tibbits J (2012) Land use influences soil fungal community composition across central Victoria, south-eastern Australia. Soil Biol Biochem 40:1724–1732
Cao CY, Jiang DM, Teng XG, Jiang Y, Liang WJ, Cui ZB (2008) Soil chemical and microbiological properties along a chronosequence of Caragana microphylla Lam. plantations in the Horqin sandy land of North. Appl Soil Ecol 40:78–85
Cao YS, Fu SL, Zou XM, Cao HL, Shao YH, Zhou LX (2010) Soil microbial community composition under Eucalyptus plantations of different age in subtropical China. Eur J Forest Res 46:128–135
Carnus JM, Parrotta J, Brockerho VEG, Arbez M, Jactel H, Kremer A, Lamb D, O’Hara K, Walters B (2006) Planted forests and biodiversity. J Forest 104:65–77
Díaz-Villa MD, Marañón T, Arroyo J, Garrido B (2003) Soil seed bank and floristic diversity in a forest–grassland mosaic in southern Spain. J Veg Sci 14:701–709
Du XJ, Qinfeng G, Gao XM, Na KP (2007) Seed rain, soil seed bank, seed loss and regeneration of Castanopsis fargesii (Fagaceae) in a subtropical evergreen broad-leaved forest. For Ecol Manag 238:212–219
Egler FE (1954) Vegetation science concepts I. Initial floristic composition, a factor in old-field vegetation development. Vegetation 4:412–417
FAO (2006) Global Forest Resources Assessment 2005—progress towards sustainable forest management. FAO Forestry Paper 147. Food and Agriculture Organization of the United Nations, Rome, Italy
FAO (2007) The state of the world's forests. ftp.fao.org/docrep/fao/009. FAO, Rome, Italy
Fenner M (2000) Seed: the ecology of regeneration in plant communities, 2nd edn. CABI, Wallingford
Garay I, Pellens R, Kindel A, Barros E, Franco AA (2004) Evaluation of soil conditions in fast-growing plantations of Eucalyptus grandis and Acacia mangium in Brazil: a contribution to the study of sustainable land use. Appl Soil Ecol 27:177–187
Gibson L, Lee TM, Koh LP, Brook BW, Gardner TA, Barlow J, Peter CA, Bradshaw CJA, Laurance WF, Lovejoy TE, Sodhi NS (2011) Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478:378–383
Halpern CB (1995) Plant species diversity in natural and managed forests of the pacific northwest. Ecol Appl 5:913–934
Hartley MJ (2002) Rationale and methods for conserving biodiversity in plantation forests. For Ecol Manag 155:81–95
Hu ZH, Yang Y, Leng PS, Dou DQ, Zhang B, Hou BF (2013) Characteristics of soil seed bank in plantation forest in the rocky mountain region of Beijing, China. J For Res 24:91–97
Huang ZL, Kong GH, Wei P, Wang JJ, Huang YJ, Zhang YC (1996) A study on the soil seed banks at the different succession stages of south subtropical forests. J Trop Subtrop Bot 4(4):42–49 (in chinese)
Humphrey JW, Newton AC, Peace AJ, Holden E (2000) The importance of conifer plantations in northern Britain as a habitat for native fungi. Biol Conserv 96:241–252
Humphrey JW, Quine CP, Watts K (2006) The influence of forest and woodland management on biodiversity in Scotland: recent findings and future prospects. In: Davison R, Galbraith CA (eds) Farming, forestry and the natural heritage: towards a more integrated approach. Scottish Natural Heritage, Edinburgh, pp 59–75
Lamb D (1998) Large-scale ecological restoration of degraded tropical forest lands: the potential role of timber plantations. Restor Ecol 6:271–279
Li X, Jiang D, Zhou Q, Oshida T (2012) Soil seed bank characteristics beneath an age sequence of Caragana Microphylla Shrubs in the Horqin sandy land region of northeastern China. Land Degrad Dev. doi:10.1002/ldr.2135
Lindenmayer DB, Franklin JF (2002) Conserving forest biodiversity: a comprehensive multiscaled approach. Island Press, Washington
Macdonald SE, Fenniak TE (2007) Understory plant communities of boreal mixedwood forests in western Canada: natural patterns and response to variable-retention harvesting. For Ecol Manag 242:34–48
Moline A (1991) Release of allelo-chemical agents from litter, through fall and topsoil in plantation of Eglobules. J Chem Ecol 17:147–159
Norton DA (1998) Indigenous biodiversity conservation and plantation forestry: options for the future. N Z Forest 43:34–39
Nsabimana D, Haynes RJ, Wallis FM (2004) Size, activity and catabolic diversity of the soil microbial biomass as affected by land use. Appl Soil Ecol 26:81–92
Parrotta JA, Turnbull J, Jones N (1997) Catalyzing native forest regeneration on degraded tropical lands. For Ecol Manag 99:1–8
Pellen R, Garay I (1999) Edaphic macroarthropod communities in fast-growing plantations of Eucalyptus grandis Hill ex Maid (Myrtaceae) and Acacia mangium Wild (Leguminosae) in Brazil. Eur J Forest Res 2:77–89
Plue J, VanGils B, Peppler-Lisbach C, DeSchrijver C, Verheyen K, Hermy M (2010) Seed-bank convergence under different tree species during forest development. Perspect Plant Ecol 12:211–218
Rosoman G (1994) The plantation effect. Greenpeace, Auckland
Sang PM, Lamb D, Bonner M, Schmidt S (2013) Carbon sequestration and soil fertility of tropical tree plantations and secondary forest established on degraded land. Plant Soil 362:187–200
Sem G, Enright NJ (1995) The soil seed bank in Agathis australis (D. Don) Lindl. (kauri) forests of northern New Zealand. N Z J Bot 33:221–235
Sicardi M, Garcia-Prechac F, Frioni L (2004) Soil microbial indicators sensitive to land use conversion from pastures to commercial Eucalyptus grandis (Hill ex Maiden) plantations in Uruguay. Appl Soil Ecol 27:125–133
Smith OH, Petersen GW, Needelman BA (2000) Environmental indicators of agroecosystems. Adv Agron 69:75–97
Stephen S, Wagner M (2007) Forest plantations and biodiversity: a fresh perspective. J Forest 105:307–313
Thompson K (1992) The functional ecology of seed banks. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities. Redwood, Melksham, pp 231–258
Thompson K (2000) The functional ecology of soil seed banks. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities. CABI, Wallingford, pp 215–235
Thompson K, Grime JP (1979) Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. J Ecol 67:893–921
Vilà M, Gimeno I (2007) Does invasion by an alien plant species affect the soil seed bank? J Veg Sci 18:423–430
Wall A, Heiskanen J (2003) Water-retention characteristics and related physical properties of soil on afforested agricultural land in Finland. For Ecol Manag 186:21–32
Wang GL, Liu GB, Xu MX (2009) Above- and belowground dynamics of plant community succession following abandonment of farmland on the Loess Plateau, China. Plant Soil 316:227–239
Wang HF, Lencinas M, Ross Friedman C, Wang XK, Qiu JX (2011) Understory plant diversity assessment of Eucalyptus plantations over three vegetation types in Yunnan, China. New Forest 42:101–116
Yan QL, Zhu JJ, Zhang JP, Yu LH, Hu ZB (2010) Spatial distribution pattern of soil seed bank in canopy gaps of various sizes in temperate secondary forests, Northeast China. Plant Soil 329:469–480
Zhang DJ, Zhang J, Yang WQ, Wu FZ (2010a) Potential allelopathic effect of Eucalyptus grandis across a range of plantation ages. Ecol Res 25:13–23
Zhang KR, Dang HS, Tan SD, Wang ZX, Zhang QF (2010b) Vegetation community and soil characteristics of abandoned agricultural land and pine plantation in the Qinling Mountains, China. For Ecol Manag 259:2036–2047
Zhang DJ, Zhang J, Yang WQ, Wu FZ (2012) The effect of afforestation with Eucalyptus grandis plantation of arable soils on their soil physicochemical and microbiological properties. Soil Res 50:167–176
Acknowledgments
The Doctoral program of the Higher Education Foundation and the National Natural Science Foundation of China (No. 30872014 and No. 31300528) financially supported this work. The authors sincerely thank Wang XQ, Zhang ZW, Zhu L and Wang XQ for their help with field and laboratory work. We are also grateful to the anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Nico Eisenhauer.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Zhang, D., Zhang, J., Yang, W. et al. Plant and soil seed bank diversity across a range of ages of Eucalyptus grandis plantations afforested on arable lands. Plant Soil 376, 307–325 (2014). https://doi.org/10.1007/s11104-013-1954-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1954-z