Abstract
Aboveground and belowground changes during vegetation restoration and vegetation successions need to be characterized in relation to their individual responses to changes in soil resources. We examined above- and belowground vegetation characteristics, soil moisture, and nutrient status at the end of the growing season in 2006 in plots with vegetation succession ages of 2, 4, 6, and 8 years (two replicates each) that had been established on abandoned cropland, where potatoes had been grown for 3 years, using hoe and plow cultivation, immediately prior to vegetation clearance and subsequent natural plant colonization. A plant community comprising pioneer species [e.g., Artemisia capillaries, (subshrub)] was characterized by low levels of species richness (7.5 ± 1.4 species m−2), plant density (35.7 ± 4.2 stems m−2), fine root length density (940.1 ± 90.1 m m−2), and root area density (2.3 ± 0.3 m2 m−2) that increased rapidly with time. Aboveground and belowground characteristics of both A. capillaries and the later successional species, Stipa bungeana (C3 perennial grass), increased in the first 6 years, but in the following 2 years A. capillaries declined while S. bungeana thrived. Thus, the fine root length density of A. capillaries, 812.4 m m−2 after 2 years, changed by a factor of 1.7, 2.0, and 0.4 in the 4th, 6th, and 8th years, whereas that of S. bungeana changed from 278.4 m m−2, after 4 years, and by 1.7 and 23.3 times in the 6th and 8th years, respectively. Secondary vegetation succession resulted in reduced soil moisture contents. Soil available P and N mainly influenced aboveground characteristics, while soil moisture mainly influenced belowground characteristics. However, soil moisture had no significant affect on S. bungeana belowground characteristics at the population level in this semiarid region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The interactions between plants and soils have received a great deal of attention (Burke et al. 1998; Tracy and Sanderson 2000; Blatt et al. 2002; Benjamin et al. 2005). On one hand, the plant community may cause an accumulation of organic matter, and may change nutrient availability and moisture contents in the soil (DiTommaso and Aarssen 1989). For example, Erica tetralix on heaths (Berendse 1998), Tsuga canadensis in temperate forests (Van-Breement et al. 1997) and Sphagnum spp. in peat bogs (Jones et al. 1994) all decrease the availability of a number of soil resources, notably N. Many plants mediate the reduction in N availability through an effect on litter chemistry and on the environment in which the litter decomposes (Breemen and Finze 1998). On the other hand, soil physical and chemical properties may influence plant composition and structure (Breemen and Finze 1998; Adema and Grootjans 2003, Blatt et al. 2005). In reviewing 150 published studies of primary succession, Connell and Slatyer (1977) found that soil resources increased the survivorship of later successional species and, therefore, changed the course of vegetation succession.
Lauenroth et al (1997) discussed key interactions between plants and soils that change in character with the extent to which water is an important limiting resource. They suggested that temperate zone ecosystems, dominated by belowground processes, occurred when annual precipitation was below 700 mm/year, while those dominated by aboveground processes occurred when rainfall was above 1,200 mm/year. Between these limits (700–1,200 mm/year) are ecosystems in which the dominant effect on belowground or aboveground processes is unclear. Based on the research by Lauenroth et al (1997), Burke et al (1998) concluded that the key influence of soil on plant communities and composition in semiarid grasslands was through water availability, and that the important influence of the plants was on soil nutrient concentration. Thus, in arid and semiarid regions, the interactions of plant and soil processes are vital to the understanding of the mechanism of vegetation succession.
The interactions between belowground characteristics and vegetation succession and soil resources are less clearly understood because the roots are not readily observable (Chapin et al. 1993; Moreno et al. 2005; Zhou and Shangguan 2007). Although a close correlation between aboveground and belowground characteristics has been reported in many studies, some recent research has indicated that these two sets of characteristics are not always in such balance (Chapin et al. 1993; Zerihun et al. 2006; Craine 2006). This has been ascribed to competition and changes in soil resources that could change the ratio of aboveground parts to those belowground (Davis et al. 1998; Hill et al. 2006; Zhou and Shangguan 2007). For example, some studies found that the ratio of aboveground to belowground biomass increased with increasing soil moisture or mean annual precipitation (Chapin et al. 1993), while others found a weak relationship, if any, between biomass ratio and water availability (Vogt et al. 1996; Schenk and Jackson 2002). Zerihun et al. (2006) believed that the conflicting evidence in these reports was due in part to a considerable change in species composition, and that interspecies differences in the ratio of aboveground to belowground biomass might mask any effect of water availability. Until now, it has not been clear how changes in soil resources, especially in soil water and nitrogen, affect changes in the above- and belowground parts of vegetation, nor how these changes would affect the ratio of above- to belowground vegetation in arid and semiarid regions.
In this paper, we hypothesize that: (1) vegetation succession may induce changes in soil resources, especially the soil moisture, in arid and semiarid regions; (2) if the soil resources change, different plant species will have different reactions, and the dominant species of earlier and later successional stages may have significantly different reactions, especially with regard to those of the belowground vegetation; and (3) the changes of aboveground and belowground characteristics are not in balance, and the ratio of aboveground to belowground biomass may change with the changing soil resources. Therefore, we investigated the changes in aboveground and belowground characteristics of a secondary succession on abandoned cropland on the Loess Plateau, China, at a site where a succession gradient existed that covered the 10-year transition period from a pioneer plant community, dominated by Artemisia capillaries, to one where the later successional species, Stipa bungeana, had replaced it. In addition, we examined the interactions between vegetation succession and soil resources at both plant population and community levels, differentiating between changes in plant characteristics of specific individual plant populations and the whole plant community, respectively.
Materials and methods
Study site
The study was conducted on the Zhifangou watershed, Shaanxi Province, China (36°51′N,109°19′E). The small watershed (8.73 km2) has the typical landforms and vegetation types of the hill-and-gully region of the Loess Plateau, and has been used to monitor vegetation restoration processes following abandonment of cropland as a field experimental base of the Institute of Soil and Water Conservation, Chinese Academy of Sciences. The watershed has a semiarid climate with an average annual precipitation of about 510 mm and a mean temperature of 8.8°C. Most of the rainfall occurs from July to September. From 1998 to 2006, the annual precipitation was 526, 500, 331, 515, 541, 578, 509, 557, and 545 mm/year, consecutively. The altitude is from 1,006 m to 1,232 m. The surface vegetation was dominated by A. capillaries in the first 6 years of abandonment and subsequently by S. bungeana. The parent material of the soil is loess. The clay, silt and sand contents are 11.7%, 23.7%, and 64.6%, respectively (USA soil taxonomy). The minimum soil depth is more than 10 m. The organic C content is 2.35 ± 0.35 g kg−1 (mean±SD); available N content, 15.73 ± 2.52 g kg−1; and available P content, 15.09 ± 3.49 g kg−1.
In 1998, two large plots on a south-facing slope with a slope gradient ranging from 46 to 58% were established for long-term monitoring of aboveground characteristics of vegetation succession following cropland abandonment. The areas of the two plots were 80 ha and 110 ha and they were considered as replicates. Prior to abandonment, the main crops grown were potato (Solanum tuberosum), Setaria italica, and Phaseolus mungo. One crop was grown in each year. Fertilizer was applied before planting. Considering the strong influence of tillage history on succession (Benjamin et al. 2005), we only selected plots where potatoes had been planted for more than 3 years before abandonment. The cropland had been cultivated using a hoe and a plow for more than 40 years. Prior to studying succession, the cropland was cleared and kept free of plants by frequently removing them by hand. Thus, the foliar coverage in the first year of abandonment was very low. After abandonment, the croplands were not subject to human interference and a natural secondary vegetation succession commenced.
In order to investigate both aboveground and belowground characteristics, and the relationship between them, smaller plots with succession ages of 2, 4, 6, and 8 years, based on our long-term monitoring records, were constructed within each of the two large plots. According to earlier research by Wang and Liu (2002) conducted at this site, the use of five quadrates, 1 m2 in area, was sufficient to characterize interspecific associations and plant community characteristics of the Artemisia sacrorum community. We reasoned that this sampling frequency would also be adequate for characterizing the similar A. capillaries community. However, based on our own long-term monitoring of the early secondary succession of this abandoned cropland, we determined to actually select six 1 × 1 m2 quadrates within each plot to study the aboveground and belowground characteristics. Therefore, at the beginning of the growing season, in April 2006, we randomly selected six 1 × 1 m2 quadrates within each succession plot for this study. Thus, our experimental design consisted of two large plots serving as the source of two replicates for each of four subplots, with areas ranging from 900 to 1,600 m2, which were selected for succession ages of 2, 4, 6, and 8 years located within them. In each subplot, six 1 × 1 m2 quadrates were randomly selected giving a total of 48 quadrates in this study.
Aboveground characterization
In each quadrate, plant density, foliar coverage and maximum plant height were recorded separately by species. Leaf area index (LAI) was measured with a LI-3000c leaf area instrument (LI-COR, USA). In addition, the entire aboveground biomass (AGB) from each quadrate was clipped and sorted into dead and live fractions, and then dried and weighed at the end of the growing season, in October 2006. The biomass for 2006 was taken to be the live fraction, which included any leaf, stem or reproductive biomass that was at least partially green. For perennial plants such as A. capillaries, we distinguished the biomass for 2006 from the previous years’ biomass by considering that latter would consist of dead material due to the intervening cold winter. For each quadrate, the importance value (IV) for each species was calculated by: [(relative height + relative aboveground biomass)/2] × 100%; where the relative values of height and aboveground biomass were derived as the value for a species expressed as a percentage of the sum of these values for all of the species present (Mueller-Dombois and Ellenberg 1974). The species richness was given by the number of species numbers in each 1 × 1 m2 quadrate. Plant density was given by the number of plants in each 1 × 1 m2 quadrate. The percentage of a given plant species’ density was the ratio of the number of that species relative to the total number of all the species in the quadrate expressed as a percentage. At the population level, the aboveground characteristics mentioned above were calculated separated by species. At the community level, the aboveground characteristics were calculated from the totals for all of the species.
Belowground characterization
In each quadrate, one soil sample, for belowground vegetation characteristics analysis, was collected from each 10 cm layer between 0 and 100 cm depth using a stainless steel auger, 9 cm in internal diameter, in April, July, and October, 2006. In addition, another such sequence of soil samples were extracted adjacent to each quadrate, at the same time, to reduce disturbance within the quadrate and to serve as replicates. Collected samples were sealed in plastic bags for later laboratory analysis. Roots were separated from the soil by hand. The separated soil samples were air-dried for soil chemical analysis. The roots of A. capillaries and S. bungeana were sorted according to color and morphology under a magnifying glass or microscope, washed free of soil with tap water, and separated into fine roots (<2 mm) and coarse roots (>2 mm). All the segments of the fine roots were dried on absorbent filter paper and then spread over a rectangular, transparent, plastic sheet so that no two segments touched. A scanner was used to scan the roots on the plastic sheet at a resolution of 300 dpi. Images of the roots were recorded in tiff format. The fine root length, area, and diameter were measured using CIAS 2.0 image analysis software (CID, USA). Before the roots were scanned, the scanner and software were calibrated with CIAS image standards for length and area. Root diameter classes were set at 0.1-mm intervals. Root diameter and length were measured and surface area was calculated. After being scanned, the roots were oven-dried at 70°C for 48 h before being weighed. The fine root length density (FRLD), fine root mass density (FRMD), and fine root area density (FRAD) were obtained by dividing the root length, root mass, and root surface area, respectively, by the inner-volume of the soil core. The specific root length (SRL) and specific root area (SRA) were obtained by dividing the root length and root area by the root masses, respectively. At the population level, the belowground characteristics mentioned above were obtained separately for the A. capillaries population, S. bungeana population, and the other populations considered as one group. The fine root diameter (FRD) was taken as the mean diameter of all the fine roots for any given species or group. At the community level, the belowground characteristics mentioned above and FRD were calculated from the totals, or mean, of all the species.
Soil characteristic
From each quadrate, soil samples for soil moisture measurement were collected from each 20 cm layer between 0 and 100 cm depth using a stainless steel auger, 4 cm in internal diameter. The soil samplings were carried out once a month from April to October 2006. The soil moisture content was determined gravimetrically by drying the samples at 105°C. In addition, from each quadrate, two soil samples for nutrient content analysis were collected from each 10 cm layer between 0 and 100 cm depth using a stainless steel auger, 4 cm in internal diameter, in October, 2006. These soil samples underwent standard analysis for nutrient contents (Lu 2000). Organic matter content was determined by K2Cr2O7 oxidation and FeSO4 titration. Total N was determined by H2SO4 digestion and H2SO4 titration. The available N was determined by the continuous alkali-hydrolyzed reduction diffusing method. Total P was measured by H2SO4-HCLO4 digestion and the molybdenum antimony-ascorbic acid colorimetric method. Available P was measured by the Olsen method.
Data analysis
One-way ANOVA tests were performed to detect significant differences among the mean values of plant density, foliar coverage, LAI, AGB, FRLD, FRAD, SRL, SRA, FRD, and fine root biomass (FRB) for succession durations of 2, 4, 6, and 8 years (independent variables). Canonical correlation analysis was carried out to examine the relationships between plant characteristics (i.e., plant density, LAI, AGB, FRLD, FRD, and FRB) and soil factors (i.e., organic matter, total N, available N, total P, available P, and soil moisture). All the statistical analyses were performed using the SPSS 13.0 statistical software package (SPSS, USA). We tested the plant data for normal distributions and, when required, we used logarithmic transformations. Homogeneity-of-variances were tested by the Levene test.
Results
At the plant community level, the species richness index increased in the first 8 years (p < 0.05) with the vegetation succession (Table 1). In addition, the plant density, foliar coverage, and LAI all increased significantly with vegetation succession time (p < 0.01) (Figs. 1a,b and Fig. 2). At the plant population level, the characteristics of each plant species changed differently with succession time. For example, the IV of A. capillaries decreased while the IV of S. bungeana increased with succession time (Table 1). In addition, the plant density, foliar coverage and LAI of A. capillaries increased in the first 6 years (p < 0.05) and then decreased significantly between the 6th and 8th years (p < 0.05). In contrast, the plant density, foliar coverage, and LAI of the S. bungeana population significantly increased over the entire succession time. The total plant density of the other populations (i.e., of all the species listed in Table 1 with the exception of A. capillaries and S. bungeana) increased in the first 6 years (p < 0.05) but then decreased over the next 2 years (p < 0.05), whereas both foliar coverage and LAI of these populations increased over the first 8 years of succession (p < 0.05).
At the plant community level, the ratio of AGB to BGB decreased significantly with succession time (p < 0.05) (Fig. 3), because the increase of BGB was more rapid than that of AGB (data not shown). The ratios for both the A. capillaries and the S. bungeana populations decreased with succession time. The decrease of the ratios of A. capillaries was rapid due to greater increases in BGB than in AGB with succession time. However, the decrease of the ratio of S. bungeana was due to the more rapid increase in BGB with succession in the first 6 years followed by a slower decrease in BGB with succession from the 6th to the 8th year. The mean ratios of the other populations fluctuated over the 8 years because they were derived from a variety of species having different trends that increased or decreased at various rates with succession time.
The changes in FRLD were similar to those in FRAD occurring over an 8-year period of vegetation succession on abandoned farmland (Fig. 4a,b). For the plant community, both the FRLD and FRAD increased significantly with succession time (p < 0.05), which was especially evident in the sharp increase between the 6th and 8th years. The FRLD and FRAD in the 8th year were 3.49 and 3.58 times those in the 6th year, respectively. At the plant population level, the dynamic changes occurring in A. capillaries, the dominant species in the early succession stage, were different from those occurring in the S. bungeana, the dominant species in the later succession stage. Both the FRLD and FRAD of the A. capillaries population increased significantly for 6 years after abandonment (p < 0.05). However, these two characteristics then decreased significantly from the 6th to the 8th year. In contrast, both the FRLD and the FRAD of the S. bungeana population increased significantly from the 4th year (p < 0.05) and, in the 8th year, comprised 78.0 and 77.9% of the community FRLD and FRAD, respectively.
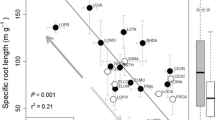
Changes in fine root length (a), fine root area (b), specific root length (c), specific root area (d), fine root diameter (e) and fine root biomass (f) with succession time (means ± SD, n = 12). Different letters indicate significant differences among the succession times for a given plant population (p < 0.05)
The dynamic changes in SRL were similar to those in SRA over the succession process (Fig. 4c,d). In the plant community, SRL increased significantly from the 2nd to the 6th year (p < 0.05); however, there was no significant difference between the SRL in the 6th year and that in the 8th year (p = 0.10). The SRA followed an increasing trend from the 2nd to th years and then a decreasing trend from the 6th to the 8th year. However, there were no significant differences among the SRAs for different succession times (p > 0.05) except for those between the 2nd and 4th years (p = 0.03). At the population level, both the SRL and SRA of A. capillaries increased significantly in the first 6 years (p < 0.05) and then decreased significantly from the 6th to the 8th year (p < 0.05). Similarly, both the SRL and SRA of the S. bungeana population increased in the first 6 years (p < 0.05). However, there was no significant difference between the SRL in the 6th year and that in the 8th year (p = 0.09), nor was there one between the SRAs for these years (p = 0.12). Furthermore, both the mean values of the SRL and SRA of the other plant populations followed an increasing trend with vegetation succession time.
At the plant community level, the mean FRD tended to decrease, but there were no significant differences among the mean FRDs over the 8-year vegetation succession period (Fig. 4e). Likewise, the FRDs of the A. capillaries and S. bungeana populations both declined but the differences among the FRDs for the four time stages were not significant (p > 0.05). The mean FRDs of other plant populations showed greater fluctuation with succession time but the differences among FRDs were also not significant (p > 0.05).
At the plant community level, the FRB increased significantly with succession time (p < 0.05) and this was especially evident in the sharp increase between the 6th and 8th years (p < 0.05) (Fig. 4 f). At the population level, the FRB of A. capillaries increased in the first 6 years of succession (p < 0.05). However, a significant decrease occurred over the next 2 years (p < 0.05). During the same period, the FRB of the S. bungeana population increased after farmland abandonment, especially so with a sharp increase between the 6th and 8th years. The total FRB of other plant populations increased with vegetation succession time (p < 0.05).
At the plant community level, among the aboveground parameters, the only significant correlation was between foliar coverage and LAI (p = 0.004). Among the belowground parameters, any pair of the parameters, FRLD, FRAD, SRL, SRA, and BGB, was significantly correlated (p < 0.05). Additionally, BGB was significantly correlated with AGB (p < 0.03).
At the plant population level, there were strong correlations among the aboveground parameters (Tables 2 and 3). Thus, the foliar coverage and LAI of the A. capillaries population (Table 2), and any pair of the parameters, plant density, foliar coverage, and LAI, of the S. bungeana population (Table 3), were significantly correlated. Among the belowground parameters, there were significant correlations between any pair of the parameters, FRLD, FRAD, SRL, SRA, and BGB of both the A. capillaries and S. bungeana populations. It was noted that the LAI of S. bungeana was significantly correlated with both its FRLD and FRAD. Additionally, the BGB was significantly correlated with AGB for both populations. Thus, the various parameters were more strongly correlated within a given plant population than within the plant community taken as a whole.
Canonical correlation analysis was carried out to study the effects of various soil factors on vegetation succession. Organic matter (g kg-1, x1), total P (g kg-1, x2), available P (g kg-1, x3), total N (g kg-1, x4), available N (g kg-1, x5), and soil moisture (%, x6) were chosen as soil factors, while plant density (plants m-2, y1), LAI (m2 m-2, y2), AGB (g m-2, y3), FRLD (m m-2, y4), mean FRD (mm, y5), and BGB (g m-2, y6) were chosen as both community and population factors. Here, LAI was used to represent the foliar coverage, given the close correlation between them, and, likewise, the FRLD was used to represent FRAD, SRL, and SRA, given the close correlation among them as reported above.
At the plant community level, canonical correlation analysis shows that the first and second pairs of correlation coefficients were significant (p < 0.05).
The first pair of canonical functions was given by:
where U 1 was the first axis, and V 1 was the second axis. The first axis (U 1 ) shows that soil moisture had a greater canonical loading, while the second axis (V 1) shows that the FRLD, FRD, and BGB had greater canonical loading. Thus, the first pair of canonical functions indicated that the development of the plant community root system (FRLD, FRD, and BGB) was mainly influenced by soil moisture.
The second pair of canonical functions was given by:
where U 1 was the first axis, and V 1 was the second axis. The first axis (U 1 ) shows that soil available P and available N have greater canonical loading, while the second axis (V 1) shows that AGB and LAI had greater canonical loading. Thus, the second pair of canonical functions indicated that development of the aboveground part of the plant community was mainly influenced by soil available P and available N.
At the population level, canonical correlation analysis within the A. capillaries population showed that only the first and second pairs of correlation coefficients were significant (the canonical functions have not been presented). The first pair of canonical functions indicated that the FRLD of the A. capillaries population was mainly influenced by soil moisture, and the second pair of canonical functions indicated that A. capillaries plant density and LAI were influenced by available N and available P.
However, canonical correlation analysis within the S. bungeana population showed that only the first pair of correlation coefficients of the S. bungeana population was significant at the 0.05 level, which indicated that S. bungeana plant density and FRLDs were mainly influenced by available N and available P.
From the analysis above, the aboveground parts were mainly influenced by soil available P and available N at both community and population levels. However, the belowground parts were mainly influenced by soil moisture at the community level. At the population level, the belowground parts of A. capillaries were significantly influenced by soil moisture, whereas the belowground parts of S. bungeana were not significantly influenced by soil moisture.
Discussion
Our research showed secondary vegetation succession in abandoned croplands may induce changes in soil resources, especially the decrease in soil moisture. In turn, changes in soil resources may have influenced vegetation succession processes. Notably, different interactions between plant species and soil moisture were observed, i.e., the above- and belowground characteristics of the earlier dominant species, A. capillaries, decreased with the lower soil moisture contents in the 6th and 8th year, while those of the later dominant species, S. bungeana, increased. In addition, aboveground and belowground characteristics were mainly influenced by soil nutrients and soil moisture, respectively, and the aboveground to belowground ratio changed with changing soil resources.
Changes of aboveground characteristics and fine root characteristics with succession time
Many researchers have observed that plant community characteristics were usually relatively lower in species richness and plant density in the earlier stages of succession following farmland abandonment (Keever 1983, Carpenter et al. 1990, El-Sheikh 2005), and our study further supports that finding. For example, we found a relatively lower species richness, plant density, FRLD, and FRAD (Table 1, Fig 4 a,b). Furthermore, we found that pioneer species, such as A. capillaries and S. viridis, have high reproductive capacities and ecological, morphological, and genetic plasticity (Denslow 1980).
At the community level, we found that species richness, plant density and foliar coverage increased rapidly with succession time. As community succession continued, later species, such as S. bungeana and Artemisia giraldii, became established and propagated quickly. Similar observations were made by Blatt et al (2005), who reported that the species number increased in the mid-successional stage.
However, while the plant density, foliar coverage, FRLD, FRAD, AGB, and BGB of the A. capillaries population tended to increase during the first 6 years, they decreased over the following 2 years. During those 2 years, the S. bungeana population propagated rapidly and became the dominant population in the 8th year. The changes in the relative dominance of A. capillaries and S. bungeana may be caused by changes in soil resources, which will be further discussed at the end of this section.
Characteristic comparison between A. capillaries and S. bungeana populations
Plants compete primarily for light aboveground and for water and nutrients belowground. The roots, especially the fine roots, are the main organs by which the plant absorbs water and nutrients, and play a key role in interspecific competition (Vogt et al. 1996, Moreno et al. 2005, Schenk and Jackson 2002). We found that S. bungeana had a stronger capability for fine root propagation than A. capillaries. Most notable was the sharp increase in the FRLD of the S. bungeana, in contrast to the sharp decrease in the FRLD of the A. capillaries, between the 6th and 8th year. Thus, the S. bungeana may have developed a stronger potential capacity for absorption of water and nutrients because the associated increased root growth and root exploration may confer an important competitive advantage (Eissenstat and Caldwell 1988). Relative plant traits occur because different plants have different reactions to decreasing moisture.
The leaf is the key organ for photosynthesis and the LAI is an important index for photosynthesis activity (Anten et al. 1998). ANOVA analysis showed that the LAI of S. bungeana was significantly greater than that of A. capillaries in the 6th and 8th years (p < 0.05). However, there was no significant difference between the LAI of the two species in the 4th year (p = 0.16). moreover, S. bungeana had a larger mean specific leaf area (LAI/AGB) than A. capillaries, i.e., 1.23 ± 0.47 m2 g-1 compared to 0.21 ± 0.13 m2 g-1, respectively.
In addition, the S. bungeana had a lower ratio of AGB to BGB than A. capillaries, i.e. S. bungeana allocated more biomass for root growth (Fig. 3), which can increase the capability for absorption of water and other limiting soil resources in arid and semiarid regions (Parrish and Bazzaz 1976, Redente et al. 1992). Craine (2006) regarded that, in general, competition could cause individual plants to allocate a greater fraction of their reserves to the acquisition of the most limiting resources compared to plants growing in the absence of competition. In this study site, the precipitations from 1998 to 2006 were 526, 500, 331, 515, 541, 578, 509, 557, and 545 mm/year, consecutively. The 331 mm/year precipitation in 2000 was exceptionally low. Soil moisture decreased significantly with succession time and declined to 8.96% in the 8th year (Table 4) although rainfall was more or less constant and typical in these years. Soil moisture obviously became a limiting factor for the growth of certain plants. Thus, subsequent increases in RLD, RAD, and the proportion of BGB may be attributed, in part, to competition among plants for soil resources, especially for the soil moisture. Alternatively, this phenomenon may be the result of plant responses in changing allocation priorities in the presence of reduced soil resources. This issue needs further research whereby competition between the two dominant species is examined under different applied soil moistures regimes.
The reciprocity of vegetation and soil resource with succession time
Many studies have found that there is strong reciprocity between vegetation and soil resources (reviewed by Burke et al. 1998; Berendse 1998). The influence of soil resources on vegetation, especially those of N, P, and soil moisture and the consequences for successional dynamics has been examined (reviewed by Wedin and Tilman 1990; Adema and Grootjans 2003). The species in a community were not generally affected equally by responses to changes in resources including available N and P, and/or soil moisture. The changes in one or more resources may induce the changes in plant community composition and structure, which then facilitates or inhibits vegetation succession (Tilman 1985, Blatt et al. 2005). At our study site, the decrease in soil moisture potentially causes competition among the plant species for the limited soil water resources (Hao et al. 2005). Moreover, although there was no significant change in either available phosphorus or available nitrogen content (Table 4), an increase in plant density could consolidate competition. Hence, soil resources, especially soil moisture, may determine the outcome of competition between plant species. The ability of the roots of A. capillaries to extract soil water was apparently affected once the soil water content ranged between 9.0 and 12.6% (Table 4). However, soil moisture contents in this range had no significant effect on S. bungeana. The replacement of A. capillaries as the dominant species by S. bungeana may be explained by the different direct response of the two species to decreasing soil moisture alone. Possibly, this change may also have occurred due to a more general interspecific competition for soil resources, or due to both of these reasons, but to definitively say so requires additional investigation along the lines suggested above.
The plant community also has a strong effect on the soil resources. For example, the plant community may facilitate the accumulation of organic materials, and change nutrient availability and soil moisture (DiTommaso and Aarssen 1989; Carrera et al. 2008). The classic theory of facilitating succession (Clements 1916) predicts that early successional species modify their environment to facilitate the replacement by other species. Furthermore, if the changes in abiotic environmental factors favor the existing vegetation, the present vegetation may stabilize or even arrest the succession, resulting in an alternative stable state (Wilson and Agnew 1992, Adema and Grootjans 2003). In this study, we found that soil moisture decreased with vegetation succession time and ascribed this to the rapid development of plant communities that vigorously extracted water from the soil. A similar conclusion has been reported by Davis et al (1998) and El-Sheikh (2005). During the same period, there were no significant changes in either available P or available N contents. This means that the succession process neither improved nor degraded the soil in this regard.
References
Adema EB, Grootjans AP (2003) Possible positive-feedback mechanisms: plants change abiotic soil parameters in wet calcareous dune slacks. Plant Ecol 167:141–149. doi:10.1023/A:1023947411605
Anten NPR, Werger MJA, Medina E (1998) Nitrogen distribution and leaf area indices in relation to photosynthetic nitrogen use efficiency in savanna grasses. Plant Ecol 138:63–75. doi:10.1023/A:1009727822617
Benjamin K, Domon G, Bouchard A (2005) Vegetation composition and succession of abandoned farmland: effects of ecological, historical and spatial factors. Landscape Ecol 20:627–647. doi:10.1007/s10980-005-0068-2
Berendse F (1998) Effect of dominant plant species on soil during succession in nutrient-poor ecosystems. Biogeochemistry 42:73–88. doi:10.1023/A:1005935823525
Blatt SE, Crowder A, Harmsen R (2005) Secondary succession in two south-eastern Ontario old-fields. Plant Ecol 177:25–41. doi:10.1007/s11258-005-2018-0
Breemen NV, Finze AC (1998) Plant-soil interaction: ecological aspects and evolutionary implications. Biogeochemistry 42:1–19. doi:10.1023/A:1005962124317
Burke IC, Lauenroth WK, Vinton MA, Hook PB, Kelly RH, Epstein HE et al (1998) Plant-soil interactions in temperate grasslands. Biogeochemistry 42:121–143. doi:10.1023/A:1005987807596
Carpenter AT, Moore JC, Redente EF, Stark JC (1990) Plant community dynamics in a semi-arid ecosystem in relation to nutrient addition following a major dynamics disturbance. Plant Soil 126:91–99. doi:10.1007/BF00041373
Carrera AL, Bertiller MB, Larreguy C (2008) Leaf litterfall, fine-root production, and decomposition in shrublands with different canopy structure induced by grazing in the Patagonian Monte, Argentina. Plant Soil. doi:10.1007/s11104-008-9655-8
Chapin FS, Autumn K, Pugnaire F (1993) Evolution of suites of traits in response to environmental stress. Am Nat 142:78–92. doi:10.1086/285524
Clements FE (1916) Plant succession: an analysis of the development of vegetation. Carnegie institute of Washington publication NO 242, Wastington DC
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111:1119–1144. doi:10.1086/283241
Craine JM (2006) Competition for nutrients and optimal root allocation. Plant Soil 285:171–185. doi:10.1007/s11104-006-9002-x
Davis MA, Wrage KJ, Reich PB (1998) Competition between tree seedlings and herbaceous vegetation: support for a theory of resource supply and demand. J Ecol 86:652–661. doi:10.1046/j.1365-2745.1998.00087.x
Denslow JS (1980) Patterns of plant species diversity during succession under different disturbance regimes. Oecologia 46:18–21. doi:10.1007/BF00346960
DiTommaso A, Aarssen LW (1989) Resource manipulation in vegetation: a review. Vegetatio 84:9–29. doi:10.1007/BF00054662
Eissenstat DM, Caldwell MM (1988) Seasonal timing of root growth in favorable microsites. Ecology 69:870–873. doi:10.2307/1941037
El-Sheikh MA (2005) Plant succession on abandoned fields after 25 years of shifting cultivation in Assuit, Egypt. J Arid Environ 61:461–481. doi:10.1016/j.jaridenv.2004.10.006
Hao WF, Liang ZS, Chen CG, Tang L (2005) Study of species diversity evolvement process during vegetation restoration of abandoned farmland in the hilly loess plateau. Pratacaltural science 22, 1–9 (In Chinese)
Hill JO, Simpson RJ, Moore AD, Chapman DF (2006) Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 286:7–19. doi:10.1007/s11104-006-0014-3
Jones CG, Lawton JH, Schachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386. doi:10.2307/3545850
Keever C (1983) Retrospective view of old-field succession after 35 years. Am Midl Nat 110:397–404. doi:10.2307/2425278
Lauenroth WK, Coffin DP, Burke IC, Virginia PA (1997) Interactions between demographic and ecosystem processes: a challenge for functional types. In: Smith TM, Shugart HH (eds) Plant functional Types. Cambridge University Press, Cambridge, UK, pp 234–254
Lu RK (2000) Chemistry analysis for agriculture soil. Chinese Agriculture Science and Technology Press, Beijing, China (in Chinese)
Moreno G, Obrador JJ, Cubera E, Dupraz C (2005) Fine root distribution in Dehesas of Central-Western Spain. Plant Soil 277:153–162. doi:10.1007/s11104-005-6805-0
Mueller-Dombois D, Ellenberg H (1974) Aims and Methods of Vegetation Ecology. John Wiley &Sons, New York
Parrish JAD, Bazzaz FA (1976) Underground niche separation in successional plants. Ecology 57:1281–1288. doi:10.2307/1935052
Redente EF, Friedlander JE, Mclendon T (1992) Response of early and late semiarid seral species to nitrogen and phosphorus gradients. Plant Soil 140:127–135. doi:10.1007/BF00012814
Schenk HJ, Jackson RB (2002) Rooting depths, lateral root spreads and below-ground/ above-ground allometries of plants in water-limited ecosystems. J Ecol 90:480–494. doi:10.1046/j.1365-2745.2002.00682.x
Tilman D (1985) The resource-ratio hypothesis of succession. Am Nat 125:827–852. doi:10.1086/284382
Tracy BF, Sanderson MA (2000) Patterns of plant species richness in pasture lands of the northeast United States. Plant Ecol 149:169–180. doi:10.1023/A:1026536223478
Van-Breement N, Finzi AC, Canham CD (1997) Canopy tree-soil interactions within temperate forests: effects of soil texture and elemental composition on species distributions. Can J For Res 27:1110–1116. doi:10.1139/cjfr-27-7-1110
Vogt KA, Vogt DJ, Palmiotto PA, Boon PO, Hara J, Asbjornsen H (1996) Review of root dynamics in forest systems grouped by climate, climate forest type and species. Plant Soil 187:159–219. doi:10.1007/BF00017088
Wang GL, Liu GB (2002) Study on interspecific Association of the Artemisia sacrorum community in loess hilly region. Grassland of China 24:1–6 (in Chinese)
Wedin DA, Tilman D (1990) Species effects on nitrogen cycling: a test with perennial grasses. Oecologia 84:433–441
Wilson JB, Agnew ADQ (1992) Positive-feedback switches in plant communities. Adv Ecol Res 23:263–337. doi:10.1016/S0065-2504(08)60149-X
Zerihun A, Montagu KD, Hoffmann MB, Bray SG (2006) Patterns of below- and aboveground biomass in Eucalyptus populnea woodland communities of northeast Australia along a rainfall gradient. Ecosystems (NY, Print) 9:501–515. doi:10.1007/s10021-005-0155-x
Zhou ZC, Shangguan ZP (2007) Vertical distribution of fine roots in relation to soil factors in Pinus tabulaeformis Carr. Forest of the Loess Plateau of China. Plant Soil 291:119–129. doi:10.1007/s11104-006-9179-z
Acknowledgements
This work was financially supported by the Second Phase of the CAS Action Plan for West Development program of China (No. KZCX2-XB2–05), the National Key Technologies R&D Program (No. 2006BAD09B03) and the frontier research fund of ISWC, CAS, China. We thank Mr. David Warrington for revising the English text. We also thank two anonymous reviewers and editors for constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tibor Kalapos.
An erratum to this article can be found at http://dx.doi.org/10.1007/s11104-009-0098-7
Rights and permissions
About this article
Cite this article
Wang, G., Liu, G. & Xu, M. Above- and belowground dynamics of plant community succession following abandonment of farmland on the Loess Plateau, China. Plant Soil 316, 227–239 (2009). https://doi.org/10.1007/s11104-008-9773-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9773-3