Abstract
Purpose
Nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) has been widely proposed to reduce nitrogen (N) loss and improve N availability in paddy soil. However, little knowledge exists regarding the optimum dose of DMPP required for inhibiting nitrification in different soil types.
Materials and methods
In undisturbed soil columns under greenhouse conditions, dynamics of ammonium (NH4+–N) and nitrate (NO3−–N) in floodwater and leachate, and ammonia (NH3) volatilization were studied in two paddy soils (hydragic and gleyed), amended with urea-N at 180 N kg/ha with DMPP applied at 0, 0.45, 0.675, and 0.90 kg/ha (0.25%, 0.375%, and 0.5% of urea-N, respectively). The source of DMPP was Entec® 46 (46% urea-N and DMPP at 0.5% of urea-N) that was mixed with pure urea (fertilizer mixture).
Results and discussion
DMPP application rates and soil types significantly influenced NH4+–N and NO3−–N concentrations in floodwater and leachate; however, DMPP application rates did not significantly impact NH4+–N concentrations in floodwater. Results indicate that concentrations of both NH4+– N and NO3−–N in leachate and floodwater were peaked between 10 and 20 days after fertilizer application. Increased DMPP application rates increased floodwater and leachate NH4+–N concentrations, while significantly decreasing NO3−–N concentrations in floodwater and leachate, with largest decrease seen in the 0.90-kg/ha DMPP treatment. NH3 emissions were observed after fertilizer was applied and decreased gradually, with no significant differences in response to the DMPP amount. The total N losses via leaching and NH3 emission were significantly decreased at treatments of 0.675 kg/ha and 0.90 kg/ha DMPP, and positively correlated with sand fraction in soil. Compared with the gleyed paddy soil, higher total N loss was observed in the hydragic paddy soil, which was related to the higher sand fraction of the hydragic paddy soil and the better behavior of DMPP in this soil type.
Conclusions
Considering economic factors, mineral N concentrations in floodwater and leachate, together with N losses via leaching and volatilization, application of 0.675 kg/ha DMPP could significantly inhibit nitrification in the hydragic paddy soil while application of 0.90 kg/ha DMPP was shown to be the best choice to inhibit nitrification in the gleyed paddy soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
N loss is a significant issue for soil nutrition and has received considerable attention from researchers. In soil, N exists as organic or/and inorganic compounds. The inorganic species have been studied intensively because they are more available for plants than organic species (Macadam et al. 2003). Inorganic forms such as NO3−–N, NH4+–N, and NH3 are commonly found in soil and can be lost via runoff, leaching, and gaseous emissions (Abalos et al. 2014; Liu et al. 2020), as well as soil respiration accompanying reduction-oxidation metabolism (i.e., denitrification and nitrification) of organisms (Rowlings et al. 2016). For instance, an estimated 30–40% of N was demonstrated to be lost by leaching, runoff, NH3 volatilization, and denitrification (Dougherty et al. 2016). It is also reported that 20% NO3−–N leaching, 13% NH3 volatilization, and 2% N2O emission were detected from urinary deposited of N by herd animals (Selbie et al. 2015).
Such heavy loss, therefore, increases demand for more N fertilizer input to the soil to enhance the N uptake by crops. However, the application of high N fertilizers causes some adverse problems including NO3−–N leaching and nitrogenous gas release (Huérfano et al. 2015; Xu et al. 2019) as well as high cost (Lam et al. 2018). Thus, it is necessary to decrease fertilizer-induced N loss and improve N use efficiency. In recent years, enhanced efficiency fertilizers (EEFs) have been frequently studied to decrease N loss from agriculture via changing N transformation rates or increasing the longevity of fertilizer granules (Suter et al. 2016). Nitrification inhibitors (NIs) such as 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD) were widely employed to decrease the N loss (Qiao et al. 2015; Yang et al. 2016). Although some studies suggested that DCD had a superior performance for N loss to DMPP, especially in the field of grain yield, DMPP was still recommended due to its lower application rate and minor eco-toxicological side impacts for plant seeding (Li et al. 2019; Vilas et al. 2019).
The use of DMPP offered a chance to reduce NO3−–N loss from agricultural fields. By applying DMPP, the NO3−–N concentrations in leachate were reduced significantly (Koci and Nelson 2016; Nair et al. 2020). In our previous study, 44.9% of NO3−–N concentrations in leachate during rice-growing season were reduced when 1% DMPP was applied with urea, compared with urea alone (Li et al. 2008a). Higher NH4+–N concentrations in leachate and surface soil are generally observed in DMPP treatment (Anderson et al. 2014; Lam et al. 2018; Vitale et al. 2013), because nitrification inhibitors can retain N in the form of NH4+–N (Di and Cameron 2005). Also, the previous study reported that DMPP-amended sandy loam soils retain relatively high contents of NH4+–N, low levels of NO3−–N, and nitrification (Barth et al. 2019; Wu et al. 2007). In our on-farm research, DMPP increased soil NH4+–N concentrations by 12.4–24.1% 10 days after the fertilizer application with rice growth (Li et al. 2008a).
The higher NH4+–N concentrations in surface soil, floodwater, or rainfall of rice fields would increase the risk of ammonia (NH3) volatilization (Harris et al. 2016). However, just a few works had been conducted to study the effects of DMPP on the loss of NH3. Recent meta-analyses demonstrated that DMPP had no significant effect on NH3 volatilization (Qiao et al. 2015; Yang et al. 2016). Lam et al. (2018) found that DMPP in conjunction with urea slightly decreased NH3 volatilization by 0.6 N kg/ha N in a subtropical pasture.
Different concentrations of DMPP may affect the efficacy of nitrification inhibition. Compared with other NIs, such as DCD and nitrapyrin (CP), the dose of DMPP was extremely low. An application of 0.5–1.5 kg/ha DMPP was sufficient under field conditions to securely inhibit nitrification for 4–10 weeks (Rodríguez et al. 2011). DMPP inhibited nitrification during the complete incubation period (95 days) averaging from 56 to 64% at DMPP application rates of 0.89 to 1.79-mg/kg soil (Chaves et al. 2005). In liquid form, however, DMPP dose did not affect the oxidation of NH4+, with the exception of the sandy loam, where at day 25, slightly more NH4+ oxidation was observed with the highest DMPP concentration of 34.6 mg/kg soil. When applied as a solution, increasing the amount of DMPP (0.7 up to 7.1 mg/kg ) to the soil did not lead to a change in the ammonium levels (Barth et al. 2001). The observation was documented that doubling the application rate of DMPP from 1.0 to 2.0 kg/ha did not significantly influence the inhibitory effect of DMPP (Xue et al. 2012). Besides, the efficacy and dose of DMPP can be highly influenced by site-specific states including soil properties such as pH, texture, water content, and temperature (Di and Cameron 2016; Florio et al. 2016). Therefore, the optimal dose of DMPP based on those factors is still unknown.
In this study, we hypothesized that differences in N losses could occur in two paddy soils treated with various doses of DMPP. To test this hypothesis, we examined the characteristics of N losses in two different types of soil. The concentrations of N species such as NH4+–N and NO3−–N in floodwater and leachate, and NH3 volatilization rates before and after the amendment of urea fertilizer with various doses of DMPP were analyzed. Finally, the optimal doses of DMPP for both types of soil were obtained based on the experimental data. These findings would greatly contribute in understanding the influence of DMPP in paddy soils.
2 Materials and methods
2.1 Experimental materials and soil sampling
Soils were collected from Qianxi village, Yuhang town (30° 21′ N, 119° 53′ E) and Jiaxing Agricultural Research Station (30° 50′ N, 120° 40′ E), respectively, in Zhejiang Province, China. The Yuhang soil is classified as hydragic paddy soil (silt loam, mixed, mesic Mollic Endoaquepts), and the Jiaxing soil type is gleyed paddy soil (clay loam, mixed, mesic Mollic Endoaquepts). The gleyed and hydragic paddy soils represented two dominant soil types in Taihu Lake basin. The orders of muddy layer thickness and pressure head at the muddy layer bottom of the paddy soils follow gleyed > hydragic, whereas the infiltration rate and saturated hydraulic conductivity follow hydragic > gleyed. The saturated hydraulic conductivity of the hydragic is about 1.5 times higher than the gleyed paddy soil (Li et al. 2008b). Properties of soils are shown in Table 1.
The undisturbed soil columns were collected similar to the method of Yu et al. (2007). Briefly, soil was excavated from around a free-standing cylindrical soil column of 30 cm diameter by 60 cm depth. When the soil column was properly shaped, a polyvinyl (PVC) pipe of 30 cm diameter and 75 cm depth was placed around the column, and liquefied soil mud was placed in the space between the soil and the PVC. An acid-washed fine sand with 2-cm thickness was spread on the bottom for filtration of soil water, and leachate collector was installed for sampling the leachate at a depth of 60 cm depth.
2.2 Experimental design
Four DMPP levels were arranged in a completely randomized design with three replicates for each soil type: urea-N (urea alone at 180 N kg/ha), 0.25% urea-N (urea 90 N kg/ha combined with Entec® 46 90 N kg/ha (0.45 kg/ha DMPP)), 0.375% urea-N (urea 45 N kg/ha combined with Entec® 46 135 N kg/ha (0.675 kg/ha DMPP)), 0.5% urea-N (Entec® 46 alone at 180 N kg/ha (0.90 kg/ha DMPP)). All the treatments were 180 kg/ha N. The source of DMPP was Entec® 46 with 46% urea-N and DMPP at 0.5% of urea-N. All the nitrogen fertilizers together with 40 kg/ha P2O5 were applied as base fertilizers.
Rice seedlings were transplanted into each undisturbed column after fertilizer application. When water levels were less than 5 millimeters (mm), all soil columns were regularly irrigated to a depth of 80 mm through day 28, excepting a period of soil drying from days 11 to 13, and then, the soil was kept moist with zero-drainage of runoff through the period. The whole experiment was conducted in an artificial greenhouse.
2.3 Sampling and analysis
In the flooding period, floodwater (surface water) was collected at an interval of 5 days, and leachate water was collected at an interval of 10 days after fertilization. Samples for NH3 analysis were collected every 2 days for the 10 days following fertilization. Soil samples were collected 60 days after experiments begin.
All the floodwater and leachate samples were stored in a refrigerator (4 °C) and analyzed within 2 days. Concentrations of NO3−–N and NH4+–N were determined using a continuous-flow analyzer (AA3, Bran+Luebbe, Germany). NH3 volatilization rate was measured using a continuous airflow enclosure method (Li et al. 2008b; Tian et al. 2001).
2.4 Statistical analysis
The experiment was a completely randomized design. We performed a two-way analysis of variance (ANOVA) to test soil types and DMPP application rate interactions. The Kruskal-Wallis method was used to identify differences in floodwater, leachate, and NH3 volatilization among the DMPP application rate (P < 0.05). Duncan’s test was used to test the significance of differences between N losses form leaching and NH3 volatilization within each soil type (P < 0.05). Spearman correlations were conducted to analyze the correlations between total mineral N losses via leaching and NH3 volatilization and soil properties.
3 Results
3.1 Effect of soil types and DMPP application rates on mineral nitrogen concentrations in floodwater
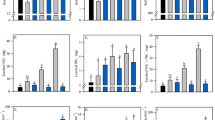
Differing DMPP application rate and soil types resulted in significant differences in the floodwater NO3−–N concentrations; however, only differing soil types significantly affected floodwater NH4+–N concentrations (Table 2). Compared with the gleyed paddy soil, the NH4+–N and NO3−–N concentrations in floodwater were significantly lower than those in the hydragic paddy soil (Figs. 1 and 2). Compared with the urea treatment, NH4+–N and concentrations in floodwater increased to about 4–13%, 9–40%, and 16–65% in the 0.45-kg/ha DMPP, 0.675-kg/ha DMPP, and 0.90-kg/ha DMPP treatments respectively in the hydragic paddy soil, and increased to about 7–13%, 18–30%, and 31–60% respectively in the gleyed paddy soil. Significant differences in NH4+–N concentrations between urea treatment and 0.90-kg/ha DMPP and 0.675-kg/ha DMPP treatments were observed, while no significant difference was observed between 0.45 kg/ha DMPP and urea treatments in the hydragic paddy soil (Fig. 1). Therefore, adding 0.675 kg/ha DMPP could significantly increase NH4+–N concentrations in floodwater of the hydragic paddy soil, and increasing the DMPP to 0.90 kg/ha may affect NH4+–N concentrations the most. For the gleyed soil, the same trend was observed except for differences between 0.675 kg/ha DMPP and urea treatments in three sampling time. Thus, 0.90 kg/ha DMPP may be the best choice to inhibit nitrification in the gleyed paddy soil.
NH4+–N concentrations in floodwater after fertilizer application in gleyed and hydragic paddy soils. Comparing across treatments within each sampling time, the lines with the asterisk are significant at P < 0.05. 0.45 kg/ha DMPP as 0.25% urea-N, 0.675 kg/ha DMPP as 0.375% urea-N, 0.90 kg/ha DMPP as 0.5% urea-N. Vertical bars indicate standard errors of the mean (n = 3)
NO3−–N concentrations in floodwater after fertilizer application in gleyed and hydragic paddy soils. Comparing across treatments within each sampling time, the lines with the asterisk are significant at P < 0.05. 0.45 kg/ha DMPP as 0.25% urea-N, 0.675 kg/ha DMPP as 0.375% urea-N, 0.90 kg/ha DMPP as 0.5% urea-N. Vertical bars indicate standard errors of the mean (n = 3)
Compared with urea treatment, NO3-−-–N concentrations in floodwater decreased to about 2–7%, 14–24%, and 41–61% in 0.45-kg/ha DMPP, 0.675-kg/ha DMPP, and 0.90-kg/ha DMPP treatments, respectively in the hydragic paddy soil (Fig. 2). The respective contents in the gleyed paddy soil decreased by 12–22%, 35–44%, and 63–70%, respectively. Significant differences were observed among 0.90-kg/ha DMPP, 0.675-kg/ha DMPP, and 0.45 -kg/ha DMPP treatments in both soils.
3.2 Effect of soil types and DMPP application rates on mineral nitrogen concentrations in leachate
Both soil types and DMPP application rates significantly affected the NH4+–N and NO3−–N concentrations in the leachate (Table 2). The NH4+–N and NO3−–N concentrations in the hydragic paddy soil were higher than those in the gleyed paddy soil (Figs. 3 and 4). With the increase of DMPP application, the NH4+–N concentrations in leachate increased significantly, while NO3−–N concentrations in leachate decreased significantly. The highest NH4+–N concentrations in leachate was found in 0.90-kg/ha DMPP treatment, with 4.85 mg/L in the gleyed paddy soil and 3.95 mg/L in the hydragic paddy soil. The significant differences were observed between 0.675 kg/ha DMPP, 0.90 kg/ha DMPP, and urea treatments in the hydragic and gleyed paddy soils, and between 0.675- and 0.90-kg/ha DMPP treatments in the gleyed paddy soil (Fig. 3). Therefore, 0.90 kg/ha DMPP worked best in the gleyed paddy soil while 0.675 kg/ha DMPP could achieve the most efficiency to inhibit nitrification in the hydragic paddy soil.
NH4+–N concentrations in leachate after fertilizer application in gleyed and hydragic paddy soils. Comparing across treatments within each sampling time, the lines with the asterisk are significant at P < 0.05 (the same in all figures). 0.45 kg/ha DMPP as 0.25% urea-N, 0.675 kg/ha DMPP as 0.375% urea-N, 0.90 kg/ha DMPP as 0.5% urea-N. Vertical bars indicate standard errors of the mean (n = 3)
NO3−–N concentrations in leachate as after fertilizer application in gleyed and hydragic paddy soils. Comparing across treatments within each sampling time, the lines with the asterisk are significant at P < 0.05. 0.45 kg/ha DMPP as 0.25% urea-N, 0.675 kg/ha DMPP as 0.375% urea-N, 0.90 kg/ha DMPP as 0.5% urea-N. Vertical bars indicate standard errors of the mean (n = 3)
For the NO3−–N concentrations, no significant differences were observed between urea and 0.5% DMPP treatments in the hydragic paddy soil; lowest concentrations were found in the 0.90-kg/ha DMPP treatment. While in the gleyed paddy soil, the NO3−–N concentrations significantly decreased with the increasing DMPP application rates (Fig. 4).
3.3 Effect of soil types and DMPP application rates on NH3 volatilization
The volatilization rates of NH3 were only significantly influenced by soil types (Table 2), with higher NH3 volatilization rates observed in the hydragic paddy soil than those in the gleyed paddy soil (Fig. 5). After 2 days of DMPP and urea application, the NH3 volatilization rates peaked, then decreased within 10 days. Although no significant differences were observed among DMPP application rates for each soil type, the highest rates of NH3 volatilization were observed when DMPP was applied to the hydragic paddy soil at a rate of 0.90 kg/ha DMPP and to the gleyed paddy soil at a rate of 0.675 kg/ha DMPP.
NH3 volatilization rates as after fertilizer application in gleyed and hydragic paddy soils. Comparing across treatments within each sampling time, the lines with the asterisk are significant at P < 0.05. 0.45 kg/ha DMPP as 0.25% urea-N, 0.675 kg/ha DMPP as 0.375% urea-N, 0.90 kg/ha DMPP as 0.5% urea-N. Vertical bars indicate standard errors of the mean (n = 3)
3.4 Effect of soil types and DMPP application rates on mineral N losses via leaching and NH3 volatilization
Two-way ANOVA revealed that N losses via leaching and NH3 emission was significantly affected by soil types, but N losses via leaching and total mineral N losses significantly changed in response to DMPP application rates (Table 2, Fig. S1). The total mineral N losses were significantly decreased in response to the increased DMPP application rate; losses were higher in the hydragic soil than those in the gleyed soil. The ratio of total N losses via leachate and NH3 were nearly 14% and 10% of the total N applied in the gleyed and hydragic paddy soils, respectively (Fig. 6). The mineral N losses via leaching and NH3 volatilization significantly and positively correlated with soil pH and sand fraction, and negatively correlated with clay fraction and soil total N, and total phosphorus (Fig. S2).
4 Discussion
Soil environmental parameters may vary significantly in different locations, affecting the performance of N fertilizer and the longevity of NIs (Florio et al. 2016; Guardia et al. 2018; Yang et al. 2016). Soil properties such as temperature, water content, pH, charge, residual carbon to nitrogen (C/N) ratio and cationic exchange capacity (CEC), and soil texture were commonly considered in the discussion of the mechanism of N preservation, and several studies had reported some of those indices. It has been reported that DMPP effectively impeded nitrification at 5–15 °C and even reduced N2O emission at 40–60% water-filled pore space after 42 days; however, this efficiency declined when temperature was elevated (25 °C in this article) (Chen et al. 2010). These results could be interpreted to mean cold weather retards microbial nitrification rates, while warmer periods contribute to the biological degradation of non-volatile DMPP. In this study, temperatures in both sites are annually recorded at 15–16 °C in the greenhouse, which indicate that other factors play important roles in the N losses.
Soil properties, such as CEC, and texture could affect the mineral nitrogen retention and water transport in soil. The observed the higher CEC of the gleyed paddy soil (Table 1) resulted in stronger retention of NH4+–N by surface soil and lower NH3 volatilization rates, corroborating our previous study (Li et al. 2008b). The gleyed paddy soil was clayey with lower sand and silt content and higher clay content (51.7%) than the sandy hydragic paddy soil. The muddy layer thickness and pressure head at the muddy layer bottom of the gleyed paddy soil were higher than those of the hydragic paddy soil, whereas the infiltration rate and saturated hydraulic conductivity of the hydragic paddy soil were higher than those of the gleyed paddy soil (Li et al. 2008b). The leaching amount in the hydragic paddy soil is 1.3–1.6 times than that of the gleyed paddy soil in this study. In addition, the leachate concentrations of NO3−–N and NH4+–N from the hydragic paddy soil were higher than those from the gleyed paddy soil, which caused the higher mineral leaching losses from the hydragic paddy soil. The results also indicated that the differences between various DMPP doses were more obvious in the hydragic paddy soil than in the gleyed paddy soil. This is consistent with findings by other researchers (Barth et al. 2019; Shi et al. 2016) that DMPP works better in sandy soil than in clayey soil, which also evidenced by the significantly positive correlations between sand concentrations with total mineral N loss in our study.
Compared with other NIs, DMPP has a higher sorption towards the soil substrate (Di and Cameron 2016). Thus, those variations between both sites could also be explained by DMPP sorption. DMPP could be attached to soil mineral surface easier than other NIs due to its negative charge and heterocyclic compound, and the fact that is not easily degradable (Barth et al. 2008; Shi et al. 2016). This sorption within the soil could be affected by the organic content and residual N level (Marsden et al. 2016). Recent studies demonstrated that the efficiency of DMPP was closely correlated to increasing levels of soil inorganic constituents and that the adsorption of DMPP to the soil clay fraction played a major role in controlling the inhibition effect (Di and Cameron 2012; Marsden et al. 2016). In our study, the mineral N losses via leaching were reduced at both sites with increased DMPP application rates with lower in more clay gleyed paddy soil. It further confirmed that the soil clay fraction would restrain the inhibition effect of DMPP.
Rice farming could be a significant source of water pollution. As shown in Fig. 1, NH4+–N concentrations in floodwater at the first 20 days after fertilization, all the samples exceed China’s surface water quality standards (class V, 2 mg/L). Figure 4 also shows that NO3−–N concentrations on day 20 in urea and 0.45-kg/ha DMPP treatments exceeded the groundwater quality standard (10 mg/L) set by China. These data indicated that without DMPP or if DMPP is less than 0.45 kg/ha, groundwater beneath the rice paddy would have NO3−–N exceedance. Further study should focus on determining the amount of urea to maintain rice yield and without contaminating groundwater.
DMPP inhibited the oxidation of ammonium in both soils, but this effect was more pronounced in the sandy loam than in the loamy soil (Barth et al. 2019). Soil types significantly affected the NH4+– and NO3−–N concentrations both in leachate and floodwater in our study, with higher effect in the hydragic paddy soil. This may be due to the higher soil sand fraction in the hydragic paddy soil. In the loamy soil, DMPP delayed NH4+ oxidation efficiency than in the sandy loam. Also, in the clayey loam soil such as vegetable soil, it impeded the accumulation of NO3−–N concentration in the leachate (Xu et al. 2005). It was observed in our previous research that DMPP could inhibit ammonia oxidization through inhibiting ammonia-oxidizing bacteria (Florio et al. 2014; Yang et al. 2012), resulting in more NH4+–N and less NO3−–N in the soil and leachate after urea application. In a column study, Wu et al. (2007) observed significantly lower NO3−–N concentrations in surface soils receiving DMPP amendment. And especially in dryland soils treated with 0.90 kg/ha DMPP, NO3−–N concentration was 23% lower than in soils treated with ammonium sulfate nitrate (ASN) alone (Weiske et al. 2001). Even when the NO3−–N concentration in leachate was higher than 10 mg/L in our study, the application of DMPP decreased it to below 5.5 mg/L. Overall, DMPP reduced NO3−–N leaching fluxes by 44.3–51.9%, potentially reducing NO3−–N migration to the groundwater. In soil columns with the same gleyed paddy soil, NO3−–N leaching loss fluxes were reduced by 69.5% when DMPP was added (Yu et al. 2007).
On the other hand, due to its high concentration in soil, higher NH4+–N concentration in leachate from the urea + DMPP plots were also observed, which was 13.0–33.3% higher than in the urea plots (Li et al. 2008a). In column experiments with the same gleyed paddy soil, NH4+–N concentration in soil solution at the top 20-cm soil was increased by 0.9 kg/ha DMPP (Yu et al. 2007). The greater amount of NH4+–N in leachate when DMPP was applied was also found by other researchers (Guardia et al. 2018; Xu et al. 2019; Yang et al. 2016). The slightly higher NH4+–N contents in the urea + DMPP plots may play an important role in enhancing N availability in soil (Yang et al. 2016). In previous studies, DMPP did not reduce or even increase crop and vegetable yields (Hu et al. 2013; Merino et al. 2005). In this study, the total N content in the gleyed paddy soil was higher than that in the hydragic paddy soil (Table 1), and for most samples in both 3sites; however, the N losses via leaching and volatilization were higher in the hydragic paddy soil than those in the gleyed paddy soil with the same N application. It may indicate that soil properties are the dominant role of in N losses when DMPP is applied.
Application of DMPP could inhibit ammonium oxidization for 4–10 weeks in farmland soils (Guardia et al. 2018), and the inhibition was more evident with more NH4+–N available soon after urea application. In 2 days after urea application, the addition of DMPP could maintain a large amount of N in NH4+–N, leading to higher NH3 volatilization rates in the urea + DMPP than those in the urea alone application. However, the observed accumulative NH3 emission losses were not significantly affected by DMPP. Qiao et al. (2015) and Yang et al. (2016) also found that DMPP had no significant effects on NH3 volatilization, which may be explained by the similar soil pH for the two soils.
5 Conclusion
Soil type significantly affected the concentration of NH4+– and NO3−–N in floodwater and leachate and the total N losses via leaching and NH3 emission; however, only NO3−–N in floodwater and leachate was affected significantly in response to DMPP application rates. The total mineral N losses in the hydragic paddy soil were more pronounced than those in the gleyed paddy soil, which was greatly related to the soil properties such as particle size distribution, CEC, and organic matter which could affect the performance of DMPP in soil, particularly the soil sandy fraction. DMPP could significantly inhibit nitrification in paddy soil: Considering economic factors, mineral N concentrations in floodwater and leachate, together with N losses via leaching and volatilization, 0.675 kg/ha DMPP could significantly inhibit nitrification in the hydragic paddy soil while 0.90 kg/ha DMPP was the best choice to inhibit nitrification in the gleyed paddy soil. This difference needs to be further explored via focusing on the soil and interaction of different types of indigenous microorganisms such as nitrite-oxidizing bacteria, ammonia-oxidizing bacteria, and archaea.
References
Abalos D, Jeffery S, Sanz-Cobena A, Guardia G, Vallejo A (2014) Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric Ecosyst Environ 189:136–144
Anderson CR, Hamonts K, Clough TJ, Condron LM (2014) Biochar does not affect soil N-transformations or microbial community structure under ruminant urine patches but does alter relative proportions of nitrogen cycling bacteria. Agric Ecosyst Environ 191:63–72
Barth G, Von Tucher S, Schmidhalter U (2001) Influence of soil parameters on the effect of 3,4-dimethylpyrazole-phosphate as a nitrification inhibitor. Biol Fertil Soils 34:98–102
Barth G, Von Tucher S, Schmidhalter U (2008) Effectiveness of 3,4-dimethylpyrazole phosphate as nitriflcation inhibitor in soil as influenced by inhibitor concentration, application form, and soil matric potential. Pedosphere 18:378–385
Barth G, Tucher S, Schmidhalter U, Otto R, Motavalli P, Ferraz-Almeida R, Meinl Schmiedt Sattolo T, Cantarella H, Vitti GC (2019) Performance of nitrification inhibitors with different nitrogen fertilizers and soil textures. J Plant Nutr Soil Sci 182:694–700
Chaves B, Opoku A, De Neve S, Boeckx P, Van Cleemput O, Hofman G (2005) Influence of DCD and DMPP on soil N dynamics after incorporation of vegetable crop residues. Biol Fertil Soils 43:62–68
Chen D, Suter HC, Islam A, Edis R (2010) Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biol Biochem 42:660–664
Di HJ, Cameron KC (2005) Reducing environmental impacts of agriculture by using a fine particle suspension nitrification inhibitor to decrease nitrate leaching from grazed pastures. Agric Ecosyst Environ 109:202–212
Di HJ, Cameron KC (2012) How does the application of different nitrification inhibitors affect nitrous oxide emissions and nitrate leaching from cow urine in grazed pastures? Soil Use Manag 28:54–61
Di HJ, Cameron KC (2016) Inhibition of nitrification to mitigate nitrate leaching and nitrous oxide emissions in grazed grassland: a review. J Soils Sediments 16:1401–1420
Dougherty WJ, Collins D, Van Zwieten L, Rowlings DW (2016) Nitrification (DMPP) and urease (NBPT) inhibitors had no effect on pasture yield, nitrous oxide emissions, or nitrate leaching under irrigation in a hot-dry climate. Soil Res 54:675–683
Florio A, Clark IM, Hirsch PR, Jhurreea D, Benedetti A (2014) Effects of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on abundance and activity of ammonia oxidizers in soil. Biol Fertil Soils 50:795–807
Florio A, Maienza A, Dell’Abate MT, Stazi SR, Benedetti A (2016) Changes in the activity and abundance of the soil microbial community in response to the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP). J Soils Sediments 16:2687–2697
Guardia G, Marsden KA, Vallejo A, Jones DL, Chadwick DR (2018) Determining the influence of environmental and edaphic factors on the fate of the nitrification inhibitors DCD and DMPP in soil. Sci Total Environ 624:1202–1212
Harris RH, Armstrong RD, Wallace AJ, Belyaeva ON (2016) Delaying nitrogen fertiliser application improves wheat 15N recovery from high rainfall cropping soils in south eastern Australia. Nutr Cycl Agroecosyst 106:113–128
Huérfano X, Fuertes-Mendizábal T, Duñabeitia MK, González-Murua C, Estavillo JM, Menéndez S (2015) Splitting the application of 3,4-dimethylpyrazole phosphate (DMPP): influence on greenhouse gases emissions and wheat yield and quality under humid Mediterranean conditions. Eur J Agron 64:47–57
Hu Y, Schraml M, Tucher S, Li F, Schmidhalter U (2013) Influence of nitrification inhibitors on yields of arable crops: a meta-analysis of recent studies in Germany. Int J Plant Prod 8:33–50
Koci J, Nelson PN (2016) Tropical dairy pasture yield and nitrogen cycling: effect of urea application rate and a nitrification inhibitor, DMPP. Crop Pasture Sci 67:766–779
Lam SK, Suter H, Bai M, Walker C, Davies R, Mosier AR, Chen D (2018) Using urease and nitrification inhibitors to decrease ammonia and nitrous oxide emissions and improve productivity in a subtropical pasture. Sci Total Environ 644:1531–1535
Li H, Liang X, Chen Y, Lian Y, Tian G, Ni W (2008a) Effect of nitrification inhibitor DMPP on nitrogen leaching, nitrifying organisms, and enzyme activities in a rice-oilseed rape cropping system. J Environ Sci 20:149–155
Li H, Liang X, Chen Y, Tian G, Zhang Z (2008b) Ammonia volatilization from urea in rice fields with zero-drainage water management. Agr Water Manag 95:887–894
Li J, Shi Y, Luo J, Li Y, Wang L, Lindsey S (2019) Effects of 3,4-dimethylpyrazole phosphate (DMPP) on the abundance of ammonia oxidizers and denitrifiers in two different intensive vegetable cultivation soils. J Soils Sediments 19:1250–1259
Liu J, Ouyang X, Shen J, Li Y, Sun W, Jiang W, Wu J (2020) Nitrogen and phosphorus runoff losses were influenced by chemical fertilization but not by pesticide application in a double rice-cropping system in the subtropical hilly region of China. Sci Total Environ 715:136852
Macadam XMB, Ad P, Merino P, Estavillo JM, Pinto M, González-Murua C (2003) Dicyandiamide and 3,4-dimethyl pyrazole phosphate decrease N2O emissions from grassland but dicyandiamide produces deleterious effects in clover. J Plant Physiol 160:1517–1523
Marsden KA, Marín-Martínez AJ, Vallejo A, Hill PW, Jones DL, Chadwick DR (2016) The mobility of nitrification inhibitors under simulated ruminant urine deposition and rainfall: a comparison between DCD and DMPP. Biol Fertil Soils 52:491–503
Merino P, Merino P, Menéndez S, Pinto M, González-Murua C, Estavillo JM (2005) 3, 4-Dimethylpyrazole phosphate reduces nitrous oxide emissions from grassland after slurry application. Soil Use Manag 21:53–57
Nair D, Baral KR, Abalos D, Strobel BW, Petersen SO (2020) Nitrate leaching and nitrous oxide emissions from maize after grass-clover on a coarse sandy soil: Mitigation potentials of 3,4-dimethylpyrazole phosphate (DMPP). J Environ Manag 260:110165
Qiao C, Liu L, Hu S, Compton JE, Greaver TL, Li Q (2015) How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Glob Chang Biol 21:1249–1257
Rodríguez VA, Luaces PA, Píccoli AB, Mazza SM, Martínez GC (2011) 3,4-Dimethylpyrazole phosphate (DMPP) efficiency in sweet orange in Argentina northeast. Rev Bras Frutic 33:1344–1349
Rowlings DW, Scheer C, Liu S, Grace PR (2016) Annual nitrogen dynamics and urea fertilizer recoveries from a dairy pasture using 15N; effect of nitrification inhibitor DMPP and reduced application rates. Agric Ecosyst Environ 216:216–225
Selbie DR, Buckthought LE, Shepherd MA (2015) The challenge of the urine patch for managing nitrogen in grazed pasture systems. Adv Agron 129:229–292
Shi X, Hu HW, Müller C, He JZ, Chen D, Suter HC (2016) effects of the nitrification inhibitor 3,4-dimethylpyrazole phosphate on nitrification and nitrifiers in two contrasting agricultural soils. Appl Environ Microbiol 82:5236–5248
Suter HC, Sultana H, Davies R, Walker C, Chen D (2016) Influence of enhanced efficiency fertilisation techniques on nitrous oxide emissions and productivity response from urea in a temperate Australian ryegrass pasture. Soil Res 54:523–532
Tian G, Cai Z, Cao J, Li X (2001) Factors affecting ammonia volatilisation from a rice-wheat rotation system. Chemosphere 42:123–129
Vilas MP, Verburg K, Thorburn PJ, Probert ME, Bonnett GD (2019) A framework for analysing nitrification inhibition: a case study on 3,4-dimethylpyrazole phosphate (DMPP). Sci Total Environ 672:846–854
Vitale L, Ottaiano L, Polimeno F, Maglione G, Amato U, Arena C, di Tommasi P, Mori M, Magliulo V (2013) Effects of 3,4-dimethylphyrazole phosphate-added nitrogen fertilizers on crop growth and N2O emissions in Southern Italy. Plant Soil Environ 59:517–523
Weiske A, Benckiser G, Herbert T, Ottow J (2001) Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biol Fertil Soils 34:109–117
Wu SF, Wu LH, Shi QW, Wang ZQ, Chen XY, Li YS (2007) Effects of a new nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on nitrate and potassium leaching in two soils. J Environ Sci 19:841–847
Xu C, Wu LH, Ju XT, Zhang FS (2005) Role of nitrification inhibitor DMPP (3, 4-dimethylpyrazole phosphate) in NO3---N accumulation in greengrocery (Brassica campestris L. ssp. chinensis) and vegetable soil. J Environ Sci 17:81–83
Xu J, Zhu T, Xue W, Ni D, Sun Y, Yang J, Xu L, Chen X, Li H, Liu M (2019) Influences of nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) and application method on nitrogen dynamics at the centimeter-scale. Eur J Agron 90:44–50
Xue Y, Wu ZJ, Zhang LL, Gong P, Dong XX, Nie YX (2012) Inhibitory effect of DMPP on soil nitrification as affected by soil moisture content, pH and organic matter. Chin J Appl Ecol 23:2663–2669
Yang J, Li X, Xu L, Hu F, Li H, Liu M (2012) Influence of the nitrification inhibitor DMPP on the community composition of ammonia-oxidizing bacteria at microsites with increasing distance from the fertilizer zone. Biol Fertil Soils 49:23–30
Yang M, Fang Y, Sun D, Shi Y (2016) Efficiency of two nitrification inhibitors (dicyandiamide and 3, 4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: a meta-analysis. Sci Rep 6:22075
Yu Q, Chen Y, Ye X, Zhang Q, Zhang Z, Tian P (2007) Evaluation of nitrification inhibitor 3,4-dimethyl pyrazole phosphate on nitrogen leaching in undisturbed soil columns. Chemosphere 67:872–878
Funding
This work was financed by National Natural Science Foundation of China (41671300), National Key Research and Development Plan of China (2016YFD0200804), and Science and technology cooperation project of agricultural and rural Department of Zhejiang Province (2018SNLF027). Shaoxian Wang is grateful for funding from Open Fund from Key Laboratory for Water Pollution Control and Environmental Safety.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies involving human participants and/or animals performed by any of the authors.
Additional information
Responsible editor: Weixin Ding
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 245 kb)
Rights and permissions
About this article
Cite this article
Li, H., Chen, X., Liu, C. et al. Effect of various doses of 3,4-dimethylpyrazole phosphate on mineral nitrogen losses in two paddy soils. J Soils Sediments 20, 3825–3834 (2020). https://doi.org/10.1007/s11368-020-02711-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02711-2