Abstract
Background and aim
Significant differences in tree growth were observed in an exotic pine plantation under different harvest residue management regimes at ages 2–10 years. However, the variations in tree growth between residue management treatments could not be explained by soil and foliar nutrient analyses, except by potassium (K) concentration. Therefore, this study determined the carbon isotope composition (δ13C) and oxygen isotope composition (δ18O) of current and archived foliar samples from the exotic pine plantation to establish relationships with foliar K concentration and tree growth indices as a means to determine changes in stomatal conductance (gs) and photosynthetic rate (Amax) or water use efficiency (WUE), and therefore understand the variations in tree growth across treatments.
Methods
The harvest residue treatments were: (1) residue removal, RR0; (2) single level residue retention, RR1; and (3) double level residue retention, RR2. Foliar δ13C and δ18O were determined for samples at ages 2, 4, 6 and 10 years, and the atmospheric 13C discrimination (Δ13C), intercellular CO2 concentration (Ci) and WUE were determined from the δ13C data. Litter needle δ13C and δ18O were also determined over 15 months between ages 9 and 10 years. These parameters or variables where correlated to each other as well as to the periodic mean annual increment of basal area (PAIB) and the periodic mean annual increment of tree diameter at breast height (PAID) across the treatments and over time. Foliar δ13C and δ18O were also related to published data of foliar K concentrations of the same trees.
Results
Significant variations of foliar δ13C, and therefore WUE and Δ13C, across treatments were only observed at ages 4 and 10 years old, and foliar δ18O at age 4 years old only. The results showed increasing foliar δ13C, δ18O and WUE, and decreasing Δ13C and Ci, from RR0 to RR2 treatments. However, while the WUE was positively related to the PAID and PAIB at age 4 years, it was negatively related to PAID and PAIB at age 10 years old. Litter needle δ13C, indicative of WUE, was also negatively related to the PAID at age 10 years old. . At age 4 years, foliar δ13C and δ18O were positively related with a steep slope of 7.70 ‰ across treatments, and that both isotopes were positively related to foliar K concentrations. Similarly, δ18O was negatively related to the Δ13C. No significant relationship can be determined between foliar δ13C, or Δ13C, and δ18O at age 10 years old. In addition, WUE was increasing (p < 0.001) and Δ13C and Ci decreasing (p < 0.001) with decreasing PAID over time.
Conclusions
The variations at age 4 years in foliar δ13C or Δ13C and δ18O and increasing WUE with increasing growth rate suggest growth induced water-stress with increasing residue-loading rate as a result of the nutritional effect of the harvest residues on tree growth. At age 10 years, the negative relationships between WUE and PAID indicate nutrient limitation has an over-riding effect on δ13C variations rather than gs. This was due to the lack of a significant relationship between foliar Δ13C and δ18O at this age, as well as over time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant organic matter carbon (C) and oxygen (O) isotopic ratios (δ13C and δ18O, respectively) are widely used to determine the influence of genetic and environmental factors on plant growth (Xu et al. 2000; Warren et al. 2001; Barbour et al. 2002; Prasolova et al. 2005; Keitel et al. 2006; Huang et al. 2008a; Hasselquist et al. 2010). They are regarded as integrative measures of eco-physiological processes over the period in which the plant material was formed, and therefore can better account for tree growth than instantaneous measurements of stomatal conductance, water use efficiencies and net photosynthesis (Zhang and Cregg 1996; Prasolova et al. 2003; Xu et al. 2003; Barbour 2007; Huang et al. 2008b).
The theory behind varying plant δ13C composition as a result of varying soil moisture conditions is well established (Farquhar et al. 1982; Farquhar and Richards 1984; Barbour et al. 2000; Warren et al. 2001). During photosynthesis, the C fixing enzyme, ribulose bisphosphate carboxylase/oxygenase (Rubisco), discriminates against the heavier 13CO2. This discrimination, however, diminishes as the leaf internal CO2 concentration (C i ) decreases (Farquhar et al. 1982; Powers et al. 2009; Hasselquist et al. 2010), such as when stomatal conductance (gs) is decreasing in response to low soil moisture. Thus, a significant correlation between plant δ13C and soil moisture availability can be established (Farquhar and Richards 1984; Xu et al. 2000; Warren et al. 2001; Huang et al. 2008c; Ibell et al. 2013).
The C i , however, can also be influenced by the photosynthetic capacity, therefore confounding the relationship between gs and δ13C of plant tissues. This limitation can be overcome by the combined determination of δ13C and δ18O, to separate the effects of gs and photosynthesis on δ13C variation, since the δ18O signature is not dependent on Rubisco activity (Scheidegger et al. 2000; Xu et al. 2000; Keitel et al. 2003; Barbour 2007). The 18O fractionation theory had been discussed extensively in Barbour et al. (2000) and Barbour (2007). In brief, it suggests, in general, that plant δ18O is negatively related to gs, and that a positive relationship between δ18O and δ13C indicates that δ13C is largely driven by gs (Saurer et al. 1997; Farquhar et al. 1998; Barbour et al. 2000; Keitel et al. 2003; Huang et al. 2008a, c). A recent study, however, showed that relationships between WUE and growth can also be used to differentiate photosynthetic capacity (Amax) from changes in gs due to water stress (Silva and Anand 2013).
A number of studies have indicated that tree growth and bulk stem wood or tree ring cellulose δ13C are related (Dupouey et al. 1993; McNulty and Swank 1995; Livingstone and Spittlehouse 1996; Garcia-G et al. 2004; Fernandez et al. 2006; Sun et al. 2010; Gomez-Guerrero et al. 2013). Furthermore, studies of conifer plantation species showed that whole leaf or foliar δ13C could be related to cumulative tree growth (Högberg et al. 1993; Xu et al. 2000). Limited studies, however, have successfully related either whole stem wood or leaf δ18O to tree growth (Xu et al. 2000, 2009). In addition, the use of leaf litter δ13C and δ18O, which are likely to be representative of longer eco-physiological history, has been largely unexplored.
A study at ages 0–6 years (Simpson et al. 2003), and our recent study at age 10 years of the same plantation (Tutua et al. 2008), on the impact of residue management on tree nutrition and growth showed significant variation among treatments of the cumulative tree growth and periodic mean annual increment (PAI) prior to the peaking of growth rate. This variation of tree growth between treatments could not be related to foliar nutrient concentrations, other than foliar potassium (K) concentration, the only nutrient that showed significant residue treatment effects in both the soil and the foliage (Tutua et al. 2008). It was speculated, however, that the variation of foliar K concentration was a response to tree water stress (Prasolova et al. 2005; Fernandez et al. 2006), where larger or fast growing trees in the residue retention treatments were much more water stressed than those in the no-residue treatments (Tutua et al. 2008), consistent with studies showing growth-induced water stress due to the high demand of faster growing trees for soil water when N and P supply are non-limiting (Högberg et al. 1993). Potassium is important for maintaining leaf water and pressure potential, allowing photosynthesis to proceed under water stressed conditions (Ashraf et al. 2001; Pervez et al. 2004). Therefore, foliar K is expected to relate to foliar δ18O and δ13C. On the other hand, the variation in foliar K concentrations could merely be due to a luxury uptake of K under the residue retention treatments.
Studies that have clearly separated the mulching or nutritional effects of logging harvest residues through their influence on gs and/or Amax and therefore foliar δ13C and δ18O and their relationships to tree growth are limited. Therefore, this study aimed to better understand the impact of harvest residues on tree growth, in relation to the soil moisture or nutrition hypotheses, through a retrospective analysis of foliar and litter needle δ13C and δ18O. The main objectives of this study were to: (1) determine the influence of residue management on the foliar δ13C and δ18O of archived and current year samples; (2) establish relationships among the δ13C, or WUE and Δ13C, δ18O, foliar K concentrations and tree growth parameters to assess whether gs andAmax or water and nutrient limitations could explain the variation in tree growth under different residue management regimes; and (3) explore the potential of using litter needle δ13C and δ18O as a tool for understanding the influence of environmental factors on tree growth in the exotic pine plantation of Subtropical Australia.
Methodology
Site description and experimental design
This study was carried out in an existing harvest residue management experiment established in July 1996. The details of this site and experimental design were as described by Simpson et al. (2003) and Chen and Xu (2005). In brief, the site was located at Toolara State Forest (26°00′S, 152° 49′E), southeast Queensland, Australia. It is generally flat, with a deep sandy soil classified as gleyic acrisols (FAO 1974). The climate is humid sub-tropical with a mean annual rainfall of 1,354 mm, with 56 % falling in December to March (Simpson et al. 2003). The July to September period is relatively dry, which may extend to November (Xu et al. 2000). The summers are hot and moist with a mid-summer mean daily temperature of 24.9 °C and a relative humidity of 70 %, while the winters are mild, with a mid-winter mean daily temperature of 14.0 °C and a mean relative humidity of 64 %. Exotic pine plantation trees in this region often experience both well-watered and water-limited conditions in a year, even in a wet summer season (Xu et al. 2000; Prasolova et al. 2005; Ibell et al. 2013). In addition, annual rainfall has been below average for the last 10 years.
The current experiment is a randomised complete block design with six treatments and four blocks. Gross plots are 12 rows by 12 trees at 3 × 3 m spacing and net plots are 8 rows by 8 trees (0.058 ha). While there are 6 treatments (Simpson et al. 2003), this study focussed on the harvest residue loading rates, which included: (1) residue removal + 50 kg P ha−1 added; (2) residues retained + 50 kg P ha−1 added; and (3) double quantities of residues retained + 50 kg P ha−1 added. Moving harvest residues from the residue removal treatments to plots with harvest residues created the double residue retention treatment (Simpson et al. 2003). The three treatments are referred to as RR0, RR1 and RR2, respectively. The plots were planted in mid 1996 with the F1 hybrid between slash pine (Pinus elliottii var. elliottii) and Carribean pine (Pinus caribeae var. hondurensis) seedling stocks from 6 different families. Each family was randomly allocated a row, with a double up of 2 families per row in 2 rows to make up the 8 rows.
Measurement of growth and foliar and litter samplings
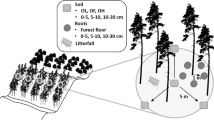
Detailed tree growth measurements and foliar and litter sampling methods were as described by Tutua et al. (2008). The growth indices, diameter at breast height (DBH), basal area (BA), tree height (HT) and periodic mean annual increments (PAI) over a 2 year period of DBH (PAID), BA (PAIB) and HT (PAIH), were determined at ages 2, 4, 6, 8 and 10 years (Tutua et al. 2008). Periodic annual increments (PAI), rather than annual increments (AI) of growth was used because tree measurements and sampling were only carried out in alternate years.
Foliar samples at age 10 years and archived samples at ages 2, 4 and 6 years (1998, 2000 and 2002, respectively; no foliar sampling in 2004) were all collected from the northward facing (sunny) side of the tree canopy (Xu et al. 2000; Tutua et al. 2008), and sampling was conducted on the same trees over the 10 years. Fifty fascicles of the most recent, fully expanded needles (approximately 1 year old) were collected from the 4 trees within a plot and bulked as one sample (Simpson et al. 2003). Even though the 4 trees within a plot may represent different families, pooling the samples was expected to minimise any variations in isotopic compositions between the families. In addition, a study of the F1 hybrid trees indicates that environmental effects on δ13C are usually greater than familial differences (Prasolova et al. 2005). Litter needles were collected quarterly from July 2005 to June 2006 by Tutua et al. (2008). All plant materials were oven dried at 60 °C for 5 days before ground to powder in a puck and ring mill.
Chemical analyses
Foliar and litter C isotope compositions (δ13C) were determined by an Eurovector 3000 elemental analyser (Milan, Italy) coupled to a GVI Isoprime mass spectrometer (Manchester, UK). The O isotope compositions (δ18O) were determined by a VARIO EL III elemental analyser (Hanau, Germany) coupled to a Sercon Hydra 20–20 mass spectrometer (Crewe, UK). The foliar and litter δ13C and δ18O were calculated relative to the PDB and IAEA VSMOW standards, respectively (Barbour et al. 2000; Xu et al. 2000; Huang et al. 2008a). Foliar K concentration data for the same trees and year was obtained from Tutua et al. (2008).
Precipitation measurements
Rainfall data for the site was obtained from January 1997 to September 2006, the last foliar sampling date. Total growth period rainfall of 12 months before the foliar sampling was carried out was also determined to correlated with the isotopic variables. This was because this rainfall would have influenced the δ13C and δ18O of the 1 year old foliar needles as they formed.
Δ13C, Ci and WUE calculations
Foliar δ13C is a good indicator of WUE for comparisons across treatments in a given year. However, since we were also interested in the long term temporal variations in isotopic compositions and tree growth parameters, we calculated foliar Δ 13 C using the foliar δ13C values to take into account the temporal variations of atmospheric CO2 and δ13C (Silva and Horwath 2013). The physiologically relevant Δ13C is expressed as:
where a is the discrimination against atmospheric 13CO2 during diffusion through the stomata (−4.4‰), b is the net discrimination due to carboxylation (−27‰), Ci is the internal CO2 concentration or partial pressures and Ca is the atmospheric CO2 concentration for a given year. The Ca value for each year of sampling was obtained from direct measurements from the South Pole at the website of the Carbon dioxide Information Analysis Centre (Sun et al. 2010). The Ci values were estimated from the following formulae (Silva and Anand 2013) expressed as:
where δ13Cplant and δ13Catmosphere are the plant and atmospheric isotopic carbon ratios, respectively. The δ13Catmosphere value for each year was estimated from the exponential function given in Feng (1998). Finally, the WUE was calculated following the derivations of Ci as follows:
where WUE, which is defined as the ratio between carboxylation and stomatal conductance, is a function of Ci and Ca, and that the conductance of CO2 molecules is 0.625 g for leaf conductance to water vapour (Silva and Anand 2013).
Statistical analyses
An analysis of variance (ANOVA) was carried out on δ13Cand δ18O and variables such as Ci, Δ13C and WUEto detect differences between the treatments at p < 0.05. The least significant difference (LSD) test at p < 0.05 was carried out to determine the degree of the variations between the treatments. Pearson’s correlation and linear regression analyses were carried out to determine relationships among tree growth indices and foliar δ13C, Ci, Δ13C, WUE and δ18O and, and . Litter δ13C sampled from July 2005 to July 2006 were regressed with foliar δ13C and δ18O sampled in July 2006 and PAID (2004–2006). The Statistix software (Version 8.0) was used for all the statistical analyses. Since we proposed that the variations in foliar K concentrations was a response to water stress, we regressed foliar K concentrations data obtained from the same trees in Tutua et al. (2008) with 13C and 18O in this present study to assess the validity of the proposition.
Results
Precipitation measurements
Figure 1 showed the rainfall distribution at the trial site from January 1997 to September 2006, the last sampling date of this study. The rainfalls for each year was below the annual mean for this site, and in general decreased over time, with the lowest 12 months rainfall in 2002. Although year 2000 had the highest rainfall, the last 2–6 months prior to sampling were relatively drier compared to the other years. In contrast, rainfall was more evenly distributed throughout the 12 months before sampling in 1998, therefore remaining relatively high within 2–6 months prior to sampling. Growth period rainfall, defined as the rainfall over the period in which the pine needles formed (1 year), also declined from 1998 to 2006.
Foliar δ13C, Δ13C, WUE and δ18O and tree growth
Significant treatment effect on foliar δ 13C occurred in 2000 and 2006 only (Table 1), where δ13C was lower in the RR0 treatment compared to the RR1 and RR2 treatments in both years. The maximum differences in δ13C variations between the treatments were 0.90 ‰ and 0.87 ‰ in 2000 and 2006, respectively. The δ18O, however, only showed significant treatment effects in 2000 when δ18O was the lowest in the RR0 treatment (p < 0.05) compared to the RR1 and RR2 treatments (Table 1). These 2 years represented the periods of peak growth (2000) and declining growth (2006) according to the PAIB and PAID trends reported in Tutua et al. (2008).
Calculations of Ci, Δ13C and WUE from the foliar δ13C data also showed significant variations among the residue management treatments in year 2000 and 2006 (Table 1) with significantly greater WUE, strongly reflecting trends in foliar δ13, of trees in the RR2 and RR1 treatments than the RR0 treatment. The results also showed a concomitant decrease in Ci and therefore Δ13C of the trees with increasing residue loading rates in both years (Table 1).
Regression analyses of PAID and PAIB with foliar δ13C and WUE in 2000 (age 4 years) showed a positive relationship with both variables (Fig. 2a–d). The results showed that WUE can explain about 36 % and 40 % of the increasing PAID and PAIB, respectively, at this stage. In contrast to year 2000, regressions of PAID and PAIB with foliar δ13C and WUE in year 2006 (age 10 years), showed a negative relationship (Fig. 3a–d). The increasing WUE explained 70 % and 49 % of the declining PAID and PAIB, respectively, with increasing residue loading rate and decreasing Δ13C in the order: RR0 > RR1 > RR2. These observations were also consistent with the decreasing Ci across the treatments (Table 1).
Relationships between foliar δ13C and periodic annual increments of tree diameter at breast height (PAID) (a) or basal area (PAIB) (b), and between tree WUE and PAID (c) or PAIB (d) at age 4 years of a F1 hybrid exotic pine plantation under three harvest residue management regimes: (1) residue removal; (2) single residue retention; and (3) double residue retention
Relationships between foliar δ13C and periodic annual increment of the basal area (PAIB) (a) or periodic annual increment of diameter at breast height (PAID) (b), and between tree WUE and PAID (c) or PAIB (d) at age 10 years of a F1 hybrid exotic pine plantation under three harvest residue management regimes: (1) residue removal; (2) single residue retention; and (3) double residue retention
Pearson’s correlations of tree growth indices and isotopic variables of the year 2000 data also yielded similar results as the regression analyses. These showed that the isotopic ratios and WUE were positively related to almost all growth indices, while the Δ13C was negatively related to the growth indices (Table 2). In 2006, foliar δ13C, Δ13C and WUE showed stronger relationships than in year 2000 as indicated by the coefficients with all growth indices, except HT. However, there were no significant correlations between δ18O and the growth indices, or between foliar δ13C and δ 18O and between Δ13C and δ18O. This was in contrast to year 2000, where there was also a significant correlation between foliar δ13C and δ 18O and between Δ13C and δ 18O (Table 2). This was further illustrated by the regression analyses of foliar δ 18O with Δ13C and foliar δ13C, indicating that these parameters could explain at least 56 % of the variations in foliar δ 18O (p < 0.005) across the treatments (Fig. 4b and c), with a slope of 7.70‰ increase in δ18O per 1.0‰ increase in δ13C (Fig. 4b). This study also found that foliar δ13C, δ18O and K concentration in 2000 were significantly related (Fig. 4a). Increasing δ13C and δ18O across the treatments coincided with the concomitant increase in foliar K concentration (Fig. 4a). In addition, both foliar K concentration and δ18O together improved the regression model and were able to explain about 67 % of the variation of δ13C (p < 0.01) in comparison to the foliar δ18O alone. This result was in contrast to year 2006 where there were no relationship between foliar δ18O and δ13C (Table 2) or between foliar δ18O and foliar K, except for foliar δ13C and K concentrations (r = 0.87; p < 0.001).
Relationships between foliar K concentration and δ18O or δ13C (a), and between foliar δ13C and δ18O (b), and Δ13C and δ18O at age 4 years (year 2000) of a F1 hybrid exotic pine plantation under three harvest residue management regimes: (1) residue removal; (2) single residue retention; and (3) double residue retention
Long –term variations in isotopic compositions and tree growth
Table 2 also shows that both foliar δ13C and δ18O increased over the last 8 years (p < 0.005), with means ranging from −30.80‰ to −28.85‰ for δ13C, and from 24.80‰ to 30.93‰ for δ18O from 1998 to 2006, respectively. Similarly, Ci and Δ13C were also decreasing (p < 0.05 and p < 0.0005, respectively), while WUE was increasing over this time period (p < 0.0001). The increase in WUE was about 45–55 % from 1998 to 2006. However, Pearson’s correlation analyses showed no significant correlation between foliar δ18O and δ13C or Δ13C, which accounted for changes in Ca and 13Catmosphere concentrations over the 8 years (Table 3). Similarly, there were no significant correlations observed between foliar δ18O and the declining PAID or PAIB, and even the foliar needle growth period rainfall. On the other hand, foliar δ13C, and therefore WUE, showed very strong negative correlations with the declining PAID and PAIB over the 8 years. These parameters were also very strongly correlated to the foliar needle growth period rainfall (Table 3), where the declining rainfall was positively related to the declining PAID or PAIB, and negatively related to the WUE.
Litter needle δ 13C and δ 18O
Litter needle δ13C ranged from −29.53‰ to −29.13‰ throughout the seasons (Table 4). Although the variations in litter needle δ13C between the treatments at each sampling were relatively small (0.27–0.33‰) compared to the foliar δ13C, they were significant (p < 0.01) in three of the four seasons assessed. In each case, δ13C increased in the following order: RR0 < RR1 < RR2, a similar trend to that seen in the foliage. Significant differences in litter needle δ18O between the treatments were observed in the January–March 2006 season only, where higher δ18O occurred in the RR0 treatment compared to the RR1 treatment (Table 4). However, this observation could not be related to litter needle δ 13C.
This study also showed that litter needle δ13C and foliar δ13C were related (Fig. 5a–b). However, only litter needle δ13C from the July – September 2005 season was significantly related (r 2 = 0.47; p < 0.05) to the PAID (2004–2006 period) (Fig. 5c). Regressing PAID with litter needle δ13C for all seasons that had significant δ13C variations showed that litter needle δ13C, in general, could be related to the PAID (r 2 = 0.26; p < 0.005) (Fig. 5d). Furthermore, Table 4 shows a temporal trend in the variation of litter needle δ13C between the treatments. The occurrence or absence of significant variations in litter needle δ13C between the treatments for each period appeared to closely follow the seasonal changes in the rainfall and temperature, where a greater variation between the treatments occurred in the periods with the lowest rainfall or highest daily temperature (Fig. 6).
Relationships between foliar δ13C sampled in 2006 and litter needle δ13C sampled from the July to September 2005 period (a) and all periods that have significant δ13C variations (Table 4) (b). Litter needle δ13C was also significantly related to the periodic annual increment of diameter at breast height (PAID) of the 2004–2006 period (age 8–10 years) as shown by the negative relationship between PAI.D and litter needle δ13C sampled in the July–September 2005 period (c) and periods that have significant litter needle δ13C variations (d)
The occurrence of litter needle δ13C variation between the treatments for each season or quarter from the 2005 to 2006 litter collection period was closely related to the rainfall (a) and temperature (b) patterns of the same periods in the previous year (2004–2005). The vertical bars are least significant differences (LSD0.05), and the sampling periods were: (1) Jul–Sept 2005, (2) Oct–Dec 2005, (3) Jan–Mar 2006, (4) Apr–Jun 2006, and (5) Jul–Sept 2006
Discussion
Foliar δ 13C and δ 18O variations
Harvest residue management clearly influenced the variations in foliar δ13C and δ18O in 2000, and δ13C in 2006 (Table 1), as indicated by the consistent increase in foliar δ13C in the order RR0 < RR1 < RR2 in both years. Whilst the maximum variations in foliar δ13C across the treatments were only 0.90‰ and 0.87‰ in 2000 and 2006, respectively, they were consistent with those studies having small variations of 1.0‰ or less, yet were useful in explaining the changes in gs, Amax and tree growth (Olbrich et al. 1993; Fischer et al. 1998; Barbour et al. 2000; Xu et al. 2000). Thus the correlation of foliar δ13C with the growth indices of the F1 hybrid exotic pine was consistent with other studies on conifer species, using foliar δ13C (Högberg et al. 1993) or Δ13C (Xu et al. 2000) and stem or wood cellulose δ13C (Dupouey et al. 1993; McNulty and Swank 1995). Relating foliar δ13C and WUE or Δ13C to growth rate indices such as PAID in this study or basal area annual increments (BAI) has also been widely used recently (Sun et al. 2010; Gomez-Guerrero et al. 2013; Silva and Anand 2013). This was because relating isotopic variations to growth rates are useful in explaining the dynamic changes in tree growth over time.
The absence of significant variations in foliar δ13C and δ18O among the treatments in some years could be due to high water availability (Keitel et al. 2003) in the 6–12 months leading to each sampling date, as reflected by the rainfall distribution (Fig. 1). In year 2000, the relatively drier 4 to 6 months prior to sampling was probably critical in the variation across the treatments, especially for δ18O, which often occur in concurrence with the variations in δ18C (Barbour et al. 2000). Rainfall distribution (Garcia-G et al. 2004), duration of drier conditions (Fernandez et al. 2006), N nutrition (Högberg et al. 1993; Huang et al. 2008b), ambient temperature and vapour pressure deficit (VPD) (Korol et al. 1999; Barbour et al. 2002) have been reported to influence tree foliar δ13C and δ18O.
Foliar isotopic compositions, WUE and K concentration and tree growth
The negative relationship between foliar δ13C or WUE and PAID or PAIB at age 10 years (2006) (Fig. 3) was similar to a study of Pinus greggii using wood cellulose δ13C (Garcia-G et al. 2004). Similarly, negative relationships between WUE, calculated from wood ring δ13C, and BAI have also been reported (Gomez-Guerrero et al. 2013). This growth response indicates an environmental stressor affecting tree growth (Silva and Anand 2013), and the effect on the growth rate is more severe on the larger trees in the RR2 (Tutua et al. 2008). The growth response was consistent with decreasing gs, which was indicated from the decreasing Ci across the treatments in this study (Table 1). Reductions in Ci is more likely due to closing stomata in response to decreasing gs, thus affecting the photosynthetic rate and therefore growth (Farquhar et al. 1982; Fischer et al. 1998; Barbour et al. 2000; Garcia-G et al. 2004). The greater WUE of trees in the RR2 treatment (with least growth rates) in year 2006 indicated that gs was probably more affected rather than C assimilation, given the decreasing Δ13C as Ci decreases (Table 1). The suggested decrease in gs with increasing residue-loading rates, however, was unexpected against the background of published work, which suggests that the residues would conserve soil moisture (Proe et al. 1999; Scott et al. 2005). In addition, the lack of a significant relationship between foliar δ13C or Δ13C and foliar δ18O in concurrence with a negative WUE/growth relationship indicated that other environmental stressors, other than reduced gs, could be more important drivers of foliar δ13C variations (Silva and Anand 2013; Gomez-Guerrero et al. 2013). Studies have shown that nutrient limitations could cause such a growth response when the photosynthetic capacity is limited by leaf nutrient content and soil nutrient availability (Silva and Anand 2013). This interpretation is consistent with the nutrient analyses of the same trees in 2006, which showed that both foliar N & P concentrations were either marginal or below critical levels (Tutua et al. 2008). Furthermore soil nutrient analyses in year 2006 showed that although total N increased and were significantly greater in the RR1 and RR2 treatments, mineral N pools (NH4 + and NO3 −) only ranged from 9.8–10.9 mg kg−1 to 0.26–0.36 mg kg−1, respectively, which were much lower than those reported at age 6 of the same plantation (Chen and Xu 2005; Tutua 2009). Similarly, labile P pools at age 10 years had decreased compared to those at age 2 years old of the same plantation (Mathers and Xu 2003; Tutua 2009). The low availability of nutrients for plant uptake, however, could be due to low mineralisation rates, which may result from soil water deficit (Högberg et al. 1993). The influence of soil water deficit could not be ruled out given the decreasing growth period total rainfall and that the total annual rainfall at this site had been below average over the 10 years of the plantation (Fig. 1). Nonetheless, nutrient limitations seemed to have an over-riding effect given the lack of a significant relationship between Δ13C and foliar δ18O (Gomez-Guerrero et al. 2013).
On the other hand, the positive relationship of foliar δ13C or WUE with tree growth (Fig. 2a–d) across increasing residue loading rate in 2000 indicates a stimulus to growth (Högberg et al. 1993; Silva and Anand 2013). This stimulus is most likely to be the unlimited nutrient availability from the decomposing residues during the early growth stages, thus enhancing the photosynthetic capacity and WUE of the trees. This proposition was consistent with our other parallel residue management study adjacent to this plantation, which showed greater levels of available or labile N and P after 18 months where harvest residues were retained (Tutua et al. 2013).
However, the decreasing Ci and Δ13C from RR0 to RR2, and the positive relationship between δ13C or the negative Δ13C and δ18O relationship (Fig. 4) indicated that gs was the main driver of the δ13C variations across the treatments in 2000 (Fischer et al. 1998; Barbour et al. 2000; Scheidegger et al. 2000). This would be inconsistent with the gs theory as a mechanism for growth, where decreasing gs would lead to reduced photosynthesis and growth (Fischer et al. 1998; Barbour et al. 2000). In addition, higher gs expected under residue retention treatments was supposed to cool the leaves, and therefore the canopy, resulting in increased internal CO2 concentration (increased Δ13C), and higher light-saturated photosynthetic rate of plants (Fischer et al. 1998). Therefore, we suspected that the increasing δ13C and δ18O with increasing tree growth rates (Fig. 4), was the result of higher growth rates in the RR1 and RR2 treatments, causing periodic water stress to which foliar δ18O was sensitive. Growth-induced water stress had been suggested by some published work (Högberg et al. 1993; Xu et al. 2000; Garcia-G et al. 2004). It has been shown that the addition of an N source to an N-limited soil would increase foliage biomass and therefore growth rate, which in turn could lead to water stress as a result of growth demands for water (Högberg et al. 1993). This effect might explain the very steep slope of the δ18O/δ13C relationship (7.70 ‰ increase in δ18O per 1‰ increase in δ13C) in this study (Fig. 4b) compared to those reported by Barbour et al. (2000) (2.9 ‰) and Keitel et al. (2003) (1.11‰), where the higher growth demands for water established a steep gradient of gs across the treatments. We would speculate that growth demands for soil water might have increased soil water deficit (SWD) over time in the RR1 and RR2 treatments as suggested for in 2006, due to the below-average rainfalls over the last 10 years, which were probably not enough to pass through the thick organic/residue layer to reach the mineral soil as observed in other studies (Huang et al. 2008b). Nonetheless, these results confirmed the nutritional value of the residues during early growth, which could not be confirmed from foliar nutrient analyses across treatments (Simpson et al. 2003; Tutua et al. 2008) possibly due to dilution effects of the trees.
The significant δ13C/δ18O/K relationships (Fig. 4a) in 2000 supported our proposition that significant K variation across treatments was probably a response to water stress (Xu et al. 2000; Tutua et al. 2008). This was due to the fact that foliar K concentrations were non-limiting in all the treatments and therefore cannot be a driver of growth variations (Tutua et al. 2008). The result, however, is consistent with studies showing enhanced K supply under decreased gs (Fernandez et al. 2006). Potassium functioned through its maintenance of leaf water and pressure potential (Ashraf et al. 2001; Pervez et al. 2004), allowing photosynthesis to proceed under water stressed conditions. The weak relationship between foliar K concentration and growth indices in 1998 when rainfall was well distributed as reported in Tutua et al. (2008) is also consistent with the proposition that the foliar K variations were a response to water stress. Although lack of direct measurements of soil moisture content is a limitation of this study, the observations parallel a separate study of the F1 hybrid exotic pine clones, showing a correlation between foliar mineral, including K, concentrations and δ13C in water stressed trees (Prasolova et al. 2005).
Long-term variations of foliar isotopic composition and WUE and declining tree growth
The long-term changes in foliar isotopic compositions and WUE over the 8 years also revealed some interesting trends relevant to long-term tree growth and climate change. The increasing δ13C, WUE and δ18O, or decreasing Δ13C, over time were consistent with the declining growth rates and rainfall over the 10 years (Table 3). Again as shown in 2006, the increasing WUE and declining PAID over time (negative coefficients) (Table 3) was consistent with reduced gs induced by SWD (Farquhar and Richards 1984; Barbour et al. 2000). This was supported by the decreasing Ci and rainfall with the declining PAID over time (Table 3). However, the lack of a significant correlation between Δ13C and δ18O over time indicated that the decrease in Ci and Δ13C or increase in WUE could not be driven solely by gs. As indicated earlier, this growth response to increasing WUE could also be due to nutrient limitation on photosynthetic rate, increasing ambient CO2 and warming associated with climate change (Sun et al. 2010; Gomez-Guerrero et al. 2013; Keenan et al. 2013). These are all possible causes given the increase in ambient CO2 by 15 ppm from 1998 to 2006 and the apparent changing climate indicated by the below average rainfalls. The nutrient limitation proposition, however, was supported by our earlier report that the decreasing foliar N and P concentrations were significantly related to the decreasing PAID over time (Tutua et al. 2008). A number of studies reported that nutrient limitation is one of the causes of declining forest growth (Gower et al. 1996; Jokela and Martin 2000; Gomez-Guerrero et al. 2013; Silva and Anand 2013). Soil nutrient and moisture interactions are also important to consider given that soil moisture could influence nutrient mineralisation, mass movement and therefore uptake by the trees (Högberg et al. 1993). Regardless, these results have significant implications for climate change adaptations. They demonstrated that increasing ambient CO2 from anthropogenic sources, warming and therefore increases in WUE do not always translate to tree growth when there are other limiting factors (Peňuelas et al. 2010; Silva and Anand 2013).
Litter δ 13C and δ 18O
The significant relationships between foliar and litter δ13C across the treatments (Fig. 5a–b), demonstrates that a significant quantity of foliar δ13C was part of leaf structural C, which remained with the litter after senescence. The apparent depletion in litter δ13C in all seasons relative to foliar δ13C in 2006, and the narrower range (0.27–0.33‰) across the treatments (Table 4), was reflective of C mobilisation or re-translocation during senescence. Regardless of this, litter needle δ13C was able to show significant variations across the treatments in at least two seasons, with the highest δ13C in the RR2 treatment, consistent with the trend of foliar δ13C in 2006. This consistency was further demonstrated in the significant relationship between litter δ13C and PAID in 2006 (Fig. 5d). The negative relationship between litter needle δ13C and tree growth in 2006 was reflective of the inverse relationship between foliar δ13C and PAID or PAIB. While this might suggest moisture stress, and therefore gs, as the controlling factor for the variation in foliar δ13C and tree growth (Farquhar and Richards 1984; Zhang and Cregg 1996; Barbour et al. 2000), this proposition was weakened by the lack of a significant relationship between litter δ 13C and δ 18O across the treatments. It is more likely due to nutrient limitation as discussed above or the interactions of both stressors. This is because the occurrence or absence of litter needle δ13C variations between the treatments across the seasons reflects the temperature and rainfall patterns of this site, whereby seasons with higher mean maximum temperatures and relatively low rainfalls of the previous year resulted in the significant variations in litter δ13C of those seasons in the present year (Fig. 6). Although the litter δ13C measurements were carried out for 15 months only, the results were consistent with studies of wood cellulose δ13C and δ18O variations, where increasing temperature and low rainfall resulted in increasing wood cellulose δ13C and δ18O (Korol et al. 1999; Barbour et al. 2002), similar to that with litter δ13C in this study . Thus, litter δ13C may be a potential indicator of environmental stressors and that its use could substitute for foliar sampling of taller trees.
This study has been able to elucidate the influence of environmental stressors on tree growth using foliar and litter isotopic compositions, including WUE, and their relationship to growth rates. However, perhaps future studies in this plantation need to carry out direct measurements of soil moisture and gas exchange to backup isotopic variations relating to environmental stressors. In addition there is a need to look into soil moisture and nutrient interactions, especially at mineralisation rates, microbial activity and understory vegetation competition for water and nutrients for a better understanding of long term growth patterns. Future work may also need to increase the sample size from 4 trees per plot in this study as well as conduct the study in more than one site for a broader understanding in subtropical conditions.
Conclusion
This study demonstrated that harvest residues can have a significant influence on the variations in foliar δ13C and δ18O of the F1 hybrid exotic pine trees. The foliar δ13C or WUE variations and their relationships with periodic tree growth rate have been helpful in understanding the mechanism that regulates tree growth and especially plantation productivity. The significant relationships among foliar δ13C, δ18O and K during early growth clearly indicated growth-induced water stress of trees in the RR1 and RR2 treatments, a situation due to greater availability of N and P where residues were retained. This suggests that harvest residue retention in these plantations had a significant nutritional effect on tree growth gain observed over the 10 years. The negative relationship between foliar and litter δ13C or WUE and growth rates at age 10 years indicated that nutrient limitation has an over-riding effect on the photosynthetic capacity of the trees. Similarly, the long term foliar isotopic compositions and WUE trends over the 10 years also indicated nutrient limitations as the cause of the decline in the growth rates. Litter needle δ13C has the potential for studying environmental conditions in relation to tree growth as demonstrated in this study.
References
Ashraf M, Ahmad A, McNeilly T (2001) Growth and photosynthetic characteristics in pearl millet under water stress and different potassium supply. Photosynthetica 39:389–394
Barbour MM (2007) Stable oxygen isotope composition of plant tissue: a review. Funct Plant Biol 34:83–94
Barbour MM, Fischer RA, Sayre KD, Farquhar GD (2000) Oxygen isotope ratio of leaf and grain material correlates with stomatal conductance and grain in irrigated wheat. Aust J Plant Physiol 27:625–637
Barbour MM, Walcroft AS, Farquhar GD (2002) Seasonal variation in δ13C and δ18O of cellulose from growth rings of Pinus radiata. Plant Cell Environ 25:1483–1499
Chen CR, Xu ZH (2005) Soil carbon and nitrogen pools and microbial properties in a 6-year-old slash pine plantation of subtropical Australia: impacts of harvest residue management. For Ecol Manag 206:237–247
Dupouey JL, Leavitt S, Choisnel E, Jourdain S (1993) Modelling carbon isotope fractionation in tree rings based on effective evapotranspiration and soil water status. Plant Cell Environ 16:939–947
FAO (1974) FAO-UNESCO soils map of the world 1:5000000. UNESCO, Paris, p 59
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11:539–552
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Farquhar GD, Barbour MM, Henry BK (1998) Interpretation of oxygen isotope composition of leaf material. In: Griffiths H (ed) Stable isotopes. Bios Scientific, Oxford, pp 27–62
Feng X (1998) Long term Ci/Ca response of trees in western North America to atmospheric CO2 concentration derived from carbon isotope chronologies. Oecologia 117:19–25
Fernandez M, Novillo C, Pardos JA (2006) Effects of water and nutrient availability in Pinus pinaster Ait. open pollinated families at an early age: growth, gas exchange and water relations. New For 31:321–342
Fischer RA, Rees D, Sayre KD, Lu ZM, Condon AG, Larque Saavedra A (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38:1467–1475
Garcia-G R, Gomez A, Lopez-U J, Vargas-H J, Horwath WR (2004) Tree growth and δ13C among populations of Pinus gregii Engelm. at two contrasting sites in central Mexico. For Ecol Manag 198:237–247
Gomez-Guerrero A, Silva LCR, Barrera-Reyes M, Kishchuk B, Velazquez-Martinez A, Martinez-Trinidad T, Plascencia-Escalante FO, Horwath WR (2013) Growth decline and divergent ring isotopic composition (δ13C and δ18O) contradict predictions of CO2 stimulation in high altitudinal forests. Global Change Biol 19:1748–1758
Gower ST, McMurtrie R, Murty D (1996) Aboveground net primary production decline with stand age: potential causes. Trends Ecol Evol 11:378–382
Hasselquist NJ, Allen MF, Santiago LS (2010) Water relations of evergreen and drought-deciduous trees along a seasonally dry tropical forest chronosequence. Oecologia. doi:10.1007/s00442-010-1725-y
Högberg P, Johannisson C, Hallgren E (1993) Studies of 13C in the foliage reveal interactions between nutrients and water in forest fertilization experiments. Plant Soil 152:207–214
Huang ZQ, Xu ZH, Blumfield TJ, Bubb K (2008a) Variations in relative stomatal and biochemical limitations to photosynthesis in a young blackbutt (Eucalyptus pilularis) plantation subjected to different weed control regimes. Tree Physiol 28:997–1005
Huang ZQ, Xu ZH, Blumfield TJ, Bubb KA (2008b) Effects of mulching on growth, foliar photosynthetic nitrogen and water use efficiency of hardwood plantation in subtropical Australia. For Ecol Manag 255:3447–3454
Huang ZQ, Xu ZH, Blumfield TJ, Bubb KA (2008c) Foliar δ13C and δ18O reveal differential physiological responses of canopy foliage to pre-planting weed control in a young spotted gum (Corymbia citriodora subsp. Variegata). Tree Physiol 28:1535–1543
Ibell PT, Xu ZH, Blumfield TJ (2013) The influence of weed control on foliar δ15N, δ13C and tree growth in an 8 year old exotic pine plantation of subtropical Australia. Plant Soil. doi:10.1007/s11104-012-1554-3
Jokela EJ, Martin TA (2000) Effects of ontogeny and soil nutrient supply on production, allocation, and leaf area efficiency in loblolly and slash pine stands. Can J For Res 30:1511–1524
Keenan TF, Hollinger DY, Bohrer G, Dragoni D, Munger JW, Schmid HP, Richardson AD (2013) Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499:324–327
Keitel C, Adams MA, Holst T, Matzarakis A, Mayer H, Rennenberg H, Geβler A (2003) Carbon and oxygen isotope composition of organic compounds in the phloem sap provides a short-term measure for stomatal conductance of European beech (Fagus sylvatica L.). Plant Cell Environ 26:1157–1168
Keitel C, Matzarakis A, Rennenberg H, Gessler A (2006) Carbon isotopic composition and oxygen isotopic enrichment in phloem and total leaf organic matter of European beech (Fagus sylvatica L.) along a climate gradient. Plant Cell Environ 29:1492–1507
Korol RL, Kirschbaum MUF, Farquhar GD, Jeffreys M (1999) Effects of water status and soil fertility on the C-isotope signature in Pinus radiata. Tree Physiol 19:551–562
Livingstone NJ, Spittlehouse DL (1996) Carbon isotope fractionation in tree ring early and late wood in relation to intra-growing season water balance. Plant Cell Environ 19:768–774
Mathers NJ, Xu ZH (2003) Solid-state 13C NMR spectroscopy: characterization of soil organic matter under two contrasting residue management regimes in a 2-year-old pine plantation of subtropical Australia. Geoderma 114:19–31
McNulty SG, Swank WT (1995) Wood δ13C as measure of annual basal area growth and soil water stress in a Pinus strobus forest. Ecology 76:1581–1586
Olbrich BW, Roux DL, Poulter AG, Bond WJ, Stock WD (1993) Variation in water use efficiency and δ13C levels in Eucalyptus grandis clones. J Hydrol 150:615–633
Peňuelas J, Canadell JG, Ogaya R (2010) Increased water use efficiency during the 20th Century did not translate into enhanced tree growth. Glob Ecol Biogeogr 20:597–608
Pervez H, Ashraf M, Makhdum MI (2004) Influence of potassium nutrition on gas exchange characteristics and water relations in cotton (Gossypium hirstum L.). Photosynthetica 42:251–255
Powers MD, Pregitzer KS, Palik BJ, Webster CR (2009) Water relations of pine seedlings in contrasting overstory environments. For Ecol Manag 258:1442–1448
Prasolova NV, Xu ZH, Lundkvist K, Farquhar GD, Dieters MJ, Walker S, Saffigna PG (2003) Genetic variation in foliar carbon isotope composition in relation to tree growth and foliar nitrogen concentration in clones of the F1 hybrid between slash pine and Caribbean pine. For Ecol Manag 172:145–160
Prasolova N, Xu ZH, Lundkvist K (2005) Genetic variation in foliar nutrient concentration in relation to foliar carbon isotope composition and tree growth with clones of the F1 hybrid between slash pine and Caribbean pine. For Ecol Manag 210:173–191
Proe MF, Craig J, Dutch J, Griffiths J (1999) Use of vector analysis to determine the effects of harvest residues on early growth of second -rotation Sitka spruce. For Ecol Manag 122:87–105
Saurer M, Aellen K, Siegwolf R (1997) Correlating δ13C and δ18O in cellulose of trees. Plant Cell Environ 20:1543–1550
Scheidegger Y, Saurer M, Bahn M, Siegwolf R (2000) Linking stable oxygen and carbon isotopes with stomatal conductance and photosynthetic capacity: a conceptual model. Oecologia 125:350–357
Scott DR, Harrington CA, Terry TA (2005) Harvest residue and competing vegetation affect soil moisture, soil temperature, N availability, and Douglas-fir seedling growth. For Ecol Manag 205:333–350
Silva LCR, Anand M (2013) Probing for the influence of atmospheric CO2 and climate change on forest ecosystems across biomes. Glob Ecol Biogeogr 22:83–92
Silva LCR, Horwath WR (2013) Explaining global increases in water use efficiency: why have we overestimated responses to rising atmospheric CO2 in natural forest ecosystems? PLOS 8:e53089
Simpson JA, Smith TE, Keay PT, Osborne DO, Xu ZH, Podberscek MI (2003) Impact of inter-rotation site management on tree growth and soil properties in the first 6.4 years of a hybrid pine plantation in subtropical Australia. In: Nambiar EKS, Ranger J, Tiarks A, Toma T (eds) Proceedings of the Fifth Workshop on Site Management and Productivity in Tropical Plantation Forests: Impacts on Soils and Options for Management Over Successive Rotations, Guangzhou, China, pp 139–149
Sun FF, Kuang YW, Wen DZ, Xu ZH, Li JL, Zuo WD, Li J, Hou EQ (2010) Long-term tree growth rate, water use efficiency and tree ring nitrogen isotope composition of Pinus massoniana in response to global climate change and local nitrogen deposition in southern China. J Soils Sediments 10:1453–1465
Tutua SS (2009) Residue management and carbon and nutrient cycling in exotic pine plantations of Southeast Queensland, PhD Thesis. Griffith University, Brisbane
Tutua SS, Xu ZH, Blumfield TJ, Bubb KA (2008) Long-term impacts of harvest residue management on nutrition, growth and productivity of an exotic pine plantation of sub-tropical Australia. For Ecol Manag 256:741–748
Tutua SS, Xu ZH, Blumfield TJ (2013) Hot water extractable phosphorus pools as indicators of soil P responses to harvest residue management in an exotic pine plantation of subtropical Australia. J Soils Sediments. doi:10.1007/s11368-013-0775-2
Warren CR, McGrath JF, Adams MA (2001) Water availability and carbon isotope discrimination in conifers. Oecologia 127:476–486
Xu ZH, Saffigna PG, Farquhar GD, Simpson JA, Haines RJ, Walker S, Osborne DO, Guinto D (2000) Carbon isotope discrimination and oxygen isotope composition in clones of the F1 hybrid between slash pine and Caribbean pine in relation to tree growth, water-use efficiency and foliar nutrient concentration. Tree Physiol 20:1209–1217
Xu ZH, Prasolova N, Lundkvist K, Beadle C, Leaman T (2003) Genetic variation in branchlet carbon and nitrogen isotope composition and nutrient concentration of 11-year-old hoop pine families in relation to tree growth in subtropical Australia. For Ecol Manag 186:359–371
Xu ZH, Chen CR, He J, Liu J (2009) Trends and challenges in soil research 2009: linking globalclimate change to local long-term forest productivity. J Soils Sediments 9:83–88
Zhang JW, Cregg BM (1996) Variation in stable carbon isotope discrimination among and within exotic conifer species grown in eastern Nebraska, USA. For Ecol Manag 83:181–187
Acknowledgments
This study was supported by Forestry Plantations Queensland (FPQ) Ltd, Australia. The authors acknowledged the collaboration and advise of Dr. Ken Bubb of FPQ and the co-operation of the Station Manager and Forestry staff at Toolara State Forest for access to the plantation, and are grateful for the assistance of Mr. Paul Keay for foliar samples, and Mr. Rene Diocares for technical support. Prof. Z.H Xu also received funds/support from the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Rafael S. Oliveira.
Rights and permissions
About this article
Cite this article
Tutua, S.S., Xu, Z. & Blumfield, T.J. Foliar and litter needle carbon and oxygen isotope compositions relate to tree growth of an exotic pine plantation under different residue management regimes in subtropical Australia. Plant Soil 375, 189–204 (2014). https://doi.org/10.1007/s11104-013-1951-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1951-2