Abstract
Aims
Soil pH is known to influence many important biochemical processes in plants and soils, however its role in salinity—boron interactions affecting plant growth and ion relations has not been examined. The purpose of this research was to evaluate the interactive effects of salinity, boron and soil solution pH on broccoli (Brassica oleracea L.) growth, yield, consumptive water use and shoot-boron accumulation.
Methods
A greenhouse experiment was conducted using a sand tank system where salinity-B-pH treatment solutions were supplemented with a complete nutrient solution. Sulfate-dominated irrigation waters, characteristic of groundwater in California’s San Joaquin valley (SJV), were tested at EC levels of 2, 5, 8, 11 and 14 dS m−1. Each salinity treatment consisted of two boron treatments (0.5 and 21 mg L−1) and each of those treatments was tested under slightly basic (pH 8.0) and slightly acidic (pH 6.0) conditions.
Results
Results included multiple salinity-boron-pH interactions affecting shoot biomass, head yield and consumptive water use. Broccoli fresh head yields were significantly reduced by salinity and boron, but the degree of yield reductions was influenced by pH. Relative head yields were substantially reduced in treatments with high pH and high B, particularly under low and high salinity where head yields were decreased by 89 % and 96 %, respectively, relative to those at low salinity and low boron. Intermediate levels of salinity were far less damaging. Increased salinity and boron reduced evapotranspiration (ET) and there were no salinity-boron associated interactions on ET. However, increased salinity and boron concentrations increased water use efficiency (shoot biomass/cumulative volume ET). Shoot B concentration increased with increased boron and was greater at pH 6 as compared to pH 8. Shoot boron concentration decreased with increasing salinity at both pH levels but particularly at the high substrate boron concentration.
Conclusions
It is likely that different mechanisms, yet unknown, are responsible for severe head-yield reductions at low and high salinity in the presence of high boron under alkaline conditions. We found that boron in the shoot did not accumulate by a simple passive process. Rather as boron increased from 0.5 to 21 mg L−1, there was a restrictive mechanism where total shoot boron (mg plant−1) was reduced by 10 to 40 times the amount potentially supplied to the shoot by passive transport via mass flow perhaps involving complex interactions with membrane channels and B exporters. Total shoot boron concentration was a poor indicator of plant growth response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic stresses due to salinity and high boron concentrations occur together naturally in many parts of the world including the Lluta Valley in Chile (Ferreyra et al. 1997), Saskatchewan, Canada (Nicholaichuk et al. 1988), the Negev region in Israel (Yermiyahu et al. 2008),Western Fresno County, California (Bañuelos et al. 2003), India (Sharma et al. 1993), Upper Eyre Peninsula, Australia (Holloway and Alston 1992), and the Rio Grande Basin, New Mexico (Picchioni et al. 2000). In addition, crops vary in the sensitivity to excess boron and salinity and those that are tolerant to salinity may not be tolerant to boron and vise versa (Grieve et al. 2012). Boron has a higher adsorption affinity to mineral soils than do common salinizing salts, with the adsorption varying greatly among soils even though salinity does not influence adsorption characteristics (Goldberg et al. 2000). Nevertheless, reclamation leaching to reduce soil salinity and boron to acceptable levels can be complicated from a crop selection perspective since greater volumes of water are needed to reduce soil boron by the same percentage as are required to reduce soil salinity (Bingham et al. 1972; Ayers and Westcot 1985) and these volumes are highly pH dependent (Suarez et al. 2012). Integration of high value vegetable crops into the schedule of rotations with more salt-tolerant agronomic crops has obvious economic advantages. However, as salt and boron concentrations are disproportionately reduced during the intermediate reclamation stages, unknown interactions between salinity and boron may affect the subsequent performance of more economically attractive vegetable crops.
Research articles related to growth and yield responses of crops to salinity and high boron as individual stresses are numerous (Alpaslan and Gunes 2001; Bingham et al. 1987; Ehret and Ho 1986; Grieve et al. 2012). However, studies addressing both stresses affecting the crop simultaneously are much more limited and the conclusions reached have been contradictory (Ben-Gal and Shani 2002; Wimmer and Goldbach 2012). The majority of the research indicates increased plant tolerance to boron with increased salinity (Ferguson et al. 2002; Grattan et al. 1996; Holloway and Alston 1992; Yadav et al. 1989; Ben-Gal and Shani 2002). However, decreased tolerance to boron in the presence of salinity-stress has also been documented in cucumber, tomato and wheat (Grieve and Poss 2000; Alpaslan and Gunes 2001; Wimmer et al. 2003, 2005).
Confounding the interpretations of salinity-boron interactions is the influence of the pH on the soil solution (Läuchli and Grattan 2012a). Soil pH on the Westside of California’s San Joaquin Valley (SJV) is generally in the neutral to alkaline range (7.0–9.0) and drainage waters are above pH 8.0. The pH in the soil solution is known to affect boron adsorption (Goldberg et al. 2000) and boron availability to plants (Marschner 1995). As pH increases, the rate of boron uptake is dramatically reduced, particularly when the pH exceeds 8 (Marschner 1995; Läuchli and Grattan 2012a) where the speciation of boron starts to progressively shift from boric acid B(OH)3 to anionic borate B(OH)4 − (equal activities of the two species occur at pH 9.3). The pH conditions recorded in previous salinity and boron experiments (Ben-Gal and Shani 2002; Sternberg et al. 2001; Supanjani 2006; Yadav et al. 1989) were either slightly acidic (pH 6.0–6.5) or slightly basic (pH 7.5–8.5) but pH was not used as an experimental variable.
Boron toxicity is rarely observed on annual crops grown in SJV soils although boron concentrations in many areas are higher than values where injury or yield losses are expected to occur (Grieve et al. 2012; Francois 1985). This apparent lack of response may be due to a salinity-induced reduction in mass-flow uptake of boron (Brown et al. 2002). At pH 8.3 only 10 % of the boron activity is represented by B(OH)4 − in these slightly basic conditions so it is not likely that this explains reduced plant boron uptake as suggested by Marschner (1995). Indeed, salinity—boron interactions are complex (Bastías et al. 2010) and adding pH variations as an additional abiotic stress places more uncertainty on the interaction of these important environmental stresses (Läuchli and Grattan 2012a). We therefore included pH in the factorial design of the experiment to provide insight into salinity-boron interactions and gain a better understanding of how the plant may tolerate higher concentrations of boron in the presence of salinity as has been observed in several other studies (Yermiyahu et al. 2008; Smith et al. 2010). Therefore our study was conducted to determine the interactive effects of salinity, boron and pH on broccoli performance including growth, yield, injury, evapotranspiration, water use efficiency and boron accumulation.
Materials and methods
A greenhouse experiment was conducted using a sand-tank system at the USDA-ARS, U. S. Salinity Laboratory located at the University of California, Riverside campus. Broccoli (Brassica oleracea L., botrytis group, cv Seminis PX511018) was selected because it is a crop commonly grown on the Westside of the SJV and is known to be moderately sensitive to salinity and moderately sensitive to B in non-saline environments (Grieve et al. 2012). The sand-tank system comprised of 60 large tanks (1.2 m × 0.6 m × 0.5 m deep) filled with washed river sand, creates a uniform rootzone and controlled environment (Wang 2002). Each tank is irrigated with a solution prepared in individual reservoirs having a volume of approximately 890 L. Salinity-B treatments were complemented with modified half-strength Hoagland’s nutrient solution. Solutions were pumped several times per day from storage reservoirs, located below the sand-tank facility, to the sand tanks and then returned to the reservoirs by gravity flow. The frequent irrigations allowed the ion concentration in the soil water to be close to that of the irrigation water as well as to avoid water stress. To confirm this hypothesis, calculations were performed to estimate the concentration factors in the soil water, above that of the irrigation water, accounting for maximum transpiration, volume of applied water and interval between irrigations. These calculations indicate that the time averaged salinity increase of the soil water relative to the irrigation water would be less than 5 % for the controls, and even less for the salinity treatments. Each reservoir irrigated three replicate tanks. Total evapotranspiration from each tank was measured by solution-volume changes in the storage reservoirs and water lost was replenished to maintain constant osmotic potentials in the treatment waters.
The irrigation treatments consisted of five salinity levels representing non-saline (2.0 dS m−1), moderately saline (5.0 and 8.0 dS m−1) and saline (11.0 and 14.0 dS m−1) conditions (Table 1). Based on soil-water (ECsw- ECe) relations for this soil, these saline treatments translate into average rootzone salinities (ECe) of 1.0, 2.5, 4.0, 5.5 and 7.0 dS m−1. Each salinity level was comprised of synthetic saline drainage water with an ion composition typical to that found in shallow, saline water tables in the western SJV (Table 1). These compositions were calculated with the ExtractChem model (Suarez and Taber 2007) www.ars.usda.gov/pwa/riverside/ussl) by concentrating a typical SJV drainage water assuming calcite and gypsum precipitation where applicable and assuming long-term equilibrium with respect to cation exchange. Each of these treatments was tested at two boron concentrations. One was low, such as that found in solution cultures (0.5 mg L−1) and the other was 21 mg L−1, a value considered on the high end of that found in drainage water from the SJV. The experiment also contained two targeted pH treatments; a slightly acidic pH of 6.0 and a slightly basic pH of 8.0. The solution treatments were maintained by frequent monitoring and pH adjustments using sulfuric acid were made to keep the pH 6 treatments near their targeted level. The pH in the respective treatments was maintained between 5.5 and 6.2 for the acidic treatments and 7.8 and 8.4 for the basic treatments. The experimental design was a 5 × 2 × 2 factorial with three replications. The data were statistically analyzed using analysis of variance (ANOVA), mean separation and regression analyses.
Broccoli was planted on 16 Sep 2005 and thinned to 30 plants per tank after emergence. Salinization began 7 days after planting when plants were developing their first true leaves. Plants were periodically harvested from each tank throughout the experiment. Broccoli was first harvested on 26 Oct 2005. Total shoot biomass was determined and plots were thinned to a density of 11 plants per tank (15 plants m−2). The plots were thinned again on 28 Nov 2005 to 8 plants per tank (11 plants m−2). The remaining plants were harvested at maturity on 13 Jan 2006 (112 days after salinization). Broccoli shoots were divided into heads, stems, and leaves. Fresh and dry weight measurements were made on all harvested biomass. Tissue boron concentration was determined on total shoots at final harvest. Total B was determined on nitric acid/hydrogen peroxide microwave digests of plant material using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES).
Standard meteorological measurements were made in the greenhouse with a Class I agro-meteorological station. Ambient daytime air temperatures in the greenhouse during the experiment ranged from 14 to 38 °C (mean 30 °C); night-time temperatures ranged from 13 to 33 °C (mean 21 °C). Relative humidity ranged from 40 % to 48 % with a mean of 44 % during the day and 45 % during the night. There were no supplemental lights, only natural sunlight through glass.
Results
Visual and sensory observations
Initial broccoli growth and appearance were similar for all treatments with respect to color and vigor. Plants in saline treatments developed a slightly darker green-blue color as compared to non-saline treatments. Stunting and reduced leaf size became apparent on plants treated with moderate to high salinity as well as those treated with excessive boron after 30 days. Plants grown in low salinity and low-boron treatments grew vigorously and matured 7 to 10 days earlier than those grown in excessive salinity and boron, which were dramatically shorter in height and had smaller heads. Symptoms, characterized by slight bleaching of the leaf tissue followed by upward cupping of leaves particularly along the leaf margins, were observed on the first true leaf and, subsequently, on newer leaves as they reached full expansion (Fig. 1). The leaf cupping was observed on plants grown at both pH levels at high solution boron but symptoms were more vividly expressed on plants treated with high boron and low salinity combinations. This symptom has been reported as a symptom associated with boron toxicity (Nable et al. 1997; Grieve et al. 2010). At harvest, all heads were healthy and free of disease. However, the flavor of heads of plants treated with excessive salinity and high boron was poor with a bitter or astringent taste as compared to the flavor of those grown in low salinity and low boron conditions. This quality may be attributed to the glucosinolate concentration associated to sulfate supply and the stress response of members of the Brassicaceae (Marschner 1995). These volatile metabolites have been identified as agents of flavor that can modify the taste of cruciferous plants rendering them favorable or unfavorable, depending on the perception of consumers (Sandell and Breslin 2006). Although this qualitative effect was not scientifically tested by a trained panel, this general observation suggests that a more thorough evaluation is warranted in future salinity-boron studies with broccoli. For example there may be some important organic compounds produced by plants under these abiotic stresses that may have negative marketable-quality characteristics.
Interactive effects of salinity, boron and pH
ANOVA of the data indicated significant interactions between salinity-boron, salinity-pH, boron-pH and salinity-boron-pH with respect to fresh weight, total biomass, yield and water use efficiency. These interactions will be described in detail but overall, interactive effects of salinity, boron concentration, and pH were not simply additive, but rather antagonistic. Accurate interpretation and prediction of salinity, boron and pH effects on plant performance requires accounting for these complex interactions.
Head yield
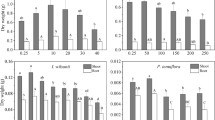
ANOVA of data representing the weights of fresh broccoli heads resulted in significant effects for salinity, boron concentration and pH and there were significant interactions for salinity-pH and boron-pH, and salinity-boron. Overall, fresh head yields decreased with both increasing salinity and boron concentration but these effects were not always significant among all treatments (Fig. 2). At pH 6.0, head yields decreased linearly as salinity increased at both boron concentrations of 0.5 and 21 mg L−1 (r 2 = 0.90 and 0.74, respectively; P < 0.05). Dramatic yield losses developed as the salinity levels reached the highest salinity levels (11 and 14 dS m−1), regardless of boron concentration, as compared to the effects observed at the intermediate salinity levels (5 and 8 dS m−1).
On the other hand, ANOVA results for head yield at pH 8.0 showed significance for salinity, boron, and the salinity-boron interaction, which required the analysis and mean separation for levels of salinity and boron. Comparison of fresh head weights between the two pH-levels with increasing salinity showed a significant interaction with respect to pH. Head yields decreased with increased pH but the degree of impact varied among treatments. Plants grown at pH 6 responded with the traditional decrease in yield with increasing salinity (De Pascale et al. 2005), but this relationship was not observed at pH 8. At pH 8, yields did follow the traditional pattern of decreasing production with the increase in salinity at the low boron concentration. However, at high boron, the response function was atypically bell shaped for fresh head yields where increases in salinity to moderate levels increased yield but further increases in salinity decreased yield. Surprisingly, head yields of plants treated with 21 mg B L−1 were significantly less (P < 0.05) at both low (2 dS m−1) and high (14 dS m−1) salinity than they were at intermediate salinity levels (5–11 dS m−1). Head yields were reduced by 89 % at the low salinity-high boron treatment and by 94 % for the high salinity-high boron concentration, in relation to the non-salinized controls at low boron.
Total shoot biomass
The effects of salinity, boron and pH on shoot biomass were similar to those on head yield, but the response was far less dramatic than for head yield (Table 2). Total shoot dry weights were adversely affected by increased boron and pH and there were significant interactions between salinity-boron, salinity-pH and boron-pH. Shoot dry weights of broccoli plants did not significantly decrease as salinity or boron concentration increased in the soil solution at pH 6. This same relationship was observed by plants in the pH 8 treatment with increased salinity at low boron (0.5 mg L−1). However, the same ‘bell-shaped’ salinity-response function observed with the fresh head yields was also found for shoot biomass of plants grown with 21 mg B L−1 at pH 8. Plants grown in the low salinity-high boron treatments were 25 % less in total biomass than those in the 5, 8 and 11 dS m−1 treatments. The total above ground biomass of plants treated at high salinity (14 dS m−1) and high boron was 50 % lower than those in the 5, 8 and 11 dS m−1 treatments.
Since the effects of salinity and boron on broccoli growth were more dramatic when analyzing fresh head yield as compared to total dry shoot biomass, broccoli is more sensitive to salinity and excess boron when salt-tolerance is defined based on reproductive growth rather than vegetative growth. Salinity and boron effects on broccoli shoot dry weight were similar to that observed in previous work (Smith et al. 2010) where there was no significant difference in shoot dry weight until levels of salinity and boron reached 18 dS m−1 and 24 mg L−1, respectively. This effect is also consistent with the results reported by De Pascale et al. (2005), who found that broccoli was more sensitive to salinity (threshold, ECe = 1.28 dS m−1; slope = 15.8 %) when expressed on a head yield basis as compared to the published salinity coefficients (threshold, ECe =2.8 dS m−1; slope = 9.2 %) where tolerance was characterized based on shoot fresh weight (Francois 1985).
Shoot biomass partitioning
Biomass of the leaf, stem and head organs as a percentage of the total shoot biomass was also examined (Fig. 3). This distribution of shoot dry matter was significantly different (P ≤ 0.05) among the plant organs and the dry matter distribution was affected by salinity, boron and pH. Overall, the leaves contributed to most of the shoot biomass ranging from 43 to 62 %, while contributions from stems and heads ranged between 25 to 39 % and 4 to 24 %, respectively. At low boron, increasing salinity shifted dry matter into the leaf biomass fraction, and at the same time reduced head and stem biomass fractions. Therefore, partitioning of the biomass towards leaf tissue and away from reproductive tissue explains why broccoli is more sensitive to salinity when tolerance is expressed on head yield rather than total shoot biomass. The ‘bell-shaped’ response relationship observed with the fresh head yields in relation to salinity at pH 8 and 21 mg B L−1 was also found in the biomass fraction of heads relative to shoot biomass for this same treatment where decreases in head dry matter resulted in a dry matter shift into leaves and stems. This effect was also observed at high boron at low pH, but the response was much less dramatic.
Cumulative evapotranspiration and water use efficiency (WUE)
Total shoot biomass was positively correlated with cumulative plant evapotranspiration (ET), as reported by Allen et al. (1998) (data not shown). Increased salinity, however, did not have a consistent effect on ET in any of the treatments except the high pH and high boron treatment (Table 3). This is in agreement with the shoot biomass data where there were no significant reductions in shoot dry weight with increases in salinity, except for the high boron, high pH treatment. Moreover, the ET from plots at slightly acidic conditions (pH 6) was not different from those in slightly basic (pH 8) conditions. Multiple regression analysis for seasonal total evapotranspiration (cm3 cm−2) as a function of salinity (ECw (dS m−1)), boron (mg L−1) and pH yielded significance (p ≤ 0.05, r 2 = 0.57) for the equation: ET = 39.1–0.61 ECw – 0.24 B – 0.74 pH.
Boron appeared to be the dominate stressor in relation to reducing broccoli ET, generating declines in water use in 9 out of 10 paired comparisons of low boron to high boron at the respective salinity and pH levels (Table 3). The effect of salinity and pH modified the extent of the ET reductions: (1) under pH 6 conditions, greater declines in ET at each salinity level occurred with increased boron but the maximum decline in ET was less for plants in the acidic environment as compared to those in basic conditions; (2) Conversely, pH 8 conditions resulted in less boron-related ET reduction at each salinity level but the reduction was greatest at the highest salinity level (14 dS m−1).
Water use efficiency (WUE) (shoot biomass divided by the cumulative ET) data were analyzed by ANOVA and found to be significant for salinity level as were the interactions between salinity-boron and salinity-boron-pH. Mean separation of WUE for salinity levels (dS m−1) and boron (mg L−1) is presented in Table 4. At low boron, increased salinity had little influence on the WUE. However at high boron and low pH, WUE increased as salinity increased up to moderate levels of salinity. Thereafter, WUE was unaffected with further increases in salinity. A repeating occurrence of the ‘bell-shaped’ response relationship found with fresh head yield and shoot dry matter with increasing salinity at combined high-B and high pH was also observed with the WUE, where at both low and high salinity the WUE was substantially lower than for plants in moderate salinity treatments. Low WUE is explained by a relatively smaller reduction in ET as compared to dry biomass. Plants at low salinity (2 dS m−1) produced 26 % less biomass while ET decreased by only a 6 %. Normally a linear response =function is observed between total biomass and cumulative ET (Allen et al. 1998). Plants treated with 14 dS m−1 water produced 50 % less shoot dry matter with a corresponding 30 % reduction in ET. Increased WUE is commonly associated with increased stress but we found in our previous study that increasing boron decreased WUE (Smith et al. 2010). In this experiment, increased salinity at pH 6 increased WUE at high boron, but an increase in substrate boron had no effect.
Shoot boron concentration
Salinity, boron and pH all had significant influences on shoot boron concentration (Fig. 4). Not surprisingly as boron increased, tissue boron increased, but as salinity increased tissue B decreased. This reduction in tissue B was much more dramatic at high substrate B concentration (21 mg/L) yet the reduction was still significant at low substrate B (0.5 mg/L). Interestingly, however, the influence of pH on shoot B concentration was dependent upon the substrate B concentration. At low, more typical solution B concentrations, an increase in pH from 6 to 8 did not affect shoot B concentration despite a hundred fold decrease in H+ activity. However when substrate B concentrations were elevated to typically toxic levels, an increase in pH reduced shoot B concentrations even though this reduction was not consistent at each level of salinity. The reduction was significant at both low and high salinity. At high substate B, shoot B concentrations were generally higher in plants treated in slightly acidic conditions.
Boron uptake: passive, energy dependent or by exclusion?
The mechanism of boron uptake by plants is complex and not fully understood. Early research, mostly conducted in the 1990’s, led to the conclusion that soluble forms of boron are primarily accumulated in the plant as a result of passive permeation and mass flow via the transpiration stream (Edelstein et al. 2005; Marschner 1995; Nable et al. 1997; Yu and Bell 1998). In a recent study on wheat by Wimmer and Goldbach (2012) the conclusion was that under high B treatments, B uptake was predominantly passive by diffusion. However in recent years, boric acid channels and B exporters have been discovered demonstrating that B uptake is much more complex than solely by passive diffusion through the lipid phase of the plasma membrane (summarized by Reid 2007; Takano et al. 2008; Fitzpatrick and Reid 2009; Miwa and Fujiwara 2010; see under Discussion). In our analysis total shoot boron accumulation (mg plant−1) was determined as the product of tissue dry weight (kg plant−1) and shoot boron concentration (mg kg−1). With the exception of the high B, high pH treatments, the total B uptake in broccoli shoots decreased linearly with increased salinity (Fig. 5). This decrease was only slight, yet significant (r2 0.46 and 0.55), for the low B treatments (low and high pH, respectively). For the high B, high pH treatment, the plants treated with the highest salinity accumulated the least B in their shoots.
These total measured values (Fig. 5) were compared to an assumed passive boron uptake, determined by multiplying the estimated volume of water transpired through the plant by the concentration of boron in the soil water. It was assumed that the B concentration in the storage reservoir was equivalent to that in the soil-water, which is a good assumption for frequently irrigated (several times daily) coarse sand. Using the stable isotope (H2018/H2016) method to separate evapotranspiration and transpiration from ET as described in a previous paper (Smith et al. 2010), we found that evaporation comprised less than 10 % the total ET in tanks where the canopy cover was over 70 % the surface area of the tanks. We found that there was no relationship between evaporation and treatment, but that evaporation on average was about 7 % of the total ET (or 93 % T). We used this value to estimate the volume of transpiration per tank and then converted this to a per plant basis.
Based on a comparison of total plant B with mass of B calculated from transpired volume of water and the concentration of the irrigation water, we conclude that boron uptake was not a simple passive process. The plants exhibited an exclusionary mechanism where the total boron uptake per plant was less than the predicted passive uptake (i.e., cumulative transpiration lost per plant multiplied by the boron concentration in the sand solution) (Table 5). Plants grown in low boron solutions accumulated only 27 to 44 % of the boron in their shoots as compared to what they would accumulate if the uptake was explained by a simple passive process. Plants at high substrate boron, on the other hand, contained only 2 to 10 % of the boron predicted by passive uptake or non-exclusionary processes. Therefore an important exclusionary process is operating and this process is enhanced under high solution boron.
Discussion
Salinity, boron and pH are dominant soil factors that affect plant performance. An understanding of the interaction of these factors is important in order to understand how plants respond to these abiotic stresses in field environments (Läuchli and Grattan 2012a, b), such as the western SJV of California where salinity and boron concentrations are high and soil pH is alkaline. In addition, clarifying these complex interactions may explain some of the observed inconsistencies concerning plant response to the combined effects of salinity and excess boron described in the literature (Yermiyahu et al. 2008).
The salinity-boron-pH interactions from this study showed the following: (1) plants grown under low salinity, high boron conditions had much greater reductions in head yields when the pH of the soil solution was slightly basic as opposed to slightly acidic; (2) moderate salinity (EC of 5 and/or 8 dS m−1) appeared to mitigate boron’s detrimental effect observed at low salinity in both acidic and alkaline pH environments; (3) high salinity (EC of 11 and 14 dS m−1) adversely affected head yield, regardless of boron or pH treatment; (4) plants treated with the combination of high salinity and high boron in slightly basic conditions suffered major losses in head yield. The most surprising finding in our study was that at very high boron concentrations (21 mg/L) and under slightly alkaline pH conditions, both low (EC 2) and high (EC 14) salinity had a large reduction in head yields. Moderate salinity was far less damaging. This bell-shaped response was also found in an earlier salinity-B interaction study with broccoli where the pH was not controlled (Smith et al. 2010), although the effect was not as dramatic as found here.
The effects of salinity and boron stresses on broccoli plant performance are usually assessed by changes in shoot biomass or fresh head yield in relation to increased salinity or boron in the substrate (Grieve et al. 2012; De Pascale et al. 2005). In our study, shoot biomass was unaffected by increased salinity and substrate boron concentration at pH 6, but at pH 8, plants displayed the more typical declines in biomass associated with salinity and boron stress. Studies indicate that broccoli is more tolerant to increased salinity when expressed as shoot biomass as opposed to fresh head yields. For example, shoot biomass of broccoli reportedly decreased 9.2 percent for every unit increase in ECe above 2.8 dS/m (Francois 1985) whereas head yield decreased 15.8 % for every unit increase in ECe above 1.28 dS m−1(de Pascale et al. 2005). Therefore when the EC of the soil solution is 8 dS/m (4 dS/m expressed as ECe), head yield would be expected to be reduced by 43 %. In our study, at this level of salinity, head yields were reduced between 20 to 45 %, at low B, depending upon solution pH. In regards to boron, studies predicte that at 21 mg L−1 boron in the soil solution, broccoli head yields would be decreased by 36 % (Grieve et al. 2012) but in our study, increasing substrate boron from 0.5 to 21 mg L−1 decreased shoot biomass by less than 20 %. Therefore our findings were somewhat similar to those reported in the literature.
Water use efficiency (WUE) in broccoli (grams total shoot biomass per liter of water) increased with increasing salinity and boron concentration, which is consistent with previous findings (Smith et al. 2010). However in this study, the trend at pH 8 was not consistent. WUE increased from low salinity to the moderate-salinity levels, and then decreased at high salinity. The overall effect of increased plant stress generating greater water use efficiency has been observed in earlier studies (Shani and Dudley 2001; Shani et al. 2005). The implication that crops growing under high saline and boron conditions have decreased transpiration requirements suggests that leaching fractions will be higher than those in non-stressed fields with the same applied water. This conflicts with a standard practice adopted by some growers of applying greater amounts of irrigation waters under these salt- and boron-stressed conditions.
Boron is more available to the plant roots in acidic conditions than slightly basic conditions (Marschner 1995), which can be advantageous in avoiding boron deficiency. However under high boron concentrations in the soil solution, plant accumulation of B could be excessive. Shoot B concentrations were higher in plants grown in acidic conditions, but plant growth and head yield were most adversely affected in alkaline conditions. Boron toxicity would be expected to be exacerbated with high soil boron levels in conjunction with acid soils due to high B uptake and accumulation. In an earlier experiment, broccoli was found to exclude boron or discriminate in boron uptake minimizing boron toxicity (Smith et al. 2010). Boron uptake was not affected in low boron treatments by pH, but it was reduced overall in the high boron treatments at pH 8. Ultimately, the alkaline soil solution pH produced the greatest reduction in dry weight biomass and fresh head yield with increasing salinity and boron concentration. Use of plant tissue boron concentration as an indicator of boron toxicity has a history of shortcomings and problems for predicting overall plant response (Nable et al. 1997). This is a consequence of variations in tissue boron concentrations between and within organs and inconsistencies in those concentrations associated with boron toxicity. Our data support this finding.
Alkaline soil pH may play an important role in aggravating salinity-boron interactions. An increase in pH will shift a percentage of boron species from [B(OH)3] to [B(OH)4 −] forms in terms of B uptake (Marschner 1995), but this does not explain our data where less than 10 % of the boron at high pH was in anionic form. Although plants preferentially take up non-ionic boric acid, it may be possible that the anionic form may be more toxic to the plant or at least be metabolically disruptive. However boron speciation within the plant is also governed by internal cellular pH, which is generally neutral to slightly alkaline in the cytoplasm and higher than the apoplastic pH. The kinetics of species conversion and pH at specific biochemical sites (e.g., membrane surfaces) is unknown and beyond the scope of this study. However boron adsorption to cell walls has been found to increase with both pH and ionic strength of the solution and complexes with phenolic groups may be involved (Goldberg and Grieve 2003). It is likely that internal boron complexation with organic groups is important and it is possible that soluble fractions of boron may be more toxic to the plant, as suggested by Wimmer et al. (2003). This reasoning follows the conclusion by Wimmer et al. (2002) that intracellular soluble B is likely responsible for boron toxicity effects. In any event more in-depth salinity-B-pH studies need to be conducted at the cellular level to better understand possible mechanisms involved.
It is also possible that this salinity-B-pH interaction may affect developmental processes in the plant that alter the timing of reproductive growth (see Wimmer et al. 2005). The rate of head development and maturity was also affected by salinity and boron. Heads were first to mature on plants in the low salinity-boron treatments and head maturity was slightly delayed on those in high salinity-boron treatments. This translated into greater fresh weight yields for the low salinity-boron treatments on the day of harvest. Therefore the influence of these interactive abiotic stresses on the phenology of plants needs further investigation.
Salinity reduced B uptake by plants but the impact was mainly evident at high substrate B concentrations, an observation found in wheat by Wimmer and Goldbach (2012). However this reduction in our study was more than what could be accounted for by salinity’s reduction in transpiration. Therefore the resulting boron uptake by shoots was less than what could be accounted for by passive uptake and mass flow to the shoot. This was observed in both acid and basic conditions.
The observed reduction of B uptake found in our study suggests that an exclusion mechanism was operating, particularly at high substrate B concentrations. This conclusion is in line with recent evidence on active or regulated B transport in plants (see reviews on B transporters by Fitzpatrick and Reid 2009; Miwa and Fujiwara 2010; Takano et al. 2008; Tanaka and Fujiwara 2008). Reid and Fitzpatrick (2009a) found in barley and wheat that B tolerant genotypes alleviated B toxicity in roots and leaves by means of B exporters expressed in both roots and leaves. Hayes and Reid (2004) had observed earlier that the high B-tolerant barley cultivar Sahara maintained low B concentrations in roots by active B efflux. The involvement of B exporters in roots and leaves is now well established and may contribute to B exclusion suggested in our study on broccoli. In addition to boric acid/borate exporters, boric acid channels at the plasma membrane have been clearly demonstrated to be in involved in maintenance of low B concentrations inside the cells (reviewed by Miwa and Fujiwara 2010; Takano et al. 2008).
The knowledge of boric acid channels and their involvement in B transport in plant cells is rapidly advancing (Fitzpatrick and Reid 2009; Miwa and Fujiwara 2010; Takano et al. 2008). However, the function of such B channels is complex and not yet completely understood. The major intrinsic protein superfamily of channel proteins (MIP) is responsible for the bidirectional transport of water and some small uncharged molecules such as silicic acid. The MIP superfamily can be divided into the aquaglyceroporins (AQGPs)/AQPS which mainly transport water or glycerol and the glycerol facilitators or non-ionic solute channels (GLPs). The aquaglyceroporins contain several sub-families including the plasma membrane intrinsic proteins (PIPs). Their subgroup PIP1 may be responsible for channel—mediated solute transport including B (Fitzpatrick and Reid 2010). Although our knowledge of the role of aquaglyceroporins in B transport is still limited, convincing evidence has recently been presented by Fitzpatrick and Reid (2009, 2010). They showed that by means of inhibitors of membrane transporters that are likely to be involved in B transport, at least 50 % of B uptake could be facilitated by transporters, and transport assays in yeast confirmed that the two barley aquaglyceroporins, HvPIP1;3 and HvPIP1;4 can transport B. Thus, passive diffusion, channel-mediated transport, and active extrusion of B (Reid and Fitzpatrick 2009b), likely contribute to the net uptake and transport of B in plants. The possible contribution of these transport processes to B uptake in broccoli remains to be elucidated.
Concluding remarks
The findings from this research reinforce and modify some of the fundamentals of soil salinity-boron relationships with respect to plant growth and development. Broccoli plants are more tolerant of boron in the presence of salinity but this tolerance is diminished in alkaline conditions. High substrate boron negatively impacted broccoli yield at both very low and very high salinity, but it was far more detrimental in alkaline pH than in acidic pH environments. At the same time, tissue boron concentrations were comparable and could not alone explain these differences in plant performance. It is possible that under alkaline pH either borate itself is directly toxic to key metabolic functions or that particular soluble fractions of boron inside the cytosol exist enhancing B toxicity, as suggested by Wimmer et al. (2002). Further complicating these unknowns is the recognition that boron transport in plants is not only mediated by passive diffusion alone but also by membrane channels and boron-efflux transporters in roots and shoots which enhance boron tolerance (Reid 2010; Miwa and Fujiwara 2010). The influence of salinity, boron and pH interact in ways that likely affect the various transport mechanisms in the plant. It is unclear why plant performance (head yield and shoot biomass) was so poor under both low and high salinity in alkaline treatments at high boron, but not under intermediate salinity. It is likely that different mechanisms, yet unknown, are operating at low and high salinity. Not only could the overall phenology of the plant be affected by interacting stresses, the mineral-nutrient relations, within the plant, is also likely affected. It is known that mineral nutrient relations are largely influenced by both salinity and pH (Läuchli and Grattan 2012a, b). The paper that follows will address ion relations in detail, particularly as they relate to different shoot organs. This forthcoming paper will hopefully shed more light on the role these interacting abiotic stresses have on the mineral nutrition of broccoli.
References
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop Evaporation: Guidelines for computing crop water requirements. Irrigation and Drainage Paper 56 U. N. Food and Agriculture Organization. Rome. 300 pp
Alpaslan M, Gunes A (2001) Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants. Plant Soil 236:123–128
Ayers RS, Westcot DW (1985) Water Quality for Agriculture. FAO Irrigation and Drainage Paper 29 Rev.1. Food and Agricultural Organization. Rome. 174 pp
Bañuelos GS, Shamarsakar S, Cone D, Stuhr G (2003) Vegetative approach for improving the quality of water produced from soils in the westside of central California. Plant Soil 249:229–236
Bastías E, Alcaraz-López C, Bonilla I, Martínez-Ballesta MC, Bolaños L, Carvajal M (2010) Interactions between salinity and boron toxicity in tomato plants involve apoplastic calcium. J Plant Physiol 167:54–60
Ben-Gal A, Shani U (2002) Yield, transpiration and growth of tomatoes under combined excess boron and salinity stress. Plant Soil 247:211–221
Bingham FT, Marsh AW, Branson R, Mahler R, Ferry G (1972) Reclamation of salt affected high B soil in Western Kern County. Hilgardia 41:195–211
Bingham FT, Strong JE, Rhoades JD, Keren R (1987) Effects of salinity and varying boron concentrations on boron uptake and growth of wheat. Plant Soil 97:345–351
Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, Pfeffer H, Dannel F, Romheld V (2002) Boron in plant biology. Plant Biology 4:205–223
De Pascale S, Maggio A, Barbieri G (2005) Soil salinization affects growth, yield and mineral composition of cauliflower and broccoli. Eur J Agron 23:254–264
Edelstein M, Ben-Hur M, Cohen R, Burger Y, Ravina I (2005) Boron and salinity effects on grafted and non-grafted melon plants. Plant Soil 269:273–284
Ehret DL, Ho LC (1986) The effects of salinity on dry matter partitioning and fruit growth in tomatoes grown in nutrient film culture. J Hortic Sci Biotechnol 61:361–368
Ferguson L, Poss JA, Grattan SR, Grieve CM, Wang D, Wilson C, Donovan TJ, Chao T (2002) Pistachio rootstocks influence scion growth and ion relations under salinity and boron stress. J Am Soc Hortic Sci 127:194–199
Ferreyra RE, Aljara AU, Ruiz RS, Rojas LP, Oster JD (1997) Behavior of 42 crop species grown in saline soils with high boron concentrations. Agric Water Manag 34:111–124
Fitzpatrick KL, Reid RJ (2009) The involvement of aquaglyceroporins in transport of boron in barley roots. Plant Cell Environ 32:1357–1365
Fitzpatrick KL, Reid RJ (2010) The ever expanding role of aquaglyceroporins. Plant Signal Behav 5:132–133
Francois LE (1985) Effect of excess boron on broccoli, cauliflower, and radish. J Am Soc Hortic Sci 111:494–498
Goldberg S, Grieve CM (2003) Boron adsorption by maize cell walls. Plant Soil 251:137–142
Goldberg S, Lesch SM, Suarez DL (2000) Predicting boron adsorption by soils using soil chemical parameters in the constant capacitance model. Soil Sci Soc Am J 64:1356–1363
Grattan SR, Shannon MC, Grieve CM, Poss JA, Suarez DL, Francois LE (1996) Interactive effects of salinity and boron on the performance and water use of eucalyptus. Acta Horticult 449:607–613
Grieve CM, Poss JA (2000) Wheat response to interactive effects of boron and salinity. J Plant Nutr 23:1217–1226
Grieve CM, Poss JA, Grattan SR, Suarez DL, Smith TE (2010) The combined effects of salinity and excess boron on mineral ion relations in broccoli. Sci Hortic 125:179–187
Grieve, C.M., Grattan, SR, Maas, EV (2012) Plant salt tolerance. In. (W.W. Wallender and K.K. Tanji, eds). Agricultural Salinity Assessment and Management (2nd edition). ASCE pp405–459
Hayes JE, Reid RJ (2004) Boron tolerance in barley is mediated by efflux of boron from the root. Plant Physiol 136:3376–3382
Holloway RE, Alston AM (1992) The effects of salt and boron on growth of wheat. Aust J Agric Res 43:987–1001
Läuchli A, Grattan SR (2012a) Soil pH extremes. In. S. Shabala (ed). Plant Stress Physiology. CAB International. pp194–209
Läuchli A, Grattan SR (2012b) Plant responses to saline and sodic conditions. In. (W.W. Wallender and K.K. Tanji, eds). Agricultural Salinity Assessment and Management (2nd edition). ASCE pp169–205
Marschner H (1995) Mineral Nutrition of Higher Plants. Academic, New York, 889
Miwa K, Fujiwara T (2010) Boron transport in plants: coordinated regulation of transporters. Ann Bot 105:1103–1108
Nable RO, Banuelos GS, Paul JG (1997) Boron toxicity. Plant Soil 193:181–198
Nicholaichuk W, Leyshon AJ, Jame YW, Campbell CA (1988) Boron and salinity survey of irrigation projects and the boron adsorption of some Saskatchewan soils. Can J Soil Sci 68:77–90
Picchioni GA, Karaca H, Boyse LG, McCaslin BD, Herrera EA (2000) Salinity, boron, and irrigated pecan productivity along New Mexico’s Rio Grande basin. J Environ Qual 29:955–963
Reid RJ (2007) Identification of boron transporter genes likely to be responsible for tolerance to boron toxicity in wheat and barley. Plant Cell Physiol 48:1673–1678
Reid R (2010) Can we really increase yields by making crop plants tolerant to boron toxicity? Plant Sci 178:9–11
Reid R, Fitzpatrick K (2009a) Influence of leaf tolerance mechanism and rain on boron toxicity in barley and wheat. Plant Physiol 151:413–420
Reid RJ, Fitzpatrick KL (2009b) Redistribution of boron in leaves reduces boron toxicity. Plant Signal Behav 4:1091–1093
Sandell MA, Breslin PA (2006) Variability in a taste-receptor gene determines whether we taste toxins in food. Curr Biol 16:R792–R794
Shani U, Dudley LM (2001) Field studies of crop response to drought and salt stress. Soil Sci Soc Am J 65:1522–1528
Shani U, Ben-Gal A, Dudley LM (2005) Environmental implications of adopting a dominant factor approach to salinity management. J Environ Qual 34:1455–1460
Sharma MK, Chhipa BR, Kumar A, Lal P, Sharma M (1993) Effects of salinity, sodicity, and boron in irrigation water on quality and forage yield of oats. Agrochimica J 37:225–232
Smith TE, Grattan SR, Grieve CM, Poss JA, Suarez DL (2010) Salinity’s influence on boron toxicity in broccoli: I. Impacts on yield, biomass distribution and water use. Agric Water Manag 97:777–782
Sternberg PD, Ulery AL, Villa CM (2001) Salinity and boron effects on growth and yield of tepary and kidney beans. HortSci 36(7):1269–1272
Suarez DL, Taber P (2007) Extract Chem model to predict major ion and boron composition of a soil solution at a desired water content. http://www.ars.usda.gov
Suarez DL, Wood J, Taber P (2012) Adsorption and desorption of B in column studies as related to pH: Results and model predictions Vadose Zone J 11:doi:10.2136/vzj2011.0073
Supanjani KDL (2006) Hot pepper response to interactive effects of salinity and boron. Plant Soil Environ 52:227–233
Takano J, Miwa K, Fujiwara T (2008) Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci 13:451–457
Tanaka M, Fujiwara T (2008) Physiological roles and transport mechanisms of boron: perspectives from plants. Pflugers Arch. Eur J Physiol 456:671–677
Wang D (2002) Dynamics of soil water and temperature in aboveground sand cultures used for screening plant salt tolerance. Soil Sci Soc Am J 66:1484–1491
Wimmer MA, Goldbach HE (2012) Boron-and-salt interactions in wheat are affected by boron supply. J Plant Nutr Soil Sci 175:171–179
Wimmer MA, Mühling KH, Läuchli A, Brown PH, Goldbach HE (2002) Boron toxicity: The importance of soluble boron. In: Goldbach et al (eds) Boron in Plant and Animal Nutrition. Klewer Academic/Plenum Publishers, New York, pp 241–253
Wimmer MA, Mühling KH, Läuchli A, Brown PH, Goldbach HE (2003) The interaction between salinity and boron toxicity affects the subcellular distribution of ions and proteins in wheat leaves. Plant Cell Environ 26:1267–1274
Wimmer MA, Bassil ES, Brown PH, Läuchli A (2005) Boron response in wheat is genotype-dependent and related to boron uptake, translocation, allocation, plant phenological development and growth rate. Funct Plant Biol 32:507–515
Yadav HD, Yadav OP, Dhankar OP, Oswal MC (1989) Effect of chloride salinity and boron on germination, growth, and mineral composition of chickpea (Cicer arietinum L.). Ann Arid Zone 28:63–67
Yermiyahu U, Ben-Gal A, Keren R, Reid RJ (2008) Combined effect of salinity and excess boron on plant growth and yield. Plant Soil 304:73–87
Yu X, Bell PF (1998) Nutrient deficiency symptoms and uptake mechanisms of rice. J Plant Nutr 21(10):2077–2088
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Robert Reid.
Rights and permissions
About this article
Cite this article
Smith, T.E., Grattan, S.R., Grieve, C.M. et al. pH dependent salinity-boron interactions impact yield, biomass, evapotranspiration and boron uptake in broccoli (Brassica oleracea L.). Plant Soil 370, 541–554 (2013). https://doi.org/10.1007/s11104-013-1653-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1653-9