Abstract
Background and aims
Al tolerance is one of the most important trait for worldwide crop production. Using microarrays, we previously identified a transcription factor belonging to the C2H2 zinc finger protein (ZFP) family associated with Al tolerance in wheat (Houde and Diallo, BMC Genomics 9:400, 2008). The current work aimed to identify specific members of the C2H2 ZFP family that are associated with Al tolerance.
Methods
Wheat ESTs were used to assemble C2H2 ZFP family members that do not contain a classical EAR repressor domain. Specific primers were designed for qRT-PCR expression profiling of wheat root tips exposed to Al and H2O2. Two Al-tolerant and sensitive wheat cultivars including a pair of near-isogenic lines differing in Al tolerance were used.
Results
We reconstituted 16 wheat Q-type C2H2 ZFP. Expression profiling identified two transcripts (TaZFP2 and TaZFP3) that accumulate rapidly upon exposure to Al or in response to H2O2 in two tolerant wheat cultivars, including the tolerant near-isogenic line.
Conclusion
The responsiveness of these transcripts to H2O2 suggests that they may represent the wheat orthologs of ZFP transcription factors ZAT7 and ZAT12 that were shown to improve ROS tolerance in Arabidopsis. Thus, they may play a crucial role in the improvement of oxidative stress tolerance in wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Al tolerance is considered to be the second most important trait for worldwide crop production after drought tolerance. Al toxicity is especially important for developing countries since acid soils in these countries account for up to 60 % of the world’s acid soils. Our ability to improve crop production in these soils can significantly contribute to increase overall crop yield. Root growth inhibition (RGI) is one of the most recognized symptoms of Al toxicity, and has a major negative impact on root biomass and uptake of water and nutrients. Although, the aluminum malate transporter was shown to be a major contributor to Al tolerance, several other QTLs were identified indicating that other genes could play specific roles in Al tolerance. Genetic studies in the Triticum aestivum wheat cultivar Atlas66 showed that two major loci on chromosome 4DL and 6A, and additional loci with additive effects are involved in Al tolerance (Ma et al. 2005; Berzonsky 1992). A minor quantitative trait locus (QTL) for Al tolerance on chromosome 3BL of Atlas66 accounts for approximately 11 % of the genetic variation for Al tolerance in a population derived from a cross of Atlas66 with the sensitive cultivar Chisholm (Zhou et al. 2007). Result in other wheat lines has shown that genes located on several chromosomes contribute to the Al tolerance trait (Aniol and Gustafson 1984; Mesdag and Slootmaker 1969; Baier et al. 1995; Ryan et al. 1995; Delhaize et al. 1993a; Johnson et al. 1997; Tang et al. 2002).
It has been reported that Al exposure leads to an exudation of a variety of organic anions such as malate, citrate, or oxalate (Delhaize et al. 1993b; Kidd et al. 2001; Kochian 1995; Ryan et al. 2009) with potential roles for other genes in their regulation (Hoekenga et al. 2006; Sasaki et al. 2006). Al tolerance of several species can be enhanced by increasing the biosynthesis of these organic acids (Anoop et al. 2003; de la Fuente et al. 1997; Koyama et al. 2000; Tesfaye et al. 2001). Over-expression of the Al-inducible malate transporter improves Al tolerance in barley (Delhaize et al. 2004) while over-expression of the SbMATE (for Multidrug And Toxic compound Extrusion) gene, encoding a putative citrate transporter, improves Al tolerance in Arabidopsis thaliana and wheat (Magalhaes et al. 2007). In wheat, the release of phosphate was also associated with Al tolerance, indicating that multiple mechanisms are involved (Pellet et al. 1996). We have previously shown that a redox biochemical activity associated with Al tolerance is rapidly inhibited upon Al exposure. This activity recovers after 24 h with the continued presence of Al, suggesting that tolerant wheat plants are able to properly adapt their physiology to maintain growth (Maltais and Houde 2002). Furthermore, cell death and turnover of root tip epidermal cells are associated with Al tolerance in wheat (Delisle et al. 2001). Al tolerance thus involves complex responses and the regulation of multiple genes/biochemical pathways.
Genes that are differentially expressed between two wheat NILs (Chisholm-T, tolerant and Chisholm-S, sensitive) were identified using suppression subtractive hybridization (Guo et al. 2007). A total of 57 genes are differentially expressed in the Al tolerant cultivar. Among these, 28 transcripts, including ALMT1 (for Al-activated Malate Transporter 1), ent-kaurenoic acid oxidase-1, β-glucosidase, lectin, histidine kinase and phosphoenolpyruvate carboxylase, were more abundant in Chisholm-T, thus correlating with Al tolerance. Genes differentially expressed between two other wheat NILs (Century-T, tolerant and Century-S, sensitive) were also identified in our laboratory using the Affymetrix GeneChip Wheat Genome Array (Houde and Diallo 2008). In this study, we were careful to use Al concentrations resulting in the same level of root growth inhibition, a hallmark of Al stress. The tolerant cultivars used (Atlas66 and the NIL Century-T) were able to grow for several days (50 % rate of control plants or a root growth inhibition (RGI) of 50 % = RGI50) in the presence of 50 μM Al. In contrast, the sensitive cultivars (Bounty and the NIL Century-S) had an RGI50 of 5 μM Al which is 10 times less than for tolerant cultivars. Using Al concentrations resulting in an RGI50 for all lines, genes that are differentially expressed after 24 h of exposure to Al were identified. Overall, 83 genes associated with Al stress and 25 genes associated with Al tolerance were identified, including genes coding for ALMT1, glutathione S-transferase, germin/oxalate oxidase, fructose 1.6-bisphosphatase, cysteine-rich proteins, cytochrome P450 monooxygenase, cellulose synthase, disease resistance response protein, F-box-containing domain proteins and a C2H2 zinc finger transcription factor. The latter gene encodes the only transcription factor associated with Al tolerance identified in the microarray experiment.
The wheat C2H2 zinc finger transcript identified may represent an ortholog of zinc finger proteins (ZFP) that are important for Al or oxidative stress tolerance in other species. The STOP1 (for Sensitive TO Proton rhizotoxicity 1) C2H2 ZFP is required for Al tolerance and induction of ALMT1 in Arabidopsis (Sawaki et al. 2009). The ART1 (for Al Resistance Transcription factor 1) zinc finger, related to STOP1 is involved in the regulation of several genes needed for Al tolerance in rice (Yamaji et al. 2009). Two zinc finger transcription factors (ZAT7 and ZAT12) are involved in the response to oxidative stress in Arabidopsis (Abercrombie et al. 2008; Chinnusamy et al. 2007; Mittler et al. 2006; Rizhsky et al. 2004).
ZFPs constitute a large family of transcription factors that are widely spread in plants. They are classified in many subfamilies with at least 176 members in the C2H2 subfamily in Arabidopsis (Ciftci-Yilmaz and Mittler 2008; Gourcilleau et al. 2011). Several ZFPs play important roles in response to biotic or abiotic stresses. The up-regulated C2H2 ZFP family member found in our study may regulate downstream genes involved in the Al tolerance trait of Atlas66 (Houde and Diallo 2008). The encoded protein contains two Q-type C2H2 domain but lacks the EAR (for Ethylene-responsive factor Associated amphiphilic Repression) repressor motif (L/F DLN L/F XP) found in most members of the Q-type two fingered C2H2 ZFPs (Gourcilleau et al. 2011; Ohta et al. 2001). In Arabidopsis, all 20 members of this subfamily, which is classified in the C1-2i subgroup, contain a functional EAR repressor motif (Ciftci-Yilmaz and Mittler 2008). Analysis of 152 two-fingered Q-type C2H2 ZFPs from 50 plants species revealed that only 11 sequences did not contain an EAR repressor motif and all of these sequences were from monocot species (Gourcilleau et al. 2011). This subfamily may represent a new subgroup that evolved recently in monocots. We aimed to assemble and characterize this new C2H2 zinc finger subgroup in wheat and to identify which members are associated with Al tolerance by following the expression kinetics of the different transcripts in two different Al-tolerant cultivars, including a near-isogenic line and their sensitive counterparts.
Materials and methods
Plant material, growth and exposure conditions
The tolerant Triticum aestivum wheat Atlas66 and sensitive Bounty cultivars and two near-isogenic lines (NILs; OK91G106, named Century-T (Al-tolerant) and OK91G108, named Century-S (Al-sensitive)) derived from a cross between Atlas66 and the sensitive cultivar Century were used in this study (Carver et al. 1993). Plants were grown as previously described and treated under conditions where Al remains mostly in the Al3+ form (Kochian et al. 2005). To reduce pH variations and ensure that Al speciation was stable throughout the experiment, at least 100 ml of solution (1 mM CaCl2 pH 4.15 with or without Al) was used for each plant. The root growth inhibition (RGI) is expressed as 100 × [1- (root growth of Al-treated seedling divided by the root growth of control seedlings)]. Four replicate experiments were performed on different days with one series of Al concentration per day. For peroxide treatment, seedlings were exposed to 100 μM hydrogen peroxide (Sigma-Aldrich) in 1 mM CaCl2 pH 7.0 for 0.5 h (Prasad et al. 1994). Controls for hydrogen peroxide treatments were exposed to 1 mM CaCl2 pH 7.0 and were collected at 0 and 30 min. As for Al treatments, four replicate experiments were performed.
Bioinformatic analysis
The EST (GenBank no. BJ220837) representing the C2H2 zinc finger transcript associated with Al tolerance on the wheat microarray (Affymetrix) (Houde and Diallo 2008) was used as query in a BLAST analysis to determine the similarity to other transcripts on the microarray and to retrieve T. aestivum C2H2 zinc finger ESTs from the GenBank databases (http://blast.ncbi.nlm.nih.gov/Blast.cgi) (Altschul et al. 1990) or assembled tentative consensus (TC) sequences from T. aestivum DFCI Gene Index database (http://compbio.dfci.harvard.edu/cgi-bin/tgi/gimain.pl?gudb=wheat). Nucleotide sequences that had ≥99 % similarity were used to assemble contigs using CAP3 (http://pbil.univ-lyon1.fr/cap3.php) (Huang and Madan 1999). The assembled sequences were analyzed using GENSCAN (http://genes.mit.edu/GENSCAN.html) (Burge and Karlin 1997), GETORF (http://www.cbib.u-bordeaux2.fr/pise/getorf.html) (Rice et al. 2000) and MyHits (http://myhits.isb-sib.ch/cgi-bin/motif_scan) (Pagni et al. 2007). The TaZFPs that encode proteins containing two Q-type C2H2 zinc finger domains and no EAR motif (L/F DLN L/F XP) as defined by Ohta et al. (Ohta et al. 2001) were retained. The deduced amino acid sequences were aligned using ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2) (Larkin et al. 2007). Neighbour-joining trees of 10,000 bootstrapped samples were constructed by MEGA (Molecular Evolutionary Genetics Analysis) version 5 (Tamura et al. 2011). Identification of domains other than zinc fingers were achieved by visually inspecting the TaZFP proteins ClustalW alignment and by using the Eukaryotic Linear Motif resource for Functional Sites in Proteins (ELM, http://elm.eu.org/) (Gould et al. 2010).
RNA isolation and quantitative RT-PCR analysis
For each time point, root tips (5–10 mm) of 50 plants were isolated and flash frozen on dry ice. Total RNA was isolated using TRIzol (Invitrogen). After assessing quality on agarose gels, RNA samples were treated with RNase-Free DNase (QIAGEN) and reverse transcribed with SuperScript III (SuperScript® III Reverse Transcriptase, Invitrogen) in the presence of oligo (dT)12–18 (Invitrogen) according to the manufacturer’s instructions. The absence of amplification in the control (cDNA without reverse transcription) qPCR reactions confirmed that there was no genomic DNA contamination in the RNA samples for all genes analyzed.
The expression level of zinc finger transcripts was assessed by quantitative Real-Time PCR. Specific primers were designed using the Primique software (http://cgi-www.daimi.au.dk/cgi-chili/primique/front.py) (Fredslund and Lange 2007). The design clearly distinguishes clades and subgroups (several bases difference in the primers, results not shown). For close members such as TaZFP1 and TaZFP2 or TaZFP15 and TaZFP16, there was a minimum of at least 2 bases of mismatch between sequences in at least one of the primers. Primer concentrations were optimized to reach 98 % to 100 % of PCR efficiency. Quantitative Real-Time PCR was performed on a LightCycler 480 instrument (Roche) using the primers described in Supplementary material 1. The cDNAs were used as template for the qRT-PCR with forward and reverse primers in a total volume of 20 μL using SYBR Green mixture (Express SYBR® GreenER™ qPCR Supermix Universal, Invitrogen). The reaction conditions were: 5 min at 95 °C followed by 40 cycles of 15 s at 95 °C, 30 s at 58 °C and 30 s at 72 °C. The 18S rRNA was used as internal reference to calculate relative transcript levels of TaZFP genes. Primer specificity was confirmed by the presence of a single sharp band on agarose gel and a single peak in melting curve analysis at the end of the Real-Time PCR reaction.
The Crossing Point (CP) values of the quadruplicates were averaged and used for quantification of transcript levels, which was performed using the comparative CT method (Livak and Schmittgen 2001). The target gene transcript level was normalized against the 18S rRNA level. The fold change in cDNA (target gene) was determined as follows: fold change = 2−∆∆CP, where ∆∆CP = (CPTarget − CP18S)Time x − (CPTarget − CP18S)Time 0. Time x is any time point and time 0 represents the expression of each gene under control conditions as indicated in each figure.
Statistical analysis
Statistical significance of differences in mRNA levels was determined by Student’s t-test, based on four biological replicates. P values of <0.05 were considered as significantly different.
Results
Assembly and characterization of wheat Q-type C2H2 zinc fingers without an EAR domain
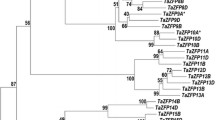
Using the newly available ESTs, we updated the assembly of wheat Q-type two-fingered C2H2 zinc fingers that do not contain an EAR domain (see Supplementary material 1). Phylogenetic analysis of the 16 protein sequences obtained is shown as a radiation tree (see Supplementary material 2). These sequences can be subdivided into at least 3 clades. Clade I contains TaZFP1 to TaZFP9, clustered in three subgroups (Ia, containing TaZFP1 to TaZFP3; Ib containing TaZFP4 to TaZFP6, and Ic containing TaZFP7 to TaZFP9). Clade II contains four sequences that are closely related (TaZFP10 to TaZFP13), while clade III contains three sequences (TaZFP14 to TaZFP16) with two of them being very similar. A detailed analysis of the different domains in Eukaryotic Linear Motif resource for Functional Sites in Proteins (ELM, http://elm.eu.org/) (Gould et al. 2010), reveals distinctive features of the different clades and subgroups of ZFP proteins (Fig. 1). In TaZFP4 and TaZFP7, the first C (in yellow) is missing in the second C2H2 domain which may affect their function. With the exception of TaZFP9, the two QALGGH domains typical of Q-type (Q in grey) zinc fingers are perfectly conserved in all sequences. All members of clade I (TaZFP1 to TaZFP9) contain a MAPK docking motif and a leucine-rich region followed by a B box (rich in basic amino acids); the latter two features are hallmarks of nuclear localization signals (NLS) (Prieve et al. 1996). TaZFP4 and TaZFP7 are incomplete sequences since no stop codon was present. The other members of this clade have strongly conserved C-terminal sequences (PVLLE/QLF) (box in Fig. 1). Analysis of the members in other clades reveals that this conserved motif is aligned with the core sequence DLN present within the EAR motif (L/F DLN L/F XP) defined by Ohta et al. (Ohta et al. 2001). This core sequence is regarded as a signature for transcriptional repressor activity in C2H2 zinc fingers (Gourcilleau et al. 2011). This DLN motif is present in TaZFP10 and TaZFP13 to TaZFP16. The absence of the DLN motif was reported in only 11 Q-type two-fingered C2H2 zinc finger sequences from 50 plant species and they were all from monocot species (Gourcilleau et al. 2011). The MAPK docking motif that is specific to clade I (see Fig. 1) was used as query with the neighboring C2H2 domain to perform a BLAST (tblastn) search of the NR and EST databases in GenBank and used to build a new phylogenetic tree (Fig. 2). We also included zinc fingers that were previously shown to be involved in Al tolerance (ART1 in rice and STOP1 in Arabidopsis) and some of their homologs in the analysis (Fig. 2). All sequences in clade I are from monocot species suggesting that this clade has evolved differently from dicots since the two groups diverged, about 200 Ma ago. Further analysis of all clade I proteins in Fig. 2 confirmed that the strongly conserved sequence (PVLLE/QLL) identified the 9 wheat sequences (Fig. 1) was also conserved in other species. Members in clade Ib proteins contain a PKA-type phosphorylation domain, and clade Ic proteins have two distinct phosphothreonine motifs known to bind a subset of FHA (forkhead-associated) domains that prefer an acidic amino-acid at position T + 3. (Fig. 1; dark grey). Members of clade Ia differ from those of clades Ib and Ic by the absence of other features. Clade II proteins do not contain a basic NLS domain but possess a recognition site for cyclin phosphorylation suggesting a role in the cell cycle. Clade III proteins contain a B box followed by a leucine-rich region. The order of these two NLS signals is thus reversed compared to clade I proteins (Fig. 1). Clade III also contains a CK1 phosphorylation site that is found at different positions within the protein. Other putative motifs such as PKC phosphorylation sites or ubiquitination sites are not shown since they are not useful to assign specific features to the clades.
Alignment of the wheat Q-type C2H2 zinc finger proteins and identification of known protein domains. The TaZFP assembled contigs (see Supplementary material 1) were translated and the coding sequences aligned using Clustal W2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html). The different protein domains were analyzed at http://www.elm.eu.org/ using wheat as model organism. The following motifs are identified:  Q-type C2H2 domain;
Q-type C2H2 domain;  leucine-rich domain;
leucine-rich domain;  B-box, basic nuclear localization signal;
B-box, basic nuclear localization signal;  MAPK docking motif;
MAPK docking motif;  PKA-type phosphorylation domain;
PKA-type phosphorylation domain;  cyclin phosphorylation;
cyclin phosphorylation;  CK1 phosphorylation site;
CK1 phosphorylation site;  phosphothreonine motifs (dark grey) known to bind a subset of FHA domains that prefer an acidic amino-acid at position T + 3;
phosphothreonine motifs (dark grey) known to bind a subset of FHA domains that prefer an acidic amino-acid at position T + 3;  phosphothreonine motif for FHA domains that prefer a large aliphatic amino acid at position T + 3;
phosphothreonine motif for FHA domains that prefer a large aliphatic amino acid at position T + 3;  core domain of EAR motifs;
core domain of EAR motifs;  new domain found in monocot species only
new domain found in monocot species only
Phylogenetic tree of Q-type C2H2 zinc finger proteins from Arabidopsis barley, maize, rice, sorghum, soybean and wheat. The NJ tree is based on full-length amino acid sequences. Bootstrap values from 10,000 replicates were used to assess the robustness of the trees. We added one new clade (clade IV) to the three ZFP clades previously described (see Online Resource 2). The proteins are identified by their usual name (see accession numbers below) or accession number followed by the species acronyms in parenthesis (At, Arabidopsis thaliana; Gm, Glycine max; Hv, Hordeum vulgare; Os, Oryza sativa; Sb, Sorghum bicolor; Ta, Triticum aestivum; Zm, Zea mays). Accession numbers: AAO65873.1; AK250895.1; ART1 (Os12g0170400); At5g22890; AZF1 (AB030731.1); AZF2 (AB030730.1); AZF3 (AB030732.1); BQ463075; BQ762731.1; EAZ29083.1; NP_001152566.1; Os02g0572900; Os03g0838800; OS04g0165200; OS08g0562300; SCOF1 (U68763.1); STOP1 (At1g34370); STZ/ZAT10 (NM_102538.2); XP_002466195.1 XP_002466196.1; ZAT5 (X98678.1); ZAT7 (NM114478.3); ZAT11 (NM_129298.2); ZAT12 (X98673.1); ZFP16-2 (NP001150897.1); ZFP150 (AA42460.1); ZFP179 (AAL76091.1); ZFP182 (AY286474.1); ZFP245 (AAP42461); ZFP252 (AAO46041.1)
Identification of zinc finger members associated with Al tolerance
Our previously published microarray study (Houde and Diallo 2008) identified a C2H2 zinc finger member of the Q-type TaZFP gene family associated with Al tolerance. The sequence (BJ220837) representing the C2H2 zinc finger transcript was used as query to determine the similarity to other transcripts on the wheat microarray. A total of seven different related ESTs were identified with e-values between e-25 and e-75. The same query also retrieved seven different wheat transcripts with e-values below e-25 in GenBank and Triticum aestivum DFCI Gene Index (TaGI) database (http://compbio.dfci.harvard.edu/cgi-bin/tgi/gimain.pl?gudb=wheat). The specificity of the oligonucleotides used on the microarrays to represent the zinc finger EST BJ220837 was tested through BLAST against the wheat zinc finger dataset published by Kam et al. (Kam et al. 2008). This analysis revealed that 16 different wheat C2H2 zinc fingers members contain sequences that could hybridize with at least one of the oligonucleotide probes on the microarray indicating that closely related members of the gene family may not be distinguished.
Expression profiling by qRT-PCR was performed for the 16 putative TaZFP transcripts from root tips of plants exposed to three different Al concentrations in Al-tolerant and sensitive cultivars. Figure 3 shows that most of the TaZFP transcripts were weakly or moderately regulated in all wheat lines when exposed to high Al concentrations suggesting that they may participate in Al stress responses. To identify genes associated with Al tolerance, a significant difference in expression between the Al-tolerant line Atlas66 exposed to 50 μM Al was compared to the Al-sensitive line Bounty at 5 μM Al AND between the Al-tolerant near-isogenic line Century-T exposed to 50 μM Al compared to the Al-sensitive near-isogenic line Century-S at 5 μM Al. These Al concentrations (50 μM in Al-tolerant lines and 5 μM in Al-sensitive lines) caused the same root growth inhibition in the four wheat lines. This physiological parameter indicates that the same level of stress is perceived by the root and was thus used to evaluate gene responses in relation to Al tolerance. The analysis revealed that several TaZFP transcripts are associated with Al tolerance in at least one of the tolerant wheat lines (Atlas66 OR Century-T) (see bars and asterisk showing a significant difference) and retained two TaZFP transcripts (TaZFP2 and TaZFP3) that are associated with Al tolerance in both tolerant wheat lines compared to the two sensitive ones. Further analysis were thus performed on TaZFP2 and TaZFP3 since these two TaZFP transcripts are associated with Al tolerance in the parental (Atlas66) and the backcrossed Century-T line.
Quantitative Real-Time PCR analysis of zinc finger genes in different wheat lines exposed to Al for 24 h. Control non-treated plants of two tolerant cultivar (Atlas66 and Century-T) and two sensitive cultivars (Bounty and Century-S) were exposed for 24 h to a solution containing 0 to 250 μM Al. RNA was extracted, reverse-transcribed and transcript levels were measured by qRT-PCR. The fold expression and statistical differences were calculated relative to the transcript level in the untreated Atlas66 plants (0 μM). Note that the scale is different for the various genes. A statistical difference (p < 0.05) is indicated by the letter “a” in the histogram columns. A statistical difference between tolerant cultivars and sensitive lines exposed to Al concentrations giving an RGI50 (50 μM Al for Atlas66 and Century-T and 5 μM for Bounty and Century-S) is indicated by brackets and an asterisk (p < 0.05)
Figure 4a shows the expression kinetics at early time points for TaZFP2 and TaZFP3 in Atlas66 and Bounty. A 5–10 fold up-regulation is observed under control conditions (pH 4.15 without Al) for TaZFP2 after 1 h of exposure (Ctr 1) in Atlas66 and Bounty. This indicates that part of the early response to Al exposure, which needs to be performed at low pH to maintain Al in its Al3+ form, results from the up-regulation caused by acidic conditions. In the presence of Al, the TaZFP2 transcript is strongly up-regulated by Al reaching nearly a 70 fold increase only in the tolerant cultivar. This accumulation occurs after only 0.5 h of Al exposure (Al 0.5) and is higher than the sensitive cultivar Bounty at all time points, confirming the association with Al tolerance. For TaZFP3, the transcript up-regulation also occurs after 0.5 h of Al exposure and accumulates specifically in the tolerant cultivar, but its accumulation is much lower at other time points compared to TaZFP2. The rapid 20 fold induction of TaZFP3 at 0.5 h of Al exposure, but much lower at other time points, suggests that it may play a role in the short term response to Al exposure or that it is part of an initial response in a cascade of events involving downstream genes that remain to be identified. Figure 4b shows that the expression level of TaZFP2 and TaZFP3 after 0.5 h of Al exposure is also associated with Al tolerance in the near-isogenic line Century-T compared to Century-S. This result confirms that these two TaZFP genes are associated with Al tolerance in both tolerant wheat lines tested.
Time course quantitative Real-Time PCR analysis of zinc finger genes in different wheat cultivars exposed to Al. Plants of Atlas66 and Bounty (a) or Century-T and Century-S (b) cultivars were treated as described in Fig. 3 for 0, 0.5, 1, 3, 6, and 24 h. The fold expression and statistical differences were calculated relative to the Atlas66 (a) or Century-T (b) initial controls (Ctr 0). A statistical difference (p < 0.05) is indicated by the letter “a” in the histogram columns
The homology of the two TaZFP genes with genes involved in the response to oxidative stress in Arabidopsis (ZAT7 and ZAT12) led us to evaluate the response of these TaZFPs to H2O2 since many different forms of free radicals are detoxified into H2O2 and it is well established that Al exposure causes oxidative stress (Richards et al. 1998). It should be noted that clade I does not contain any gene homologs from dicot species. Clade II thus contains the closest homologs based on the phylogenetic analysis (see also the Discussion section). In these experiments, we used a neutral pH to reduce the impact of acid conditions on the up-regulation of these genes. Results in Fig. 5 show that in the control, a stable expression is observed (control 0.5). TaZFP2 and TaZFP3 accumulate strongly in response to H2O2 exposure (H2O2 0.5) in the tolerant cultivar Atlas66. The up-regulation of these two genes is associated with Al tolerance (* in Fig. 5a) indicating that these two genes are up-regulated by Al through a ROS responsive pathway. Figure 5b shows that the expression level of TaZFP2 and TaZFP3 after 0.5 h of Al exposure is also associated with Al tolerance in the near-isogenic line Century-T compared to Century-S. This result confirms that these two TaZFP genes associated with Al tolerance are responsive to H2O2 in both Al-tolerant wheat lines tested but not in the Al-sensitive lines.
Quantitative Real-Time PCR analysis of zinc finger genes in wheat cultivars exposed to H2O2. Al tolerant (Atlas66 and Century-T) and Al-sensitive (Bounty and Century-S) cultivars were treated with 100 μM H2O2 in 1 mM CaCl2 at pH 7 for 0.5 h. Controls were collected at 0 and 0.5 h. The fold expression and statistical differences were calculated relative to the initial controls (Ctr 0). A statistical difference between the two Al-tolerant cultivars and the two Al-sensitive cultivars is indicated by brackets and an asterisk (p < 0.05)
Discussion
Our previous work using microarrays identified 25 genes associated with Al tolerance in both Atlas66 and Century-T including the constitutively expressed ALMT1 gene that was expressed more than 5 fold more in the tolerant lines compared to the sensitive lines (Houde and Diallo 2008). The encoded protein is involved in the extrusion of malate which has been suggested to play a role in Al exclusion. It is well known that Al stress causes several symptoms including oxidative stress (Richards et al. 1998) and the association of different other genes with Al tolerance suggest that some of these genes could contribute to the alleviation of different stress components associated to Al exposure (see Houde and Diallo 2008). Among the other genes associated to Al tolerance, we identified only one transcription factor, that was identified as a member of the C2H2 ZFP family. In the present work, we used bioinformatic analyses to reconstruct closely related cDNAs, establish their phylogenetic relationship and identify the members that are associated with Al tolerance using two Al-tolerant cultivars, including a near-isogenic wheat line (Atlas66 and Century-T) and their sensitive counterparts (Bounty and Century-S).
We reconstructed a total of 16 different Q-type ZFPs that do not contain the conserved DLN motif (L/F DLN L/F XP) found in most proteins of this class (Gourcilleau et al. 2011). These proteins were subdivided in three different clades with clade I further subdivided in three sub-clades containing specific protein domains. All proteins of clade I contain a MAPK docking motif suggesting that members of this clade interact with a MAPK signaling cascade. MAPK signaling pathways were proposed to be associated with Al responses in Coffea arabica cell suspension cultures (Arroyo-Serralta et al. 2005) and in the response to Al associated to malate efflux in the wheat root apex (Osawa and Matsumoto 2001). Furthermore, we have previously identified at least two genes induced by Al in wheat (WCI-5 and WAS-2) that were previously shown to be induced through a MAP kinase pathway in response to pathogens in Arabidopsis (Houde and Diallo 2008). Interestingly, the MAP kinase SLT2 was shown to be important for Al tolerance in yeast (Schott and Gardner 1997). The MAPK domain in TaZFP2 and TaZFP3 may thus play an important role in the regulation of their activity through this signaling pathway. Furthermore, the proteins in clade I possess a novel sequence (PVLLE/QLV) that is highly conserved in wheat (Fig. 1) and other monocot species (from Fig. 2). The high conservation of this sequence suggests that it is a monocot specific domain with an important function. Its presence at the C-terminus in a position similar to the DLN motif (Fig. 1) may indicate that this motif is a monocot specific repressor motif. Further investigations, such as mutations/deletions of this domain in transgenic plants, will be needed to elucidate this putative function.
The expression kinetics revealed that two of the clade Ia ZFPs accumulate rapidly upon Al exposure in Al tolerant cultivars but not in sensitive cultivars. The use of a near-isogenic line derived from the cultivar Atlas66 (backcrossed 6 times) greatly reduces the risk of identifying a false positive gene as associated with Al tolerance. The same approach was used in our microarray analyses where single comparisons between one Al tolerant and one Al-sensitive cultivar (Atlas66 versus Bounty; OR Century-T versus Century-S) identified over 1,000 differentially expressed transcripts. However, when only genes that are common to both pairs of Tolerant/Sensitive comparisons were retained, the number of genes was reduced to only 25 genes associated with Al tolerance (Houde and Diallo 2008).
The Al-tolerance protein STOP1 identified in Arabidopsis (Sawaki et al. 2009) and ART1 from rice (Yamaji et al. 2009) were compared in a new phylogenetic analysis and they were clearly separated in a new clade (we named this clade as clade IV in our phylogenetic tree, Fig. 2) that is very distinct from the other clades. This separation in a different clade is mostly because these two Al tolerance genes are in a distinct family which is different from the Q-type subfamily. ART1 and STOP1 are clearly related and play an important role in Al tolerance (Sawaki et al. 2009; Yamaji et al. 2009). The presence of at least three rice genes that are of the Q-type within clade Ia indicates that these genes play a distinct role within the ZFP family. Furthermore, similar genes from different monocot species are found within this clade (Fig. 2). ZFP182, a rice gene member of clade I, is inducible by salt, cold and ABA and was shown to improve salt tolerance in transgenic tobacco and rice plants (Huang et al. 2007). ZFP179, a member of clade II, is a rice salt- and drought-inducible zinc finger that increases the expression of a number of stress-related genes and can improve tolerance to salt and oxidative stress in transgenic rice (Sun et al. 2010). Clade II also contains different ZAT proteins associated with oxidative stress tolerance in Arabidopsis and contains four wheat ZFP proteins (ZFP10 to ZFP13) represented by ESTs isolated from normal or stress exposed roots (salt, Al). Clade III contains proteins (ZFP245 and ZFP252) associated with abiotic stress in rice (Huang et al. 2005; Xu et al. 2008). In Arabidopsis, proteins that are part of the C1 C2H2-type zinc finger family were shown to play key roles in different developmental pathways, as well as in the defense and stress response pathways (Ciftci-Yilmaz and Mittler 2008). Since all abiotic stresses cause more than one type of stress (oxidative stress, dehydration, osmotic stress, wounding, etc.) which in turn activate distinct signaling pathways, the specific features associated with the protein domains of the different clades and subgroups may confer specificity to different stress components and signaling pathways leading to their induction. However, it is premature to speculate on the specific roles of clade members since genes from different clades participate in similar stress responses. Furthermore, the information currently available on these transcripts cannot be associated with specific wheat genomes (A, B or D) and the copies assembled represent bioinformatic predictions. Cloning of the different gene copies will be needed to identify sequence polymorphism and to associate them with specific genomes. It should be noted that the sequence homology between the different TaZFP transcripts is lower than 98 % while homeologs generally have around 99 % homology or higher.
Al is well known to cause oxidative stress and various oxidative damages leading to the over-expression of several oxidative stress associated genes (Kochian 1995; Richards et al. 1998). However, few studies focused on early responses to Al exposure. The H2O2 stimulated inositol 1,4,5-triphosphate pathway was shown to be rapidly (1 min) inhibited by Al and was proposed to be caused by the inhibition of phospholipase C (Jones and Kochian 1995). Similarly, a redox activity associated to root growth, detected by nitro-blue tetrazolium (NBT) staining (which is normally reduced by a superoxide anion), is rapidly inhibited (within 1 min) in the root elongation zone that is highly sensitive to Al (Maltais and Houde 2002). The mechanism of root growth was described in recent years and involves the production of hydroxyl radicals (●OH) that can non-enzymatically cleave the bonds between sugar molecules and requires a reduced metal such as Cu+ (or Fe2+), O ●−2 , H2O2 and ascorbate (Liszkay et al. 2003; Liszkay et al. 2004; Schopfer 2001). The exact biochemical steps involved in the reduction of metal ions remains to be established but ascorbate is an important substrate for this reaction (Supplementary material 3; box after the arrow from ascorbate). However, as described by Liszkay et al. (Liszkay et al. 2004) the use of different scavengers, inhibitors, and stimulators are consistent with the concept that ●OH is formed in a peroxidase-catalyzed reaction from apoplastic O ●−2 and H2O2 produced by an NAD(P)H oxidase located in the plasma membrane of root cells. The discovery that different ROS (H2O2, O ●−2 ) are needed to generate hydroxyl radicals during the normal process of cell wall elongation and root growth indicates that the redox activity detected by NBT staining is a superoxide radical. The recent finding that the pro-oxidant activity of Al can be explained by its ability to react with the superoxide anion and form a stable superoxide Al radical ([Al(O2•)(H2O)5]2+) (Mujika et al. 2011) strongly supports the possibility that Al could bind superoxide anions produced during root growth and thus interfere with the cell wall loosening needed for root elongation growth. The pro-oxidant activity of the superoxide Al radical could then cause oxidative damages to different molecules including phospholipids and sensitive enzymes (such as phospholipase C mentioned above). Similarly, the accumulation of this superoxide Al radical in the cytoplasm can cause intracellular ROS damages. Even though, it is difficult to confirm which ROS is produced after initial exposure to Al, it is well known that detoxification pathways will rapidly lead to H2O2 production. This molecule is more stable than many other free radicals and is a well known signal transduction intermediate. Our results have shown that Al specifically up-regulates TaZFP2 and TaZFP3 in the two Al tolerant lines analyzed. However, their up-regulation appears to be stronger in the parental line Atlas66 (especially for TaZFP2, Fig. 3) compared to the line Century-T. We do not know whether the level of expression at the transcript level is directly related to the level of Al tolerance or other stress components that are associated to Al tolerance and detailed characterization of these genes is needed to better understand dosage effects. The finding that H2O2 also specifically up-regulate these two ZFP transcripts, in Al tolerant cultivars only, suggests that up-regulation of these transcription factors during Al exposure is mediated by a ROS responsive pathway. The up-regulation by H2O2 indicates that TaZFP2 and TaZFP3 may represent the wheat orthologs of the two C2H2 zinc fingers transcription factors ZAT7 and ZAT12 that were shown to improve ROS tolerance in Arabidopsis thaliana.
The two TaZFP wheat transcription factors associated with Al tolerance could also play an important role in the improvement of oxidative stress which is an important component of Al stress. Furthermore, the different TaZFPs may also be involved in the response to other stresses since all clades contain ZFP proteins that respond to similar stresses. For example, TaZFP10-13 are in the same clade as the Arabidopsis ZAT10 and ZAT12 that are associated to oxidative stress tolerance suggesting that they may also play a role in the response to oxidative stress. Similarly, genes responding to oxidative stress or salinity are present in different clades indicating that clades are not regrouping proteins based on stress types but regroup proteins that share similar features such as regulatory pathways. This hypothesis is supported by the specific protein domains that can distinguish the clades as described above. However, since the TaZFP10-13 transcripts are not associated to Al tolerance, they were not analyzed further for their potential response to oxidative stress. The use of a segregating population will be useful as genetic tool to determine how these the TaZFP2/TaZFP3 genes are associated to Al or oxidative stress and will allow us to confirm whether their expression is associated with genetic loci from the A, B and D genomes involved in Al tolerance. The over-expression of TaZFP2/TaZFP3 and the characterization of specific protein domains will help to determine their specific roles in wheat Al/ROS tolerance and to identify downstream genes that are part of their regulon.
References
Abercrombie JM, Halfhill MD, Ranjan P, Rao MR, Saxton AM, Yuan JS, Stewart CN Jr (2008) Transcriptional responses of Arabidopsis thaliana plants to As (V) stress. BMC Plant Biol 8(87):87
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Aniol A, Gustafson JP (1984) Chromosome location of genes controlling aluminum tolerance in wheat, rye, and triticale. Can J Genet Cytol 26:701–715
Anoop VM, Basu U, McCammon MT, McAlister-Henn L, Taylor GJ (2003) Modulation of citrate metabolism alters aluminum tolerance in yeast and transgenic canola overexpressing a mitochondrial citrate synthase. Plant Physiol 132(4):2205–2217
Arroyo-Serralta GA, Ku-Gonzalez A, Hernandez-Sotomayor SM, Zuniga Aguilar JJ (2005) Exposure to toxic concentrations of aluminum activates a MAPK-like protein in cell suspension cultures of Coffea arabica. Plant Physiol Biochem 43(1):27–35
Baier AC, Somers DJ, Gustafson JP (1995) Aluminum tolerance in wheat: correlating hydroponic evaluations with field and soil performances. Plant Breed 114:291–296
Berzonsky WA (1992) The genomic inheritance of aluminum tolerance in Atlas-66 wheat. Genome 35:689–693
Burge C, Karlin S (1997) Prediction of complete gene structures in human genomic DNA. J Mol Biol 268(1):78–94
Carver BF, Whitmore WE, Smith EL, Bona L (1993) Registration of four aluminum-tolerant winter wheat germplasms and two susceptible near-isolines. Crop Sci 33:1113–1114
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12(10):444–451. doi:10.1016/j.tplants.2007.07.002
Ciftci-Yilmaz S, Mittler R (2008) The zinc finger network of plants. Cell Mol Life Sci 65(7–8):1150–1160
de la Fuente JM, Ramirez-Rodriguez V, Cabrera-Ponce JL, Herrera-Estrella L (1997) Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science 276(5318):1566–1568
Delhaize E, Craig S, Beaton CB, Bennet RJ, Jagadish VC, Randall PJ (1993a) Aluminum tolerance in wheat (Triticum aestivum L.) I. Uptake and distribution of aluminum in root apices. Plant Physiol 103:685–693
Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ (1993b) Aluminum tolerance in wheat (Triticum aestivum L.) (I. Uptake and distribution of aluminum in root apices). Plant Physiol 103(3):685–693
Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H (2004) Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc Natl Acad Sci U S A 101(42):15249–15254. doi:10.1073/pnas.0406258101
Delisle G, Champoux M, Houde M (2001) Characterization of oxalate oxidase and cell death in Al-sensitive and tolerant wheat roots. Plant Cell Physiol 42(3):324–333
Fredslund J, Lange M (2007) Primique: automatic design of specific PCR primers for each sequence in a family. BMC Bioinforma 8(369):369
Gould CM, Diella F, Via A, Puntervoll P, Gemund C, Chabanis-Davidson S, Michael S, Sayadi A, Bryne JC, Chica C, Seiler M, Davey NE, Haslam N, Weatheritt RJ, Budd A, Hughes T, Pas J, Rychlewski L, Trave G, Aasland R, Helmer-Citterich M, Linding R, Gibson TJ (2010) ELM: the status of the 2010 eukaryotic linear motif resource. Nucleic Acids Res 38(Database issue):D167–D180
Gourcilleau D, Lenne C, Armenise C, Moulia B, Julien JL, Bronner G, Leblanc-Fournier N (2011) Phylogenetic study of plant Q-type C2H2 zinc finger proteins and expression analysis of poplar genes in response to osmotic, cold and mechanical stresses. DNA Res 18(2):77–92
Guo P, Bai G, Carver B, Li R, Bernardo A, Baum M (2007) Transcriptional analysis between two wheat near-isogenic lines contrasting in aluminum tolerance under aluminum stress. Mol Genet Genomics 277(1):1–12
Hoekenga OA, Maron LG, Pineros MA, Cancado GM, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T, Matsumoto H, Yamamoto Y, Koyama H, Kochian LV (2006) AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci U S A 103(25):9738–9743
Houde M, Diallo AO (2008) Identification of genes and pathways associated with aluminum stress and tolerance using transcriptome profiling of wheat near-isogenic lines. BMC Genomics 9:400. doi:10.1186/1471-2164-9-400
Huang X, Madan A (1999) CAP3: a DNA sequence assembly program. Genome Res 9(9):868–877
Huang J, Wang JF, Wang QH, Zhang HS (2005) Identification of a rice zinc finger protein whose expression is transiently induced by drought, cold but not by salinity and abscisic acid. DNA Seq 16(2):130–136
Huang J, Yang X, Wang MM, Tang HJ, Ding LY, Shen Y, Zhang HS (2007) A novel rice C2H2-type zinc finger protein lacking DLN- box/EAR-motif plays a role in salt tolerance. Biochim Biophys Acta 1769:220–227
Johnson JP, Carver BF, Baligar VC (1997) Expression of aluminium tolerance transfered from Atlas66 to hard winter wheat. Crop Sci 37:103–108
Jones DL, Kochian LV (1995) Aluminum inhibition of the inositol 1,4,5-Trisphosphate signal transduction pathway in wheat roots: a role in Aluminum toxicity? Plant Cell 7(11):1913–1922
Kam J, Gresshoff PM, Shorter R, Xue GP (2008) The Q-type C2H2 zinc finger subfamily of transcription factors in Triticum aestivum is predominantly expressed in roots and enriched with members containing an EAR repressor motif and responsive to drought stress. Plant Mol Biol 67(3):305–322. doi:10.1007/s11103-008-9319-3
Kidd PS, Llugany M, Poschenrieder C, Gunse B, Barcelo J (2001) The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52(359):1339–1352
Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46:237–260
Kochian LV, Pineros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274:175–195
Koyama H, Kawamura A, Kihara T, Hara T, Takita E, Shibata D (2000) Overexpression of mitochondrial citrate synthase in Arabidopsis thaliana improved growth on a phosphorus-limited soil. Plant Cell Physiol 41(9):1030–1037
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948
Liszkay A, Kenk B, Schopfer P (2003) Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta 217(4):658–667
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O(2)(.-), H(2)O(2), and (.)OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136(2):3114–3123, discussion 3001
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25(4):402–408
Ma HX, Bai GH, Carver BF, Zhou LL (2005) Molecular mapping of a quantitative trait locus for aluminum tolerance in wheat cultivar Atlas 66. Theor Appl Genet 112(1):51–57
Magalhaes JV, Liu J, Guimaraes CT, Lana UG, Alves VM, Wang YH, Schaffert RE, Hoekenga OA, Pineros MA, Shaff JE, Klein PE, Carneiro NP, Coelho CM, Trick HN, Kochian LV (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39(9):1156–1161
Maltais K, Houde M (2002) A new biochemical marker for aluminium tolerance in plants. Physiol Plant 115(1):81–86
Mesdag J, Slootmaker LAJ (1969) Classifying wheat varieties for tolerance to high soil acidity. Euphytica 18:36–42
Mittler R, Kim Y, Song L, Coutu J, Coutu A, Ciftci-Yilmaz S, Lee H, Stevenson B, Zhu JK (2006) Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett 580(28–29):6537–6542. doi:10.1016/j.febslet.2006.11.002
Mujika JI, Ruiperez F, Infante I, Ugalde JM, Exley C, Lopez X (2011) Pro-oxidant activity of aluminum: stabilization of the aluminum superoxide radical ion. J Phys Chem A 115(24):6717–6723
Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M (2001) Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13(8):1959–1968
Osawa H, Matsumoto H (2001) Possible involvement of protein phosphorylation in aluminum-responsive malate efflux from wheat root apex. Plant Physiol 126(1):411–420
Pagni M, Ioannidis V, Cerutti L, Zahn-Zabal M, Jongeneel CV, Hau J, Martin O, Kuznetsov D, Falquet L (2007) MyHits: improvements to an interactive resource for analyzing protein sequences. Nucleic Acids Res 35:W433–W437
Pellet DM, Papernik LA, Kochian LV (1996) Multiple aluminum-resistance mechanisms in wheat (roles of root apical phosphate and malate exudation). Plant Physiol 112(2):591–597
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6(1):65–74
Prieve MG, Guttridge KL, Munguia JE, Waterman ML (1996) The nuclear localization signal of lymphoid enhancer factor-1 is recognized by two differentially expressed Srp1-nuclear localization sequence receptor proteins. J Biol Chem 271(13):7654–7658
Rice P, Longden I, Bleasby A (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16(6):276–277
Richards KD, Schott EJ, Sharma YK, Davis KR, Gardner RC (1998) Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiol 116(1):409–418
Rizhsky L, Davletova S, Liang H, Mittler R (2004) The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J Biol Chem 279(12):11736–11743. doi:10.1074/jbc.M313350200
Ryan PR, Delhaize E, Randall PJ (1995) Malate efflux from root apices and tolerance to aluminium are highly correlated in wheat. Aust J Plant Physiol 22:531–536
Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E (2009) A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol 149(1):340–351
Sasaki T, Ryan PR, Delhaize E, Hebb DM, Ogihara Y, Kawaura K, Noda K, Kojima T, Toyoda A, Matsumoto H, Yamamoto Y (2006) Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant Cell Physiol 47(10):1343–1354
Sawaki Y, Iuchi S, Kobayashi Y, Ikka T, Sakurai N, Fujita M, Shinozaki K, Shibata D, Kobayashi M, Koyama H (2009) STOP1 regulates multiple genes that protect arabidopsis from proton and aluminum toxicities. Plant Physiol 150(1):281–294
Schopfer P (2001) Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J 28(6):679–688
Schott EJ, Gardner RC (1997) Aluminum-sensitive mutants of Saccharomyces cerevisiae. Mol Gen Genet 254(1):63–72
Sun SJ, Guo SQ, Yang X, Bao YM, Tang HJ, Sun H, Huang J, Zhang HS (2010) Functional analysis of a novel Cys2/His2-type zinc finger protein involved in salt tolerance in rice. J Exp Bot 61(10):2807–2818
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28(10):2731–2739
Tang Y, Garvin DF, Kochian LV, Sorrells ME, Carver BF (2002) Physiological genetics of aluminum tolerance in the wheat cultivar Atlas 66. Crop Sci 42:1541–1546
Tesfaye M, Temple SJ, Allan DL, Vance CP, Samac DA (2001) Overexpression of malate dehydrogenase in transgenic alfalfa enhances organic acid synthesis and confers tolerance to aluminum. Plant Physiol 127(4):1836–1844
Xu DQ, Huang J, Guo SQ, Yang X, Bao YM, Tang HJ, Zhang HS (2008) Overexpression of a TFIIIA-type zinc finger protein gene ZFP252 enhances drought and salt tolerance in rice (Oryza sativa L.). FEBS Lett 582(7):1037–1043
Yamaji N, Huang CF, Nagao S, Yano M, Sato Y, Nagamura Y, Ma JF (2009) A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell 21(10):3339–3349
Zhou LL, Byeon IJ, Ma HX (2007) Quantitative trait loci for aluminum resistance in wheat. Mol Breeding 19:153–161
Acknowledgments
The authors thank Dr N. Chevrier (UQAM) for bulking up the Atlas66 seed stock, and Dr F. Ouellet and F. Sarhan for critical reading of the manuscript. This work was supported by a Natural Sciences and Engineering Research Council of Canada grant (OGP0138557) to M.H.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jian Feng Ma.
Rights and permissions
About this article
Cite this article
Ali-Benali, M.A., Badawi, M., Houde, Y. et al. Identification of oxidative stress-responsive C2H2 zinc fingers associated with Al tolerance in near-isogenic wheat lines. Plant Soil 366, 199–212 (2013). https://doi.org/10.1007/s11104-012-1417-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1417-y