Abstract
Background and aims
Biomechanical properties of cereal root systems largely control both resistance to root lodging and their ability to stabilise soil. Abiotic stresses can greatly modify root system growth and form. In this paper the effect of waterlogging and moderate mechanical impedance on root biomechanics is studied for both lateral roots and the main axes of barley.
Methods
Barley (Hordeum vulgare) plants were subjected to transient water-logging and moderate mechanical impedance in repacked soil columns. Roots were excavated, separated into types (nodal, seminal or lateral) and tested in tension to measure strength and elastic modulus.
Results
Water-logging and mechanical impedance substantially changed root system growth whilst root biomechanical properties were affected by waterlogging. Root strength was generally greater in thin roots and depended on root type. For example, seminal roots 0.4–0.6 mm in diameter were approximately seven times stronger and five times stiffer than lateral roots of the same diameter when mechanically impeded. Root sample populations typically exhibited negative power-law relationships between root strength and diameter for all root types. Mechanical impedance slowed seminal root elongation by approximately 50 % and resulted in a 15 % and 11 % increase in the diameter of in nodal and seminal roots respectively. Power-law relationships between root diameter and root biomechanical properties corresponded to the different root types. Coefficients for between root diameter, strength and elastic modulus improved when separated by root type, with R2 values increasing in some roots from 0.05 to 0.71 for root strength and 0.08 to 0.74 for elastic modulus.
Conclusions
Moderate mechanical impedance did not influence the tensile strength of roots, but, waterlogging diminished the relationship between root strength and diameter. Separation of root type improved predictions of root strength and elastic modulus using power-law regressions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The practical benefits of understanding and manipulating the root systems of agricultural crops are potentially large, ranging from improved production for food security to decreased soil degradation, but aspects such as lodging resistance and soil stabilisation are often overlooked. Much research has investigated contributions to soil stabilisation by tree roots (Abe and Ziemer 1991; Bischetti et al. 2009; Genet et al. 2008; Mickovski et al. 2009; Stokes and Mattheck 1996) where large roots act as reinforcing rods. The biomechanical properties of woody roots has been linked with internal cell structures (Niklas et al. 2002, 2006), with other research examining tissue composition impacts on the biomechanical properties of herbaceous plant stems (Hepworth and Vincent 1998).
Studies on plant anchorage have been performed for sunflower (Ennos 1989), wheat (Crook and Ennos 1993; Ennos 1991a, b), maize (Ennos et al. 1993) and rice (Oladokun and Ennos 2006) amongst other species. Models developed in these studies generally use single values of root strength and elastic modulus for the entire root system with these values typically derived from the coronal or nodal roots only. Work on soil stabilisation by roots, however, has established that the biomechanical properties of roots depend substantially on root diameter (Bischetti et al. 2005; Genet et al. 2005; Mickovski et al. 2009; Pollen and Simon 2005). Other research has shown that mechanical vibration of stems (Hepworth and Vincent 1999) and the properties of soil influence the biomechanical properties of roots.
Studies into the influence of internal structures on fibrous root biomechanical properties are relatively limited despite fibrous roots offering the potential for significant contributions to soil shear resistance, particularly near the soil surface. Compared with tree roots, the thin roots associated with fibrous systems usually have greater root length densities with individual fibrous roots after having greater tensile strength (defined here as stress; force per unit cross-sectional area at breakage) than thicker woody roots. Fibrous root systems in soil are akin to fibre reinforced composites, whereas individual woody roots are more analogous to reinforcing rods. Models in the literature for predicting root contributions to soil stabilisation range from simple tensile reinforcement by uniform roots (Waldron 1977) to reinforcement by populations of roots of varying strength acting as a fibre bundle (Daniels 1945; Pollen and Simon 2005). Key to the accuracy of such models is an understanding of root biomechanics and the potential effects of abiotic stresses on root tensile strength and stiffness (elastic or Young’s modulus defined as stress per unit strain). Natural variability in soil properties, and topographic location, result in roots growing in very different soil physical conditions. Root growth is often limited by adverse soil physical conditions, if the soil is hard or dry, or is waterlogged (Bengough et al. 2006; Sahnoune et al. 2004). Such abiotic stresses may contribute to root lodging within cereal crops (Berry et al. 2004). Lodging may occur through the stem failing (stem lodging) or if the root system can no longer anchor the plant when soils are very wet (root lodging) underlying a need to understand the effects of abiotic stresses on root biomechanics. Climate change may affect the soil environment through changed patterns of rainfall intensity. More intense rainfall events may increase soil erosion (Favis-Mortlock and Guerra 1999) and also incidences of waterlogging and the likelihood of crop lodging.
Hypoxia, mechanical impedance and water stress can all influence the anatomy and morphology of roots (Bengough et al. 2006; Iijima and Kato 2007). Hypoxia and mechanical impedance decrease root elongation rate with mechanical impedance also increasing root diameter, cortex thickness and the diameter of the xylem vessel in pea, maize and cotton (Iijima and Kato 2007). Root diameter has a large effect on root strength with negative power-law relationships between root strength and diameter (Bischetti et al. 2005; Genet et al. 2005; Loades et al. 2010; Mickovski et al. 2009; Pollen and Simon 2005). If mechanical impedance increases root diameter then it is possible that root strength will also decrease. Similarly the presence of aerenchyma within waterlogged roots is likely to decrease their tensile strength.
The aim of this paper is to unravel the effects of two abiotic stresses (transient waterlogging and moderate mechanical impedance) and root type on root biomechanical properties using barley (Hordeum vulgare) as a model cereal. Barley plants were grown within a controlled environment for 21 day in either a control soil, soil exerting a mechanical impedance to root elongation or a transiently water logged soil, after which root tensile strength and root elasticity (modulus) were measured.
Materials and methods
Soil grown plants
Three experimental treatments, (i) control, (ii) moderate mechanical impedance, and (iii) transient water-logging were established to investigate the impact of abiotic stress on root growth and root biomechanical properties. The experiment was repeated three times, with four replicate tubes for each treatment. The experiment ran concurrently, each staggered by 7 day at the start to allow sufficient time for root sampling and testing with minimal sample storage. All tests used barley (Hordeum vulgare cv. Bowman) because of its global importance for agricultural production and its susceptibility to root lodging.
The plants were grown in soil columns contained in plastic pipes 1 m long × 50 mm diameter. Each soil column was packed with an arable sandy loam soil (Eutric Cambisol, FAO) that was first passed through a 4 mm sieve at field water content. The soil was sampled from 0 to 10 cm depth and consisted of 71 % sand, 19 % siltand 10 % clay, with a pH of 6.2. Land management prior to sampling was spring barley grown for two years and the field (Bullion Field) was located at the James Hutton Institute, Mylnefield Farm (Lat 56°27′36.44″N; Long 3°4′21.74″W).
The plastic pipes were lined with a 0.2 mm thick polythene sheet so that soil columns could be removed with minimal disturbance. Pea gravel was placed in the bottom of each tube to a depth of 20 mm, with 980 mm of soil packed above this layer. Gravimetric soil water content was measured by oven drying four subsamples at 105 °C for 72 h prior to packing. De-aired distilled H2O was added as a fine mist to the sieved soil to achieve a water content of 0.20 g g−1. Following the addition of water, the soil was left to equilibrate for 24 h to ensure uniform water distribution and then packed to a dry bulk density of 1.2 g cm−3 (control and waterlogged treatments) or 1.4 g cm−3 for the mechanically impeded treatment. Packing was performed using a metal plunger weighing 2.78 kg dropped from a height of 20 mm for control treatments and 80 mm for mechanically impeded treatments. To achieve uniform soil packing, soil surfaces between each layer were roughened prior to packing the subsequent layer to ensure a homogenous column of soil. The soil was packed in 25 layers for 1.2 g cm3 and 50 layers for 1.4 g cm3. The energy required for packing was 7 kJ m−3 and 113 kJ m−3 for soil densities of 1.2 g cm3 and 1.4 g cm3 respectively. Batches of four tubes were placed in 160 mm diameter pipes that could be filled with water to enable the waterlogging treatment to be applied. Each batch of four tubes comprised a block of samples.
Before planting, barley (cv. Bowman) grains were sterilised in 2 % saturated Ca(ClO2) solution for 15 min, rinsed five times in distilled water and germinated on filter paper for 3 day at 12 °C . Seedlings were then transferred to soil columns with grains inserted at a depth of 10 mm. Small holes were bored into the soil to plant the seedlings, with soil replaced after planting. All plants were grown in a growth room with 16 h of light (300 μmol m−2 and 18 °C) and 8 h of darkness within each 24 h period.
After 7 day of growth water was added to the containers containing the soil tubes to a depth 50 mm below the soil surface. Tubes subjected to water-logging were open at the base to allow for water ingress. Those not subjected to water-logging were sealed at the base. Waterlogged conditions in the tubes with an open base were maintained for a period of 7 day before water was drained. Tubes not water-logged were also immersed in water to ensure soil temperature was the same in all tubes.
Plant height was recorded every 4th day after sowing (DAS) by measuring the distance from the soil surface to the tip of the longest leaf. After 21 DAS growth the plants were cut at the base of the stem and roots were extracted from the soil column (described below). Above ground biomass data was collected following drying of plant leaves at 60 °C for 48 h.
Hydroponically grown plants
Plants were grown hydroponically for assessment of root biomechanical properties free from the influence of abiotic stress. They were grown in 1 m long tubes similar to those used for the soil grown plants. Tubes were sealed at their base using a rubber bung and supported vertically within a controlled environment chamber. Tubes were filled with 2 l of a complete nutrient solution comprising of: 1.059 mM ammonium chloride; 1.412 mM calcium nitrate; 1.412 mM potassium nitrate; 1.059 mM magnesium sulphate; 0.353 mM ethylenediaminetetraacetic acid iron; 3.53 mM potassium phosphate and micronutrients: 0.006 mM manganese chloride; 0.023 mM boric acid 0.0006 mM zinc chloride; 0.0016 mM copper sulphate 0.001 mM sodium molybdate; 0.001 mM cobalt chloride (Dietrich et al. 2012). Nutrient solution in tubes was topped up daily to replace losses through evaporation.
Grains were pre-germinated as described for the soil experiment and then wrapped in plastic non-toxic foam and suspended so roots touched the hydroponic solution. Air stones, attached to an air pump, were placed 80 cm below the surface of the solution within each tube to aerate the solution. Day length, lighting and temperature were the same as those reported for the soil grown plants. The root systems from two plants were used for biomechanical testing and with tensile testing performed 21 DAS.
Bio-mechanical testing of roots
Soil grown plant roots were removed by sliding the plastic lining from the pipes and washing gently with tap water over a 2 mm sieve. Care was taken when washing roots to minimise potential root breakage or separation from the main root system. Following washing, roots were stored at 5 °C on moist blotting paper, with bio-mechanical testing performed within 72 h of root collection. For hydroponically grown plants the root washing process was not necessary.
Immediately prior to testing, individual root types were identified as originating either from the base of the grain (seminal roots) or from plant stem (nodal roots). Lateral roots produced from the main axis of seminal roots were also isolated. Lateral roots were not produced by nodal roots in all treatments. Each root type was segregated and subsamples of seminal, lateral and nodal roots were collected. Roots were segmented into 60 mm lengths and root diameter recorded at the midpoint of the root axis using a Leica MZFLIII stereo microscope and graticule at 10× magnification (Leica, Milton Keynes, United Kingdom).
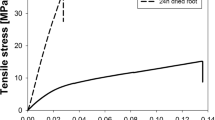
Tensile tests of individual plant roots were performed using a universal testing frame (Instron 5544, Norwood, MA, USA) at an extension rate of 1 mm min−1. Tensile load was measured using a 50 N load cell accurate to ±2 mN at maximum load. Full details of the mechanical testing of roots maybe found in Loades et al. (2010). The tensile load measured was converted to tensile stress (σ) using:
where F = breaking force and A = root cross-sectional area.
Elastic modulus (E) was measured for the initial linear portion of the stress-strain relationship (Fig. 1) using the equation:
where F = force; L o = original sample length; A o = root cross-sectional area and ∆L = change in root length.
Statistical analysis
All data were tested for normality followed by linear regression analysis using GenStat (Tenth Edition) statistical analysis software (GenStat for Windows, (2007) 10th Edition, VSN International Ltd., Hemel Hempstead, UK). Relationships between root diameter and strength were fitted with power-law curves. Significance testing for the relationship between root strength and diameter was performed using a linear regression with groups following log transformation of tensile strength, elastic modulus and root diameter data. The impact of treatment on plant height was assessed using repeated measures ANOVA with significant differences reported for P < 0.05. Effects of water-logging and soil porosity on root diameters within the three types was analysed using a general ANOVA.
Results
The height of soil grown plants increased over the growth period in all treatments (Fig. 2a) (P < 0.001, F = 167.6, df = 36) though the only treatment effect was measured at 20 day where waterlogged plants were significantly shorter than the mechanically impeded treatment (P < 0.01, F = 1.57, df = 3).
Mean plant height measured every four days for each of the four replicates (±SE) (a). All significant differences reported at the P < 0.05 level, significant differences in plant height over time indicated by capitalised letters. Treatment effect within each measurement interval indicated by lower case letters. Dry weights of total shoot biomass for each of the four replicates after 21 days growth (±SE),bars with the same letter indicate no significant differences at P < 0.05 level (b)
Plant shoot biomass in the control soil was approximately double that in the waterlogged soil (Fig. 2b). Biomass produced was not significantly affected by the mechanical impedance treatment.
Seminal root length was affected by soil treatment (Fig. 3; P < 0.001, F = 46.01, df = 26) with seminal roots subjected to transient waterlogging being only one fifth as long as those in the control treatment. Seminal root length in the mechanically impeded treatment was only about half of that of roots in the control treatment. Treatment also affected nodal root length (P < 0.01, F = 6.15, df = 28) with nodal roots longest in waterlogged soil (268 ± 35 mm) and shortest in the mechanically impeded soil treatment (98 ± 10 mm). Lateral root length was not significantly affected by treatment.
In most treatments the tensile strength (σ) and elastic modulus of roots (E) decreased with increasing root diameter (Fig. 4). Within all treatments nodal roots were the largest in diameter (0.91 mm to 1.05 mm), lateral roots smallest (0.37 mm to 0.54 mm) and seminal roots intermediate (0.61 mm to 0.68 mm; Table 1). Nodal root diameters were significantly thicker than seminal root diameters (P < 0.001, F = 18.63, df = 264). Plants grown in waterlogged soil had the thinnest nodal and seminal roots, whilst the thickest nodal and seminal roots were found in the mechanically impeded treatment (Table 1).
Significant differences in the elastic modulus of roots were observed between the waterlogged roots and those grown in the control and mechanically impeded treatments, and also those grown hydroponically (P < 0.001, F = 10.45, df = 146; P < 0.001, F = 9.78, df = 162 and P < 0.05, F = 11.85, df = 141 respectively; Table 2). Elastic modulus was smaller in waterlogged soils, with no relationship with root diameter in contrast to the other treatments where the tensile strength of roots increased with decreasing diameter (Fig. 4). Hydroponically grown nodal roots had a greater elastic modulus than those of either control (P < 0.001, F = 12.12, df = 33) or waterlogged plants (P < 0.01, F = 12.08, df = 81; Fig. 4). Seminal roots of waterlogged plants had the smallest elastic modulus of all treatments. Different root types within the same diameter range showed approximately a 5 fold increase in stiffness when grown in the mechanically impeded treatment. Lateral and seminal roots 0.4–0.6 mm in diameter had an average Young’s modulus of 7.03 (±1.2) MPa and 35.08 (±5.8) MPa respectively. Hydroponically grown lateral and seminal roots 0.4–0.6 mm in diameter had an average Young’s modulus of 18.65 (±1.7) MPa and 72.51 (±7.7) MPa respectively representing just under a 4 fold increase in stiffness (Fig. 4).
Moderate mechanical impedance or transient water-logging did not influence the tensile strength of seminal roots grown in the soil treatments. However, nodal roots grown hydroponically had a greater tensile strength than roots subjected to waterlogging (P < 0.05, F = 15.43, df = 81; Fig. 4), suggesting that root types are best studied as separate populations. Different root types within the same diameter range showed approximately a 7 fold increase in strength when grown in the mechanically impeded treatment. Lateral and seminal roots 0.4–0.6 mm in diameter had an average strength of 0.66 (±0.1) MPa and 4.58 (±0.5) MPa respectively. Hydroponically grown lateral and seminal roots 0.4–0.6 mm in diameter had an average strength of 2.11 (±0.3) MPa and 8.89 (±0.7) MPa respectively representing just over a 4 fold increase in strength (Fig. 4).
Relationships between the tensile strength or elastic modulus of roots and either their diameter or cross-sectional area were fitted with power-law relationships. The R2 values, between strength and diameter, were poor and between 0.05 and 0.15 when fitted to all roots in the population. When fits were applied to each root type, R2 values improved to between 0.22 and 0.71 (Tables 3 and 4). Similar trends were found in elastic modulus, with root population R2 values of 0.07 to 0.14 for the all root types, before improving to 0.13–0.74 when fitted for each root type.
Discussion
Root type and biomechanical properties
Root type was a key factor influencing tensile strength and elastic modulus. Each root type exhibited different tensile strength and elastic modulus relationships with root. Correlation coefficients between tensile strength and diameter were relatively poor for combined populations of roots from an individual soil treatment (Table 3). Considerable previous research has correlated the tensile strength and elastic modulus of roots with root diameter using a negative power-law relationship. Whilst these show reasonable fits for many woody species (Bischetti et al. 2005; Genet et al. 2005; Mickovski et al. 2009; Pollen and Simon 2005), results for fibrous roots are more poorly correlated (Loades et al. 2010). Roots extracted from individual soil samples are likely to contain a mixture of root types, and ages, and this will probably increase the scatter of the root strength—diameter relationship.
Correlation co-efficients between root tensile strength/modulus and diameter improved when root type was included in the model. Nodal root structure differs from that of seminal roots (Watt et al. 2008). Seminal roots have central and multiple peripheral xylem tracheary elements (XTE), whilst nodal roots have central pith surrounded by both inner and peripheral XTE. Root internal structure is very different within each of the three root types, seminal, nodal and lateral. The observed dependence of mechanical properties on root type is therefore to be expected from the root anatomy, so needs to be considered when attempting to predict the tensile strength and elastic modulus of roots from diameter data alone. Environmental factors will affect the root structure and potentially root biomechanical properties. Hales et al. (2009) found increasing cellulose content in woody roots with decreasing soil water content influenced by topographic location. Hales et al. (2009) also found that relationships between strength and diameter had R2 values less than 0.35. In this study R2 values were between 0.05 and 0.15 when fits were to all roots in the population, however, when fits were restricted to root type this improved to between 0.22 and 0.71. Similar trends were found in elastic modulus with root population R2 values of 0.07 to 0.14 improved to 0.13–0.74 when fitted dependant on root type.
Variations in power-law relationships between either the tensile strength or elastic modulus of roots with their diameter has been questioned previously due to low R2 values of 0.22 reported (Beek et al. 2005) however, root type may explain at least part of the reason for such poor fits.
Hales et al. (2009) suggested an alternative fitting of peak load to root cross-sectional area to reduce potential auto correlation effects (Hales et al. 2009). This alternative approach gave similar correlation co-efficients for our data as between strength and diameter with values ranging from 0.05 to 0.19 for the whole root populations in each treatment. Negative power-law fits appear to provide useful predictions of root strength from root diameter, especially if allowance is made for root type.
Root response to water-logging and mechanical impedance
The tensile strength of roots was not influenced by mechanical impedance, but waterlogging eliminated the relationship between root diameter and both tensile strength and elastic modulus. Poor aeration associated with waterlogging can stimulate aerenchyma development (Evans 2004) and this may explain the impact on root modulus and strength. Aerenchyma formation increases air spaces within roots to help the plant survive in anoxic conditions and this may also decrease root structural integrity (Engelaar et al. 1993). Increasing air spaces results in increasing root porosity. Root strength, measured by compressing the root surface, decreasing in some species with increasing root porosity (Striker et al. 2007). If root tissue is replaced by air spaces that portion of the root cross-sectional area will not contribute to either root strength or stiffness. Secondary effects of waterlogging may be responsible for changes observed in root strength and modulus and not solely aerenchyma development. The primary response of plants to waterlogging is controlled by ethylene and auxin which initiates programmed cell death, in the development of aerenchyma, and also adventitious root growth (Visser and Voesenek 2005). Increases in the availability of plant genotypes with differing amounts of aerenchyma, not formed through exposure to abiotic stresses such as waterlogging (Zhu et al. 2010), offer the potential to examine whether aerenchyma are specifically responsible for changes in root biomechanics. It is possible that other alterations in cell wall composition or tissue structure may also contribute to observed changes in root strength and elastic modulus.
Waterlogging decreased above ground biomass when compared to the control grown plants. These findings are in agreement with results from others (Sahnoune et al. 2004). Malik et al. (2002) found biomass significantly reduced after 7 day of waterlogging. Other studies have shown that final yield is reduced with increasing mechanical impedance (Ishaq et al. 2001; Whalley et al. 2008). The mechanical impedance was selected to approximately halve the root elongation rate, whilst ensuring sufficient root material would be available for mechanical testing. Schmidt et al. (2013) found in the same soil, equilibrated to a similar water content (0.2 g g−1), penetrometer resistances of 0.42 MPa and 0.81 MPa for soils packed to densities of 1.2 g cm−3 and 1.4 g cm−3 respectively. Mechanical impedance did not affect root strength or stiffness in this study, though more severe impedance may double root diameter and so have a greater affect.
Relevance of root biomechanical properties to soil stability and crop lodging
The role of internal root structures on plant tissue has mainly been studied in woody species (Niklas et al. 2002, 2006). Waterlogging and mechanical impedance influenced the amount of lateral, seminal and nodal roots highlighting the potential of abiotic stress to affect root architecture within different growth environments. One of the difficulties in understanding root lodging within cereal crops is the complexity of environmental processes likely to affect roots. Compaction is thought to increase the lodging resistance of plants due to increases in anchorage strength resulting from greater soil strength and reduced plant competition (Scott et al. 2005). With no change in root strength, enhanced soil strength and root-soil adhesion is likely to decrease lodging in compacted soil. The mechanisms responsible for increased lodging in waterlogged conditions may be attributed to both weaker soil and to weaker and more compliant roots.
From a soil stabilisation perspective, root elastic modulus is a key parameter for soil stability modelling (Schwarz et al. 2010) with changing elastic modulus potentially affecting soil stability. Transmission of stress along an individual root is determined by its relative elasticity in relation to the soil and to other roots though it is a topic that is only recently being incorporated into root reinforcement models.
Waterlogging was found to influence the biomechanics of nodal roots through elimination of the relationship with diameter and decreases in both strength and stiffness. A change in nodal root modulus is most likely to influence plant resistance to lodging (Ennos 1991b). Root lodging resistance of wheat has also been linked to the size of the root-soil cone (Berry et al. 2004), however, this relationship is not explicit with root bending resistance shown to be of greater importance in one variety (Crook and Ennos 1993). Although root bending resistance was not measured explicitly, through three point bend tests, tensile modulus infers some measure of the materials ability to resist tensile bending or twisting forces (Niklas 1998). With bending resistance critical to lodging resistance, waterlogging is likely to increase lodging both from a root biomechanical perspective, due to decreased elastic modulus, and also from inherent failure due to weak adhesion between root and already weakened soil.
There is scope to use information on the biomechanics of different root types to improve root anchorage and soil stability models. Root architecture models maybe adapted to include predictions of root strength for each element of the root system. Understanding each element of the root system may increase accuracy within soil stability models and also increase our knowledge of factors influencing root lodging of cereal crops. Although simple root analogues have shown the influence of root architecture on pull-out resistance (Mickovski et al. 2007), more information on real root systems is required.
Conclusions
Root type had a large effect on the relationships between tensile strength and root diameter, and, root stiffness and root diameter. For a given root type, thinner roots were generally stiffer and stronger. Seminal roots were found to be seven times stronger than lateral roots when mechanically impeded with four time’s greater strength when grown hydroponically. Root stiffness showed a similar trend with just under 5 and 4 fold increases in stiffness between lateral and seminal roots when mechanically impeded or grown hydroponically respectively. This dependence on root type may partly explain the large scatter observed in many experimental studies on populations of mixed root types. More work is required to fully understand whether changes in root strength and stiffness are associated with changes in cell wall structure, composition or tissue density. The effect of root age on biomechanical properties is another parameter which may also influence root strength and stiffness. Waterlogging had a substantial effect on root biomechanical properties, and this may have implications for crop lodging following intense rainfall events.
References
Abe K, Ziemer RR (1991) Effect of tree roots on a shear zone—modeling reinforced shear-stress. Can J For Res 21:1012–1019
Beek L, Wint J, Cammeraat L, Edwards J (2005) Observation and simulation of root reinforcement on abandoned Mediterranean slopes. Plant Soil 278:55–74
Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA (2006) Root responses to soil physical conditions; growth dynamics from field to cell. J Exp Bot 57:437–447
Berry PM, Sterling M, Spink JH, Baker CJ, Sylvester-Bradley R, Mooney SJ, Tams AR, Ennos AR (2004) Understanding and reducing lodging in cereals. Elsevier Academic Press Inc, San Diego, p 271
Bischetti GB, Chiaradia EA, Simonato T, Speziali B, Vitali B, Vullo P, Zocco A (2005) Root strength and root area ratio of forest species in Lombardy (Northern Italy). Plant Soil 278:11–22
Bischetti GB, Chiaradia EA, Epis T, Morlotti E (2009) Root cohesion of forest species in the Italian Alps. Plant Soil 324:71–89
Crook MJ, Ennos AR (1993) The mechanics of root lodging in winter-wheat, triticum-aestivum l. J Exp Bot 44:1219–1224
Daniels HE (1945) The statistical theory of the strength of bundles of threads.1. Proc Roy Soc A-Math Phys 183:405–435
Dietrich RC, Bengough AG, Jones HG, White PJ (2012) A new physical interpretation of plant root capacitance. J Exp Bot 63:6149–6159
Engelaar WMHG, Jacobs MHHE, Blom CWPM (1993) Root-growth of Rumex and Plantago species in compacted and waterlogged soils. Acta Bot Neerlandica 42:25–35
Ennos AR (1989) The mechanics of anchorage in seedlings of sunflower, Helianthus-annuus l. New Phytol 113:185–192
Ennos AR (1991a) The mechanics of anchorage in wheat triticum-aestivum l.1. the anchorage of wheat seedlings. J Exp Bot 42:1601–1606
Ennos AR (1991b) The mechanics of anchorage in wheat triticum-aestivum l.2. Anchorage of mature wheat against lodging. J Exp Bot 42:1607–1613
Ennos AR, Crook MJ, Grimshaw C (1993) The anchorage mechanics of Maize, Zea-mays. J Exp Bot 44:147–153
Evans DE (2004) Aerenchyma formation. New Phytol 161:35–49
Favis-Mortlock DT, Guerra SJT (1999) The implications of general circulation model estimates of rainfall for future erosion: a case study from Brazil. Catena 37:329–354
Genet M, Stokes A, Salin F, Mickovski S, Fourcaud T, Dumail JF, van Beek R (2005) The influence of cellulose content on tensile strength in tree roots. Plant Soil 278:1–9
Genet M, Kokutse N, Stokes A, Fourcaud T, Cai X, Ji J, Mickovski S (2008) Root reinforcement in plantations of Cryptomeria japonica D. Don: effect of tree age and stand structure on slope stability. For Ecol Manag 256:1517–1526
Hales TC, Ford CR, Hwang T, Vose JM, Band LE (2009) Topographic and ecologic controls on root reinforcement. J Geophys Res-Earth 114:FO3013
Hepworth DG, Vincent JFV (1998) The mechanical properties of xylem tissue from tobacco plants (Nicotiana tabacum ‘Samsun’). Ann Bot 81:751–759
Hepworth DG, Vincent JFV (1999) The growth response of the stems of genetically modified tobacco plants (Nicotiana tabacum ‘Samsun’) to flexural stimulation. Ann Bot 83:39–43
Iijima M, Kato J (2007) Combined soil physical stress of soil drying, anaerobiosis and mechanical impedance to seedling root growth of four crop species. Plant Prod Sci 10:451–459
Ishaq M, Hassan A, Saeed M, Ibrahim M, Lal R (2001) Subsoil compaction effects on crops in Punjab, Pakistan: I. Soil physical properties and crop yield. Soil Tillage Res 59:57–65
Loades KW, Bengough AG, Bransby MF, Hallett PD (2010) Planting density influence on fibrous root reinforcement of soils. Ecol Eng 36:276–284
Malik AI, Colmer TD, Lambers H, Setter TL, Schortemeyer M (2002) Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol 153:225–236
Mickovski SB, Bengough AG, Bransby MF, Davies MCR, Hallett PD, Sonnenberg R (2007) Material stiffness, branching pattern and soil matric potential affect the pullout resistance of model root systems. Eur J Soil Sci 58:1471–1481
Mickovski S, Hallett PD, Bransby MF, Davies MCR, Sonnenberg R, Bengough AG (2009) Mechanical reinforcement of soil by willow roots: impacts of root properties and root failure mechanism. Soil Sci Soc Am J :1276–1285
Niklas KJ (1998) Effects of vibration on mechanical properties and biomass allocation pattern of Capsella bursa-pastoris (Cruciferae). Ann Bot-London 82:147–156
Niklas KJ, Molina-Freaner F, Tinoco-Ojanguren C, Paolillo DJ (2002) The biomechanics of Pachycereus pringlei root systems. Am J Bot 89:12–21
Niklas KJ, Spatz HC, Vincent J (2006) Plant biomechanics: an overview and prospectus. Am J Bot 93:1369–1378
Oladokun MAO, Ennos AR (2006) Structural development and stability of rice Oryza sativa L. var. Nerica 1. J Exp Bot 57:3123–3130
Pollen N, Simon A (2005) Estimating the mechanical effects of riparian vegetation on stream bank stability using a fiber bundle model. Water Resour Res 41:W07025
Sahnoune M, Adda A, Soualem S, Harch MK, Merah O (2004) Early water-deficit effects on seminal roots morphology in barley. C R Biol 327:389–398
Schmidt S, Gregory PJ, Grinev DV, Bengough AG (2013) Contrasting responses of maize and lupin root growth to compact and dry soil are similar in their association of root elongation rate with length of the non-root hair—bearing apical region. Accepted with minor revisions in Plant and Soil
Schwarz M, Preti F, Giadrossich F, Lehmann P, Or D (2010) Quantifying the role of vegetation in slope stability: a case study in Tuscany (Italy). Ecol Eng 36:285–291
Scott DI, Tams AR, Berry PM, Mooney SJ (2005) The effects of wheel-induced soil compaction on anchorage strength and resistance to root lodging of winter barley (Hordeum vulgare L.). Soil Tillage Res 82:147–160
Stokes A, Mattheck C (1996) Variation of wood strength in tree roots. J Exp Bot 47:693–699
Striker GG, Insausti P, Grimoldi AA, Vega AS (2007) Trade-off between root porosity and mechanical strength in species with different types of aerenchyma. Plant Cell Environ 30:580–589
Visser EJW, Voesenek LACJ (2005) Acclimation to soil flooding—sensing and signal-transduction. Plant Soil 274:197–214
Waldron LJ (1977) Shear resistance of root-permeated homogeneous and stratified soil. Soil Sci Soc Am J 41:843–849
Watt M, Magee LJ, McCully ME (2008) Types, structure and potential for axial water flow in the deepest roots of field-grown cereals. New Phytol 178:135–146
Whalley WR, Watts CW, Gregory AS, Mooney SJ, Clark LJ, Whitmore AP (2008) The effect of soil strength on the yield of wheat. Plant Soil 306:237–247
Zhu JINM, Brown KM, Lynch JP (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ 33:740–749
Acknowledgments
We thank the BBSRC for funding a PhD studentship (BBS/S/K/2005/12211A) for Kenneth Loades. The James Hutton Institute receives funding from the Scottish Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric J. W. Visser.
Rights and permissions
About this article
Cite this article
Loades, K.W., Bengough, A.G., Bransby, M.F. et al. Biomechanics of nodal, seminal and lateral roots of barley: effects of diameter, waterlogging and mechanical impedance. Plant Soil 370, 407–418 (2013). https://doi.org/10.1007/s11104-013-1643-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1643-y