Abstract
Aims
Phosphorus (P) export with harvested grains is a key step of the P cycle in agroecosystems. In wheat, the accumulation of P in grains originates from both exogenous and endogenous P sources. We investigated the effects of different post-anthesis P supply on P partitioning and P remobilization in two durum wheat cultivars with contrasting biomass allocation.
Methods
Wheat plants were grown on a complete nutrient solution with sufficient P until anthesis. Thereafter, half of the plants were deprived of P and the other half was maintained on the complete nutrient solution. P uptake, allocation, remobilization, and traits related to yield and grain P were determined.
Results
Modifications of post-anthesis P supply had no effect on grain yield. Grain P concentrations at maturity for deprived P supply ranged from 2.2 to 3.4 mg P g DW− 1. Without P, net P fluxes to grains essentially came from leaves (35%), roots (28%) and stems (17% ). With P, net P fluxes came mainly from post-antheis P uptake.
Conclusions
Our results suggest that when the P nutrition of durum wheat is limited after anthesis, endogenous P remobilization can sustain grain growth with minor yield penalties if the plants are well supplied during vegetative growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is an essential element for plant growth because of its pivotal role in many metabolic processes (Vance et al. 2003). The use of P fertilizers in agriculture is a widespread practice to maintain soil fertility and to ensure the sustainability of crop production (Tilman et al. 2002). However, given the non-renewable nature of this resource, P supply to agriculture will be limited by mineable reserves in the future (Cordell and White 2014). Thus, reducing the use of mineral P fertilizers and improving P recycling while sustaining crop productivity are crucial to secure future food demand and to preserve our environment from eutrophication problems (Cordell and White 2014). As it is the case for nitrogen, improving the P efficiency of farming systems can be achieved through the use of P-efficient cultivars and the optimization of crop P management (Veneklaas et al. 2012). The traits associated with P use efficiency (PUE) can be divided into two categories: the traits associated with the P acquisition efficiency and those associated with the internal use efficiency of the acquired P (Rose and Wissuwa 2012). Another emerging solution is the use of low P grain genotypes that export less P from the field without impairing grain yield and consequently contributing to the sustainability of the farming system (Raboy 2001; Rose et al. 2013; Wang et al. 2016). Targeting this trait in crops could be part of the solution to minimize P exportation and its associated environmental problems. Therefore, all these approaches require a deep understanding of the processes that determine the relationship between grain yield and grain P content (Veneklaas et al. 2012; Wang et al. 2016).
The demand for P changes over the annual cycle of the wheat plant (Römer and Schilling 1986). During the vegetative stage, P is required for shoot and root growth (Engels et al. 2012). After the onset of anthesis, the grain becomes the main sink for P (Grant et al. 2001; Gregersen et al. 2008). Thus, the P demand of developing grain can be sustained from continuous post-anthesis P uptake and/or from the remobilization and translocation of endogenous P (Wang et al. 2016).
Wheat grain development can be divided into three main phases (Shewry et al. 2012). In the first 14 days following anthesis, the grain grows very rapidly as a result of high cell division rate. During this period, P is required for energy transfer, nucleic acid synthesis and other metabolic and cellular processes (Iwai et al. 2012). Subsequently, grain filling is then initiated and consists of progressive storage of starch, proteins and nutrients (Shewry et al. 2012). This stage lasts between 14 and 20 days. Finally, the last phase is characterized by rapid water loss and a decrease in grain metabolic activity (Ferreira et al. 2012).
Plant vegetative organs play an important role in the whole plant P budget. They could act as transition storage of P before its subsequent translocation to the growing grains (Wang et al. 2016). Depending on P availability, the proportion of total plant P that is found in grains has been reported to vary from 30% to 90% at maturity (Batten 1992). In cereals, about 50 to 90% of grain P was estimated to be derived from re-mobilized P from vegetative organs depending on genotypes and growth conditions (Batten et al. 1986; Peng and Li 2005; Veneklaas et al. 2012). Prior to its translocation to the grain, P must first be remobilized. Several studies showed that senescence in vegetative organs such as leaves induces nutrient remobilization (Engels et al. 2012; Etienne et al. 2018). However, P remobilization may occur also during vegetative growth when plant face P deficiency (Grant et al. 2001). Given its mobility in the phloem and xylem, the P becomes readily available to be transported to sink organs (Engels et al. 2012; Peng and Li 2005). While extensive studies focused on the effect of early P limitation on plant growth, less effort has been devoted to the understanding of the effect of late P limitation and its consequence on yield and PUE, especially in wheat (Wang et al. 2016; White and Veneklaas 2012).
In addition, up to 70% of grain carbon (C) is originated from C assimilated during grain filling (Masoni et al. 2007). During the vegetative stage, an insufficient P supply to plants can lead to a reduction in plant growth and to a greater root to shoot ratio (Plénet et al. 2000; Vance et al. 2003). Such a change in biomass allocation pattern is one of the strategies that plants use to increase access to a larger P sources in the soil. Decrease in grain yield and grain number are also reported in cereals (Colomb et al. 2007; Plénet et al. 2000). However, late season P limitation is known to have less impact on grain yield (Batten et al. 1986; Peng and Li 2005). Batten and Wardlaw (1987) have shown that foliar or root applications of phosphate fertilizers during grain development delay leaf senescence without a significant increase in grain yield in wheat. Accordingly, it can be assumed that grain growth will not be limited by P supply in the post-anthesis period and that P remobilization would be enough to sustain the growing grains. One approach to test this hypothesis is to force the plant to rely on endogenous P source during grain filling. This can be achieved by removing the exogenous P supply during the post-anthesis period and then assessing the potential of P remobilization from wheat organs and their contribution to final grain P. To date, many studies have been done with rice but little information has been reported regarding P remobilization and P partitioning among different organs in wheat under different P supply over the post-anthesis period. For example, Julia et al. (2016) studied P partitioning and remobilization in rice plants and found that post-anthesis P uptake is a critical contributor to grain P content since it represented 40 to 70% of the above-ground P accumulated at maturity.
Durum wheat is one of the most important cereal crops in the Mediterranean area (Villegas et al. 2001). Although genotypic variation for P efficiency has been documented to occur in wheat (Deng et al. 2018; Ozturk et al. 2005; Wang et al. 2010), there are little insights concerning the possible mechanisms that may underlie these variations, especially during post-anthesis period. A plant’s ability to cope with P limitation does not only depend on its capacity to take up and store P but also involves mechanisms of P transport and P remobilization to sink organs (Hammond et al. 2004; Veneklaas et al. 2012). If large root systems are important for efficient P acquisition, P remobilization is decisive, especially in the case of P limitation during grain development (Wang et al. 2010). In our experiment, we used one cultivar with high shoot to root ratio and another one with low shoot to root ratio. We hypothesized that the two cultivars with different shoot to root ratio would have different P remobilization profiles during the grain filling period. We further hypothesized that P accumulation during the vegetative stage would be sufficient to supply the durum wheat grain production. Therefore, this study aimed i) to assess the effects of different post-anthesis P supply on the dynamics of P uptake, P partitioning and P remobilization in two durum wheat cultivars with contrasting shoot to root ratio, ii) test whether these processes are driven by biomass allocation, iii) to evaluate to which extent P remobilization could sustain grain growth.
Materials and methods
Experimental design
Two locally adapted French spring durum wheat cultivars (Tritium turgidum L. subsp. durum) cv. Dakter and cv. Sculptur were used in this experiment.Sculptur has previously been identified as having higher shoot to root ratio than Dakter (Perrier et al. 2016). The two cultivars were grown to the stage of anthesis in complete nutrient solution. After anthesis, half of the plants were P deprived until grain maturity while the other half were kept to grow on a complete nutrient solution (Online Resource 1). Phosphorus content and dry biomass of wheat organs (grains, spikelets, rachis & peduncles, stems, flag leaves and lower leaves, and roots) were determined at four stages during grain filling to quantify the net transfer of P from organs that play the role of P source to organs that play the role of P sink.
Plant growth conditions
Uniformly weighed seeds were surface sterilized in 6% (v/v) sodium hypochlorite (H2O2) solution for 10 min and rinsed thoroughly with ultra-pure water. The seeds were then pre-germinated on moist paper and kept in darkness for 3 days at 25 ∘C. The germinated seeds were then transferred to 50 mL Falcon\(^{{\circledR }}\) tubes filled with modified Hoagland nutrient solution (1/4 strength for macronutrients and full strength micronutrients). After third leaf emergence, 48 seedlings were transferred to 7.5 L plastic pots (1 plant per pot) containing 5.5 L of modified Hoagland’s nutrient solution: 0.125 mM KH2PO4, 0.625 mM KNO3, 0.85 mM KCl, 1.25 mM Ca(NO3)2, 0.5 mM MgSO4, 46.25 μM H3BO3, 1μM MnCl2, 10 μM ZnSO4, 2μM CuSO4, 0.03 μM (NH4)6Mo7O24, 100 μM NaFe EDTA, 50 mg L− 1 SiO2, 25 μM HEDTA and 2 mM MES buffer. The pH was adjusted to 6 ± 0.5 with KOH. The nutrient solution was aerated and refreshed everyday using an overflow-type system. The pot was arranged in randomized complete block design.
To limit the high rate of tillering associated to hydroponic condition (Perrier et al. 2016), only the first five tiller were allowed to grow while the late emerging tillers were removed every 2-3 days.
At the onset of flowering, half of the wheat plants (24 plants) were maintained in P sufficient nutrition (0.125 mM KH2PO4) and the other half were transferred to a P deprivation treatment (0 mM KH2PO4) until maturity (Online Resource 1).
Wheat plants were grown under greenhouse conditions, with a photo-period of 16h/8h day/night. Photosynthetically active radiation (PAR), air temperature and relative humidity were recorded every 30 min using a data logger (CR32X sensor, Campbell Scientific). Natural light was supplemented with high-pressure sodium lamps supplying an average photosynthetic photon flux density of 353.5 μ mol photons m− 2 s− 1 to limit spatial diferences in light intensity. The average of air temperature and relative humidity were 22 ∘C and 58%, respectively. Details on environmental conditions are given in Online Resource 2.
Plant sampling and measurements
Phenological stages were closely recorded on main stems for each single plant after head emergence. Durum wheat plants are considered at anthesis stage when 50% of flowers are visible. Leaf senescence was monitored from anthesis until maturity by a non-destructive method, using a hand-held chlorophyll meter (SPAD-502, Minolta, Milton Keynes, Japan).
Harvest days were chosen to cover main grain development phases in wheat (Shewry et al. 2012). Wheat plants were harvested four times: Anthesis, 10, 20, and 42-49 (maturity) days after anthesis according to P supply and cultivar.
Harvested plants (44 plants, n = 3 at each harvest except at maturity for Sculptur where n = 4) were cut with scissors and separated into seven parts: grains, spikelets, rachis & peduncles, stems, flag leaves (including leaf sheaths), lower leaves, and roots (Online Resource 1). Stems includes nodes and inter-nodes. Lower leaves are the remaining leaves. Roots were washed 3 times with distilled water to remove the adhered nutrient solution.
Plants organs were oven dried for 3 days at 60 ∘C, and dry biomass was assessed. At maturity, fresh and dry weights of grains were measured to determine the grain moisture content (GMC). Yield components including grain number (GN) and thousand grain weight (TGW) were measured.
Plant samples were calcined at 550 ∘C for 5 h. After cooling, ashes were dissolved in 2.5 mL of concentrated nitric acid and slowly heated on a hotplate to evaporate. Then, 5 ml of ultra-pure water was added until only a few drops were left. Mineralized solutions were filtered through ash-free filter paper and diluted to a final volume of 50 ml with ultra-pure water.
Phosphorus concentrations in diluted digests (mg P g DW− 1) were determined using a malachite green colorimetric method (Van Veldhoven and Mannaerts 1987). For each given plant compartment, the amount of P content was calculated by multiplying the dry biomass and its measured P concentration. The C and N concentration in the grains were assayed with an elemental analyzer (Flash EA112, ThermoFisher), according to the Dumas method.
Whole plant P content (mg plant− 1) was determined by summing the P content of grains, spikelets, rachis & peduncles, stems, flag and lower leaves, and roots. Post-anthesis P uptake (mg plant− 1) was calculated as the difference between whole plant P at maturity and whole plant P at anthesis. Harvest index (HI) and P harvest index (PHI) were calculated as the ratio between grain weight or grain P content to above-ground biomass or above-ground P content respectively. The PHI is a measure of the efficiency of the allocation of P to the grains.
To characterize the dynamics of P in every organ during the post-anthesis period, net P remobilization from different organs was calculated as the difference between P contents in each organ at maturity and at anthesis. P utilization efficiency (PUtE, g DW mg P− 1) was estimated as grain yield per unit of P in the above-ground parts. P uptake efficiency (PUptE, mg P g root DW− 1) was calculated as post-anthesis P uptake per unit of root dry biomass.
Statistical analyses
Reported values are mean ± SE of 3-4 replicates. To determine the statistical significance of the effect of P treatment or cultivar, the means of the different parameters were compared by conducting a two-way analysis of variance (ANOVA). When significant differences are observed (P < 0.05), multiple comparisons between treatment and cultivar at each harvest were conducted using Least Significant Difference (LSD) test. All statistical analyses were performed using ⒸR 3.4.4 statistical software (R Core Team 2018).
Results
Plant growth and biomass partitioning between organs during grain filling period
Durum wheat plants reached anthesis on 53 and 46 days after germination for Dakter and Sculptur, respectively. The most notable visual difference between the two treatments was the delayed senescence in the plant grown in P sufficient supply. This was confirmed by SPAD measurements on flag leaves. Both cultivars had similar chlorophyll contents during the first two weeks. Thereafter, chlorophyll content decreased more rapidly in P deprivation treatment, especially for Sculptur (Online Resource 3). As a result, wheat plants grown under P deprivation treatment reached maturity earlier (42 days after anthesis for both cultivars) than plants under sufficient P supply (48 and 49 days after anthesis for Dakter and Sculptur, respectively). At maturity, grain moisture content was below 20 % for all plants, indicating that plants were harvested after grain filling ended, as no further grain filling occurs below 45% (Online Resource 4).
The two cultivars differed significantly in total biomass accumulation with the smallest values in Sculptur. At maturity, total biomass averaged 26 g for Dakter and 22 g for Sculptur (Table 1). During grain filling, Dakter accumulated more biomass than Sculptur (P < 0.05). In contrast, no significant differences in total biomass were observed between P treatments for each cultivar except for Dakter at maturity where total biomass increased by 14% in P sufficient plants (P < 0.05).
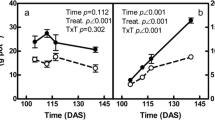
Dakter and Sculptur have similar patterns of grain development (Fig. 1a). Grain weight increased slowly during the first 10 days after anthesis. Thereafter, grain weight increased until maturity. Except leaves, all other organs showed a small increase in weight. In general, Dakter had a higher organ biomass than Sculptur. Regardless of P treatment, no differences in biomass accumulation among organs were observed except for lower leaves in Dakter (P < 0.05).
The two cultivars had different biomass partitioning patterns as shown in Fig. 2a. Post-anthesis P supply did not influence the partitioning of biomass between organs (P < 0.05). As expected, Dakter allocated more biomass to the root than Sculptur (22.3% and 10.9% respectively). The opposite pattern was observed for grain where it accounts for more than 53% in Sculptur and less than 38% in Dakter. The biomass partitioning within organs was not affected by P treatments although the total biomass was reduced by post-anthesis P deprivation for Dakter.
P concentration in durum wheat organs
Starting from 10 days after anthesis (DAA), grain P concentration decreased in both cultivars immediately in the P deprivation treatment and about 20 DAA for the P sufficient treatment (Fig. 1b). At maturity, grain P concentration was 3.4 to 4.4 mg P g− 1 DW for Dakter and 2.2 to 3.1 mg P g− 1 DW for Sculptur in the deprivation and sufficient P treatment, respectively.
For Sculptur, P concentrations decreased slightly throughout the post-anthesis period in rachis and peduncle, flag and lower leaves, and roots. In stems, P concentrations decreased rapidly only after 20 DAA. Roots P concentration showed a small decrease 10 DAA and then remained constant until maturity. For Dakter, flag leaves and spikelets P concentrations decreased in the first 20 DAA and then increased significantly. Lower leaves P increased throughout the post-anthesis period. P concentration in stems showed the same pattern as in Sculptur.
Under P deprivation treatment, P concentration decreased strongly in in all organs during the post-anthesis period for both cultivars (Fig. 1b).
Post-anthesis P remobilization and redistribution
Under P sufficient supply, whole P contents increased from 50.9 to 162.2 mg P plant− 1 and from 35.9 mg P plant− 1 to 77.7 mg P plant− 1 from anthesis to maturity for Dakter and Sculptur, respectively (Table 1). Without P in the nutrient solution during grain filling, whole P contents decreased over the post-anthesis period as a consequence of the lack of P in the nutrient solution. This indicates that no P was taken up during this period. The small decrease might be due to P loss and efflux. Higher P contents in Dakter compared to Sculptur at the P sufficient treatment was due to higher P uptake during the post-anthesis period (111.4 vs 41.7 mg P plant− 1, respectively). The large part of accumulated P in Sculptur was absorbed between anthesis and 20 days after anthesis, whereas Dakter accumulated P during the whole post-anthesis period.
Grain P contents increased as grains matured in both treatments (Fig. 1c). It was higher under P sufficient than under P deprivation treatment. Stem P contents increased during the first 10 and 20 days after anthesis for P deprivation and P sufficient treatment respectively before a huge decrease towards maturity. For Dakter under post-anthesis P sufficient treatment, P contents in all organs increased. Contrariwise for Sculptur, P contents in flag and lower leaves and roots were constant between anthesis and maturity.
As expected, P deprivation treatment led to a strong decrease in P content in all organs except grains indicating that there was a net export of P from these organs to the grain (Fig. 1c). Spikelets was a sink for P at maturity for both cultivars at sufficient P supply. Also for Dakter, flag and lower leaves were still accumulating P throughout the post-anthesis period for this treatment.
The two cultivars had different P partitioning patterns as shown in figure Fig. 2b. Under P sufficient supply, Sculptur allocated more than 47% of total P to the grain whereas Dakter allocated only 30%.
The P partitioning during the grain filling period between the different organs is significantly affected by the lack of P in the nutrient solution. The difference between cultivars was smaller under P deprivation treatment where 86 and 79% of P were allocated to grain for Dakter and Sculptur, respectively, which indicates that all organs acted as source of P for grain during this period. This enhanced P remobilization indicates that P could be remobilized from all organs.
Grain yield, its components and the indicator of P use efficiency
The yield and yield components varied among treatments and cultivars (Table 2). Post-anthesis P treatment had no impact on grain yield and grain number (P < 0.05). Grain yield averaged 10.2 and 11.8 g per plant for Dakter and Sculptur, respectively. Thousand grain weight was reduced significantly only for Sculptur. Dakter had larger grain but less grain per head in comparison to Sculptur with smaller grain but more grain per head. The P utilization efficiency (PUtE) differed between cultivars and P supply. Regardless of the post-anthesis P supply, Sculptur had a higher PUtE compared to Dakter, 0.17 and 0.28 g DW mg− 1 respectively, indicating that Sculptur is more efficient in using the acquired P to produce grain yield than Dakter. In contrast, P uptake efficiency (PUptE) did not differ significantly among cultivars (18 mg P g root DW− 1) indicating that they have the same capacity to take up P during the post-anthesis period. Thus, in-between cultivar differences are largely explained by the greater root system size for Dakter in comparison with Sculptur.
C:N ratio was 11 and 15 for Dakter and Sculptur, respectively, indicating that Dakter has a higher grain protein content than Sculptur. There were no significant impacts of post-anthesis P deprivation on grain C:N ratio.
The HI was 50% and 61% for Dakter and Sculptur, respectively (Table 2). The phosphorus harvest index (PHI) was 86 and 41% for Dakter under P deprivation and P sufficient treatment, respectively, and from 89 and 53% for Sculptur. The shoot to root ratio was 3.5 in Dakter and 8.2 in Sculptur.
P budget at whole plant level during grain filling
The pattern of P uptake and remobilization differed between cultivars and post-anthesis P supply (Fig. 3). Post-anthesis sufficient supply lowered P remobilization while it was enhanced in the P deprivation treatment. Under sufficient post-anthesis P supply, P uptake was important for both cultivars accounting for 68% and 51% of total P for Dakter and Sculptur, respectively. Dakter grain P mainly originated from post-anthesis P uptake while more than 20% originated from remobilized P from stems, roots and leaves for Sculptur. The P taken up by Dakter were mainly allocated to roots, leaves and spikelets (Fig. 3a).
Net P fluxes (mg P plant− 1) from anthesis to maturity among organs of durum wheat cultivars Dakter and Sculptur grown under P sufficient (a) and P deprivation (b) treatments throughout the post-anthesis period. Net fluxes of P from remobilization (–) or post-anthesis P uptake (+) in the different organs are expressed as mg P organs− 1. The percentage (%) are the contribution of P remobilization in each organs to grain P contents. Efflux refer to the loss of P by the roots. R & P are rachis and peduncle. Data are means of 3-4 replicates
In contrast, under post-anthesis P deprivation, no exogenous P uptake was possible. Thus, all P accumulated in the grain came from P remobilization of plant organs (Fig. 3b). One important result is that all vegetative organs and roots acted as a net source for P. Organs contribution to grain P followed this ranking: leaves > roots > stems > spikelets and finally rachis and peduncle. Phosphorus efflux accounted for 28% and 14% of total P remobilized at maturity, for Dakter and Sculptur, respectively.
Discussion
We assessed the effect of different post-anthesis P supply on the dynamics of P uptake, P partitioning and P remobilization in two durum wheat cultivars with contrasting biomass allocation. This study attempts to answer the question to what extent endogenous P could sustain grain growth. Phosphorus deprivation treatments were imposed during the grain filling period which allowed us to calculate the net P fluxes from vegetative organs towards the grains. The quantification of these net P fluxes is a key element for developing more efficient P management in cropping systems.
Impact of different post-anthesis P supply on biomass accumulation and grain yield
At anthesis, P concentrations in the plant organs were above the critical value of 4 mg P g DW− 1 reported for wheat (Römer and Schilling 1986; Veneklaas et al. 2012), indicating that plants were well supplied with P during vegetative stages. Dakter continued to take up P during the whole post-anthesis period while Sculptur maintained P uptake only between anthesis and 20 days after anthesis (Table 1). Different post-anthesis P treatments had no effect on grain yield. This result is a direct demonstration that P remobilization could be sufficient to sustain grain growth if plants are well supplied with P during the the vegetative stage. Since the number of tillers is determined before anthesis, the exogenous sufficient P supply is more critical during the vegetative stage than during the grain filling stage when the potential yield is already determined.
This finding is consistent with that of Peng and Li (2005) who found that withdrawing P from the nutrient solution during flag leaf expansion did not influence grain growth even if there was a reduction in total biomass accumulation. In addition, late foliar applications of P delayed leaf senescence without a significant increase in grain yield (Batten and Wardlaw 1987). These studies and our results support the idea that P absorbed throughout the post-anthesis period does not contribute significantly to increase grain yield (Batten et al. 1986; Grant et al. 2001; Peng and Li 2005). In fact, previous studies have reported that P uptake is more critical during vegetative stages than during grain filling in wheat (Grant et al. 2001; Rose et al. 2007). Indeed, since P treatments started after flowering, yield potential for both treatments is assumed to be the same. Hence, grain number is already set up whereas grain weight will rely on post-anthesis carbon (C) supply (González et al. 2011). To maintain C supply to grains as long as possible, plants might remobilize less essential P compounds. This hypothesis is consistent with the results of Jeong et al. (2017) who showed that rice plants first remobilize less essential P pools such as vacuolar phosphate and phospholipids. In that way P can be recycled within the plant organs without affecting grain yield.
In addition, the P deprivation treatment has no effect on grain yield. Consequently, the requirements of P during early grain development is fulfilled through P remobilization. At later stage, P is stored in the form of phytic acid in grains (Iwai et al. 2012; Raboy 1997). This would imply that the reduction of grain P content found under P deprivation treatment is due to a decrease in phytic acid content. This is found to be the case for bread and durum wheat (Batten et al. 1986), barley, maize and soybean where grain P and phytic acid P are found to be highly correlated (Raboy 1997, 2001). Thus, the variation in grain P found in our study is likely due to the variation in phytic acid P content.
Net P fluxes at whole plant level
Under post-anthesis P sufficient supply, grain P was largely fulfilled by continuous P uptake and to a lesser extent by remobilization of endogenous P (Fig. 3a). This finding was confirmed by remarkable increases in P concentration found in some organs over the post-anthesis period, especially for Dakter, indicating that P is preferentially distributed to the apical organs (Fig. 1c). Similarly, the increase of stem P contents during the first 20 days after anthesis and also the increase of P content in spikelets at maturity may suggest that stems and spikelets act as a temporary storage organs of P before its translocation to the grain. In line with this hypothesis, Julia et al. (2016) reported little direct P allocation to grain throughout the post-anthesis period in rice grown under hydroponic condition. In the present study, the wheat plants received ample P during the vegetative stage. Thus, high P remobilization would be the major processes determining grain P under P deprivation treatment. In the fields, this may be related to the limitation of post-anthesis P uptake due to lower P availability (e.g. drier soil, depletion of P around the roots) or lower demand because plants can efficiently remobilize the acquired P before anthesis. However, this is not the case for all species because other studies conducted with canola showed that post-anthesis P supply can be critical if the plants have not accumulated enough P during the vegetative stage (Rose et al. 2007).
As mentioned earlier, post-anthesis P uptake was not associated with any increase in grain yield. P is a mobile element in both phloem and xylem and its transport within the plant organs depend on developmental stage and P supply (Engels et al. 2012). In our study, the cultivar with low shoot to root ratio continued P uptake until a later stage while the other cultivar slowed its uptake earlier. This finding is in contrast with some field studies that showed that P uptake does not always occur in wheat at later stages after anthesis (Batten 1992; Masoni et al. 2007; Rose et al. 2007). One explanation could be that environmental conditions, especially under field conditions, are generally not favorable for post-anthesis P uptake rather than the plant’s ability itself. Furthermore, the grain filling period often coincides with drought period which limit water and nutrient availability, including P (Villegas et al. 2001). Another explanation could be that under hydroponic conditions, the decline of root activity is less marked than in field conditions which may lead to an overestimation of the contribution of post-anthesis root P uptake. However, the regulation of root activity and root nutrient remobilization during this period is not fully understood (Wang et al. 2016).
On the other hand, under post-anthesis P deprivation treatment, P grain requirements are mainly coming from remobilization and re-translocation of pre-anthesis P in different organs (Fig. 3b). As a result, P distribution at the whole plant level was strongly affected, leading to a high P remobilization efficiency. The main contributors to grain P were in decreasing order leaves > roots > stems and then spikelets, indicating that P remobilization can occur in all organs for both cultivars. The optimization of the internal P use is a common response of plants to P limitations (Hammond et al. 2004). As P deprivation sets in, plants start to remobilize P from non metabolic P compounds such as vacuolar P. The replacement of membrane phospholipids by sulfolipids and galactolipids is also an important mechanism in recycling P within the plant to supply growing tissues (Jeong et al. 2017; Veneklaas et al. 2012). In addition, considerable amounts of P between anthesis and maturity was lost for both cultivars. This P loss was attributed to efflux of P from the roots. P efflux in wheat is reported to changes in the presence of different levels of P supply (Cogliatti and María 1990). These authors found that P efflux from wheat roots has increased from 28% of influx at 50 μM to 90% at 5 mM.
The differences in post-anthesis P uptake between the two cultivars resulted in differences in grain P content at maturity (P < 0.05). The cultivar with high shoot to root ratio remobilized more than 7 mg of P while the other cultivar remobilized only 1.6 mg of P per plant. This means that with a high shoot to root ratio, the reduced P uptake due to small root system was compensated by a greater P remobilization of P from vegetative organs and roots. This difference for post-anthesis root P uptake suggests that root activity may be regulated independently from remobilization which has major implications for crop breeding. These results indicate that an improved P remobilization during the post-anthesis period could be an important lever to maximize the whole plant P use efficiency.
Biomass and P allocation relationship
To answer the question whether the difference in biomass allocation has an impact on P remobilization, we looked at the net fluxes of P at whole plant level. We found no difference between the two cultivars regarding the contribution of the different organs to the remobilization of P even if the allocation patterns of biomass were different. In addition, the P harvest index greatly increased under P deprivation treatment without any increase in harvest index.
Overall, these results suggest an impairment between C and P allocation to the grain. The reason behind this impairment may be that the allocation of C and P to grain is regulated independently in the P deprivation treatment. First, P and C flux into grain is largely provided by different sources (Masoni et al. 2007). In wheat, grain C is mainly supplied via photosynthesis and stem C remobilization while grain P is provided via P remobilization from other plant tissues (Masoni et al. 2007; Peng and Li 2005). Secondly, although the transport via xylem is possible, the exportation of P to the grain is mostly covered through the phloem unlike C which is only transported via phloem (Engels et al. 2012; Maillard et al. 2015; Peng and Li 2005).
In the present study, post-anthesis P supply had no effect on grain C:N ratio (Table 2). Wheat grain N is known to be mainly supplied by the remobilization of leaf N providing up to 90% of grain N (Distelfeld et al. 2014; Masclaux-Daubresse et al. 2008). In our study, P remobilization has occurred from all organs. Therefore, this suggest that P remobilization is not necessarily associated with N remobilization, contrary to what we initially expected, or that N remobilization from the leaves was enough to meet grain N demand. In addition, root P remobilization contributed up to 28% of the remobilized P under P deprivation treatment. Nevertheless, under field conditions, the contribution of root N remobilization to grain N is found to be very low (Allard et al. 2013). This variation could be useful in modulating the accumulation of P in wheat grain.
We found also that P deprivation accelerated leaf senescence and shortened the period of grain filling. Our results are in accordance with Maillard et al. (2015), who showed that nutrient deficiency, including P, modulate nutrient remobilization during leaf senescence. In wheat and monocarpic crops, upon the onset of senescence, vegetative organs shift from being a sink to be a source for phloem mobile nutrients (Etienne et al. 2018; Gregersen et al. 2008). Previous studies with bean plants also reported that P remobilization in leaves occurred earlier under low P supply in comparison to high P supply (Snapp and Lynch 1996).
Grain P and its implications for P management
Without exogenous post-anthesis P supply, the range of grain P concentrations found in the two cultivars was 2.2 to 3.4 mg P g− 1 DW. These concentrations are similar to those found by Batten (1992) in tetraploid wheats grown under low P conditions. In fact, grain is a net sink for P after anthesis. Therefore, P and other nutrients are allocated in priority to sustain grain growth (Veneklaas et al. 2012). These concentrations are above those required for normal functioning for a cereal crop. Recent studies have shown that grain P concentrations as low as 0.9 mg P g DW− 1 were sufficient for normal functioning during seedling growth of rice (Pariasca-Tanaka et al. 2015). In addition, with almost equal yield, wheat plants grown under continuous post-anthesis P supply would export more P than those grown under P deprivation conditions. Assuming that mean durum wheat yield in France is around 5.7 t ha− 1 (AGRESTE, 2019), P export would be equivalent to 22 and 16 kg P ha− 1respectively, which mean an exportation of P 25% higher under continuous P supply. Lowering grain phosphorus concentration is particularly important when one considers that 75% of total P in grain is in the form of phytate (Raboy 2001). This storage form of P, which is mainly found in cereal aleurone layer, is indigestible by human and monogastric animals. Furthermore, it is also an anti-nutrient due to its capacity to chelate zinc, iron and other micronutrients that are essential to human nutrition (Iwai et al. 2012; Raboy 2001). This finding suggests that an improved P remobilization while reducing post-anthesis P uptake could be an important way to maximize the whole plant P efficiency and contribute to the optimization of field P balance. In addition to this, recently Yamaji et al. (2017), described a P transporter, SULTR-like phosphorus distribution transporter (SPDT), located in the rice nodes that is involved in P allocation to the grain. Loss of function of this transporter reduced total P content by 20% without yield penalty. This is an interesting aspect because even under high P availability, efficient P remobilization is required to minimize soil P depletion, without neglecting that roots are of great importance for uptake of water and other nutrients. Screening for P transporters responsible for P allocation to the grain is a promising approach to reduce P exportation from the field.
Conclusion
Our study demonstrated that even under a situation of total limitation of external P supply during durum wheat grains filling, grain P nutrition can be fully ensured by the internal transfer of P from leaves, roots and stems. Our results showed also that all vegetative organs can act as a reserve of P for optimal grain production. Thus, an enhancement of P remobilization during the grain filling while reducing post-anthesis P uptake could be an important lever to maximize the whole plant P use efficiency. Further research will concentrate on determining the relative contribution of exogenous and endogenous P source to grain P using isotope tracer technique to quantify the gross P fluxes between the different plant organs during grain filling.
References
Allard V, Martre P, Le Gouis J (2013) Genetic variability in biomass allocation to roots in wheat is mainly related to crop tillering dynamics and nitrogen status. Eur J Agron 46:68–76. https://doi.org/10.1016/j.eja.2012.12.004
Batten GD (1992) A review of phosphorus efficiency in wheat. Plant Soil 146(1):163–168. https://doi.org/10.1007/BF00012009
Batten GD, Wardlaw IF (1987) Senescence of the flag leaf and grain yield following late foliar and root applications of phosphate on plants of differing phosphorus status. J Plant Nutr 10(7):735–748. https://doi.org/10.1080/01904168709363605
Batten GD, Wardlaw IF, Aston MJ (1986) Growth and the distribution of phosphorus in wheat developed under various phosphorus and temperature regimes. Aust J Agric Res 37(5):459–469. https://doi.org/10.1071/ar9860459
Cogliatti DH, María GES (1990) Influx and efflux of phosphorus in roots of wheat plants in non-growth-limiting concentrations of phosphorus. J Exp Bot 41(5):601–607. https://doi.org/10.1093/jxb/41.5.601
Colomb B, Debaeke P, Jouany C, Nolot JM (2007) Phosphorus management in low input stockless cropping systems: crop and soil responses to contrasting P regimes in a 36-year experiment in southern France. Eur J Agron 26(2):154–165. https://doi.org/10.1016/j.eja.2006.09.004
Cordell D, White S (2014) Life’s bottleneck: sustaining the world’s phosphorus for a Food secure future. Annu Rev Environ Resour 39(1):161–188. https://doi.org/10.1146/annurev-environ-010213-113300
Deng Y, Teng W, Tong YP, Chen XP, Zou CQ (2018) Phosphorus efficiency mechanisms of two wheat cultivars as affected by a range of phosphorus levels in the field. Front Plant Sci, 9. https://doi.org/10.3389/fpls.2018.01614
Distelfeld A, Avni R, Fischer AM (2014) Senescence, nutrient remobilization, and yield in wheat and barley. J Exp Bot 65(14):3783–3798. https://doi.org/10.1093/jxb/ert477
Engels C, Kirkby E, White P (2012) Chapter 5 - mineral nutrition, yield and source–sink relationships. In: Marschner’s mineral nutrition of higher plants. 3rd edn. Academic Press, San Diego, pp 85–133, DOI https://doi.org/10.1016/B978-0-12-384905-2.00005-4, (to appear in print)
Etienne P, Diquelou S, Prudent M, Salon C, Maillard A, Ourry A (2018) Macro and micronutrient storage in plants and their remobilization when facing scarcity: the case of drought. Agriculture 8(1):14. https://doi.org/10.3390/agriculture8010014
Ferreira MSL, Martre P, Mangavel C, Girousse C, Rosa NN, Samson MF, Morel MH (2012) Physicochemical control of durum wheat grain filling and glutenin polymer assembly under different temperature regimes. J Cereal Sci 56(1):58–66. https://doi.org/10.1016/j.jcs.2011.11.001
González FG, Miralles DJ, Slafer GA (2011) Wheat floret survival as related to pre-anthesis spike growth. J Exp Bot 62(14):4889–4901. https://doi.org/10.1093/jxb/err182
Grant CA, Flaten DN, Tomasiewicz DJ, Sheppard SC (2001) The importance of early season phosphorus nutrition. Can J Plant Sci 81(2):211–224. https://doi.org/10.4141/P00-093
Gregersen PL, Holm PB, Krupinska K (2008) Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol 10(s1):37–49. https://doi.org/10.1111/j.1438-8677.2008.00114.x
Hammond JP, Broadley MR, White PJ (2004) Genetic responses to phosphorus deficiency. Ann Bot 94(3):323–332. https://doi.org/10.1093/aob/mch156
Iwai T, Takahashi M, Oda K, Terada Y, Yoshida KT (2012) Dynamic changes in the distribution of minerals in relation to phytic acid accumulation during rice seed development. Plant Physiol 160(4):2007–2014. https://doi.org/10.1104/pp.112.206573
Jeong K, Julia CC, Waters DLE, Pantoja O, Wissuwa M, Heuer S, Liu L, Rose TJ (2017) Remobilisation of phosphorus fractions in rice flag leaves during grain filling: implications for photosynthesis and grain yields. PLOS ONE 12(11):e0187521. https://doi.org/10.1371/journal.pone.0187521
Julia C, Wissuwa M, Kretzschmar T, Jeong K, Rose T (2016) Phosphorus uptake, partitioning and redistribution during grain filling in rice. Ann Bot 118(6):1151–1162. https://doi.org/10.1093/aob/mcw164
Maillard A, Diquélou S, Billard V, Laîné P, Garnica M, Prudent M, Garcia-Mina JM, Yvin JC, Ourry A (2015) Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front Plant Sci, 6. https://doi.org/10.3389/fpls.2015.00317
Masclaux-Daubresse C, Reisdorf-Cren M, Orsel M (2008) Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol 10(s1):23–36. https://doi.org/10.1111/j.1438-8677.2008.00097.x
Masoni A, Ercoli L, Mariotti M, Arduini I (2007) Post-anthesis accumulation and remobilization of dry matter, nitrogen and phosphorus in durum wheat as affected by soil type. Eur J Agron 26(3):179–186. https://doi.org/10.1016/j.eja.2006.09.006
Ozturk L, Eker S, Torun B, Cakmak I (2005) Variation in phosphorus efficiency among 73 bread and durum wheat genotypes grown in a phosphorus-deficient calcareous soil. Plant Soil 269(1):69–80. https://doi.org/10.1007/s11104-004-0469-z
Pariasca-Tanaka J, Vandamme E, Mori A, Segda Z, Saito K, Rose TJ, Wissuwa M (2015) Does reducing seed-P concentrations affect seedling vigor and grain yield of rice? Plant Soil 392 (1):253–266. https://doi.org/10.1007/s11104-015-2460-2
Peng Z, Li C (2005) Transport and partitioning of phosphorus in wheat as affected by P withdrawal during flag-leaf expansion. Plant Soil 268(1):1–11. https://doi.org/10.1007/s11104-004-0297-1
Perrier F, Yan B, Candaudap F, Pokrovsky OS, Gourdain E, Meleard B, Bussière S, Coriou C, Robert T, Nguyen C, Cornu JY (2016) Variability in grain cadmium concentration among durum wheat cultivars: impact of aboveground biomass partitioning. Plant Soil 404(1):307–320. https://doi.org/10.1007/s11104-016-2847-8
Plénet D, Mollier A, Pellerin S (2000) Growth analysis of maize field crops under phosphorus deficiency. II. Radiation-use efficiency, biomass accumulation and yield components. Plant Soil 224 (2):259–272. https://doi.org/10.1023/A:1004835621371
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna,Austria
Raboy V (1997) Accumulation and storage of phosphate and minerals. In: Cellular and molecular biology of plant seed development, advances in cellular and molecular biology of plants. Springer, Netherlands, pp 441–477, DOI https://doi.org/10.1007/978-94-015-8909-3_12, (to appear in print)
Raboy V (2001) Seeds for a better future: ’low phytate’ grains help to overcome malnutrition and reduce pollution. Trends Plant Sci 6(10):458–462. https://doi.org/10.1016/S1360-1385(01)02104-5
Römer W, Schilling G (1986) Phosphorus requirements of the wheat plant in various stages of its life cycle. Plant Soil 91(2):221–229. https://doi.org/10.1007/BF02181789
Rose TJ, Wissuwa M (2012) Chapter five - Rethinking internal phosphorus utilization efficiency: a new approach is needed to improve PUE in grain crops. In: Advances in agronomy. Academic Press, pp 185–217, DOI https://doi.org/10.1016/B978-0-12-394277-7.00005-1, (to appear in print)
Rose TJ, Rengel Z, Ma Q, Bowden JW (2007) Differential accumulation patterns of phosphorus and potassium by canola cultivars compared to wheat. J Plant Nutr Soil Sci 170(3):404–411. https://doi.org/10.1002/jpln.200625163
Rose T, Liu L, Wissuwa M (2013) Improving phosphorus efficiency in cereal crops: Is breeding for reduced grain phosphorus concentration part of the solution? Front Plant Sci, 4. https://doi.org/10.3389/fpls.2013.00444
Shewry PR, Mitchell RAC, Tosi P, Wan Y, Underwood C, Lovegrove A, Freeman J, Toole GA, Mills ENC, Ward JL (2012) An integrated study of grain development of wheat (cv. Hereward). J Cereal Sci 56(1):21–30. https://doi.org/10.1016/j.jcs.2011.11.007
Snapp SS, Lynch JP (1996) Phosphorus distribution and remobilization in bean plants as influenced by phosphorus nutrition. Crop Sci 36(4):929–935. https://doi.org/10.2135/cropsci1996.0011183X003600040019x
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418(6898):671. https://doi.org/10.1038/nature01014
Van Veldhoven PP, Mannaerts GP (1987) Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem 161(1):45–48. https://doi.org/10.1016/0003-2697(87)90649-X
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. Phytol 157(3):423–447. https://doi.org/10.1046/j.1469-8137.2003.00695.x
Veneklaas EJ, Lambers H, Bragg J, Finnegan PM, Lovelock CE, Plaxton WC, Price CA, Scheible WR, Shane MW, White PJ, Raven JA (2012) Opportunities for improving phosphorus-use efficiency in crop plants. Phytol 195(2):306–320. https://doi.org/10.1111/j.1469-8137.2012.04190.x
Villegas D, Aparicio N, Blanco R, Royo C (2001) Biomass accumulation and main stem elongation of Durum Wheat grown under Mediterranean conditions. Ann Bot 88(4):617–627. https://doi.org/10.1006/anbo.2001.1512
Wang X, Shen J, Liao H (2010) Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops? Plant Sci 179(4):302–306. https://doi.org/10.1016/j.plantsci.2010.06.007
Wang F, Rose T, Jeong K, Kretzschmar T, Wissuwa M (2016) The knowns and unknowns of phosphorus loading into grains, and implications for phosphorus efficiency in cropping systems. J Exp Bot 67(5):1221–1229. https://doi.org/10.1093/jxb/erv517
White PJ, Veneklaas EJ (2012) Nature and nurture: the importance of seed phosphorus content. Plant Soil 357(1–2):1–8. https://doi.org/10.1007/s11104-012-1128-4
Yamaji N, Takemoto Y, Miyaji T, Mitani-Ueno N, Yoshida KT, Ma JF (2017) Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature 541(7635):92–95. https://doi.org/10.1038/nature20610
Acknowledgments
This work has been carried out with financial support from the French National Institute for Agriculture, Food and Environment (INRAE), Bordeaux Sciences Agro and in the framework of the Cluster of Excellence COTE. We thank Mark Bakker for internal revision and valuable comments on the manuscript. The authors are grateful to Jean-Yves Cornu for his help in the experimental set-up and to Sylvie Milin for plant chemical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: James Rose
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
El Mazlouzi, M., Morel, C., Robert, T. et al. Phosphorus uptake and partitioning in two durum wheat cultivars with contrasting biomass allocation as affected by different P supply during grain filling. Plant Soil 449, 179–192 (2020). https://doi.org/10.1007/s11104-020-04444-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04444-0