Abstract
Phosphorus (P) deficiency is a major problem for Australian agriculture. Development of new perennial pasture legumes that acquire or use P more efficiently than the current major perennial pasture legume, lucerne (Medicago sativa L.), is urgent. A glasshouse experiment compared the response of ten perennial herbaceous legume species to a series of P supplies ranging from 0 to 384 µg g−1 soil, with lucerne as the control. Under low-P conditions, several legumes produced more biomass than lucerne. Four species (Lotononis bainesii Baker, Kennedia prorepens F.Muell, K. prostrata R.Br, Bituminaria bituminosa (L.) C.H.Stirt) achieved maximum growth at 12 µg P g−1 soil, while other species required 24 µg P g−1. In most tested legumes, biomass production was reduced when P supply was ≥192 µg g−1, due to P toxicity, while L. bainesii and K. prorepens showed reduced biomass when P was ≥24 µg g−1 and K. prostrata at ≥48 µg P g−1 soil. B. bituminosa and Glycine canescens F.J.Herm required less soil P to achieve 0.5 g dry mass than the other species did. Lucerne performed poorly with low P supply and our results suggest that some novel perennial legumes may perform better on low-P soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many soils in Australia and elsewhere contain too little phosphorus (P) for productive agriculture and fertiliser P needs to be applied for profitable crop and pasture production. Large-scale use of P fertiliser has led to the rapid depletion of global P reserves with current P reserves predicted to be halved by 2060 (Steen 1998). In Australia, particularly in agricultural areas of south-western Australia, the replacement of deep-rooted native perennial species by annual crop and pasture species has led to rising water tables and dryland salinisation (Dunin 1970; George et al. 2008). Lucerne (Medicago sativa L.) is an alternative to shallow-rooted annual pasture species in this region (Cocks 2001). However, lucerne is poorly adapted to acidic sandy soils, to the seasonal waterlogging encountered in many texture-contrast soils, and to salinisation (Humphries and Auricht 2001). In addition, lucerne is poorly adapted to the dry and hot conditions from November to April in areas with <350 mm average annual rainfall, and to the set-stocking grazing management common in the region (Loo et al. 2006).
A potential advantage of novel perennial pasture species may be their adaptation to challenging environmental conditions (low nutrients, low rainfall, acid soils) (Dear and Ewing 2008). Novel legumes that are adapted to low-nutrient soils are likely to have greater nutrient-acquisition and -use efficiency, including acquisition and use of P, when compared with conventional species such as lucerne. Novel native and exotic perennial pasture legumes are being considered as alternatives to lucerne, and adaptation to lower P soils and higher P-use efficiency are considered to be beneficial traits for their selection. For instance, many Australian native plant species have evolved in P-impoverished soils (Handreck 1997) and have developed various mechanisms to acquire P from such soils, e.g., root clusters, mycorrhizal symbioses, exudation of carboxylates and phosphatases (Lambers et al. 2006). However, for many native Australian plant species, P-uptake capacity is poorly regulated when the P supply in soil is increased (Shane et al. 2004), and these species are sensitive to P toxicity (Handreck 1997). Previous studies on P toxicity have mainly focused on woody perennials in the Proteaceae (Handreck 1997; Shane et al. 2004) and little is known about the impact of high soil P on the performance of herbaceous perennial legumes.
The present glasshouse study compared responses of ten Australian native and exotic perennial species with pasture potential to applied P, with lucerne used as a “control”. In particular, we were interested in their response to low-P (P-response efficiency) and their capacity to grow under high-P, where toxicity might be expected. The impact of P supply on perennial legumes was measured in terms of components of plant dry mass, P concentration in shoots, leaf area and P-response efficiency (amount of dry mass produced per unit of P applied).
Materials and methods
Plant material and growth conditions
The eleven herbaceous perennial legume species studied are listed in Table 1. Seeds were scarified and pre-germinated in Petri dishes at staggered times according to their pre-determined germination time using a 1:100 dilution of smoke water to enhance germination (Tieu et al. 2001). Three germinated seedlings were planted in each pot and thinned to one plant after one week. All seedlings were inoculated with an appropriate strain of rhizobium provided by Rutherglen Center, Department of Primary Industries, Victoria, Australia, as listed in Table 1. The experiment was carried out in a temperature-controlled glasshouse at The University of Western Australia, Perth, Australia with an average daytime temperature of 24°C and average night time temperature of 21°C.
Non-drained pots (8.5 cm × 8.5 cm × 18 cm) were filled with 1.2 kg sterilised washed coarse river sand as growth substrate to minimise interference with P availability (phosphorus-retention index 1.5, measured as described by Allen and Jeffery 1990). Soil samples were air-dried and passed through a 2 mm sieve. Soil analyses performed on subsamples of soil were conducted by CSBP FutureFarm analytical laboratories (Bibra Lake, Western Australia). The washed river sand contained 1 μg g−1 of nitrate-N and 4 μg g−1 of ammonium-N (Searle 1984), 3 μg g−1 of bicarbonate-extractable P (Colwell 1963), 6 μg g−1 of total P (Allen and Jeffery 1990), and had a pH (CaCl2) of 6.3 (Rayment and Higginson 1992). All essential nutrients other than P were provided with final concentrations being (μg g−1 dry soil): N 30, S 50, Ca 24, Mg 10, Cu 0.5, Zn 2, Mn 4, B 0.119, Mo 0.4, Fe 5, Cl 23. Nitrogen was added as a mixture of NH4NO3 and Ca(NO3)2 (at equal molar concentrations) to provide an initial supply after germination, prior to nodulation. There were eight P treatments; P (KH2PO4) was added to the initial nutrient solution to provide final concentrations of 0, 6, 12, 24, 48, 96, 192 and 384 μg P g−1 dry sand. K was balanced by supplying KCl to the same level among different P treatments. The sand was initially moistened to 50% water-holding capacity with nutrient solution; thereafter, soil water content was maintained by watering pots to weight with deionised water every second day. The experiment was conducted during June–August 2007.
Plant measurements
Plants were harvested at 8 weeks. Roots were washed carefully and plants were separated into leaves, stems and roots. Leaf area was measured immediately after harvest using an Epson 1680 scanner and the Winrhizo version 4.1c (Regent Instructions, Quebec, Canada) scanner program. Shoots and roots were dried in an oven at 70°C for 72 h and dry weights recorded. To determine shoot P concentration, all above-ground parts were combined and dried material was ground to a fine powder using a stainless ball mill. Approximately 200 mg subsamples were digested using a hot concentrated nitric-perchloric (3:1) acid mixture. Total P concentration was determined by the malachite green method (Motomizu et al. 1983) using a UV-VIS spectrophotometer (Shimadzu Corporation, Japan). Seed P content was determined using the same procedure.
Yield responses of total growth (calculated by adding the dry mass (DM) of leaves, stems, roots and flowers) to applied P was calculated at P supplies of 6, 12 and 24 μg P g−1 dry sand. The yield responses to applied P were calculated for the following ranges of P application: 1) subtracting yields for the nil-P treatment from yields when 6 μg P g−1 was applied; 2) subtracting yields for the 6 μg P g−1 treatment from yields when 12 μg P g−1 was applied; 3) subtracting yields for the 12 μg P g−1treatment from yields produced when 24 μg P g−1was applied. Yield responses were then divided by the difference between amounts of P supplied to provide P-response efficiency values (DM produced per unit of P applied in each of the three ranges of P application). That is, P-response efficiency = ∆ dry mass/∆ applied soil P. As seed P content was highly related to plant growth in the nil-P treatment in this study, P-response efficiency between 0 and 6 μg P g−1 was therefore referred to as “apparent” P-response efficiency.
Statistics
The experiment was a two-factorial (species and soil P application level) randomised complete block design. Data for growth and other parameters were analysed by general analysis of variance (ANOVA) in Genstat version 10.2 (Lawes Agricultural Trust, Rothamsted Experimental Station, UK, 2007). Where significant effects were found, the l.s.d. values at P = 0.05 are shown in legends for each figure.
Results
Plant growth
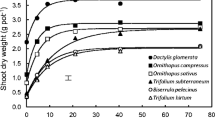
Plant growth responses to applied soil P differed among legume species. When no P was supplied, total DM of all species was low, ranging from 0.01 to 0.34 g (Figs. 1 and 2a). When no P was applied, Bituminaria bituminosa, Kennedia prostrata, Glycine canescens and K. prorepens had slightly higher total DM than the other species, while Lotononis bainesii and Medicago sativa had the lowest total DM (Fig. 2a). There was a high correlation between seed P content and plant total DM when no P was applied (Fig. 2b, r 2 = 0.77, P < 0.001).
Dry mass (DM) of 11 perennial legumes grown for 8 weeks in washed river sand supplied with 8 levels of P, ranging from 0 to 384 μg P g−1 dry soil. Data are means (n = 6). Error bars on Total DM represent s.e. (n = 6). There was a significant interaction between soil P supply level and species (p < 0.001, LSD0.05 = 0.39 g for total DM). Note x-axis break and different scales for y-axis in different species
a Dry mass of 11 perennial legumes grown for 8 weeks in washed river sand when no P was supplied or P supply was 6 μg P g−1 dry soil (means ± s.e., n = 6). b Correlation between plant dry mass and seed P content when no P was supplied to soil (means ± s.e., n = 3). c Correlation between plant dry mass and seed P content with addition of 6 μg P g−1 dry soil (means ± s.e., n = 3). Species abbreviations are: LA (L. australis, closed triangle), LB (L. bainesii, closed diamond in grey), MB (M. bracteatum, open triangle), CT (C. tenax, open circle), KPA (K. prostrata, closed diamond in black), KPS (K. prorepens, closed square in black), CA (C. australasicum, dash), LC (L. corniculatus, closed square in grey), MS (M. sativa, closed circle), GC (G. canescens, open square), and BB (B. bituminosa, open diamond)
When plants were supplied with 6 μg P g−1 dry soil, total DM increased (P < 0.05) in all species, ranging from 0.17 to 1.00 g plant−1 (Figs. 1 and 2a). B. bituminosa and G. canescens had the highest total DM, followed by M. sativa, Lotus corniculatus, Cullen australasicum and K. prorepens, while Lotus australis and L. bainesii had the lowest total DM at 6 μg P g−1 dry soil. The correlation between seed P content and total DM weakened when soil P supply was increased to 6 μg P g−1 soil (r 2 = 0.24, P > 0.05, Fig. 2c).
Total DM continued to increase (P < 0.05) as soil P application increased from 6 μg P g−1 soil to 12 μg P g−1 soil. L. bainesii, K. prorepens, K. prostrata and B. bituminosa reached maximum total DM at 12 μg P g−1 soil, while the other seven species reached maximum total DM at 24 μg P g−1 soil (Fig. 1). M. sativa had the highest total DM among all species at 24 μg P g−1 soil, followed by B. bituminosa, while L. australis and K. prostrata had the lowest total DM (Fig. 1).
When soil P application was increased above 24 μg P g−1 soil, total DM either remained the same or decreased (Fig. 1). Total DM for L. bainesii and K. prorepens decreased significantly when soil P supply was ≥24 μg P g−1 soil (P < 0.05), whereas that of K. prostrata decreased when soil P supply was ≥48 μg P g−1 soil, that of G. canescens and L. corniculatus decreased at ≥192 μg P g−1 soil, and that of C. tenax, B. bituminosa and Macroptilium bracteatum decreased at 384 μg P g−1 soil (Fig. 1). There was little change in total DM in response to soil P supply ≥24 μg g−1 for L. australis, C. australasicum and M. sativa (Fig. 1).
M. bracteatum was flowering at harvest, while other species were at a vegetative growth stage. The DM of leaves, stems, roots and flowers all followed a similar pattern to total DM in response to soil P supply (Fig. 1). Leaf area also showed a similar trend to total DM in response to soil P supply (data not shown). The root:shoot ratios were significantly higher in all species when no P was supplied to soil compared with P-supplied treatments (data not shown). There was little change in the root:shoot ratio among different P treatments, except in K. prostrata and M. bracteatum where the root:shoot ratio was also higher at 6 μg P g−1 soil than in other P treatments.
When no P was supplied, old leaves at the bottom gradually showed chlorosis and later dropped off in all species except for K. prostrata, K. prorepens and G. canescens in which no such phenomenon was observed in 8 weeks (data not shown). G. canescens and K. prorepens showed necrosis in the old leaves when P supply was ≥96 μg g−1, while K. prostrata showed toxicity symptoms at P ≥ 192 μg g−1 (data not shown). There was no obvious visual toxicity symptom at high P supply in other species.
P-response efficiency
P-response efficiency differed among the legume species. B. bituminosa and G. canescens showed the highest apparent P-response efficiency as P increased from 0 to 6 μg P g−1 soil, followed by M. sativa, while L. australis and K. prostrata showed the lowest apparent P-response efficiency (Fig. 3). When supplied P increased from 6 to 12 μg P g−1 soil, L. australis and K. prostrata still had the lowest P response efficiency, but the order for the other species changed. M. sativa had the highest P-response efficiency with 0.29 g DM mg−1 P, followed by B. bituminosa and K. prorepens. When P increased from 12 to 24 μg P g−1 soil, P-response efficiency decreased in all species, except C. australasicum. It was still highest in M. sativa, while lower in all the other species and even negative for L. bainesii, K. prostrata and K. prorepens (Fig. 3).
Phosphorus-response efficiency (yield response per unit of P applied) measured for 3 P application ranges (0–6, 6–12, and 12–24 μg P g−1 dry soil) for 11 perennial legume species. Data are means ± s.e. (n = 6). There was a significant interaction between soil P-supply level and species (p < 0.01, LSD0.05 = 0.07 g DM mg−1 P). Species abbreviations are: LA (L. australis), KPA (K. prostrata), LB (L. bainesii), MB (M. bracteatum), CT (C. tenax), KPS (K. prorepens), CA (C. australasicum), LC (L. corniculatus), MS (M. sativa), GC (G. canescens), and BB (B. bituminosa)
Most species had the highest P-response efficiency when supplied P increased from 6 to 12 μg P g−1 soil, except for C. australasicum, L. corniculatus and G. canescens, which had higher values, between 0 and 6 μg P g−1 soil. When more than 24 μg P g−1 soil was applied, P-response efficiency seldom surpassed that at 24 μg P g−1 soil for all species tested.
To minimise the influence of seed P on the apparent P-response efficiency at low P-supply, the external P-supply level required to achieve 0.5 g and 1 g dry mass was derived from the dry mass curve in response to soil P-supply (Fig. 1), when seed P influences were minimal. The required level of P supplied to soil to achieve a 0.5 g and 1 g dry mass differed greatly among species (Table 2). The level of P-supply to achieve 0.5 g dry mass ranged between 1.5 and 18 μg P g−1 soil. Among all species, B. bituminosa and G. canescens required the lowest external P-supply level, followed by M. sativa and L. corniculatus, while L. australis required the highest level of external P-supply. A very similar trend in external P-supply level was found among species to achieve 1 g dry mass (Table 2). B. bituminosa had the lowest external P-supply requirement, followed by M. sativa and G. canescens, while other species required much higher external P supply (Table 2).
Shoot P concentration
Shoot P concentration increased with increasing P supply for all species (Fig. 4). Shoot P concentration continued to increase as P supply reached 384 μg P g−1 (P < 0.05) in L. australis, M. sativa, K. prorepens, C. tenax and M. bracteatum, while shoot P concentration remained the same as that at 192 μg P g−1 in L. bainesii, B. bituminosa, G. canescens and L. corniculatus, and decreased in C. australasicum and K. prostrata (Fig. 4). At maximum total DM, the shoot P concentration was <10 mg g−1 DM for all species. At maximum total DM, C. australasicum and L. australis had the highest shoot P concentration (>7 mg g−1 DM), while G. canescens, M. sativa and B. bituminosa had the lowest shoot P concentration (<3 mg g−1 DM) (Fig. 4).
Comparison of shoot P concentration of 11 perennial legumes grown for 8 weeks in washed river sand supplied with 8 levels of P ranging from 0 to 384 μg P g−1 dry soil (means ± s.e., n = 3). There was a significant interaction between soil P-application level and species (p < 0.001, LSD0.05 = 3 mg g−1 DM). Note x-axis break
Discussion
A comparison of the response to P application of 11 perennial legumes (six native to Australia and five exotic) showed large differences among species in response to P. The implications of this result, and other major results of this study, are discussed below.
Performance of legumes under low-P conditions
All species grew relatively poorly without addition of P (Figs. 1 and 2a). However, B. bituminosa (exotic), K. prostrata, G. canescens and K. prorepens (all natives) had slightly higher total biomass than all other species when no P was supplied. The high correlation between total biomass and seed P content (Fig. 2b) when no P was applied to soil indicates a high dependence of perennial legume seedling growth on seed P reserves as found previously (Denton et al. 2006; Milberg et al. 1998). Seed P content of the perennial legume species used in this study varied between 0.002 and 0.098 mg P seed−1. B. bituminosa (exotic), K. prostrata, G. canescens and K. prorepens (all native) had much higher seed P contents than the other species (Table 1). Many plants inhabiting nutrient-poor soils in south-western Australia have seeds with high concentrations of several nutrients (Denton et al. 2007; Kuo et al. 1982). Milberg and Lamont (1997) found that N and P translocated from seeds to seedlings growing in infertile sandy soils made up a substantial part of the total amount of N and P for two Eucalyptus and two Hakea species. The high content of mineral nutrients and their translocation from cotyledons during early growth enables large-seeded species to produce large seedlings (Milberg et al. 1998). The chlorosis of old leaves when no P was supplied which we observed in most of the present legume species, except in K. prostrata, K. prorepens and G. canescens, reflects a process of translocation of P and other nutrients to young leaves. It is unclear what effect on performance seed P will have once plants become established in the field. However, other factors such as root fineness, root hairs and mycorrhizas would likely have important effects on P status of older plants.
The influence of seed P on biomass accumulation diminished when applied soil P increased from 0 to 6 μg P g−1 soil (Fig. 2c) which supports the results of many other studies. For instance, in annual pasture legumes (Bolland and Paynter 1990) and in 18 wheat genotypes (Liao et al. 2008), the relative increase in shoot dry matter due to higher seed P concentrations decreased with increasing P supply to the soil and with plant age. The influence of seed mass on seedling growth decreased with increasing nutrient availability in the soil for 21 species from three woody genera in Australia, indicating that seed mass exert a large influence on seedling performance only in extremely nutrient-impoverished situations (Milberg et al. 1998).
The ability to acquire P from soil and use it efficiently for biomass production is an important characteristic for adaptation to low-P soils. For P supply between 0 and 6 μg P g−1 soil, B. bituminosa and G. canescens were better at using applied P than other species were, as seen in their higher apparent P-response efficiency, while L. australis and K. prostrata had the lowest apparent P-response efficiency. The higher seed P content in these species may partly account for their high P-response efficiency. For P supply between 6 and 12 μg P g−1 soil, M. sativa had the highest P-response efficiency (Fig. 3). In order to minimise the influence of seed P on plant growth, the external soil P-supply level required to achieve 0.5 and 1 g dry mass was used to investigate P-use efficiency. It further indicated that B. bituminosa and G. canescens used P more efficiently as they required a lower soil P-supply than M. sativa to achieve 0.5 g dry mass. B. bituminosa also needed less soil P to achieve 1 g dry mass than M. sativa and G. canescens, which required a similar level of P-supply (Table 2).
The legume species in the present study differed in the soil P application level required for maximum growth. Previous reports have found that plant species, and even cultivars of a species, respond differently to added P and require different amounts of applied P fertiliser to achieve maximum growth. Andrew and Robins (1969) found that nine tropical and one temperate pasture legume species differed in their response to added P. In their study, Stylosanthes humilis, L. bainesii and M. sativa reached maximum dry mass at 20 kg P ha−1 (∼15 μg P g−1) while other species such as Glycine javanica and Vigna luteola required 120 kg P ha−1 (∼90 μg P g−1). The differences in external P requirement might be due to different intrinsic growth rates, P-acquisition efficiency, internal P requirements, different root-soil contact areas possibly resulting from differences in root fineness, root hairs and mycorrhizal associations (Hill et al. 2006).
At maximum total DM, C. australasicum and L. australis had a higher shoot P concentration (>7 mg g−1) than the other species, while G. canescens, M. sativa and B. bituminosa accumulated <3 mg g−1 (Fig. 4). The shoot P concentration giving the maximum biomass in our study was close to the critical foliar P concentrations of six temperate perennial pasture species during the late spring-early summer period in New Zealand (2.3–3.0 mg g−1) and of lucerne (2.6–7.0 mg g−1) (Reuter and Robinson 1997). Both the ability to maximise P acquisition and to use P efficiently are important mechanisms for plants to thrive on low-P soils (Handreck 1997). Higher shoot P concentrations at maximum total DW in C. australasicum and L. australis may reflect different aspects of thriving under P stress than found in other species. Further studies are required to clarify this.
P toxicity
P toxicity at high P supply is common for Australian woody Proteaceae that have evolved on P-impoverished soils (Heddle and Specht 1975; Shane et al. 2004). However, little information is available on plant P accumulation in the perennial legumes used in this study. Apart from L. australis, C. australasicum and M. sativa, total DM of the species in this study was reduced when soil P supply was high, although most species, except G. canescens, K. prorepens and K. prostrata, did not show obvious visual P-toxicity symptoms at high P supply. Maximum growth for most species occurred when soil P application was 24 μg g−1 soil (Fig. 1). However, at this level total DM decreased for L. bainesii (exotic), K. prorepens and K. prostrata (both native) (Fig. 1). Total DM at the highest soil P supply in K. prorepens and K. prostrata was close to total DM when no P was supplied (Fig. 1). However, total DM of L. australis, C. australasicum and M. sativa was relatively constant when P supply was >24 μg P g−1 soil, indicating a better ability to grow under high P. Species differences in response to high soil P supply have been reported previously (Islam et al. 1999). In the present study, Kennedia species were less tolerant to high P supply than the other species; indeed, they are the only species likely to suffer large growth decrease in response to P levels commonly found in agriculture soils (ca. <50 μg P g−1 soil).
Some species, including natives such as C. australasicum, L. australis and C. tenax did not show visual symptoms of P toxicity, even though they accumulated quite high shoot P concentrations. Similarly, shoot P concentration steadily increased to ∼40 mg g−1 dry weight in Ptilotus polystachyus, an Australian native forb, with the addition of soil P up to 100 mg kg−1, suggesting a poor ability to down-regulate P uptake, but a remarkable tolerance of high shoot P concentrations (Ryan et al. 2009). Some Proteaceae that are adapted to low-P soils in a Mediterranean climate acquire and store P in their roots and stems during winter (wet season) and remobilise P for growth in summer (dry season) which contributes to highly efficient P use (Handreck 1997; Jeschke and Pate 1995; Parks et al. 2000). A number of processes act to prevent the accumulation of inorganic P (Pi) reaching toxic concentrations when plants have an adequate supply of Pi and absorb it at rates exceeding demand (Schachtman et al. 1998). These processes include the conversion of Pi into organic storage compounds, e.g., phytic acid, a reduction in Pi-uptake rate from the outside solution, and Pi loss by efflux, which can be between 8 and 70% of the influx (Schachtman et al. 1998). In the present study, leaves and stems were not separated and the storage forms of P and localisation of P in tissues and at the cellular level for these species requires further research.
Trends in shoot P concentration differed among species when soil P supply was high. Some species, including L. australis, M. sativa, K. prorepens, C. tenax and M. bracteatum, were apparently unable to down-regulate P accumulation, as shoot P concentrations steadily increased with increasing soil P supply up to 384 μg P g−1. However, other species were able to maintain shoot P concentration, e.g., L. bainesii, B. bituminosa, G. canescens and L. corniculatus, or reduce shoot P concentration, e.g., C. australasicum and K. prostrata (Fig. 4). The decreased biomass of some species such as L. corniculatus and K. prostrata grown at the highest P supply was not related to the further increases in shoot P concentration; in contrast, a lower P concentration at the highest P levels was found.
The possibilities of introducing novel perennial legumes into low-P farming systems
Although lucerne is increasingly being used in the wheatbelt in south-western Australia, there are areas where lucerne is not suitable and other perennial legumes need to be identified as possible alternatives (Humphries and Auricht 2001). Understanding the P requirements of plants is important in plant introduction, selection and breeding. In the present study, some novel perennial legume species, such as B. bituminosa and G. canescens, grew better under low-P stress and required less external P-supply to achieve 0.5 g dry mass than lucerne did. Although maximum dry mass production was less than in lucerne, this might be improved by breeding and selection of superior cultivars and a search to find the existence of ecotypic variation in our study provided important background. Results of this eight-week glasshouse study need to be confirmed in long-term field studies in order to identify if: 1) novel perennial legumes are able to take up toxic amounts of P from heavily fertilised, high-P soils; and, 2) novel perennial legumes can use P already present in soil more efficiently for herbage production, reducing requirements for freshly-applied fertiliser.
Conclusions
Some species, particularly B. bituminosa, K. prostrata, G. canescens and K. prorepens, grew better than M. sativa and other species under low-P stress, partly related to their higher seed P content. B. bituminosa and G. canescens used applied soil P more efficiently than did other species under low-P stress, as shown by higher apparent P-response efficiency and lower external soil P-supply requirement to achieve 0.5 g dry mass. Species apparently differed in their capacity to down-regulate P uptake at a high soil P supply. Kennedia species (K. prostrata and K. prorepens) showed the most severe P toxicity at high soil P supply with reduced DM production, close to that when no soil P was applied. Our results suggest that some Australian native as well as novel exotic perennial legumes have the potential to fit low-P niches where lucerne production is poor and further study of mechanisms to do well at low-P is warranted.
References
Allen DC, Jeffery RC (1990) Methods for the analysis of phosphorus in Western Australian soils. In: Report on Investigation No 37. Chemistry Center of Western Australia, Perth
Andrew CS, Robins MF (1969) Effect of phosphorus on growth and chemical composition of some tropical pasture legumes. I. growth and critical percentages of phosphorus. Aust J Agric Res 20:665–685
Bolland MDA, Paynter BH (1990) Increasing phosphorus concentration in seed of annual pasture legume species increases herbage and seed yields. Plant Soil 125:197–205
Cocks PS (2001) Ecology of herbaceous perennial legumes: a review of characteristics that may provide management options for the control of salinity and waterlogging in dryland cropping systems. Aust J Agric Res 52:137–151
Colwell JD (1963) The estimation of the phosphorus fertiliser requirements of wheat in southern New South Wales by soil analysis. Aust J Exp Agric Anim Husb 3:190–197
Dear BS, Ewing MA (2008) The search for new pasture plants to achieve more sustainable production systems in southern Australia. Aust J Exp Agric 48:387–396
Denton MD, Sasse C, Tibbett M, Ryan MH (2006) Root distributions of Australian herbaceous perennial legumes in response to phosphorus placement. Funct Plant Biol 33:1091–1102
Denton MD, Veneklaas EJ, Freimoser FM, Lambers H (2007) Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilisation of phosphorus. Plant Cell Environ 30:1557–1565
Dunin FX (1970) Changes in water balance components with pasture management in south-eastern Australia. J Hydrol 10:90–102
George R, Clarke J, English P (2008) Modern and palaeogeographic trends in the salinisation of the Western Australian wheatbelt: a review. Aust J Soil Res 46:751–767
Handreck KA (1997) Phosphorus requirements of Australian native plants. Aust J Soil Res 35:241–289
Heddle EM, Specht RL (1975) Dark Island Heath (90-Mile Plain, South-Australia). 8. Effect of fertilizers on composition and growth, 1950–1972. Aust J Bot 23:151–164
Hill JO, Simpson RJ, Moore AD, Chapman DF (2006) Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 286:7–19
Humphries AW, Auricht GC (2001) Breeding lucerne for Australia’s southern dryland cropping environments. Aust J Agric Res 52:153–169
Islam M, Turner DW, Adams MA (1999) Phosphorus availability and the growth, mineral composition and nutritive value of ephemeral forbs and associated perennials from the Pilbara, Western Australia. Aust J Exp Agric 39:149–159
Jeschke WD, Pate JS (1995) Mineral nutrition and transport in xylem and phloem of Banksia prionotes (Proteaceae), a tree with dimorphic root morphology. J Exp Bot 46:895–905
Kuo J, Hocking PJ, Pate JS (1982) Nutrient reserves in seeds of selected Proteaceous species from southwestern Australia. Aust J Bot 30:231–249
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713
Liao M, Hocking PJ, Dong B, Delhaize E, Richardson AE, Ryan PR (2008) Variation in early phosphorus-uptake efficiency among wheat genotypes grown on two contrasting Australian soils. Aust J Agric Res 59:157–166
Loo C, Dolling PJ, Mokhtari S (2006) Lucerne. In: Moore G, Sanford P, Wiley T (eds) Perennial pastures for Western Australia, bulletin 4690. Department of Agriculture and Food Western Australia, South Perth, pp 59–75
Milberg P, Lamont BB (1997) Seed/cotyledon size and nutrient content play a major role in early performance of species on nutrient-poor soils. New Phytol 137:665–672
Milberg P, Perez-Fernandez MA, Lamont BB (1998) Seedling growth response to added nutrients depends on seed size in three woody genera. J Ecol 86:624–632
Motomizu S, Wakimoto T, Toei K (1983) Spectrophotometric determination of phosphate in river waters with molybdate and malachite green. Analyst 108:361–367
Parks SE, Haigh AM, Cresswell GC (2000) Stem tissue phosphorus as an index of the phosphorus status of Banksia ericifolia L. f. Plant Soil 227:59–65
Reuter DJ, Robinson JB (1997) Plant analysis: an interpretation manual, 2nd edn. CSIRO, Collingwood
Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata, Melbourne
Ryan MH, Ehrenberg S, Bennett RG, Tibbett M (2009) Putting the P in Ptilotus: a phosphorus accumulating herb native to Australia. Ann Bot 103:901–911
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453
Searle PL (1984) The berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. Analyst 109:549–568
Shane MW, McCully ME, Lambers H (2004) Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae). J Exp Bot 55:1033–1044
Steen I (1998) Phosphorus availability in the 21st century: management of a non-renewable resource. Phosphorus Potassium 217:25–31
Tieu A, Dixon KW, Meney KA, Sivasithamparam K (2001) The interaction of heat and smoke in the release of seed dormancy in seven species from southwestern Western Australia. Ann Bot 88:259–265
Acknowledgements
This work was funded by the Australian Research Council (ARC), Department of Agricultural and Food Western Australia, and Heritage Seeds. We thank Daniel Real (DAFWA), Richard Snowball (DAFWA), Ron Yates (DAFWA), Steve Hughes (SARDI), and Leonard Song (Heritage seeds) for providing legume seeds and rhizobium. Richard Bennett provided technical advice, and Tammy Edmonds-Tibbett and Tim Morald provided technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tim Simon George.
Rights and permissions
About this article
Cite this article
Pang, J., Tibbett, M., Denton, M.D. et al. Variation in seedling growth of 11 perennial legumes in response to phosphorus supply. Plant Soil 328, 133–143 (2010). https://doi.org/10.1007/s11104-009-0088-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-009-0088-9