Abstract
Background and aims
Phosphorus (P) nutrition is very important during early maize seedling growth. Remobilization of endogenous seed P and uptake of exogenous P are therefore of prime importance during this period. Our objectives were to study the effect of the availability of endogenous and exogenous P on i) remobilization of endogenous seed P, ii) the beginning of exogenous P uptake and its intensity, iii) their interaction and effect on seedling development.
Methods
Seeds with high and low reserves of endogenous seed P were cultivated at three rates of availability of exogenous P (0, 100, 1,000 μM) over a growth period of 530 cumulated degree days after sowing. Exogenous P was labeled with radioactive P (32P) to distinguish the two fluxes of P in seedlings, one due to remobilization of seed P and the other to uptake of exogenous P.
Results
Initially, 86% of endogenous seed P was localized in the scutellum, mainly in the form of phytate, regardless of initial endogenous seed P. At 89 cumulated degree days after sowing (base temperature: 10°C), 98% of seed phytate was hydrolyzed in all treatments. In treatments with available exogenous P, significant uptake of exogenous P started at 71 cumulated degree days after sowing. Efficient uptake of exogenous P depended on its availability, but was independent of phytate hydrolysis and seedling P status. Significant loss of P from germinating seeds due to efflux was observed and was also independent of the availability of exogenous P.
Conclusions
Our results show that hydrolysis of seed P was not influenced by the availability of exogenous P, and conversely, that uptake of exogenous P was not influenced by endogenous P in the seed. This suggests that remobilization of endogenous seed P and uptake of exogenous P by seedling roots are controlled independently.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a component of key molecules like nucleic acid, phospholipids, or ATP, phosphorus nutrition is one of the most significant factors in the plant life cycle, particularly during germination and early growth stages (Grant et al. 2001; Assuero et al. 2004; Pellerin et al. 2000; Plénet et al. 2000; Colomb et al. 2000; Mollier and Pellerin 1999). Although crops absorb only small quantities of P during the first 2–3 weeks of growth, this early accumulation of P is extremely important for the final crop yield (Römer and Schilling 1986; Barry and Miller 1989; Gavito and Miller 1998; Römer et al. 1988).
The main metabolic events associated with seed germination are the remobilization of nutrients stored in seeds and their subsequent translocation and utilization in different seedling compartments. In the seed, phosphorus is stored primarily in the form of phytate (Park et al. 2006; Lott et al. 1995). The phytate content of cereal seed is highly correlated with total phosphorus (Lockhart and Hurt 1986). In maize, 86% of total endogenous seed P is localized in the scutellum and the remaining 14% in the endosperm (Nadeem et al. 2011).
Previous studies showed that the phytate stored in the scutellum started to hydrolyze from the 1st day after sowing and reached a plateau between the 5th and 7th day; phytate was converted into organic phosphorus and mineral cations that become available for the young maize seedlings (Nadeem et al. 2011; Laboure et al. 1993). It was also shown that 4-day-old maize seedlings contained only P originating from the seed and that significant uptake of exogenous P by roots only started after the 5th day after sowing. During the first 2–3 weeks of growth, seedling P requirements were largely met by remobilization of endogenous seed P with a little uptake of exogenous P (Nadeem et al. 2011). Consequently, during the first 2–3 weeks after germination, the remobilization of endogenous seed P is of prime importance along with uptake of exogenous P.
As the remobilization of endogenous seed P and the uptake of exogenous P overlap in time (Nadeem et al. 2011), the question arises whether these processes have an effect on each other or if they are independently controlled. Our objectives were to study the effect of the availability of endogenous and exogenous P on i) the remobilization of endogenous seed phosphorus, ii) the beginning of uptake of exogenous P and its intensity, iii) their interaction and its effect on the development of young maize seedlings during germination and early growth. Exogenous P was labeled with 32P to distinguish the two fluxes of P, (one remobilized from endogenous seed reserves and the other being the uptake of exogenous P) in young maize seedlings and their effect on each other according to their origin.
Materials and methods
Seed treatment and seedling establishment
The study was conducted in a growth chamber in November and December 2010, at INRA Bordeaux, France. The homogenous maize seeds (cv. DKC-5783) with low (LS) and high (HS) reserves of endogenous seed P were harvested as part of a long term P fertilization experiment with irrigated maize at the experimental site of Pierroton in southwest France (lat. 44° 44′ 30″; long. 0° 46′ 59″; alt. 55 m). Homogenous seeds from LS and HS treatments with the same initial seed weight (0.33 g ± 0.001) were used for this study. The initial seed endogenous P was determined by an adaptation of malachite green colorimetric technique after P mineralization with HNO3 (Van Veldhoven and Mannaerts 1987). The initial endogenous seed P was 506 μg P seed−1 and 952 μg P seed−1 for LS and HS seeds, respectively. The LS and HS seeds were sown in opaque polypropylene pots (10*10*14 cm width, length and height, respectively) with three rates of availability of exogenous P: 0, 100 and 1,000 μM for the no P treatment (0P), low (LP) and high (HP) exogenous P availability, respectively. The exogenous P was labeled with radioactive P (32P) to quantify uptake of exogenous P and to monitor the remobilization kinetics of endogenous seed P and the partitioning of seedling P in different seedling compartments during germination and early growth stages. Out of a total 183 pots i) 180 pots with 32P labeling were used to monitor the kinetics of remobilization of the reserves of endogenous P in the seed and of the uptake of exogenous P and ii) three pots without 32P labeling sown with HS and HP to measure radioactivity induced background noise at final seedling harvest.
Composition of the exogenous nutrient solution of P and labeling with 32P
Three complete nutrient solutions containing P and all the necessary micro and macro nutrients were used to grow the maize seedlings (Bhadoria et al. 2004) with three concentrations of exogenous P. The nutrient solution consisted of 1 mM NO3 as Ca(NO3)2.4H2O, 0.2 mM K as KCl, 0.1 mM Mg as MgSO4.7H2O, 46 μM B as H3BO3, 9.1 μM Mn as MnCl2.4H2O, 0.8 μM Zn as ZnSO4.7H2O, 0.3 μM Cu as CuSO4.5H2O, 0.5 μM Mo as (NH4)6Mo7O24.4H2O, 2 mg L−1 Fe was added as Sequestrene-138 Fe (water soluble granules with 6% Fe in chelated form). The concentrations of exogenous P were 0, 100 or 1,000 μM as NaH2PO4.2H2O for 0P, LP and HP, respectively. The pH of all three nutrient solutions was adjusted to 6.4 with addition of NaOH. An initial measured quantity (R t0), 1,561 kBq and 1,669 kBq of 32P were added in 16 L of the 0P and LP solutions, respectively, while 1,667 kBq were added in 15 L of the HP solution for labeling of exogenous P. The initial specific activity (SA t0) was 1.043 kBq (μmol P)−1 and 0.111 kBq (μmol P)−1 in LP and HP labeled nutrient solutions, respectively.
Seedling growth conditions
The LS and HS seeds were sown with three rates of availability of exogenous P: 0, 100 and 1,000 μM for the no P treatment (0P), low (LP) and high (HP) exogenous P availability, respectively. Before sowing, the LS and HS seeds were separated into groups of nine for each endogenous and exogenous P treatment. Out of a total 183 pots, 90 were planted with LS seeds and remaining 90 pots with HS seeds. On November 9, 2010, the seeds were sown in perlite (120 g pot−1) at a sowing depth of 2 cm, and 210 mL labeled nutrient solution was added to each pot to reach 90% saturation capacity of the perlite. Out of a total 90 LS seed pots, each set of 30 pots was irrigated with a labeled nutrient solution of 0P, LP, and HP, respectively. Similarly, out of 90 pots planted with HS seeds, each set of 30 pots was irrigated with a labeled nutrient solution of 0P, LP, and HP, respectively (210 mL pot−1). The labeled nutrient solution (0P, LP and HP) was added to all 180 pots. The same volume (210 mL pot−1) of HP nutrient solution without labeling was added to the three control pots sown with HS seeds to measure background noise induced by radioactivity.
All the pots were placed in plastic trays. The photoperiod was 18 h of light and 6 h of darkness throughout the seedling growth period (Palomo et al. 2006). Air temperature and the temperature inside the pots were measured with copper-constantan thermocouples (0.2 mm diameter). Air relative humidity was measured with a relative humidity probe (HMP35AC, Vaisala, Finland). Photosynthetically active radiation (PAR) was recorded with a PAR Sensor (SKP 215, Skye Instruments, Llandrindod Wells, UK and JYP-1000, SDEC, France). All sensors were connected to a data logger (21X, Campbell Scientific, UK). Measurements were taken every 15 min, but only hourly average values were recorded. During the experiment, the average air temperature, relative humidity and light intensity were 28°C, 49% and 657 μmol m−2 sec−1, respectively. Thermal time (TT) in cumulated degree days after sowing was calculated on a daily basis as follows:
where \( \overline {{T_d}} \) is the daily air temperature (in °C) as the average of the hourly air temperatures recorded each day. Any hourly air temperature exceeding 30°C was set at 30°C (Bonhomme et al. 1994). T b (10°C) is the base temperature (Eagles and Hardacre 1979).
Throughout the experiment, the pots were irrigated with water on the basis of water lost from the pots by evapotranspiration. Pots destined for the last seedling harvest were weighed (g) daily to calculate average weight loss due to evapotranspiration.
Seedling harvest, growth measurements and chemical analysis
The experiment was carried out for 530 cumulated degree days after sowing and seedlings were harvested 10 times during the first 4 weeks of growth. The initial seed biomass, total P, phytate and phytate-P reserves were determined in 18 seeds (3 replications of 6 seeds) in each LS and HS seed. The seedlings were harvested at 16, 34, 52, 71, 89, 126, 163, 199, 309 and 530 cumulated degree days after sowing. At each seedling harvest, three pots were selected for each treatment and six homogenous seedlings were sampled from each pot. For all treatments, the six selected seedlings were first washed with a 400 mL solution of 0.5 mM CaSO4 at 4°C for 1 min to remove all the perlite from the seedling roots. Next, the seedlings were placed in a 400 mL solution containing P (0, 100 or 1,000 μM, depending upon the exogenous P treatment) and CaSO4 (0.5 mM) for the desorption of 32P from the seedling roots (4 min at 4°C) (Rubio et al. 2004). After washing, the seedlings were placed on a glass plate and separated with a spatula into five different seedling compartments: endosperm, scutellum, roots, coleoptile + mesocotyle and leaves. The following compartments were also used for data analysis: the seed (comprising endosperm and scutellum), the seedling (comprising leaves, roots and coleoptile + mesocotyle), and the total seedling (comprising leaves, roots, coleoptile + mesocotyle, endosperm and scutellum). All the results in this study are expressed on the basis of each seedling in each pot.
The fresh biomass (g) of each seedling compartment (endosperm, scutellum, leaf, root, coleoptile + mesocotyle) was determined, and samples were then lyophilized for 24 h and their lyophilized weight (g) recorded.
P determination in each seedling compartment
Phosphorus contents in all seedling compartment were determined by an adaptation of malachite green colorimetric technique (Van Veldhoven and Mannaerts 1987). Each seedling compartment was ground separately in a micro-vibrant grinder (Retsch MM 400 mixer mill, Retsch GmbH, Haan, Germany) and divided into two parts. One part of each ground sample was weighed and then reduced to ashes at 550°C for 5 h. The resulting ash was dissolved in 5 mL distilled water and left on the hotplate to evaporate until only a few drops remained. After P mineralization with HNO3, P contents were measured colorimetrically (Van Veldhoven and Mannaerts 1987). Total seedling P contents were the sum of the respective amounts of P in each seedling compartment.
Phytate and phytate-P determination
The second part of the lyophilized ground seedling samples was used to determine phytate and phytate-P content in the endosperm and scutellum compartments of seedlings only. Phytate contents were determined in the scutellum and endosperm at each seedling harvest. The phytate was extracted from grounded samples with HCl and determined with ion chromatography, (Nadeem et al. 2011).
Loss of endogenous seed P
The loss of endogenous seed P was calculated based on the difference between endogenous P in the seed at the beginning of the experiment and endogenous P in the seed at each seedling harvest in all P treatments.
Determination of exogenous P uptake using 32P labeling
Assuming that no 32P/31P-fractionation occurred during exogenous P uptake by seedling roots (Fardeau 1993; Schjørring and Jensén 1984), exogenous P uptake from the nutrient solution and its allocation toward seedling compartments were calculated from measured 32P activity as explained in Nadeem et al. (2011). As no significant 32P activity was detected in the HS seedling compartments grown in the HP nutrient solution without labeling, background noise induced by radioactivity was considered to be zero.
P efflux from germinating seeds and growing maize seedling roots
The P efflux from germinating seeds and growing seedling roots was calculated considering uptake of exogenous P and remobilization of endogenous P in the seed at each seedling harvest. The cumulated P efflux was calculated as the difference between the P lost from the seed plus accumulated exogenous P uptake assessed by 32P activity measured in the seedling, and accumulated P in the seedling.
Experimental design and statistical analysis
Treatments were defined as the factorial combination of two levels of endogenous P in the seed (LS, HS) and three rates of availability of exogenous P (0P, LP, HP) in the nutrient solutions. The treatments were applied in a completely randomized block design with three replicates and 10 seedling harvest dates. Data were analyzed by ANOVA using the R environment for statistical computing and graphics, version 2.9.1 (R Development Core Team, 2009). Means were compared using Tukey’s test at the 0.05 probability level.
Results
Early seedling growth
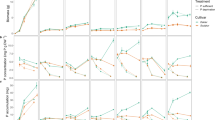
The percentage of germinated seeds on 71 cumulated degree days after sowing was 83%. The percentage of germinated seeds was not significantly affected by P treatments. Prior to germination, the seed dry biomass reserves were largely localized (90%, w/w) in the endosperm rather than in the scutellum. This proportion of biomass in scutellum was independent of initial endogenous seed P reserves in LS and HS seeds. Remobilization of the seed dry biomass reserves started at 34 cumulated degree days after sowing in all P treatments as shown in Fig. 1a. The availability of endogenous or exogenous P had no significant effect on the remobilization of seed dry biomass during germination and early growth stages.
a Seed biomass remobilization (g) and b seedling biomass accumulation (g) during germination and early growth stages: Low endogenous seed P seedlings (LS) with no exogenous P (white circle), low exogenous P availability (white square), high exogenous P availability (white triangle), high endogenous seed P seedlings HS with no exogenous P (black circle), low exogenous P availability (black square) high exogenous P availability (black triangle) treatments. Data are means and vertical bars indicate ±SE for n = 3 replications
Seedling biomass started to accumulate on 34 cumulated degree days after sowing (Fig. 1b) with the emergence of seedling radicle. The emergence of the seedling radicle was independent of the availability of either endogenous or exogenous P. The first seedling leaf became visible at 71 cumulated degree days after sowing and at 530 cumulated degree days, seedlings had five visible leaves (Table 1). A regular increase in accumulated seedling biomass was observed throughout the growth period and the availability of endogenous or exogenous P had no significant effect on the accumulation of seedling dry biomass (Table 1) during germination and early growth stages.
Accumulation of P in seedlings
The seedlings started to accumulate P with the emergence of the seedling radicle. P accumulated rapidly in LS seedlings from 34 cumulated degree days after sowing to 199 cumulated degree days after sowing in all exogenous P treatments and thereafter an increase in HP was only observed in the exogenous P treatment (Fig. 2a). A regular increase in accumulated P in the seedling was observed in HS seedlings from 34 cumulated degree days after sowing until 530 cumulated degree days after sowing (Fig. 2b). The growing seedlings accumulated significantly more P in HP than in 0P or LP treatments with available exogenous P despite endogenous P reserves in the seed. Less P accumulated in the seedling than the initial amount of endogenous seed P in LS and HS seedlings in 0P and LP treatments with exogenous P, whereas more accumulated P in the seedling in LS and HS seedlings treated with HP exogenous P, indicating net P accumulation at whole plant level. Accumulation of P in the seedling was significantly affected by the availability of endogenous and exogenous P at 530 cumulated degree days. HS seedlings had significantly more accumulated P (959 μg, 637 μg and 596 μg in 0P, LP and HP exogenous P treatments, respectively) than LS seedlings (267 μg, 324 μg and 582 μg P in 0P, LP and HP exogenous P treatments, respectively) as shown in Table 1.
Phosphorus accumulation (μg) in maize seedlings during germination and early growth stages: a Low endogenous seed P seedlings (LS) with no exogenous P (white circle), low exogenous P availability (white square), high exogenous P availability (white triangle) and b high endogenous seed P seedlings HS with no exogenous P (black circle), low exogenous P availability (black square) high exogenous P availability (black triangle) treatments, grown for 530 cumulated degree days after sowing. Data are means and vertical bars indicate ± SE for n = 3 replications
The initial P concentration was 8 mg g−1 seedling DW in LS and HS seedlings (Fig. 3). Concentrations of P in the seedling decreased from 8 mg g−1 to 1–4 mg g−1 seedling DW in LS and HS seedlings up to 309 cumulated degree days after sowing. P concentrations in the seedling were higher in HP than in 0P and LP exogenous P treatments despite endogenous P seed P reserves. The final P concentrations in different seedling compartments at 530 cumulated degree days after sowing are given in Table 2. The leaf, root and coleoptile + mesocotyle concentrations are higher in HPHS seedlings as compared to other P treatments.
Seedling phosphorus concentrations (mg g–1) in maize seedlings during germination and early growth stages. Low endogenous seed P seedlings (LS) with no exogenous P (white circle), low exogenous P availability (white square), high exogenous P availability (white triangle) and high endogenous seed P seedlings (HS) with no exogenous P (black circle), low exogenous P availability (black square) high exogenous P availability (black triangle) treatments, grown for 530 cumulated degree days after sowing. Data are means and vertical bars indicate ± SE for n = 3 replications
Seed phytate-P hydrolysis
Before germination, the LS and HS seeds had 1.8 mg g−1 DW and 2.7 mg g−1 DW phytate-P, respectively. The phytate-P was mainly localized in the scutellum rather than in the endosperm (79% w/w in LS scutellum, vs. 91% w/w in HS scutellum) and one reverse case of seed dry biomass distribution among seed compartments (91% w/w in LS endosperm, vs. 88% w/w in HS endosperm). Phytate-P in the scutellum and endosperm started to hydrolyze at 16 cumulated degree days after sowing. In LS and HS seeds, a sharp increase in hydrolysis of phytate-P reserves in seedling scutellum was observed up to 89 cumulated degree days after sowing (Fig. 4). Almost 98% of phytate-P in the scutellum and endosperm was hydrolyzed at 89 cumulated degree days after sowing despite the availability of endogenous or exogenous P. The absolute rate of hydrolysis of phytate-P in the seed was faster in HS than in LS seed, but was clearly independent of exogenous P availability (Fig. 4).
Scutellum phytate-P hydrolysis (μg) in maize seeds during germination and early growth stages. Inset, Phytate-P in the scutellum (ratio of scutellum phytate-P content to initial scutellum phytate-P content) as a function of cumulated degree days after sowing. Low endogenous seed P seeds with no exogenous P (white circle), low exogenous P availability (white square), high exogenous P availability (white triangle) and high endogenous seed P seeds with no exogenous P (black circle), low exogenous P availability (black square) high exogenous P availability (black triangle) treatments, grown for 530 cumulated degree days after sowing. Data are means and vertical bars indicate ± SE for n = 3 replications
Endogenous seed P remobilization
The scutellum is the main P storing compartment of maize seed (86%; w/w) (before the endosperm) irrespective of reserves of endogenous seed P (LS or HS seeds). Remobilization of endogenous P in LS and HS seeds and losses started from 16 cumulated degree days after sowing in all exogenous P treatments (Fig. 5). The loss of endogenous seed P was similar up to 89 cumulated degree days after sowing despite the availability of endogenous or exogenous P; subsequent differences were due to initial endogenous seed P stocks. More than 90% of initial endogenous P in the seed was lost earlier in LS seeds than in HS seeds (Fig. 5). Phosphorus lost from seed displayed exactly the same pattern regardless the availability of exogenous P. The difference between LS and HS seeds reflected the differences of initial endogenous seed P stocks.
Quantity of endogenous seed P (μg) lost from the seed during germination and early growth stages. Low endogenous seed P seedlings with no exogenous P (white circle), low exogenous P availability (white square), high exogenous P availability (white triangle) and high endogenous seed P seedlings with no exogenous P (black circle), low exogenous P availability (black square) and high exogenous P availability (black triangle) grown for 530 cumulated degree days after sowing. Data are means and vertical bars indicate ± SE for n = 3 replications
Exogenous P uptake by growing seedling roots
There was no significant uptake of exogenous P in the zero exogenous P treatment (0P), (Fig. 6). No 32P activity was observed in seedling roots until 52 cumulated degree days after sowing in either endogenous or exogenous P treatments. At 71 cumulated degree days after sowing, significant 32P activity was observed in seedling roots in treatments with available exogenous P despite the availability of endogenous seed P, indicating that uptake of exogenous P was already underway, and increased thereafter (Fig. 6). At the last sampling date, uptake of exogenous P was slightly higher in the HS than in the LS treatment, but the difference was not statistically significant. The uptake of exogenous seedling P was significantly affected by the availability of exogenous P, while endogenous P in the seed had no effect on the uptake of exogenous P during germination and early growth. The seedlings took up significantly more exogenous P in HP than in LP.
Exogenous phosphorus (μg) uptake in maize seedlings during germination and early growth stages. Low endogenous seed P seedlings with no exogenous P (white circle), low exogenous P availability (white square), high exogenous P availability (white triangle) and high endogenous seed P seedlings with no exogenous P (black circle), low exogenous P availability (black square) high exogenous P availability (black trianlge) grown for 530 cumulated degree days after sowing. Data are means and vertical bars indicate ± SE for n = 3 replications
P efflux and whole seedling P budget
The whole P budget in growing maize seedlings was calculated considering the P lost from germinating seeds, exogenous P uptake and seedling P accumulation. In all treatments, the amount of P that accumulated in the seedling was less than the sum of P lost from the seed plus the P that was taken up. This result shows that a proportion of P lost from the seed was not allocated to the growing seedling but was released outside. The P efflux started to increase from 16 cumulated degree days after sowing with the imbibition of seeds and was higher at 52 cumulated degree days after sowing (Fig. 7). The P efflux was 127 μg, 98 μg and 109 μg P seedling−1 in LS seedlings whereas it was 184 μg, 181 μg and 150 μg P seedling−1 in HS seedlings up to 52 cumulated degree days after sowing in 0P, LP and HP treatments, respectively. At 530 cumulated degree days after sowing, the P efflux was 205 μg, 189 μg and 172 μg P seedling−1 in LS seedlings and 192 μg, 304 μg and 282 μg P seedling−1 in HS seedlings in 0P, LP and HP treatments with available exogenous P, respectively.
Phosphorus efflux (μg) from germinating maize seeds and growing seedling roots during germination and early growth stages. a Low endogenous seed P seedlings with no exogenous P (white circle), low exogenous P availability (white square), high exogenous P availability (white triangle) and b high endogenous seed P seedlings with no exogenous P (black circle), low exogenous P availability (black square) high exogenous P availability (black triangle) treatments, grown for 530 cumulated degree days after sowing. Data are means and vertical bars indicate ± SE for n = 3 replications
Discussion
Effect of endogenous and exogenous P availability on remobilization of seed P and seedling growth
Early seedling growth occurs at the expense of seed reserves and their allocation among different tissues of the new seedling. As the scutellum is in close contact with the embryo (Fincher and Stone 1986), scutellum reserves were remobilized first while endosperm reserves remain unchanged up to 34 cumulated degree days after sowing in all P treatments. The seed biomass reserves displayed exactly the same remobilization kinetics irrespective of the availability of endogenous or exogenous P. In the present study no significant effect of endogenous or exogenous P was observed on remobilization of dry biomass reserves in the seed or on the accumulation of dry biomass in the seedling.
Seedling growth was similar in all P treatments. This result is consistent with the fact that the concentration of P in the seedling was higher than the level (1 mg g−1 DW) at which the increase in leaf area and in leaf length would be impaired due to P shortage during early growth stages (Plénet et al. 2000; Assuero et al. 2004). Although exogenous P was absent in the 0P treatment, the P supply from remobilization of endogenous seed P satisfied seedling P requirements during at least 530 cumulated degree days after sowing. Despite similar seedling growth, HP seedlings accumulated much more P than LP seedlings, clearly demonstrating P accumulation in excess of the plant’s immediate requirements.
Phytate was found to be a stored form of P in maize seeds and was localized mainly in the scutellum rather than in the endosperm or embryo as reported by other authors (Nadeem et al. 2011; Park et al. 2006; Lorenz et al. 2007; Lott et al. 1995; Modi and Asanzi 2008). The initial concentration of endogenous P in the seed only slightly affected the initial distribution of phytate between the scutellum and the endosperm. The rate of hydrolysis of seed phytate-P was not affected by the availability of exogenous P and very slightly affected by the amount of endogenous P (Fig. 4, inset). Hydrolysis of phytate-P depends on the synthesis of phytase and on phytase activity (Laboure et al. 1993; Wyss et al. 1999), which are controlled by seed soaking, imbibition (Lestienne et al. 2005; Centeno et al. 2001; Egli et al. 2002) and temperature (Sung et al. 2005).
The loss of endogenous P in the seed in LS and HS seeds was similar up to 89 cumulated degree days after sowing irrespective of the availability of endogenous or exogenous P (Fig. 5). The difference in the loss of endogenous P in LS and HS seeds was only due to the concentration of endogenous P in the seed at 89 cumulated degree days after sowing. Hall and Hodges (1966) suggested that the breakdown of phytate in germinating seeds accounts primarily for the increase in inorganic P in the roots and shoot. The hydrolyzed forms of P were temporarily stored in seeds (Nadeem et al. 2011) and were then translocated towards growing maize seedlings. The rapid remobilization of endogenous P in the seed during early growth corresponds to the dramatic increase in the demand for P by the seedling during periods of rapid cell division, such as seed germination and early growth (Hegeman and Grabau 2001).
Effect of endogenous and exogenous P availability on uptake of exogenous P
Significant P uptake was observed in seedling roots at 71 cumulated degree days after sowing in treatments with available exogenous P (in LP and HP exogenous P treatments). The seedling roots started to take up exogenous P soon after the radicle emerged, in accordance with previous results (Nadeem et al. 2011). The intensity of exogenous P uptake was mainly dependent on the availability of exogenous P (Elliott et al. 1984; Bhadoria et al. 2004) and on the root surface area exposed to exogenous P (Anghinoni and Barber 1980). In the 0P treatment, no significant P uptake was detectable using labeling. In this treatment, as the concentration of exogenous P was lower than the concentration at which net P influx ceases (e.g. Cmin ~ 0.04–4 μM), P efflux from roots may have occurred (Anghinoni and Barber 1980; Bhadoria et al. 2004). Because of higher concentrations of exogenous P (1,000 μM) in the HP treatment, exogenous P uptake was higher than in the LP treatment (Fig. 6). In HP and LP exogenous treatments, similar P absorption fluxes were observed in LS and HS seedlings, although concentrations of P were higher in HS seedlings than in LS seedlings (Fig. 3). There was no negative effect of the concentration of P in the seedling on the intensity of absorption of exogenous P. We did not find any correlation between seedling P status and exogenous P influx during 530 cumulated degree days after sowing. This result supports the conclusion that the demand for P by the seedlings was fully satisfied during germination and early growth in LP and HP treatments.
Whole seedling P budget
By comparing accumulated P in the seedling and loss of P from the seed plus uptake of exogenous P, we demonstrated that a significant amount of P was lost via efflux. Losses of P reached 40%, 37% and 33% in LS seedlings and 20%, 32% and 30% of initial endogenous seed P in HS seedlings in the 0P, LP and HP exogenous P treatments, respectively. Lestienne et al. (2005) showed that 21% of initial seed phytate was lost when whole maize seeds were soaked for 24 h, which released inorganic P from the seeds. The amount of P efflux depended on initial concentration of endogenous P in the seed and occurred mainly during the first 52 cumulated degree days after sowing. This result suggests that P efflux from seeds occurs during imbibition and germination, as seedlings have small root systems. Bewley (1997) suggested that the influx of water into cells of dry seeds during imbibition results in temporary disturbance of the structure, particularly that of membranes, leading to immediate and rapid leakage of solutes and low molecular weight metabolites into the surrounding imbibition solution. The structural disturbances associated with seed imbibition combined with high release of inorganic P due to phytate hydrolysis during germination may contribute to P release into the external medium.
As soon as the seedlings started to accumulate biomass after leaf emergence, the P efflux decreased, suggesting allocation of inorganic P towards seedling organs. P efflux is a component of P uptake in whole plants (Elliott et al. 1984) and is at least partially under metabolic control. P efflux from roots is part of the mechanism whereby plants maintain a P balance (Bieleski and Ferguson 1983). P releases due to phytate hydrolysis and to the loss of inorganic P from the seed were greater than seedling P requirements. The period of P losses from germinating seeds and growing seedling roots was similar in LS and HS seedlings despite the availability of exogenous P, as already reported by Elliott et al. (1984).
Up to 52 cumulated degree days after sowing, remobilization of endogenous seed P satisfied seedling P requirements in all exogenous P treatments. Similar results were reported by Barry and Miller (1989) and Nadeem et al. (2011). From 71 cumulated degree days after sowing, both sources of P (including endogenous and exogenous P) started to fulfill concomitantly seedling P requirements, whereas only endogenous seed P reserves supported seedling growth in the treatment with no exogenous P (0P). The seedling P originating from endogenous or exogenous P sources was largely translocated towards seedling leaves rather than seedling roots as already explained in Nadeem et al. (2011). This high translocation rate suggests high P requirements by seedling leaves during early growth.
Further research is needed to improve our understanding of the remobilization of P in the seed and its allocation towards the seedling. Our results suggest that seed P remobilization is closely related to germination processes independently of both seed P content and external P availability. Consequently, we need to study how external factors controlling germination (i.e. water availability and temperature) affect both seed P remobilization and seedling P requirements. Seedling P requirements during early growth stages also need to be characterized for better prediction of how long seed P remobilization -including seed P release into the external medium- is able to sustain the seedling growth.
Conclusion
Our results showed that remobilization of endogenous P in the seed is independent of exogenous P availability and is not a limiting step in maize seedling P nutrition during germination and early growth. Uptake of exogenous P started soon after the radicle emerged and was mainly controlled by exogenous P availability despite the availability of endogenous P in the seed. It can thus be concluded that seed P remobilization and exogenous P uptake by the roots are independently controlled. A significant loss in P was observed via efflux, mainly from germinating seeds. Moreover, the loss of P was independent of exogenous P availability. On the whole, these results suggest that although hydrolysis of seed phytate-P is not a limiting step to providing P to the growing seedling, the rapid hydrolysis of phytate favors P losses probably by diffusion during germination. This may explain the often reported P deficiencies in maize seedlings during germination and early stages, especially in low P soils where root uptake is limited by exogenous P availability.
Abbreviations
- LS seeds:
-
Seeds with low endogenous seed P
- HS seeds:
-
Seeds with high endogenous seed P
- LS seedlings:
-
Seedlings grown from LS seeds
- HS seedlings:
-
Seedlings grown from HS seeds
- 0P:
-
No exogenous P
- LP:
-
Low exogenous P availability
- HP:
-
High exogenous P availability
- DW:
-
Dry weight
- Endo-P:
-
Endogenous seed P
- Exo-P:
-
Exogenous P uptake
References
Anghinoni I, Barber SA (1980) Phosphorus influx and growth characteristics of corn roots as influenced by phosphorus supply. Agron J 72(4):685–688
Assuero SG, Mollier A, Pellerin S (2004) The decrease in growth of phosphorus-deficient maize leaves is related to a lower cell production. Plant Cell Environ 27(7):887–895
Barry DAJ, Miller MH (1989) Phosphorus nutritional-requirement of maize seedlings for maximum yield. Agron J 81(1):95–99
Bewley JD (1997) Seed germination and dormancy. Plant Cell 9(7):1055–1066
Bhadoria PS, El Dessougi H, Liebersbach H, Claassen N (2004) Phosphorus uptake kinetics, size of root system and growth of maize and groundnut in solution culture. Plant Soil 262(1–2):327–336
Bieleski RL, Ferguson IB (1983) Physiology and metabolism of phosphate and its compounds. In: Läuchli A, Bieleski RL (eds) Encyclopedia of Plant Physiology. New Series, vol 15A. Springer, Berlin, pp 422–449
Bonhomme R, Derieux M, Edmeades GO (1994) Flowering of diverse maize cultivars in relation to temperature and photoperiod in multilocation field trials. Crop Sci 34(1):156–164
Centeno C, Viveros A, Brenes A, Canales R, Lozano A, de la Cuadra C (2001) Effect of several germination conditions on total P, phytate P, phytase, and acid phosphatase activities and inositol phosphate esters in rye and barley. J Agric Food Chem 49(7):3208–3215
Colomb B, Kiniry JR, Debaeke P (2000) Effect of soil phosphorus on leaf development and senescence dynamics of field-grown maize. Agron J 92(3):428–435
Eagles HA, Hardacre AK (1979) Genetic variation in maize (Zea mays L.) for germination and emergence at 10°C. Euphytica 28(2):287–295
Egli I, Davidsson L, Juillerat MA, Barclay D, Hurrell RF (2002) The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feeding. J Food Sci 67(9):3484–3488
Elliott GC, Lynch J, Läuchli A (1984) Influx and efflux of P in roots of intact maize plants -double labeling with 32P and 33P. Plant Physiol 76(2):336–341
Fardeau JC (1993) Available soil phosphate—Its representation by a functional multiple compartment model. Agronomie 13(4):317–331
Fincher GB, Stone BA (1986) Cell walls and their components in cereal grain technology. In: Pomeranz Y (ed) Advences in cereal science and technology, vol 8. American Association of Cereal Chemists, St. Paul, pp 207–295
Gavito ME, Miller MH (1998) Early phosphorus nutrition, mycorrhizae development, dry matter partitioning and yield of maize. Plant Soil 199(2):177–186
Grant CA, Flaten DN, Tomasiewicz DJ, Sheppard SC (2001) The importance of early season phosphorus nutrition. Can J Plant Sci 81(2):211–224
Hall JR, Hodges TK (1966) Phosphorus metabolism of germinating oat seeds. Plant Physiol 41:1459–1464
Hegeman CE, Grabau EA (2001) A novel phytase with sequence similarity to purple acid phosphatases is expressed in cotyledons of germinating soybean seedlings. Plant Physiol 126(4):1598–1608
Laboure AM, Gagnon J, Lescure AM (1993) Purification and characterization of a phytase (myo-Inositol-hexakisphosphate phosphohydrolase) accumulated in maize (Zea mays) seedlings during germination. Biochem J 295:413–419
Lestienne I, Icard-Verniere C, Mouquet C, Picq C, Treche S (2005) Effects of soaking whole cereal and legume seeds on iron, zinc and phytate contents. Food Chem 89(3):421–425
Lockhart HB, Hurt HD (1986) Nutrition of oats. In: Webster FH (ed) Oats: chemistry and technology. American Association of Cereal Chemists, Inc., St Paul, Minnesota, USA, pp 297–308
Lorenz AJ, Scott MP, Lainkey KR (2007) Quantitative determination of phytate and inorganic phosphorus for maize breeding. Crop Sci 47(2):600–606
Lott JNA, Greenwood JS, Batten GD (1995) Mechanisms and regulation of mineral nutrient storage during seed development. In: Kigel J, Galili G (eds) Seed Development and Germination. Marcel Dekker, New York, pp 215–235
Modi AT, Asanzi NM (2008) Seed performance of maize in response to phosphorus application and growth temperature is related to phytate-phosphorus occurrence. Crop Sci 48(1):286–297
Mollier A, Pellerin S (1999) Maize root system growth and development as influenced by phosphorus deficiency. J Exp Bot 50(333):487–497
Nadeem M, Mollier A, Morel C, Vives A, Prud’homme L, Pellerin S (2011) Relative contribution of seed phosphorus reserves and exogenous phosphorus uptake to maize (Zea mays L.) nutrition during early growth stages. Plant Soil 346(1):231–244
Palomo L, Claassen N, Jones DL (2006) Differential mobilization of P in the maize rhizosphere by citric acid and potassium citrate. Soil Biol Biochem 38(4):683–692
Park SH, Sung JK, Lee SY, Park JH, Lee JY, Jang BC, Lee BH, Kim TW (2006) Early growth, carbohydrate, and phytic acid contents of germinating rice seeds under NaCl stress. Korean J Crop Sci 51(2):137–141
Pellerin S, Mollier A, Plenet D (2000) Phosphorus deficiency affects the rate of emergence and number of maize adventitious nodal roots. Agron J 92(4):690–697
Plénet D, Etchebest S, Mollier A, Pellerin S (2000) Growth analysis of maize field crops under phosphorus deficiency:I. Leaf growth. Plant and Soil 223(1–2):117–130
Römer W, Augustin J, Schilling G (1988) The relationship between phosphate absorption and root length in 9 wheat cultivars. Plant Soil 111(2):199–201
Römer W, Schilling G (1986) Phosphorus requirements of the wheat plant in various stages of its life-cycle. Plant Soil 91(2):221–229
Rubio G, Sorgona A, Lynch JP (2004) Spatial mapping of phosphorus influx in bean root systems using digital autoradiography. J Exp Bot 55(406):2269–2280
Schjørring JK, Jensén P (1984) Phosphorus nutrition of barley, buckwheat and rape seedlings. I. Influence of seed-borne P levels and external P levels on growth, P content and 32P/31P-fractionation in shoots and roots. Physiol Plant 61(4):577–583
Sung HG, Shin HT, Ha JK, Lai HL, Cheng KJ, Lee JH (2005) Effect of germination temperature on characteristics of phytase production from barley. Bioresour Technol 96(11):1297–1303
Van Veldhoven PP, Mannaerts GP (1987) Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem 161(1):45–48
Wyss M, Brugger R, Kronenberger A, Remy R, Fimbeld R, Oesterhelt G, Lehman M, Van Loon AP (1999) Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolyses): Catalytic properties. Appl Environ Microbiol 65:367–373
Acknowledgement
This study was funded by a project grant from the Higher Education Commission (HEC), Pakistan and benefited from the financial support from the INRA (French National Institute for Agricultural Research). M. Nadeem thanks the Pakistan Higher Education Commission for funding his PhD studentship at the University of Bordeaux I, France. The authors acknowledge the technical help and the useful advice offered by Anne Gallet-Budynek and Sylvie Milin.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
Nadeem, M., Mollier, A., Morel, C. et al. Maize (Zea mays L.) endogenous seed phosphorus remobilization is not influenced by exogenous phosphorus availability during germination and early growth stages. Plant Soil 357, 13–24 (2012). https://doi.org/10.1007/s11104-011-1111-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-1111-5