Abstract
Aims
The thiosulphate induced accumulation of mercury by the three plants Brassica juncea var.LDZY, Brassica juncea var.ASKYC and Brassica napus var. ZYYC and the transformation of mercury fractionation in the rhizosphere of each plant was investigated in the field.
Methods
Experimental farmland was divided into control and thiosulphate plots. Each plot was divided into three subplots with each planted with one of the plants. After harvesting, the mercury concentration in plants, mercury fractionation in rhizosphere soil before and after phytoextraction, and the vertical distribution of bioavailable mercury in bulk soil profiles was analyzed.
Results
The cultivar B. juncea var.LDZY accumulated a higher amount of mercury in shoots than the other two plants. Thiosulphate treatment promoted an increase in the concentration of metal in plants and a transformation of Fe/Mn oxide-bound and organic-bound mercury (potential bioavailable fractions) into soluble and exchangeable and specifically-sorbed fractions in the rhizosphere. The observed increase in bioavailable rhizosphere mercury concentration was restricted to the root zone; mercury did not move down the soil profile as a function of thiosulphate application to soil.
Conclusions
Thiosulphate-induced phytoextraction has the potential to manage environmental risk of mercury in soil by decreasing the concentration of mercury associated with potential bioavailable fraction that can be accumulated by crop plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soils contaminated with mercury represent a major source of environmental and human health risk around the world. Mercury exists in soil through natural and anthropogenic processes. Vectors for anthropogenic contamination include coal combustion, mercury and gold-mining activities, as well as industrial activities (Feng et al. 2002; Hassett et al. 2009; Mukherjee et al. 2004; Wu et al. 2006).
The widespread contamination of soil with mercury causes public anxiety (Järup 2003). Under anaerobic conditions, mercury in soil can be transformed to methylmercury (MeHg), which can be readily accumulated by rice (Oryza sativa) (Zhang et al. 2010). Increased scientific understand of the risks associated with mercury contamination of soil and food has led to increasing demand for remediation of this element in the environment.
Remediation methods involve excavation and disposal, stabilization/solidification, electro-remediation, soil washing and thermal desorption (Wang et al. 2012a). However, these conventional methods generally fail to achieve public acceptance and/or economic viability. A key environmental risk associated with mercury in the environment is metal accumulation by crops, and the transfer of mercury into the food chain. Some researchers have proposed that this risk may be managed by growing crops that exhibit low potential to accumulate mercury (Chen and Yang 2012). However, metal will still remain in soil and may transfer through runoff or leaching, which will cause secondary pollution to the surrounding environment or groundwater. In addition, mercury-enriched soil can readily emit gaseous mercury to the atmosphere. This gaseous mercury will deposit onto surrounding environment and cause diffuse pollution (Wang et al. 2007).
An alternative technology, phytoextraction, may be a more viable option to remediate heavy metal contaminated soils (Wang et al. 2012a). The aim of phytoextraction is to remove high-risk metals from the soil environment through plant uptake and subsequent harvest. However, no mercury hyperaccumulators have been identified, and those plant species that are reported to accumulate mercury generally suffer from ineffective translocation of mercury from roots to shoots (Rodriguez et al. 2003, 2007). The use of chemicals to increase the bioavailable concentration of heavy metals in soil and to enhance plant accumulation of these metals has therefore been widely reported (Blaylock et al. 1997; Huang et al. 1997; Luo et al. 2005). Chelators and ligands such as ammonium thiosulphate ((NH4)2S2O3), sodium thiosulphate (Na2S2O3), ethylenediaminetetraacetic acid (EDTA), urease and potassium iodide (KI) have been used to enhance the mobility of mercury in soil (Moreno et al. 2005a; Smolińska and Cedzyńska 2007; Wang and Greger 2006; Wang et al. 2011a). Of these chemicals, ammonium thiosulphate has been widely used to enhance plant uptake of mercury from soil due to its well-defined efficiency to transport mercury from soil to aboveground plant biomass (Lomonte et al. 2010; Moreno et al. 2005a, b).
The feasibility of phytoextraction as a technique for mercury remediation has been questioned. At mercury mining areas, soils have become heavily contaminated with mercury due to extensive mining and refining activities (Wang et al. 2012a). For example, at the Almadén mercury mine in Spain and at the Wanshan mercury mine in China, total mercury concentrations in soil have been reported as high as 9,000 mg kg−1 (Higueras et al. 2003) and 1,972 mg kg−1 (Wang et al. 2011b) respectively. Based on previously reported phytoextraction models, phytoextraction to remove mercury from such heavily mercury-contaminated soil to an acceptable level would be a long process (Rodriguez et al. 2007; Wang et al. 2011a). But not all mercury in the soil is available to the plants, animals and humans. The bioavailable fraction of mercury in soil is the most important fraction due to the potential of this phase of mercury to enter the food chain and to be transported in the environment. Mercury present in non-available forms is relatively stable and less likely to move within the environment. Non-available mercury therefore presents lower environmental risk. From the point view of food safety, it perhaps seems prudent that the focus of remediation should be on the bioavailable forms of mercury in soil, and not the non-bioavailable forms of this metal (Hamon and McLaughlin 1999; Pedron et al. 2013).
Many efforts have been made to investigate specific plant species that can be used for phytoextraction. The species Rumex induratus and Marrubium vulgare have been reported to accumulate mercury from soil (soil Hg 122–550 mg kg−1) with phytoextraction yields of 12.9 and 27.6 g ha−1 respectively (Moreno-Jiméneza et al. 2006). Rodríguez et al. (2003, 2007) reported that the phytoextraction yield for Hordeum spp., Lens culinaris, Cicer arietinum, Lupinus polyphyllus and Triticum aestivum was 4.7, 2.8, 0.4, 0.4 and 0.28 g ha−1 respectively when grown in soil with mercury concentration of 18–32 mg kg−1. However, these phytoextraction yields are negligible in comparison to the magnitude of the total mercury concentration in soils.
One species that has been extensively used to accumulate a range of metals from soil, including cadmium (Cd), copper (Cu), nickel (Ni), zinc (Zn), lead (Pb), chromium (Cr) and selenium (Se), is Brassica juncea L. due to the apparent ability of this plant to effectively transfer metals from roots to above-ground biomass. (Haag-Kerwer et al. 1999; Han et al. 2004; Rio et al. 2000). Brassica napus L. has been investigated as a candidate for phytoextraction for similar reasons, but with the added advantage that this species can produce an economic oil yield at crop maturity. Any commodity recovered from biomass creates the potential for turning phytoextraction into a profitable enterprise (Grispen et al. 2006).
In the present study, the capacity of two cultivars of Brassica juncea (B. juncea var.LDZY, B. juncea var.ASKYC) and one cultivar of Brassica. napus (B. napus var. ZYYC) were used in conjunction with thiosulphate to extract mercury from heavily mercury-contaminated soils at the Wanshan mercury mine. The three plants used in this study were chosen as candidate plants for phytoextraction due to their ready availability, suitability for growth in the Wanshan climate, and their potential for high biomass production. This investigation was conducted under field conditions. The phytoextraction yield of the plants and the effect of cropping on the mercury fractionation in the rhizosphere soil before and after the experiment were investigated. Detailed consideration of the fractionation in soil was conducted to quantify the effect of thiosulphate-induced phytoextraction on the distribution of bioavailable mercury in the bulk soil profile. Specifically our interest was to examine the remediation effect of phytoextraction on both total and bioavailable mercury in soil under cropping land use.
Materials and methods
Field trials
The field experiment was carried out in mercury-contaminated farmland in the vicinity of the Wanshan mercury mine from June to August 2010 (Fig. 1). The environment surrounding the Wanshan mercury mine has become seriously contaminated with mercury due to extensive mining and retorting activities that have occurred in the mining district over the past 2,000 years (Table 1). The experimental design of the phytoextraction field trial has been described previously (Wang et al. 2012b). Briefly, an experimental area of 30 m2 was established. The area was divided into three plots (5 m × 2 m), with each plot planted with one of two cultivars of Brassica juncea L or one cultivar of Brassica. napus L. Each plot was further divided into two equal sized subplots (5 m × 1 m), which were designated for either control or thiosulphate treatment. Each subplot contained three equal-sized grids (1.5 m2) with 50–60 plants per grid. Agronomic management techniques at each field plot were performed manually as required. The plants were maintained for 75 days. Seventy days after seeding, at the point of crop maturity, a thiosulphate solution was irrigated onto treatment subplot at a rate of 8 g of thiosulphate per kg of soil. A target treatment soil depth of 15 cm was assumed. The soil mass of each treated subplot was calculated as soil depth × area × soil density. The total soil mass in the subplot treatment area of B. juncea var. LDZY, B. juncea var. ASKYC and B. napus var. ZYYC was calculated to be 0.25 t, 0.23 t and 0.27 t respectively, and therefore these areas received 3.3 kg, 3 kg and 3.6 kg of 60 % (w/w) thiosulphate solution respectively. Five days after treatment, three individual plant samples were randomly collected from each of the three replicate thiosulphate-treated grids and the three replicate control grids for each species within each subplot. Samples of rhizosphere soil were collected using a corer with an internal volume of 32 cm3 from the root zone of the sampled plants. Additional bulk soil cores to 30 cm depth were collected and divided into intervals of 0–5 cm, 5–10 cm, 10–15 cm, 15– 20 cm, 20–25 cm and 25–30 cm from the control and thiosulphate-treated subplots. The root and shoot biomass was separated, and washed in running tap and then deionised water. All plant samples were freeze dried and ground to powder. Soil samples were air dried, ground in a ceramic disc mill, and sieved to 200 mesh.
Analytical procedures
Soil analysis
The following soil sample properties were measured. Soil density samples were collected using a stainless steel cylinder with volume of 100 cm3. The soil moisture was determined after drying in an oven at 105 °C for 48 h. Soil density was calculated as the ratio of the oven-dried soil mass to the cylinder volume. pH: 5 g of each air-dried and ground soil sample was weighted into a 50 ml polytetrafluoroethylene tube and 12.5 ml de-ionized water was added (1:2.5 w:w). The tubes were shaken on a reciprocal shake (HY-2, Baidian Instruments, China) at150 rpm min−1 for 30 min. The pH of the supernatant was measured using a pH meter (Hanna HI3M, Hanna instruments®, USA). Soil texture was determined using a Malvern Mastersizer 2000 (Malvern Ltd., UK) (Yang et al. 2008) and organic matter (OM) was determined according to the method of Lu (2000). Briefly, 0.1 g of soil samples were weighed into 50 ml glass tubes and 5 ml of 1 M K2Cr2O7 and 10 ml of concentrated sulfuric acid was added. The samples were digested on a water bath at 100 °C for 15 min to dissolve organic materials, and then the solution was transferred into 250 ml-Erlenmeyer flasks. Phenanthroline was added to the flask as an indicator, and the ferrous sulfate was used to titrate the remained K2Cr2O7 in solution. Therefore, the volume of ferrous sulfate before and after titrating can be applied to calculate the amount of K2Cr2O7 used for digesting organic materials through which the concentration of organic matter can be calculated. For total carbon, total nitrogen, and total sulfur content determination, 0.02–0.03 g of air-dried soil sample was weighed into a tin boat and subsequently sealed. The total carbon, total nitrogen, and total sulfur of soil samples in tin boat were directly measured using an Elemental Analyzer (Perkin Elmer model 2400-II, MA, USA). Bioavailable mercury in bulk soil profiles collected from control and thiosulphate-treated plot at the end of the experiment was determined as follows. 1 g of air-dried soil sample was weighed into a 50 ml polytetrafluoroethylene tube and 5 ml of 0.1 M dilute hydrochloric acid was added. The tubes were shaken on a reciprocal shake at 150 rpm min−1 for 30 min, and then centrifuged at 3,000 rpm min−1 for 15 min. The mercury concentration of the supernatant was measured by cold vapor atomic absorption spectrometry (CVAAS) (Jing et al. 2008). The total mercury concentration in the soil was determined by cold vapor atomic absorption spectrometry (CVAAS) using a F732-S mercury analyzer (Huaguang Ltd. China) after sample digestion in fresh aqua regia as described in Wang et al. (2011b). The geochemical fractionation of mercury in rhizosphere soil samples (initial soil, and soil from the control plot and thiosulphate-treated plot at the end of the experiment) was determined using the sequential extraction procedure described by Wang et al. (2011b). Mercury in soil was defined using this procedure as belonging to one of the five operational fractions: soluble and exchangeable, specifically sorbed, Fe/Mn oxide bound, organic bound and residual fractions.
Plant analysis
The total mercury concentration in all plant samples was directly measured (solid sample) using a Lumex RA915+ mercury analyzer equipped with a Pyro 915+ pyrolysis attachment by way of thermal decomposition to Hg0 (Sholupov et al. 2004). The detection limit of the equipment is 0.2–5 ng g−1.
Quality control and quality assurance
Standard reference materials (SRM) and reagent blanks were used for analytical QC protocol. The concentration of mercury in reagent blank was below the determination limit of the mercury analyzer. The average total mercury concentration of the soil standard GBW (E) 070009 (Manufactured by the Institute of Geophysical and Geochemical Exploration, China) was 2.04 ± 0.07 mg kg−1 (n = 7), which is comparable with the certified value of 2.2 ± 0.4 mg kg−1. The plant standard GBW10020 was used for plant analytical QC. The average total mercury concentration of the standard was 0.14 ± 0.01 mg kg−1 (n = 7), which is comparable with the certified value of 0.15 ± 0.02 mg kg−1. The relative percentage difference of sample replicates for soil and plant were < 12 % and < 9 % respectively.
Statistical analyses
Data were examined by one-way ANOVA followed by LSD (Equal Variance Assumed) or Tamhane’s T2 (Equal Variance not Assumed) test as available in the SPSS 17.0 statistical package.
Results
Physico-chemical properties of soils
The main physico-chemical properties of the soils constituting the field area are shown in Table 2. Data is reported for each of the planted plots. The soils could be classified as a sandy loam with a soil density of 1.1–1.2 g cm−3 across the experimental area. The soil pH was alkaline, with consistent organic matter, total carbon and total sulfur content. However, the total nitrogen content was variable across the plot area. The average total mercury concentration of the soil was 374 mg kg−1, a level which greatly exceeds the maximum allowable concentration of 1.5 mg kg−1 set by the Chinese government (CNEPA 1995).
The biomass of the plants
The root and shoot biomass (dry weight) of the three plants at harvest is shown in Table 3. The application of thiosulphate did not affect the biomass of the three plants. For both the control and thiosulphate treatments, the shoot and root biomass of the three plants ranged from 1.6 to 4.9 t ha−1 and 0.2 to 0.8 t ha−1, respectively, with B. juncea var.LDZY showing higher shoot biomass than the other two plants (p < 0.05), and B. napus var.ZYYC showing higher root biomass than the other two plants (p < 0.05).
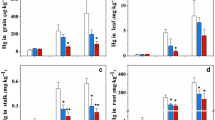
Mercury concentration in plant
The root and shoot mercury concentration of the three plants is shown in Fig. 2. For the control plot, the roots and shoots of all plants had an average mercury concentration below 1 mg kg−1. The application of thiosulphate significantly increased (p < 0.05) the root and shoot mercury concentration of the three plants. The mercury concentration in the roots and shoots of the treated plants ranged from 12 to 40 mg kg−1 and 5 to 34 mg kg−1, respectively. Thiosulphate treatment increased the accumulation of mercury by a factor of 34–128 for root and 60–179 for shoot biomass in the three plants.
After thiosulphate amendment, the three plants exhibited a differential ability to transport mercury from root to shoot. Brassica juncea var.LDZY exhibited a significantly greater (p < 0.05) root mercury concentration than B. juncea var.ASKYC and B. napus var.ZYYC. Brassica juncea var.ASKYC had a significantly higher shoot mercury concentration than B. juncea var.LDZY and B. napus var.ZYYC (p < 0.05). The concentration of mercury in the shoot of B. juncea var. ASKYC was significantly higher than that in root (p < 0.05), however, the other two plants showed a greater concentration of mercury in roots relative to shoots. This phenomenon indicates that the cultivar B. juncea var. ASKYC may be more effective at transporting mercury from root to shoot than the other two plants.
Total amounts of mercury accumulated in plants
The total amounts of mercury extracted by the three plants are shown in Table 3. For the control plots, the total amount of mercury accumulated by the roots and shoots of the three plants ranged from 0.04 to 0.3 g ha−1 and 0.1 to 1.4 g ha−1, respectively. The shoot biomass of B. juncea var.LDZY accumulated a significantly higher amount of mercury than the other two plants (p < 0.05), while the amount of mercury accumulated in the roots of the B. juncea var.LDZY and B. napus var.ZYYC was higher than B. juncea var.ASKYC. The application of thiosulphate significantly increased the accumulation of mercury in the roots and shoots of the three plants relative to each control. The total amount of mercury accumulated by the roots and shoots of the three plants after treatment ranged from 3.6 to 20 g ha−1 and 12 to 133 g ha−1, respectively. Among the three tested plants, the root and shoot of B. juncea var.LDZY accumulated a higher amount of mercury than the other two plants.
The effect of remediation on the total mercury concentration and mercury fractionation in rhizosphere soil
The total mercury concentration in soil and the relative mercury concentration in each of five geochemical soil fractions before and after the experiment is shown in Table 4. Across the three plots, the total mercury concentration in the thiosulphate-treated rhizosphere soil at harvest was significantly lower than that in both the initial soil and in the control soil at the conclusion of the experiment (p < 0.05). The difference between the initial soil and the control soil was statistically insignificant (p > 0.05). These comparisons demonstrate that thiosulphate-assisted phytoextraction can reduce the mercury concentration in the rhizosphere. However, the magnitude of the phytoextraction effect varied as a function of the species used. The least effective species was B. napus var. ZYYC plot, where the average total mercury concentration (obtained from single digestion) for the thiosulphate-treated soil was not statistically different (p > 0.05) to the concentration for the initial and control soils.
The mercury fractionation in the sampled soils was studied using a sequential extraction method (SEP). A SEP can separate soil metal into operationally defined chemical associations according to the solubility of soil metal in different chemical reagents. Although metal fractionation defined by SEP varies as a function of the reaction conditions used during extraction, SEP is a useful tool to evaluate the bioavailability of metal in soil (Fayiga et al. 2007).The concentration of soluble and exchangeable, and specifically-sorbed mercury in the thiosulphate-treated soil was significantly higher than the corresponding concentration in the initial soil and the control soil (p < 0.05). These two fractions are generally considered to define the concentration of bioavailable metal in soil (Wang et al. 2012b). However, the difference for these fractions between the initial soil and the control soil was statistically insignificant (p > 0.05).
In each of the three treated subplots, the concentration of Fe/Mn oxide-bound mercury and organic-bound mercury was dramatically decreased as a function of thiosulphate treatment relative to the initial soil and control soil, suggesting that the addition of thiosulphate to soil can induce a redistribution of mercury between soil geochemical phases. We believe that the primary effect of thiosulphate is to solubilize a portion of mercury bound to organic matter and to Fe/Mn oxides, and to thereby transform mercury in these phases to the more available fractions (i.e. the soluble and exchangeable and specifically-sorbed fractions).
The concentration of Fe/Mn oxide-bound mercury was dramatically decreased in the control soil relative to the initial soil, indicating that the cultivars of B. juncea and B. napus used may naturally induce the solubilization of mercury associated with the Fe/Mn oxide-bound fraction independent of thiosulphate. The concentration of mercury associated with the organic-bound fraction in the control soil of B. juncea var. ASKYC was significantly lower than that in the initial soil, indicating that this cultivar can, to some extent, solubilize mercury associated with organic matter. Moreover, for the B. juncea var.ASKYC plot, the concentration of residual mercury was decreased in both the control and thiosulphate-treated plots relative to the initial soil, although there was no difference between the control and thiosulphate-treated plots.
The distribution of bioavailable mercury in bulk soil profiles
In the scenario where thiosulphate- or plant-solubilized mercury is only partially adsorbed by the plants, residual soluble mercury may remain in the rhizosphere. The presence of excess soluble mercury may create a potential risk situation where mercury can leach into the groundwater. To assess the environmental risk that may result from the use of thiosulphate as described in this paper, bulk soil samples were taken at different sampling depths to generate a profile of mercury concentration in soil as a function of depth for both the control and thiosulphate-treated plots. Figure 3 presents the distribution of the concentration of bioavailable mercury (defined as 0.1 M HCl soluble) in the control and thiosulphate-treated bulk soil profiles. Any difference between the control plot and the treated plot was confined to the top 10 cm. The thiosulphate-treated plot had a higher concentration of bioavailable mercury in the top 5 cm relative to the control plot. However, the concentration for the 5–10 cm soil profile interval was lower in the thiosulphate-treated soil relative to the control plots. Below 10 cm there was no significant difference between the soil sampled from the control and thiosulphate-treated plots.
The bioavailable mercury concentration in the bulk soil profiles was nearly one order of magnitude less than the bioavailable mercury concentration in the rhizosphere soil samples. This indicates that bioavailable mercury may concentrate in the root zone (rhizosphere soil samples 0.4 mg kg−1; bulk soil samples 4–25 ng g−1).
Discussion
Mercury is very toxic to plants. Exposure to excessive levels of mercury will damage the antioxidative and photosynthesis systems, as well as inhibit plant growth (Israr and Sahi 2006; Patra and Sharma 2000; Patra et al. 2004). In the present study, although the soils had a high mercury concentration, no visual toxicity symptoms, such as water loss and wilting, were observed at any time throughout the growing season prior to treatment with thiosulphate. However, toxicity symptoms such as water loss and wilting were observed two days after application of this treatment to the soil. The apparent toxicity may be attributed to the increased salt concentration in the soil caused by adding thiosulphate (Wang et al. 2012b).
Under field condition, the thiosulphate showed great potential to enhance plant uptake of mercury from soil, the results are similar with the previous greenhouse studies (Wang et al. 2011a; Pedron et al. 2013). Brassica juncea var.LDZY accumulated the highest amount of mercury in shoot (133 g ha−1) of the three plants investigated in our research. Assuming a soil density of 1.1 g cm−3 and target depth of remediation of 15 cm, the total mass of soil per hectare was 1,650 t. The average concentration of total mercury for the three plots at the start of remediation was 373 mg kg−1, and therefore the total mass of mercury in the target soil volume was 615 kg. Using this figure, the calculated removal rate of mercury by B. juncea var. LDZY was 0.1 %, and it therefore seems impossible to apply phytoextraction technology to remove total mercury from Wanshan soil to meet the Chinese environmental standard (1.5 mg kg−1).
However, investigation of the effect of phytoextraction on the bioavailable concentration of metal in soil shows that this technology has more promise. Although the mercury concentration of Wanshan soil greatly exceeds the Chinese environmental standard, the majority of mercury in the soil is present as the residual form and presents relatively low risk. The most hazardous form of mercury in soil is that associated with the bioavailable pools of mercury which can readily be taken up by crop plants as either inorganic mercury or organic mercury (Bishop et al. 1998). In this study, the mercury concentration in shoots of each control plant exceeded the maximum allowable mercury concentration in foodstuffs (20 ng g−1) as defined by the Chinese government, indicating that bioavailable mercury in Wanshan soil is associated with environmental risk through potential incorporation of this pollutant into the food chain. In general, the soluble and exchangeable and specifically-sorbed fractions of soil metal have higher bioavailability than other fractions. However, in our study, these fractions presented very low concentration and exhibited no differences between the initial soil and control soil.
Mercury was removed by plants, and this mercury uptake corresponded with a significant decrease in the concentration of Fe/Mn oxide-bound mercury for all control plots relative to the initial soils. Root metabolism is known to release amino acids, organic acids, and other low-molecular-weight organic acid anions (OAAs) into the rhizosphere, which play an important role in maintaining root growth and ensure the survival of plants (Jones 1998). Many OAAs have been reported to have a high capacity to mobilize manganese and iron oxides (Jones 1998). Jones et al. (1996) reported that the presence of malate and citrate in the rhizosphere can dissolve substantial amounts of Fe from Fe-bearing solid phases to meet a plant’s demand. Therefore, the exudation of OAAs by B. juncea and B. napus may lead to a decrease in the concentration of high-surface area Fe/Mn oxides in the rhizosphere, and therefore indirectly release mercury bound to these soil constituents. In addition, the concentration of organic-bound mercury in the rhizosphere of B. juncea var. ASKYC was significantly decreased compared to the initial soil, indicating that mercury associated with organic matter is also, to some extent, bioavailable to plants. Many researchers have found that OAAs can increase the concentration of dissolved organic carbon (DOC) that is released through the breakdown of soil organic matter (SOM). This increase may be attributable to the degradation of SOM as a result of enhanced microbial activity induced by OAAs (Dessureault-Rompré et al. 2008; Hauser et al. 2005; Yang et al. 2001).
We therefore propose that the Fe/Mn oxide-bound and organic-bound soil fractions may better define bioavailable and thus high-risk mercury in soil, and that remediation of mercury associated with these fractions may be sufficient to mitigate the risk of mercury transfer into crop plants. Remediation of the total mercury concentration of the Wanshan soil may be unnecessary to address environmental risk.
Thiosulphate treatment enhanced the magnitude of mercury accumulation in plants and induced a change in the relative distribution of mercury fractionation in the rhizosphere soil. The concentration of Fe/Mn oxide-bound and organic-bound mercury was greatly decreased compared to the initial soil and control soil (p < 0.05). Pedron et al. (2013) reported similar results for a greenhouse pot experiment where B. juncea was used to remove mercury from soil containing 15 mg kg−1 Hg. Pedron et al. (2013) used thiosulphate to establish a constant bioavailable pool of mercury in soil. After one growth cycle, nearly 96 % of bioavailable mercury in soil was removed. The residual mercury in soil was present as non-available forms which could not be mobilized by thiosulphate or taken up by the plant. Pedron et al.’s conclusions support our hypothesis that thiosulphate-assisted phytoextraction can effectively decrease the concentration of mercury associated with bioavailable pool (Fe/Mn oxide-bound and organic-bound soil fractions) in soil, and that this may potentially address environmental risk over a realistic timeframe.
Extensive literature evidence suggests that mercury associated with the residual soil fraction is mainly in the form of HgS (Issaro et al. 2009), which is relatively stable in soil and is resistant to changes in soil chemistry and biology. However, in the present study, for the B. juncea var.ASKYC plot, the concentration of residual mercury was decreased in both the control and thiosulphate-treated plots relative to the initial soil, although there was no difference between the control and thiosulphate-treated plots. It appears that B. juncea var. ASKYC can mobilize unknown forms of mercury in the residual fraction. In a previous study, Han et al. (2006) found that HgS in soil is to some extent bioavailable to Chinese brake fern (Pteris mayii). The possible mechanism of mobilization was interpreted to be surface complexation of mercury and oxidation of surface sulfur species by DOC (Ravichandran et al. 1998; Ravichandran 2004).
The concentration of soluble and exchangeable and specifically-sorbed mercury in the rhizosphere soil increased as a function of remediation. This elevated mercury concentration could pose a secondary risk to the environment under the scenario described here. However, an increased mercury flux corresponding to these fractions was only apparent in the root zone (rhizosphere soil) not in the bulk soil. When thiosulphate was added to the mercury-contaminated field soil, a portion of the mercury associated with the Fe/Mn oxide bound and organic bound fraction was solubilized by thiosulphate, and transformed to a soluble mercury-thiosulphate complex (Hg(S2O3)2 2−), which can be easily taken up by plants (Wang et al. 2012b). We propose that the species Hg(S2O3)2 2− in the rhizosphere can be preferentially taken up by plants relative to other soluble mercury complexes (Wang et al. 2011a). Once the concentration of Hg(S2O3)2 2− in the rhizosphere soil was decreased, bulk soil Hg(S2O3)2 2− will move to the rhizosphere as a function of mass flow.
The formation of Hg(S2O3)2 2− can occur under natural conditions (1). However, the Hg(S2O3)2 2− will decompose to form cinnabar and sulfate (2) (Ullah 2008).
Thus, in the bulk soil samples, the residual soluble Hg(S2O3)2 2− complex may be decomposed, subsequently decreasing the concentration of bioavailable Hg. However, in the rhizosphere soil, due to the continuous transportation of soluble Hg(S2O3)2 2− complex from bulk soil to rhizosphere soil, the concentration of this soluble mercury species may remain relatively high. We propose that the elevated concentration of mercury associated with these mobile fractions could be reduced though continuous planting with a crop such as B. juncea, or through adding thiosulphate at a lower dose, but over a longer time frame. Further research should be conducted to verify these proposals.
Conclusions
Under field conditions, three plants (Two cultivars of Brassica juncea and one cultivar of Brassica napus) naturally accumulated a small amount of mercury in their tissues. However, the mercury concentration in plant tissues was greatly enhanced after the application of thiosulphate to the soil. Among the three tested plants, the cultivar B. juncea var.LDZY accumulated a higher concentration of mercury in its shoots than the other two plants. The total mercury concentration in the rhizosphere soil was significantly decreased in the thiosulphate-treated soil at harvest. Analysis of the relative soil mercury fractionation showed that the concentration of mercury associated with Fe/Mn oxides and organic matter was decreased as a function of phytoextraction. In contrast, the concentration of mercury associated with the soluble and exchangeable mercury and specifically-sorbed fractions in bulk soil was not affected by crop development. We therefore propose that the Fe/Mn oxide and organic matter fractions may better define the concentration of bioavailable mercury in soil. Our results indicate that thiosulphate-enhanced phytoextraction can be used to specifically target and to reduce environmental risk associated with bioavailable Fe/Mn oxide-bound and organic-bound mercury in soil. The concentration of soluble and exchangeable and specifically-sorbed mercury (conventional bioavailable fraction) was increased in the rhizosphere soil after remediation. However, we believe that any risk associated with an increase in this fraction of soil mercury may be mitigated though continuous planting to extract this mercury from soil, or by adding thiosulphate at a lower dose over a longer time frame.
References
Blaylock MJ, David E, Dushenkov S, Zakharova O, Gussman C, Kapulnik Y, Ensley BD, Raskin I (1997) Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ Sci Technol 31:860–865
Bishop KH, Lee YH, Munthe J, Dambrine E (1998) Xylem sap as a pathway for total mercury and methylmercury transport from soils to tree canopy in the boreal forest. Biogeochemistry 40:101–113
Chen J, Yang ZM (2012) Mercury toxicity, molecular response and tolerance in higher plants. BioMetals 25:847–857
CNEPA (Chinese National Environment Protect Agency) (1995) Environmental quality standard for soils, GB15618-1995, pp.1–6 (In Chinese)
Dessureault-Rompré J, Nowack B, Schulin R, Tercier-Waeber ML, Luster J (2008) Metal solubility and speciation in the rhizosphere of Lupinus albus cluster roots. Environ Sci Technol 42:7146–7151
Fayiga AO, Ma LQ, Zhou QX (2007) Effects of plant arsenic uptake and heavy metals on arsenic distribution in an arsenic-contaminated soil. Environ Pollut 147:737–742
Feng X, Li P, Qiu G, Wang S, Li G, Shang L, Meng B, Jiang H, Bai W, Li Z (2008) Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province, China. Environ Sci Technol 42:326–332
Feng X, Sommar J, Lindqvist O, Hong Y (2002) Occurrence, emissions and deposition of mercury during coal combustion in the province Guizhou, China. Water Air Soil Pollut 139:311–324
Grispen VMJ, Nelissen HJM, Verkleij JAC (2006) Phytoextraction with Brassica napus L.: a tool for sustainable management of heavy metal contaminated soils. Environ Pollut 144:77–83
Haag-Kerwer A, Schäfer HJ, Heiss S, Walter C, Rausch T (1999) Cadmium exposure in Brassica juncea causes a decline in transpiration rate and leaf expansion without effect on photosynthesis. J Exp Bot 50:1827–1835
Hamon RE, McLaughlin MJ (1999) Fifth International Conference on the Biogeochemistry of Trace Elements (ICOBTE), Vienna. pp. 908–909
Han FX, Sridhar B, Monts DL, Su Y (2004) Phytoavailability and toxicity of trivalent and hexavalent chromium to Brassica juncea. New Phytol 162:489–499
Han FX, Su Y, Monts DL, Waggoner CA, Plodinec MJ (2006) Binding, distribution, and plant uptake of mercury in a soil from Oak Ridge, Tennessee, USA. Sci Total Environ 368:753–768
Hassett DJ, Heebink LV, Pflughoeft-Hassett DF (2009) Potential for mercury vapor release from coal combustion by-products. Fuel Sci Technol 85:613–620
Hauser L, Tandy S, Schulin R, Nowack B (2005) Column extraction of heavy metals from soils using the biodegradable chelating agent EDDS. Environ. Sci Technol 39:6819–6824
Higueras P, Oyarzun R, Biester H, Lillo J, Lorenzo S (2003) A first insight into mercury distribution and speciation in soils from the Almadén mining district, Spain. J Geochem Explor 80:95–104
Huang JW, Chen J, Berti WR, Cunningham SD (1997) Phytoremediation of lead-contaminated soils: role of synthetic chelates in lead phytoextraction. Environ Sci Technol 31:800–805
Israr M, Sahi SV (2006) Antioxidative responses to mercury in the cell cultures of Sesbania Drummondii. Plant Physiol Biochem 44:590–595
Issaro N, Abi-Ghanem C, Bermond A (2009) Fractionation studies of mercury in soils and sediments: a review of the chemical reagents used for mercury extraction. Anal Chim Acta 631:1–12
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Jing YD, He ZL, Yang XE, Sun CY (2008) Evaluation of soil tests for plant-available mercury in a soil-crop rotation system. Commun Soil Sci Plan 39:3032–3046
Jones DL (1998) Organic acids in the rhizosphere–a critical review. Plant soil 205:25–44
Jones DL, Darah PR, Kochian LV (1996) Critical evaluation of organic acid mediated iron dissolution in the rhizosphere and its potential role in root iron uptake. Plant soil 180:57–66
Lomonte C, Doronila AI, Gregory D, Baker AJM, Kolev SD (2010) Phytotoxicity of biosolids and screening of selected plant species with potential for mercury phytoextraction. J Hazard Mater 173:494–501
Lu R (2000) Chemical analysis method of agricultural soil. China Agricultural Science Press, Beijing (In Chinese)
Luo C, Shen Z, Li X (2005) Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere 59:1–11
Moreno F, Anderson C, Stewart R, Robinson B, Nomura, Ghomshei M, Meech JA (2005a) Effect of thioligands on Plant-Hg accumulation and volatilisation from mercury-contaminated mine tailings. Plant Soil 275:233–246
Moreno FN, Anderson CWN, Stewart RB, Robinson BH (2005b) Mercury volatilisation and phytoextraction from base-metal mine tailings. Environ Pollut 136:341–352
Moreno-Jiméneza E, Gamarrab R, Carpena-Ruiza RO, Millánc R, Peñalosaa JM, Esteban E (2006) Mercury bioaccumulation and phytotoxicity in two wild plant species of Almadén area. Chemosphere 63:1969–1973
Mukherjee AB, Zevenhoven R, Brodersen J, Hylander LD, Bhattacharya P (2004) Mercury in waste in the European Union: sources, disposal methods and risks. Resour Conserv Recycl 42:155–182
Patra M, Bhowmik N, Bandopadhyay B, Sharma A (2004) Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ Exp Bot 52:199–223
Patra M, Sharma A (2000) Mercury toxicity in plants. Bot Rev 66:379–422
Pedron F, Petruzzell G, Barbafier M, Tassi E (2013) Remediation of a mercury-contaminated industrial soil using bioavailable contaminant stripping. Pedosphere 23:104–110
Qiu G, Feng X, Wang S, Fu X, Shang L (2009) Mercury distribution and speciation in water and fish from abandoned Hg mines in Wanshan, Guizhou province, China. Sci Total Environ 407:5162–5168
Qiu G, Feng X, Wang S, Shang L (2005) Mercury and methylmercury in riparian soil, sediments, mine-waste calcines, and moss from abandoned Hg mines in east Guizhou province, southwestern China. Appl Geochem 20:627–638
Ravichandran M (2004) Interactions between mercury and dissolved organic matter––a review. Chemosphere 55:319–331
Ravichandran M, Aiken GR, Reddy MM, Ryan JN (1998) Enhanced dissolution of cinnabar (mercuric sulfide) by dissolved organic matter isolated from the Florida Everglades. Environ Sci Technol 32:3305–3311
Rio M, Font R, Fernandez-Martinez J, Domínguez J, Haro A (2000) Field trials of Brassica carinata and Brassica juncea in polluted soils of the Guadiamar river area. Fresen Environ Bull 9:328–332
Rodriguez L, López-Bellido F, Carnicer A, Alcalde-Morano V (2003) Phytoremediation of mercury-polluted soils using crop plants. Fresen Environ Bull 12:967–971
Rodriguez L, Rincon J, Asencio I, Rodriguez-Castellanos L (2007) Capability of selected crop plants for shoot mercury accumulation from polluted soils: phytoremediation perspectives. Int J Phytoremediat 9:1–13
Sholupov S, Pogarevb S, Ryzhovb S, Mashyanovb V, Stroganova A (2004) Zeeman atomic absorption spectrometer RA-915+ for direct determination of mercury in air and complex matrix samples. Fuel Process Technol 85:473–485
Smolińska B, Cedzyńska K (2007) EDTA and urease effects on Hg accumulation by Lepidium sativum. Chemosphere 69:1388–1395
Ullah MB (2008) Mercury Stabilization using Thiosulphate and Thioselenate. Dissertation, University of British Columbia
Wang J, Feng X, Anderson CWN, Qiu GL, Ping L, Bao ZD (2011a) Ammonium thiosulphate enhanced phytoextraction from mercury contaminated soil-results from a greenhouse study. J Hazard Mater 186:119–127
Wang J, Feng X, Anderson CWN, Zhu W, Yin R, Wang H (2011b) Mercury distribution in the soil–plant–air system at the Wanshan mercury mining district in Guizhou, Southwest China. Environ Toxicol Chem 30:2725–2731
Wang J, Feng X, Anderson CWN, Xing Y, Shang L (2012a) Remediation of mercury contaminated sites-a review. J Hazard Mater 221-222:1–18
Wang J, Feng X, Anderson CWN, Wang H, Zheng L, Hu T (2012b) Implications of mercury speciation in thiosulphate treated plants. Environ Sci Technol 46:5361–5368
Wang SF, Feng XB, Qiu GL, Fu XW, Wei ZQ (2007) Characteristics of mercury exchange flux between soil and air in the heavily air-polluted area, eastern Guizhou, China. Atmos Environ 41:5584–5594
Wang YD, Greger M (2006) Use of iodide to enhance the phytoextraction of mercury-contaminated soil. Sci Total Environ 368:30–39
Wu Y, Wang S, Streets DG, Hao J, Chan M, Jiang J (2006) Trends in anthropogenic mercury emissions in China from 1995 to 2003. Environ Sci Technol 40:5312–5318
Yang Y, Fang J, Tang Y, Ji C, Zheng CY, He JS, Zhu B (2008) Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Global Change Biol 14:1592–1599
Yang Y, Ratte D, Smets B, Pignatello J, Grasso D (2001) Mobilization of soil organic matter by complexing agents and implications for polycyclic aromatic hydrocarbon desorption. Chemosphere 43:1013–1021
Zhang H, Feng X, Larssen T, Qiu G, Vogt R (2010) In inland China, rice, rather than fish is the major pathway for methylmercury exposure. Environ Health Perspect 118:1183–1188
Acknowledgments
This research was financed by the Natural Science Foundation of China (41030752, 41021062).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Henk Schat.
Rights and permissions
About this article
Cite this article
Wang, J., Feng, X., Anderson, C.W.N. et al. Thiosulphate-induced mercury accumulation by plants: metal uptake and transformation of mercury fractionation in soil - results from a field study. Plant Soil 375, 21–33 (2014). https://doi.org/10.1007/s11104-013-1940-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1940-5