Abstract
In this study, individual and combined effects of boron and sodium chloride salinity on growth, photosynthetic pigments (chlorophyll and carotenoids content), enzymatic activities (catalase and ascorbate peroxidase), hydrogen peroxide content, malondialdehyde content, proline accumulation, and some ion contents, such as B–, Na+, Cl–, K+, Ca2+, Mg2+ of purslane (Portulaca oleracea L.) were investigated. Five B levels (0, 4, 8, 16, 24, 40 mg/kg) and 100 mM NaCl were applied to the soil and mixed before sowing. Results showed that purslane growth was reduced significantly by higher B levels and salinity due to ion toxicity and osmotic stress. Also, content of photosynthetic pigments increased with both higher B levels and salinity, but they were decreased with combined effects of them. Tissue B–, Na+, Cl–, K+ and Ca2+ levels in shoot increased with applied NaCl, but B levels applied together with NaCl caused a decrease in B– content due to antagonistic effects between B– and Cl– ions. The MDA content, proline accumulation, and H2O2 content increased with higher B levels, but salinity caused a decrease in MDA content. The catalase and ascorbate peroxidase activities increased with B and salinity combination, but did not change with salinity. Increasing B reduced the catalase activity. It is suggested that purslane has the potential to manage the amount of soluble boron and also it has a promising potential that can be grown in B-rich and saline soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Purslane (Portulacca oleracea L.) is one of the most widespread weed species in the world and has a rich potential in terms of ω-3 fatty acid, α-linoleic acid, and some antioxidants (α-tocopherol, β-carotenes, ascorbic acid and glutathione) [1]. This crop was rated as a moderately salt tolerant with a threshold value of 6.3 dS/m [2], and could keep its productive capacity, even if irrigated with waters that have sulphate salinity and may be contaminated with potentially toxic trace elements [3].
Boron is an essential micronutrient for plants and has a vital role in many physiological processes, such as synthesis and structure of the cell wall, sugar transport, same metabolisms (carbohydrate, ribonucleic acid, indole acetic acid, phenol etc.), lignification, and respiration [4, 5]. But, excess B levels may cause a considerable crop loses in B-rich soils, especially in arid and semi-arid regions of the world or in soils exposed to B-rich irrigation water, fertilizers, sewage sludge, fly ashes [6].
Salinity affects some of the soil properties, reducing the productivity of soils and causing yield losses in agricultural crops. Many studies have revealed that high concentration of soluble salt in soils plays a critical role in the mechanisms and strategies that controlling uptake and accumulation of ions, osmotic regulation, antioxidant metabolism, and stress signaling [2, 7–11].
Boron toxicity was often associated to saline soils and crops grown in arid and semi-arid regions are frequently exposed to both stresses simultaneously [4]. Although the relationship between B and salinity is complex [12], the responses of different plant species to concurrent behaviour of excess B and salinity has been reported by many researchers. These stress factors may cause a reduction in shoot and root growth in wheat [13]; an increase in membrane damage in lettuce [14] or in tomato and cucumber [15].
When plants are exposed to abiotic stress conditions such as ion toxicity, drought, extreme temperatures, high salinity, pesticide treatments etc., the balance between reactive oxygen species (ROS) production and defensive activity of antioxidants disrupts and results in oxidative damage. The ROS production in plants increases with impairment of electron transport processes in photosynthesis and respiration [16]. Ion toxicity and osmotic stress due to excess B and salinity induces oxidative damage by lipid peroxidation and H2O2 accumulation; thereby, causes free proline accumulation in the cell [8]. Catalase (CAT) and ascorbate peroxidase (APX) reduce cellular H2O2 to water [17], but APX is better H2O2 scavenger than CAT due to higher affinity for H2O2 [16]. Most publications regarding plant stress tolerance mechanisms indicate a correlation between the resistance to environmental stresses and more effective antioxidative systems [8, 14].
The main objectives of this study are to evaluate physiological responses of the purslane plant to salinity and toxicity (i.e., B, Na, and Cl) and also, to offer a solution proposal related to the management of saline and B-rich soils.
MATERIALS AND METHODS
Experimental design and treatments. Seeds of purslane (Portulaca oleracea L. cv. Halkapınar) were grown in a naturally lighted greenhouse (day/night average temperature of 27/18°C, and relative average humidity of 70%). The seeds soaked 4 h in de-ionized water at room temperature. Then 30 seeds were sown into each plastic pot (16 cm deep, with diameters of 17.5 cm at the top and 12 cm at the bottom) lined with polyethylene bags, containing 2 kg of air-dried soil under greenhouse conditions.
Six levels of B (0, 4, 8, 16, 24, and 40 mg/kg) and one level of salt (100 mM) were applied to the soil from boric acid (H3BO3) and NaCl, respectively. All these application levels were prepared as a solution and added separately to the soil just before seed sowing and then thoroughly mixed. For basal fertilization, 100 mg/kg nitrogen (N), 50 mg/kg phosphorus (P), and 100 mg/kg potassium (K) were applied to the soil. Ammonium nitrate (NH4NO3), ammonium dihydrogen phosphate (NH4H2PO4), and potassium sulfate (K2SO4) were used for N, P, and K sources. Purslane seeds were sown at the rates of 30 seeds to each pot. After a good stands of plants had developed they were thinned to 20 plants per pot. During the experiment, soil in the pots was kept at approximately 70% of the field capacity using B-free tab water.
Some properties of the experimental soil were as follows: loam texture (sand : clay, 35.8 : 21.7 by dry wt); pH 7.34 (1 : 2.5, soil : water); electrical conductivity (EC), 508 μS/cm (saturation extract); calcium carbonate (CaCO3), 17.29 g/kg; organic carbon, 6.25 g/kg and total N, 0.86 g/kg. Ammonium acetate (NH4OAc)-extractable K, Ca, Mg and Na were 100, 2151, 124 and 64 mg/kg, respectively. Also, sodium bicarbonate (NaHCO3)-available P concentration and hot water-extractable B were 12.43 and 1.64 mg/kg, respectively. Diethylenetriamine penta-acetic acid (DTPA)-extractable iron (Fe), manganese (Mn), zinc (Zn) and copper (Cu) were 24.28, 65.27, 2.09, and 1.17 mg/kg, respectively. The soil characteristics were determined according to methods detailed by Page et al. [18].
Sampling and harvest of plants. After eight weeks, plants were harvested and separated into shoots and roots for determining fresh and dry weight of biomass. To obtain fresh weights the shoots and roots were washed with running tap water and three-times rinsed with deionized water to remove any soil particles attached to the plant surfaces and dried with paper. Then all samples were dried in an air-forced oven at 70°C until constant mass was reached. After cooling down till room temperature they were weighted for shoot and root dry weights and subsequently ground to powder for nutrient ion analysis.
Enzyme extraction and assay. For extraction and assay of enzymes, fully matured leaves (1.0 g) were homogenized by using Heidolph DIAX 900 homogenizer (Kelheim, Germany) with 5 mL of extraction buffer (100 mM Na-phosphate buffer, pH 7.5) containing 0.5 mM EDTA-Na2 at 4°C. Also, 1 mM ascorbate was included to extraction buffer for ascorbate peroxidase due to instability of APX in the absence of ascorbate [17]. The homogenate was centrifuged at 10 000 g for 5 min. The supernatant was used for determining the enzymes activity analyses and a spectrophotometer (Shimadzu UV/VIS 1201, Japan) was used for all colorimetric measurements (including enzyme activities) at 25°C. The activity of CAT (EC 1. 11.1.6) was determined by using a reaction solution (2.5 mL per 0.2 mL supernatant, pH 7) containing 50 mM potassium dihydrogen phosphate (KH2PO4) and 1.5 mM H2O2 as a decrease in absorbance at 240 nm for 1 min following the decomposition of H2O2 [19] and calculated using the extinction coefficient (ε = 40 mM–1 cm–1) for H2O2. The activity of APX (EC 1.11.1.11) was determined by using a reaction solution (3.0 mL per 0.1 mL supernatant, pH 7) containing 50 mM KH2PO4, 0.05 mM ascorbic acid, 0.1 mM EDTA-Na2, and 1.5 mM H2O2 as a decrease of ascorbate and measuring the change in absorbance at 290 nm for 1 min [20] and calculated using the extinction coefficient (ε = 2.8 mM–1 cm–1) for ascorbate.
Determination of membrane damage and non-enzymatic antioxidants. Lipid peroxidation of leaves is a good indicator for assessing membrane damage and was estimated by the content of MDA, the end product of lipid peroxidation, described by Hodges et al. [21]. In brief, mature leaf samples (0.25 g) was homogenized by using Heidolph DIAX 900 homogenizer (Kelheim, Germany) in 5 mL 0.1% trichloroacetic acid (TCA) and the homogenate was centrifuged at 5000 g for 5 min. After that, 4 mL of 20% TCA containing 0.5% thiobarbituric acid (TBA) were added to 1 mL aliquot of the supernatant. The mixture was heated in boiling water bath (95°C) for 15 min and allowed to cool in ice bath quickly. The supernatant was centrifuged at 10 000 g for 5 min and resulting supernatant was used for spectrophotometric determination of MDA. The absorbance at 532 nm was recorded and corrected for nonspecific absorbance at 600 nm. The MDA concentration was calculated by means of an extinction coefficient of (ε = 155 mM–1 cm–1).
The H2O2 content of the leaves was extracted and estimated as described by Mukherjee and Choudhuri [22]. Leaf samples (0.25 g) were homogenized by a homogenizer (Heidolph, Diax 900) in 5 mL with cold acetone and filtered. An aliquot (1 mL) of the extracted solution was mixed with 4 mL of titanium dioxide (TiO2) reaction solution in TiO2 (0.06%, w/v), K2SO4 (0.6%, w/v), and H2SO4 (10%, v/v) and added 5 mL concentrated ammonia (NH3) solution. The mixture was centrifuged at 10 000 g for 5 min. The intensity of yellow color of the supernatant was measured at 415 nm. The concentration of H2O2 was calculated from a standard curve plotted with the range of 100–1000 nM H2O2.
Free proline content was extracted from 0.25 g fresh leaf samples homogenized with 5 mL of 3% (w/v) sulfosalicylic acid at 4°C and estimated by ninhydrin reagent [23].
Determination of photosynthetic pigments. Photosynthetic pigments were measured in the youngest fully expanded fresh leaves before harvest. The fresh leaf samples (0.2 g) were cut into small pieces and were extracted in 10 mL of acetone (90% (v/v)) with a homogenizer. The extract was then filtered and the absorbance of the extract was measured at 663, 645, and 470 nm using a spectrophotometer (Shimadzu UV-1201). The contents of Chl a, Chl b, total chlorophyll a and b, and carotenoids were calculated according to the formula reported by Lichtenthaler [24].
Determination of nutrient ions. For the measurement of nutrient ion concentrations, 500 mg of each of the shoot sample was dry-ashed in a muffle furnace at 500°C for 6 h and then the cooled ash was dissolved in 5 mL, 0.1 M hydrochloric acid (HCl) solution. The nutrient ions were analyzed using Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES, Perkin Elmer Optima 2100 DV; Waltham, MA). Chloride (Cl) concentration of the sample was determined by titration with silver nitrate (AgNO3) using potassium chromate (K2CrO7) indicator according to the Mohr’s method described by Johnson and Ulrich [25].
Statistical analysis. The experimental design was a completely randomized factorial design with three replicates. Obtained data were analyzed by ANOVA and the differences were compared by Duncan’s multiple-range test (α = 0.05). The levels of significance are represented by * at P < 0.05, ** at P < 0.01, *** at P < 0.001, and ns: not significant. The statistical tests were performed by using MINITAB Statistical Software (Minitab Corp., State Collage, PA).
RESULTS
Boron Toxicity
The symptoms of boron toxicity appeared at 8 mg/kg boron level and increased at higher B levels. Typical symptoms were visible in leaf burn (chlorotic and necrotic patches in older leaves), decreased leaf number, small younger leaves, and delayed development in purslane [6].
Salinity has been reported to affect plant growth in two phases. These are (1) the osmotic phase that affects mainly young leaves and (2) the ionic phase that causes a reduction in the photosynthetic area in older leaves due to accumulation of Na+ and Cl– ions [26].
The visible leaf damage due to B toxicity was reduced by salinity application. But, plant height shortened, leaf size shrinks, and leaf color became darker (Fig. 1).
Plant Growth
The changes in shoot and root fresh and dry weights of purslane plant are exhibited on the Table 1. The fresh and dry weights in both shoot and root decreased significantly with increasing B levels, especially in the higher B levels (16, 24, and 40 mg/kg), compared to control. The decreases in these levels were found by 37.7, 52.3 and 75.9% in shoot fresh weight; 52, 64.8, and 81.6% in root fresh weight; 50.17, 66.4, and 84.1% in shoot dry weight; and 60.2, 68.1, and 87.4% in root dry weight, respectively. In the presence of salinity, decreases in growing parameters were not significant; except for in shoot fresh weight with the highest B level. Besides, regardless of B applications, plant biomass decreased by 62.3% in shoot fresh weight; 65.5% in root fresh weight; 68% in shoot dry weight; and 63.6% in root dry weight, respectively (Table 1).
Photosynthetic Pigments
The interaction between increasing B and salinity on photosynthetic pigments (chlorophyll and carotenoids contents) were significant (Fig. 2). The Chl a, Chl b, total chlorophyll a and b, and carotenoids (Car) contents increased remarkably with the higher B levels and salinity, compared to the control. In the presence of salinity, Chl b and Car contents decreased with all B levels, except for 4 mg/kg B level. Also, Chl a and Chl (a + b) did not change with 4, 8 and 40 mg/kg B levels, but 16 and 24 mg/kg B level caused a decrease in Chl a by 11.0 and 24.7%; in Chl (a + b) 9.6 and 29.7% contents, respectively (Figs. 2a, 2c).
The effects of increasing B levels on the contents of Chl a (a), Chl b (b), Chl (a + b) (c), and Car (d) of purslane leaves in the absence and presence of salinity. 1—Control, 2—4 mg/kg B, 3—8 mg/kg B, 4—16 mg/kg B, 5—24 mg/kg B, 6 —40 mg/kg B. The values (means ± SD, n = 3) followed by the different letter on the bars indicates significant differences according to the Duncan Multiple Range Test (α < 0.05).
Ion Concentrations
A significant interaction between B levels and salinity application on the ion concentration was seen in the Table 2. In the absence and presence of salinity, increasing B levels caused a notable increase in B content. But, salinity application decreased considerably the endogenous B contents in all B levels. These decreases were by 10.2, 56.3, 69.1, 71.4, 58.2, and 57.4% in the application of 0, 4, 8, 16, 24, and 40 mg/kg B, respectively. On the other hand, non-significant increases in the Na and the Cl concentrations were found with increasing B levels in the absence of salinity. However, with the combined effect of B and salinity, B content decreased significantly, whereas Na and Cl contents increased (Table 2).
As seen in Table 3, the K concentration in purslane increased considerably with 8, 16, 24, and 40 mg/kg B levels in the absence of salinity; however, in the presence of salinity, 4 and 8 mg/kg B levels caused a notable increase in K concentration by 42.7 and 25.1%, respectively. Besides, shoot Ca concentration in purslane decreased considerably with only 16 and 24 mg/kg B levels, compared to control. But, in the presence of salinity, it increased significantly with 4, 8, 16, 24, and 40 mg/kg B levels and these increases were found by 75.0, 40.0, 91.6, 66.7, and 34.9%, respectively. On the other hand, the changes in shoot Mg concentration showed differences depending on increasing B levels. While 4, 16, and 24 mg/kg B levels decreased the Mg content, 40 mg/kg B level increased considerable it by 25.0%. But, salinity application caused significant decreases in Mg content with all B levels. These decreases were by 42.7, 33.6, 50.4, 49.5, 44, and 60% in 0, 4, 8, 16, 24, and 40 mg/kg B levels, respectively.
MDA Content and Proline Accumulation
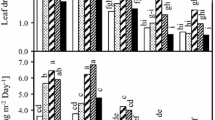
The effects of increasing B levels and salinity on MDA content and proline accumulation of purslane leaves were exhibited in Fig. 3. In the absence of salinity, the levels of 8, 16, and 24 mg/kg B increased significantly the MDA content by 33, 22, and 33%, respectively. But, it was decreased with the highest B level by 45%. When B applied together with NaCl, the MDA content decreased significantly with 4, 8, 16, 24, and 40 mg/kg B levels by 61.3, 60.1, 59.3, 65.3 and 36.1%, respectively. Also, salinity application caused a notable decrease in MDA content by 72.1%, compared to control (Fig. 3a).
The effects of increasing B levels on the MDA content (a) and proline accumulation (b) of purslane leaves in the absence and presence of salinity. 1—Control, 2—4 mg/kg B, 3—8 mg/kg B, 4—16 mg/kg B, 5—24 mg/kg B, 6—40 mg/kg B. The values (means ± SD, n = 3) followed by the different letter on the bars indicates significant differences according to the Duncan Multiple Range Test (α < 0.05).
Proline accumulation in purslane increased significantly in both excess B levels and salinity. These increases were 4-, 9-, 12-, 10-, and 14-fold with 4, 8, 16, 24, and 40 mg/kg B levels and applied NaCl, respectively. In the presence of salinity, it also increased considerably with all B levels, except for 8 mg/kg B (Fig. 3b).
Hydrogen Peroxide Content, Catalase and Ascorbate Peroxidase Activities
A significant interaction between increasing B levels and salinity was found in the H2O2 content and antioxidant features (CAT and APX activities) of purslane leaves (Fig. 4). The H2O2 content was increased considerably with increasing B levels and salinity. When B applied together with NaCl, 4, 8, 16, 24, and 40 mg/kg B levels increased remarkably the H2O2 content by 7.7-, 4.5-, 3.0-, 2.0-, 1.8-, and 1.7-fold, respectively (Fig. 4a).
The effects of increasing B levels on the content of H2O2 (a), activities of CAT (b) and APX (c) of purslane leaves in the absence and presence of salinity. 1—Control, 2—4 mg/kg B, 3—8 mg/kg B, 4—16 mg/kg B, 5—24 mg/kg B, 6—40 mg/kg B. The values (means ± SD, n = 3) followed by the different letter on the bars indicates significant differences according to the Duncan Multiple Range Test (α < 0.05).
On the other hand, the CAT activity was diminished with higher B levels. But, in the presence of salinity, it increased significantly 2.1-, 1.9-, 2.5-, 2.2- and 2.1-fold in 4, 8, 16, 24, and 40 mg/kg B levels, respectively (Fig. 4b). The APX activity tended to decrease in the absence of salinity and it showed a tendency of increase with salinity. However, it increased significantly in all B levels applied together with salinity (Fig. 4c).
DISCUSSION
Excess B and salinity could cause simultaneous stress in plants grown in saline and B-rich soils. Both stress factors are likely to occur in the soils characterized by low rainfall and poor drainage in both arid and semiarid regions [6]. Our results showed that increasing B levels and applied NaCl caused significant decreases in shoot and root fresh and dry weights of purslane (Table 1). Ion toxicity and osmotic stress are the problem sourced by salt stress and excess B [14, 25]. Moreover, excess B causes a reduction of leaf area and also inhibited the load photosynthetic products to growing parts [6]. On the other hand, salinity caused a significant reduction in biomass production of purslane as a parallel to findings reported by Kafi and Rahimi [11] and Grieve and Suarez [3]. But, salinity application alleviated the toxic effects of B in biomass production (Table 1). This result could be associated with the reduction in shoot B contents with salinity (see Fig. 1 and Table 2). Also, Baetz et al. [27] revealed sequestering of Na+ and Cl− into the vacuole to help maintain cytosolic ion homeostasis and avoid cellular damage during salt stress. Besides, applied NaCl to the root media reduces the ability of plants to take up water, causing a rapid decrease in growth parameters [26].
Chlorophyll content, a good indicator for determination of abiotic stress effects [10], was measured in young leaves of the plants. Considering B toxicity and salinity effect on purslane physiology, increase in chlorophylls content was seen at higher B levels (Fig. 2). Alam et al. [9] revealed an increase in chlorophyll content of purslane at 16 dS/m salinity level. Also, purslane exhibited a pattern of deeper green color combined with thicker and narrow leaves in presence of salinity (Fig. 1). This indicates that purslane could better adapt to stress conditions and has the ability to grow and reproduce at even high salinity conditions as already reported by Munns and Tester [26].
The B contents of purslane were increased with all B levels in the absence and presence of salinity (Table 2). However, in the presence of salinity, increasing B levels decreased B content. Reduction of B toxicity in the presence of salinity could be explained with antagonistic effects between B and the salt ions (Na+ and Cl–). Eraslan et al. [14] explained that stomatal resistance, and also B uptake and transpiration rate could be influenced negatively by salinity in rooting media of lettuce.
In the absence of salinity, the tendency of the concentrations of Na (at high boron levels) and Cl (at low boron levels) to increase can be explained by the toxic effects of B on plasma membrane (Table 2). Excess B may reduce toxic effects of Cl– with the anion competition as reported by Kafi and Rahimi [11] in purslane. High Na+ concentration sourced by salinity not only interferes with K uptake at root level but also causes alterations in the integrity and selectivity of membranes [2].
K+ plays a critical role for cell expansion, osmoregulation and homeostasis in cell and whole plant [11]. Purslane has high content of K, Mg, and Ca in stems and leaves, even if K and Ca level reduced by high salinity [2]. Our results showed that excess B increased the K content of purslane, but it was not changed with both excess B and salinity applications (Table 3). Apparently, Na+ in the rooting media affected the K+ uptake by plants. And also, the Na+ competes with K+ in plant uptake due to their similar physicochemical properties, especially through high-affinity potassium transporters and nonselective cation channels [28]. Furthermore, membrane injury may accelerate increase the K efflux from roots to growth media [15].
Both B and Ca stabilize the pectin fractions in cell wall and these two ions must co-exist in the cell wall [12]. The Ca content was decreased with excess B in the absence and presence of salinity; however, it was increased significantly with salinity application (Table 3).
The observed increase in Ca2+ content in the presence of salinity may be alternation of the Ca2+ content in the apoplastic space where mainly Ca2+ localization area and there could be a tendency of increase in Ca2+ content under both salinity and B-toxic conditions [13]. In addition, an increase in Na+ around the root causes increase in cytosolic free Ca2+ [26]. The other reason could also be a notable role of Ca in maintaining the membrane integrity, to control membrane permeability, osmoregulation, and to improve resistance of plants to salt stress [5].
Magnesium is a component of chlorophyll and required for photosynthesis and protein synthesis, and also it serves as a regulator of cation–anion balance in plant cell [5]. The Mg content showed differences depending on B levels; however, salinity application caused significant decrease (Table 3). Reductions in Mg content could be often associated with ion imbalance resulted from high content of Ca in purslane leaves. Excess B and salinity increased the Ca2+ in leaves; thereby, Mg2+ was excluded by Ca2+ from root plasma membrane binding sites [5]. Non-significant increase in Mg content with salinity reported by Teixeira and Carvalho [2] for purslane is parallel to our results.
The MDA level is a good indicator for membrane integrity and lipid peroxidation [8]. Results showed that MDA level was increased considerably with higher B due to detrimental effects of B on cell membrane. But, the highest B level and salinity application caused a significant decrease in MDA level (Fig. 3a). This result is in line with the report of Sudhakar et al. [7]. They reported that MDA content increased in susceptible mulberry variety, but tolerant one did not exhibit any increase in lipid peroxidation with exposure to salinity stress. The other reason also could be related to its rich antioxidant capacity as already revealed by Yazıcı et al. [8] for purslane. Also, this plant contains α-tocopherol, ascorbic acid, β-carotene and glutathione [1] and these antioxidants may act as a scavenger of ROS for mitigating the injury on biomembranes under salt stress. Besides, previous studies showing a toxic ion- and/or salt-induced decrease of MDA contents in barley [29] and lettuce [14] are in agreement with our study.
Proline is one of the most accumulated osmolytes in plants exposed to abiotic stress [11] and due to its intracellular osmotic adjustment it might be playing a critical role in protecting photosynthetic activity under salt stress [10]. Results showed that higher B levels caused an increase in proline accumulation due to detrimental effects of excess B [14]. Similarly, salinity application remarkably increased proline accumulation, compared to non-saline conditions (Fig. 3b). These increases in proline accumulation depending on stress factors may be explained by the plant’s ability to protect itself against osmotic potential changes due to NaCl toxicity. Bonilla et al. [30] stated that the high proline accumulation and Ca content (Table 3) in both salinity conditions and toxic B levels may be related to enhancing plant tolerance to salinity and toxicity.
The H2O2 is a non-radical ROS and its concentration can be given rise by biotic and abiotic stress condition, resulting in oxidative damage at cellular level [17]. At the same time, plants are equipped with H2O2-metabolizing enzymes (CAT, APX, etc.) and these scavenging enzymes reduce H2O2 to water in these cell organelles [16, 17].
The results revealed notable increases in the H2O2 content in both stress conditions (excess B and salinity) due to detrimental effects of toxicity (Fig. 4a). Significant decreases in CAT activity (Fig. 4b) and tendency of decrease in APX activity (Fig. 4c) indicated the peroxidation of lipid membranes in purslane leaves due to excess B. Considerable increases in CAT and APX activities under salinity conditions possibly showed that purslane, a moderately salt tolerant plant, exhibited a better protection mechanism against salinity-induced oxidative damage of the cell membrane. Similar results in relation with enhanced enzyme activity and improved protection against stress conditions were revealed by researchers for lettuce [14] and purslane [8] under salinity stress.
In conclusion purslane has the potential for becoming a highly nutritious vegetable for human and animal. This plant is able to survive under stress conditions derived from toxic B levels and salinity. Higher B levels caused a significant increase in shoot B, K, and Mg contents. The Na+ and Cl– contents increased with the application of B and NaCl together and this caused ion toxicity and osmotic stress. Toxic effects of B on biomass production and mineral composition in purslane were partly ameliorated by NaCl application. On the other hand, the positive or negative relationships between B and salinity come from the increase of Na+ and Cl– ions in the rooting media. Other than the antagonistic effects between B and Cl, there are no satisfactory findings explaining uptake mechanism of these ions in plants. Our result revealed that purslane responded to excess B and salinity stress by enhancing their antioxidative capacity (CAT and APX activity) and proline accumulation. Purslane is a candidate to further investigation because of rich nutrient content, having antioxidative capacity, and coping with various stress conditions. In addition, it is suggested that purslane has the potential to manage the amount of soluble boron and also it has a promising potential that can be grown in B-rich saline soils.
REFERENCES
Gonnella, M., Charfeddine, M., Conversa, G., and Santamaria, P., Purslane: a review of its potential for health and agricultural aspects, Eur. J. Plant Sci. Bi-otech., 2010, vol. 4, pp. 131–136.
Teixeira, M. and Carvalho, I.S.D., Effects of salt stress on purslane (Portulaca oleracea) nutrition, Ann. Appl. Biol., 2009, vol. 154, pp. 77–86.
Grieve, C.M. and Suarez, D.L., Purslane (Portulaca oleracea L.): a halophytic crop for drainage water reuse systems, Plant Soil, 1997, vol. 192, no. 2, pp. 277–283.
Gupta, U.C., Jame, Y.W., Campbell, C.A., Leyshon, A.J., and Nicholaichuk, W., Boron toxicity and deficiency: a review, Can. J. Soil Sci., 1985, vol. 65, pp. 381–409.
Marschner, H., Marschner’s Mineral Nutrition of Higher Plants, London: Elsevier, 2012.
Nable, R.O., Bañuelos, G.S., and Paull, J.G., Boron toxicity, Plant Soil, 1997, vol. 193, pp. 181–198.
Sudhakar, C., Lakshmi, A., and Giridarakumar, S., Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity, Plant Sci., 2001, vol. 161, no. 3, pp. 613–619.
Yazici, I., Türkan, I., Sekmen, A.H., and Demiral, T., Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation, En-viron. Exp. Bot., 2007, vol. 61, pp. 49–57.
Alam, M., Juraimi, A.S., Rafii, M.Y., and Abdul Hamid, A., Effect of salinity on biomass yield and physiological and stem–root anatomical characteristics of purslane, BioMed. Res. Int., 2015, vol. 2015: 105695.
Silva-Ortega, C.O., Ochoa-Alfar, A.E., Reyes-Agüero, J.A., Aguado-Santacruz, G.A., and Jiménez-Bremont, J.F., Salt stress increases the expression of p5cs gene and induces proline accumulation in cactus pear, Plant Physiol. Biochem., 2008, vol. 46, pp. 82–92.
Kafi, M. and Rahimi, Z., Effect of salinity and silicon on root characteristics, growth, water status, proline content and ion accumulation of purslane (Portulaca oleracea L.), Soil Sci. Plant Nutr., 2011, vol. 57, pp. 341–347.
Bastías, E., Alcaraz-López, C., Bonilla, I., Martínez-Ballesta, M.C., Bolaños, L., and Carvajal, M., Interactions between salinity and boron toxicity in tomato plants involve apoplastic calcium, J. Plant Physiol., 2010, vol. 167, pp. 54–60.
Wimmer, M.A., Mühling, K.H., Läuchli, A., Brown, P.H., and Goldbach, H.E., The interaction between salinity and boron toxicity affects the subcellular distribution of ions and proteins in wheat leaves, Plant Cell Environ., 2003, vol. 26, pp. 1267–1274.
Eraslan, F., Inal, A., Savasturk, O., Gunes, A., Changes in antioxidative system and membrane damage of lettuce in response to salinity and boron toxicity, Sci. Hortic., 2007, vol. 14, pp. 5–10.
Alpaslan, M. and Gunes, A., Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants, Plant Soil, 2001, vol. 236, pp. 123–128.
Asada, K., Ascorbate peroxidase—a hydrogen peroxide-scavenging enzyme in plants, Physiol. Plant., 1992, vol. 85, pp. 235–241.
Shigeoka, S., Ishikawa, T., Tamoi, M., Miyagawa, Y., Takeda, T., Yabuta, Y., and Yoshimura, K., Regulation and function of ascorbate peroxidase isoenzymes, J. Exp. Bot., 2002, vol. 53, no. 372, pp. 1305–1319.
Page, A., Miller, R., and Keeney, D., Methods of Soil Analysis, Wisconsin: Am. Soc. Agron., 1982.
Chance, B. and Maehly, A.C., Assay of catalases and peroxidases, Methods Enzymol., 1955, vol. 2, pp. 764–775.
Nakano, Y. and Asada, K., Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts, Plant Cell Physiol., 1981, vol. 22, pp. 867–880.
Hodges, D.M., de Long, J.M., Forney, C.F., and Prange, R.K., Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds, Planta, 1999, vol. 207, pp. 604–611.
Mukherjee, S.P. and Choudhuri, M.A., Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings, Physiol. Plant., 1983, vol. 58, pp. 166–170.
Bates, L.S., Waldren, R.P., and Teare, I.D., Rapid determination of free proline for water-stress studies, Plant Soil, 1973, vol. 39, pp. 205–207.
Lichtenthaler, H.K., Chlorophylls and carotenoids: pigments of photosynthetic biomembranes, Methods Enzymol., 1987, vol. 148, pp. 350–382.
Johnson, C.M. and Ulrich, A., Analytical Methods for Use in Plant Analysis-II, New York: Calif. Agric. Exp. Stn. Bull., 1959.
Munns, R. and Tester, M., Mechanisms of salinity tolerance, Annu. Rev. Plant Biol., 2008, vol. 59, pp. 651–681.
Baetz, U., Eisenach, C., Tohge, T., Martinoia, E., and de Angeli, A., Vacuolar chloride fluxes impact ion content and distribution during early salinity stress, Plant Physiol., 2016, vol. 172, no. 2, pp. 1167–1181.
Wakeel, A., Potassium–sodium interactions in soil and plant under saline–sodic conditions, J. Plant Nutr. Soil Sci., 2013, vol. 176, no. 3, pp. 344–354.
Karabal, E., Yücel, M., and Öktem, H.A., Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity, Plant Sci., 2003, vol. 164, pp. 925–933.
Bonilla, I., El-Hamdaoui, A., and Bolaños, L., Boron and calcium increase Pisum sativum seed germination and seedling development under salt stress, Plant Soil, 2004, vol. 267, pp. 97–107.
ACKNOWLEDGMENTS
This research was supported by Kocaeli University, Scientific Research Projects Coordination Unit (BAP) (project no. 2015/022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
The article is published in the original.
Abbreviations: APX—ascorbate peroxidase; CAT—catalase.
Rights and permissions
About this article
Cite this article
Samet, H., Çıkılı, Y. Response of Purslane (Portulaca oleracea L.) to Excess Boron and Salinity: Physiological Approach. Russ J Plant Physiol 66, 316–325 (2019). https://doi.org/10.1134/S1021443719020110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443719020110