Abstract
Positive effects of sugar beet (SB) application on soil properties and performance of several woody shrub legumes have been described under heavy metal stress and in diverse degraded environments, especially when combined with arbuscular mycorrhiza (AM). However, information on the combined effect of SB amendments and AM symbiosis in horticultural crop plants under drought stress is scarce. Thus, the main objective of this work was to determine if the combination of treated SB waste and AM fungi results in improved drought tolerance of an horticultural food crop such as lettuce and whether or not the effects observed are linked to enhanced antioxidant activities and regulation of two stress-related genes. Lettuce plants inoculated or not with Glomus intraradices and grown on soil amended or not with a treated SB waste were cultivated under well-watered conditions or subjected to drought stress. Plant growth, expression of two drought responsive genes encoding for Δ1-pyrroline-5-carboxylate synthetase and 9-cis-epoxycarotenoid dioxygenase, oxidative damage to lipids and the activity of four antioxidant enzymes were measured. Results showed that the application of treated SB waste resulted negative for the development of AM and nonAM plants (both under well-watered and under drought stress conditions). This effect can not be ascribed to the impairment of specific plant antioxidant defenses. In contrast, a lack of induction of a gene from the ABA biosynthetic pathway was observed in SB-treated plants, which could have contributed to the low performance of these plants. The positive effects of combined application of treated SB waste as amendment and AM fungi have not been shown for a horticultural food crop such as Lactuca sativa. Thus, before starting a program aimed at the utilization of different amendments based on transformed wastes, basic studies on functional and physiological compatibility between the plant and the amendment are necessary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lack of adequate water in soil is an important problem faced by plants in order to grow and develop properly. Soils too dry for crop production have been estimated to cover 28% of the Earth’s land surface (Bray 2004). Nevertheless, plants have developed several physiological, biochemical and molecular mechanisms in order to cope with drought stress (Bray 2004). The arbuscular mycorrhizal (AM) symbiosis improves many plant nutritional, biochemical, physiological and morphological plant responses and, thus, it enhances the plant resistance to biotic and abiotic stresses (Barea and Jeffries 1995; Barea et al. 2002; Vivas et al. 2003).
Studies on drought stress alleviation by the AM symbiosis have suggested several mechanisms by which this symbiosis can alleviate drought stress in host plants (for reviews see Augé 2001, 2004; Ruiz-Lozano 2003: Ruiz-Lozano et al. 2008). The most important ones include: direct uptake and transfer of water through the fungal hyphae to the host plant (Hardie 1985; Ruiz-Lozano and Azcón 1995), changes in soil water retention properties (Augé et al. 2001), better osmotic adjustment of AM plants (Augé et al. 1992; Ruiz-Lozano et al. 1995a; Kubikova et al. 2001), enhancement of plant gas exchange and water use efficiency (Augé et al. 1992; Ruiz-Lozano et al. 1995a, b) and protection against the oxidative damage generated by drought (Ruiz-Lozano et al. 2001; Porcel et al. 2003; Porcel and Ruiz-Lozano 2004). This last mechanism has been recognized as crucial (Ruiz-Lozano 2003). In fact, many of the degenerative reactions associated with several environmental stresses, including water deficit, result in the production of reactive oxygen species (ROS). These ROS cause an additional oxidative stress in plants. Indeed, ROS such as singlet oxygen, superoxide anion (O2.-), hydrogen peroxide and hydroxyl radical (-.OH) are inevitable by-products of the interaction between oxygen and electrons leaked from the electron transport chains in chloroplast and mitochondria during normal aerobic metabolism. During water deficit, the induced stomatal closure leads to photosynthesis inhibition and subsequent leakage of electrons towards O2, resulting in enhanced ROS generation (Basu et al. 2010). Thus, it has been proposed that a major function of AM fungi could be to protect plants against induced oxidative stress (Schutzendubel and Polle 2002; Porcel et al. 2003).
It has also been noted that AM and non AM plants differently regulate the expression of several genes in root tissues related to water stress (Ruiz-Lozano et al. 2006). These stress related genes include a P5CS gene (Porcel et al. 2004), encoding for Δ1-pyrroline-5-carboxylate synthetase. P5CS is the enzyme that catalyzes the rate-limiting step in the biosynthesis of proline, which is a robust osmotic and antioxidant agent (Aral and Kamoun 1997). Regulated genes by AM symbiosis under drought conditions also include nced genes (Jahromi et al. 2008; Aroca et al. 2008a, b). The nced gene family encodes for 9-cis-epoxycarotenoid dioxygenase (NCED), the key enzyme for the biosynthesis of ABA (Schwartz et al. 2003). ABA plays a major role in plant responses to several stresses and accumulates in plant tissues under stress conditions (Zhang et al. 2006). Such ABA accumulation promotes stomatal closure to minimize transpirational water loss, but it also mitigates stress damage through the activation of many stress-responsive genes which collectively increase plant stress tolerance (Bray 2002).
As soil quality deteriorates in soils subjected to adverse environmental conditions, the use of an organic amendment is recommended. The agronomic utilization of agrowastes, such as sugar beet (SB), dry olive cake (DOC) and urban organic wastes has increased steadily in recent years as an alternative source of nutrients and organic matter. At the same time, its utilization is an acceptable method for their disposal (Caravaca et al. 2006). After treatment with Aspegillus niger added to a rock-phosphate (RP) medium, SB, an inexpensive lignocellulosic residue, can be used as an effective amendment for improving soil characteristics (Vassilev et al. 1996; Medina et al. 2006; Azcón et al. 2009). This SB residue is transformed by A. niger into more simple sugar compounds that can be used by rhizosphere microorganisms for metabolic activities and growth (Bowen and Rovira 1999). Previous studies have proposed the use of AM fungi and A. niger-treated SB amendments as alternative strategies for alleviating plants heavy metal resistance (Medina et al. 2005, 2006; Azcón et al. 2009), as well as, for improving soil properties in diverse degraded areas (Alguacil et al. 2003; Medina et al. 2004a, b; Caravaca et al. 2004, 2005, 2006). Furthermore, Medina et al. (2007) described stimulatory effects of A. niger-treated SB on the AM fungal growth and P uptake by external mycelium. In contrast, the information on the combined effect of SB amendments and AM inoculation under drought stress is scarce, mainly in horticultural crop plants. Drought affects the plant metabolic activities by adversely affecting cell integrity and functioning. This phenomenon also involves biochemical adaptations such as changes in the cell enzymatic activities. The effects of composted SB in combination with AM fungi on antioxidant plant defense and expression of stress-related genes under drought stress are not well known, and this study represents the first attempt to determine the usefulness of these values in detecting changes in the drought tolerance of plants growing in dry environments. Thus, the main objective of this work was to determine if the combination of composted SB waste and AM fungi results in improved drought tolerance in an horticultural crop plant such as lettuce, and whether or not the effects observed are linked to enhanced antioxidant activities and regulation of two stress-related genes. This would allow validation of SB waste as a general amendment for improving plant development in many arid and semiarid environments, especially when combined with AM fungi.
Materials and methods
Experimental design and statistical analysis
The experiment consisted of a factorial design with three factors: (1) inoculation or not with the AM fungus Glomus intraradices (Schenck and Smith) BEG 121, (2) water regime involving plants cultivated under well-watered conditions or plants subjected to drought stress and (3) amendment or not of soil with a composted SB waste. These factors totaled eight treatments that were replicated fives times giving a total of 40 pots.
Data were subjected to analysis of variance (ANOVA) with inoculation treatment, water regime, soil amendment and their interactions as sources of variation, and followed by Duncan’s multiple range test (Duncan 1955). Percentage values were arcsin transformed before statistical analysis.
Soil and biological material
Loamy soil was collected from the Zaidin Experimental Station (Granada, Spain), sieved (2 mm), diluted with quartz-sand (<1 mm) (1:1, soil:sand, v/v) and sterilized by steaming (100°C for 1 h on three consecutive days). The original soil had a pH of 8.1 (water); 1.81% organic matter, nutrient concentrations (mg kg-1): N, 2.5; P, 6.2 (NaHCO3-extractable P); K, 132.0. The electrical conductivity of soil was 0.7 dS m−1. The soil texture was made up of 35.8% sand, 43.6% silt and 20.5% clay.
Three seeds of lettuce (Lactuca sativa L. cv. Romana) were sown in pots containing 750 g of the same soil/sand mixture as described above and thinned to one seedling per pot after emergence.
Mycorrhizal inoculum was bulked in an open-pot culture of Zea mays L. and consisted of soil, spores, mycelia and infected root fragments. The AM species used was Glomus intraradices (Schenck and Smith) isolate BEG 121. Ten grams of inoculum with about 60 infective propagules per gram (according to the most probable number test), were added to appropriate pots at sowing time just below lettuce seeds.
Uninoculated control plants received the same amount of autoclaved mycorrhizal inoculum together with a 2-ml aliquot of a filtrate (<20 μm) of the AM inoculum in order to provide a general microbial population free of AM propagules.
The treatments receiving the SB waste were amended with composted SB waste. The amendment was mixed at a rate of 5% with half of the soil/sand mixture and left for equilibration for 3 weeks at room temperature, before starting the experiment.
Preparation of SB amendment
Sugar beet waste, a lignocellulosic material, was ground in an electrical grinder to 1 mm fragments. It was mixed at a concentration of 10% with 50 ml of Czapek’s solution (described in Fluka Chemica, catalogue no. 70185) containing (g/litre of distilled water): FeSO4, 0.01; MgSO4.7H2O, 0.5; KCl, 0.5; NaNO3, 3.0; sucrose, 30; K2HPO4, 1.0, and with a final pH of 7.3 ± 0.2 for static fermentation in 250 ml Erlenmeyer flasks. Rock phosphate (RP) at a concentration of 3 g L−1 was added to the ground SB material before fermentation. The SB waste was inoculated with 3 ml of Aspergillus niger spore suspension (1.2 × 106 spores). The NB2 strain of A. niger was used in this study. It had previously been selected as it produces citric acid on complex substrates (Vassilev et al. 1986). Static fermentation was performed at 28°C for 20 days. Rodriguez et al. (1999) analyzed the organic matter and nutrient contents of SB waste + RP after the fermentation period, resulting in 51% organic matter, 0.18% P and 1.4% N.
Growing conditions
The experiment was carried out under glasshouse conditions with temperatures ranging from 19 to 25°C, 16/8 light/dark period and a relative humidity of 70%–80%. A photosynthetic photon flux density of 800 μE m−2 s−1 was measured with a light meter (LICOR, Lincoln, NE, USA, model LI-188B).
Soil moisture was measured with a ML2 ThetaProbe (AT Delta-T Devices Ltd., Cambridge, UK) as previously described (Porcel and Ruiz-Lozano 2004). Water was supplied daily to maintain soil at field capacity during the first 6 weeks after planting. Then, half of the plants were allowed to dry until soil water content reached 70% field capacity, while the other half were maintained at field capacity. The soil water content was daily measured with the ThetaProbe ML2 before rewatering (at the end of the afternoon), reaching a minimum soil water content ranging from 65% to 70% field capacity. The amount of water lost was added to each pot in order to keep the soil water content at the desired level of 70% field capacity (Porcel and Ruiz-Lozano 2004). Plants were maintained under such conditions for additional 10 days before harvesting.
Parameters measured
Biomass production
At harvest (52 days after planting), the shoot and root system were separated and the shoot dry weight (DW) was measured after drying in a forced hot-air oven at 70°C for 2 days.
Symbiotic development
The percentage of mycorrhizal root length infected was estimated by visual observation of fungal colonization after clearing washed roots in 10% KOH and staining with 0.05% trypan blue in lactic acid (v/v) (Phillips and Hayman 1970). Quantification was carried out using the grid-line intersect method (Giovannetti and Mosse 1980).
Proline content
Free proline was extracted from 1 g of fresh root or shoot tissues that was ground with 6 ml of methanol and 6 ml of chloroform (Blig and Dyer 1959). After that, 3 ml of a 0.9% NaCl solution was added and mixed. The resulting mixture was centrifuged at 5000 rpm for 10 min at 1°C. Proline was estimated by spectrophotometric analysis of four replicates of the methanolic phase at 515 nm of the ninhydrin reaction, according to Bates et al. (1973). Four replicates per treatment were used.
Hydrogen peroxide content
Hydrogen peroxide content was determined by Patterson’s method (Patterson et al. 1984), with slight modifications as described previously by Aroca et al. (2003). Five hundred milligrams of root or shoot fresh weights were homogenized in a cold mortar with 5 ml 5% (w/v) TCA containing 0.1 g of activated charcoal and 1% (w/v) PVPP. The homogenate was centrifuged at 18,000 g for 10 min. The supernatant was filtered through a Millipore filter (0.22 μm). A volume of 1.2 ml of 100 mM potassium phosphate buffer (pH = 8.4) and 0.6 ml of the colorimetric reagent were added to 130 μl of the supernatant. The colorimetric reagent was freshly made by mixing 1:1 (v/v) 0.6 mM potassium titanium oxalate and 0.6 mM 4–2 (2-pyridylazo) resorcinol (disodium salt). The samples were incubated at 45°C for 1 h and the absorbance at 508 nm was recorded. The blanks were made by replacing leaf extract by 5% TCA.
Oxidative damage to lipids
Lipid peroxides were extracted by grinding 500 mg of root or leaf tissues with and ice-cold mortar and 6 ml of 100 mM potassium phosphate buffer (pH 7). Homogenates were filtered through one Miracloth layer and centrifuged at 15,000 g for 20 min. The chromogen was formed by mixing 200 μl of supernatants with 1 ml of a reaction mixture containing 15% (w/v) Trichloroacetic acid (TCA), 0.375% (w/v) 2-thiobarbituric acid (TBA), 0.1% (w/v) butyl hydroxytoluene, 0.25 N HCl and by incubating the mixture at 100°C for 30 min (Minotti and Aust 1987). After cooling at room temperature, tubes were centrifuged at 800 g for 5 min and the supernatant was used for spectrophotometric reading at 532 nm. Lipid peroxidation was estimated as the content of 2-thiobarbituric acid-reactive substances (TBARS) and expressed as equivalents of malondialdehyde (MDA) according to Halliwell and Gutteridge (1989). The calibration curve was made using MDA in the range of 0.1–10 nmol. A blank for all samples was prepared by replacing the sample with extraction medium, and controls for each sample were prepared by replacing TBA with 0.25 N HCl. In all cases, 0.1% (w/v) butyl hydroxytoluene was included in the reaction mixtures to prevent artifactual formation of 2-thiobarbituric acid-reactive substances (TBARS) during the acid-heating step of the assay.
Antioxidant enzymatic activities
Total superoxide dismutase (SOD) activity (EC 1.15.1.1) (Beyer and Fridovich 1987) was measured on the basis of SOD’s ability to inhibit the reduction of nitroblue tetrazolium (NBT) by superoxide radicals generated photochemically. One unit of SOD was defined as the amount of enzyme required to inhibit the reduction rate of NBT by 50% at 25°C. Catalase (CAT) activity (EC 1.11.1.6) was measured (Aebi 1984). Consumption of H2O2 (extinction coefficient of 39.6 mM−1 cm−1) at 240 nm for 1 min was monitored. The reaction mixture consisted of 50 mM phosphate buffer (pH 7.0) containing 10 mM H2O2 and 100 μl of enzyme extract in a 2 ml volume. Ascorbate peroxidase (APX) activity (EC 1.11.1.11) was measured in a 1-ml reaction volume containing 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM hydrogen peroxide and 0.5 mM ascorbate. The H2O2 was added to start the reaction, and the decrease in absorbance at 290 nm was recorded for 1 min to determine the oxidation rate for ascorbate (Amako et al. 1994). Glutathione reductase (GR) activity (EC 1.20.4.2.) was estimated by measuring the decrease of absorbance at 340 nm and 25°C due to the oxidation of NADPH (Carlberg and Mannervik 1985). The reaction mixture (1 ml) contained 0.1 M HEPES-NaOH 100 mM (pH 7.8), 1 mM EDTA, 3 mM MgCl2, 0.5 mM oxidized glutathione, 150 μl enzyme extract, and 0.2 mM NADPH was added and mixed thoroughly to begin the reaction. The activity was calculated from the initial speed of reaction and the molar extinction coefficient of NADPH (ε340 = 6.22 mM−1 cm−1).
Northern blot analysis
Total RNA was isolated from lettuce roots and leaves by phenol/chloroform extraction according to the method described by Kay et al. (1987). RNA (10 μg) was separated by electrophoresis on 1.2% agarose gel containing 2.2 M formaldehyde and blotted onto Hybond-N + nylon membranes (Amersham, Little Chalfont, UK) by capillarity (Sambrook et al. 1989). Blots were prehybridized 2–3 h at 42°C in 6X Denhardt’s solution, 5X SSC, 0.5% SDS and hybridized with lsp5cs or lsnced specific probes (from genes encoding for Δ1-pyrroline-5-carboxylate synthetase, accession AJ715852 and 9-cis-epoxycarotenoid dioxygenase, accession AB120109) obtained by radioactive PCR labelling of plasmid inserts. Unincorporated 32P was removed using Mini Quick SpinTM columns (Boehringer Manheim, Indianapolis, IN). A total of 107 cpm probe was heat-denatured and used to hybridize the blots overnight at 65°C under standard conditions (Sambrook et al. 1989). After washing twice for 5 min at room temperature in 2X SSC and 0.1% SDS, and twice for 15 min at 65°C with 0.5X SSC and 0.1% SDS, membranes were exposed to phosphorimager (Molecular Dynamics Inc). The amount of rRNA in the membranes was quantified using Quantity One software (BioRad, Hemel Hempstead, UK) in ethidium bromide-stained membranes. After the northern blot, the hybridization signals were analyzed with a phosphorimager and quantified using the same software. Transcript accumulation levels for each gene probe (in arbitrary units) were divided by the corresponding amount of rRNA in the membrane (also in arbitrary units). Each quantification of signals on screens and of rRNA in the membranes was repeated three times and the average value for each was used for normalization.
Results
AM colonization and plant growth
No AM colonization was observed in roots of the non inoculated treatments. AM fungal inoculation resulted in about 35% of mycorrhizal root length in absence of SB waste and in 50% of mycorrhizal root length in presence of SB waste. The water regime applied did not affect the mycorrhizal root colonization (data not shown).
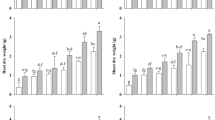
Under well-watered conditions and in absence of SB-waste AM plants grew 23% more than nonAM plants (Fig. 1). When the SB-waste was added to the growing medium all plants grew less than in absence of SB. However, in presence of SB-waste and drought stress conditions AM plants grew 25% more than nonAM plants. Drought stress decreased plant growth both in presence and in absence of SB.
Shoot dry weight of lettuce plants cultivated under well-watered (WW) conditions or subjected to drought stress (DS). Plants remained as uninoculated control (C) or were inoculated with the AM fungus Glomus intraradices (Gi). The soil substratum of half of plants was amended with a composted sugar beet waste (sugar beet) while the other half remained unamended (−). Means followed by the same letter are not significantly different (P < 0.05) as determined by Duncan’s multiple range test
Proline accumulation and expression of lsp5cs gene
Drought induced the accumulation of proline in AM and nonAM plants as compared to well-watered plants (Fig. 2). It is noticeable that under drought stress nonAM plants accumulated more proline in shoots than AM plants and this effect was evidenced both in presence (increase by 130%) and in absence of SB-waste (increase by 72%). In contrast, in roots, AM plants subjected to drought accumulated more proline than nonAM plants (by 40% and 113% in presence and absence of SB waste, respectively).
Proline accumulation in shoots (a) and roots (b) of lettuce plants. See legend for Fig. 1
We analyzed the expression of lsp5cs gene, which encodes the protein Δ1-pyrroline-5-carboxylate synthetase (P5CS), involved in the biosynthesis of proline. In absence of SB waste, the expression of that gene was induced by drought stress, both in shoots and in roots, although it was more clearly visible in shoots than in roots (Fig. 3). In shoots, the highest transcript accumulation was observed in nonAM plants subjected to drought. In presence of SB waste, the highest transcript accumulation was observed in roots of nonAM and AM plants subjected to drought. In contrast, in shoots, the expression was almost unaffected by the different treatments.
Northern blot analysis of lsp5cs gene expression in shoots and roots of lettuce plants. See legend for Fig. 1. Numbers close to each northern represent the relative gene expression after normalization to rRNA and “nq” means not quantifiable
Expression of lsnced gene
The expression of lsnced gene was observed only in shoots of nonAM plants subjected to drought and cultivated without SB waste, while AM plants subjected to drought did not induce the expression of the gene (Fig. 4). The application of SB almost prevented lsnced gene expression both in shoots and in roots. In fact, only nonAM plants subjected to drought showed a very slight expression of this gene in roots. In contrast, when no SB was applied the expression of this gene was observed in roots of nonAM plants cultivated under well-watered conditions and also in the roots of AM and nonAM plants subjected to drought. In that case, the expression in roots of nonAM plants was higher than in those of AM plants.
Northern blot analysis of lsnced gene expression in shoots and roots of lettuce plants. See legend for Fig. 1. Numbers close to each northern represent the relative gene expression after normalization to rRNA and “nq” means not quantifiable
Hydrogen peroxide accumulation and oxidative damage to lipids
In shoots hydrogen peroxide accumulation was considerably lower (by 69%) in AM plants than in nonAM plants when cultivated under well-watered conditions, but not when subjected to drought stress (Fig. 5). The application of SB maintained the hydrogen peroxide concentration in similar values for AM and nonAM plants cultivated under well-watered conditions. Drought stress decreased the hydrogen peroxide concentration both in AM and nonAM plants, but the decrease was only significant in nonAM plants (decrease by 44%).
Hydrogen peroxide accumulation in shoots (a) and roots (b) of lettuce plants. See legend for Fig. 1
In roots the hydrogen peroxide concentration was always significantly higher in AM plants than in nonAM ones, regardless of water regime and presence or absence of SB waste.
The oxidative damage to lipids varied considerably depending on the different treatments and the plant tissue considered (Fig. 6). In shoots, under well-watered conditions and absence of SB waste it was 72% higher in AM plants, but in presence of SB waste, it was 41% higher in nonAM plants. Under drought stress conditions, AM plants exhibited significantly higher oxidative damage to lipids only in presence of SB waste (33% of increase). In root tissues the oxidative damage to lipids showed an almost opposite trend as compared to shoots. In fact, under well-watered conditions and absence of SB waste it was 74% lower in AM plants, but in presence of SB waste it increased by 42% in AM plants. Under drought stress conditions AM plants showed enhanced lipid peroxidation (by 240%) in absence of SB waste as compared to nonAM plants. Under these water conditions, the application of SB decreased lipid peroxidation both in AM and nonAM plants, but the decrease was higher (by 70%) in AM plants.
Oxidative damage to lipids in shoots (a) and roots (b) of lettuce plants. See legend for Fig. 1
Plant antioxidant enzyme activities
SOD
In shoots the SOD activity did not show important changes as a consequence of the treatments applied (Fig. 7). Only nonAM plants subjected to drought in presence of SB waste showed 45% enhanced SOD activity as compared to AM plants.
SOD activity in shoots (a) and roots (b) of lettuce plants. See legend for Fig. 1
In roots the SOD activity was 45% lower in AM plants than in nonAM ones, both under well-watered and under drought stress conditions. The SOD activity of AM and nonAM plants increased as a consequence of drought. The application of SB waste almost equalized SOD activity in all treatments, with the exception of AM plants cultivated under well-watered conditions, which showed slightly lower SOD activity than the corresponding nonAM plants.
APX
In shoots the APX activity increased by 145% in AM plants as a consequence of drought, but the application of SB waste avoided such enhancement and resulted in a clear reduction of 39% in APX activity of AM plants subjected to drought (Fig. 8). The application of SB waste itself enhanced APX activity in well-watered plants and in nonAM plants subjected to drought.
APX activity in shoots (a) and roots (b) of lettuce plants. See legend for Fig. 1
In roots and in absence of SB the APX activity decreased in AM roots, both under well-watered (by 53%) and under drought stress conditions (by 59%). In presence of SB waste APX activity increased by 287% in AM plants subjected to drought, as compared to droughted nonAM plants.
CAT
The CAT activity could be measured only in shoots. No activity was detected in roots (Fig. 9). Thus, drought stress enhanced CAT activity of nonAM plants both in presence (increase by 38%) and in absence (increase by 149%) of SB waste. In contrast, AM plants did not increase their CAT activity under drought stress, regardless of SB application.
CAT activity in shoots of lettuce plants. See legend for Fig. 1
GR
The GR activity showed just an opposite trend in shoots than in roots (Fig. 10). In fact, in shoot tissues it was enhanced (by 400%) by AM symbiosis under well-watered conditions and it decreased (by 67%) in root tissues. Drought stress enhanced GR activity by 813% in shoots of nonAM plants, while it enhanced the activity in roots of AM plants by 406%. In presence of SB waste and well-watered conditions, the GR activity increased in shoots of AM plants by 84% and it decreased non significantly in their roots, while under drought stress conditions it was similar in shoots of AM and nonAM plants, but showed a 332% of enhancement in roots of AM plants.
GR activity in shoots (a) and roots (b) of lettuce plants. See legend for Fig. 1
Discussion
In this study, we have investigated the effects of microbial treatments (AM fungus) and A. niger-treated SB amendment on the responses of a food crop plant such as lettuce under drought stress conditions.
A. niger-treated SB waste provides an organic amendment rich in polysaccharide compounds and available P through the RP applied during the fermentation process. This amendment has been shown to significantly increase biomass production in a variety of plants (Alguacil et al. 2003; Medina et al. 2004a, b; Caravaca et al. 2004, 2005, 2006). In our study, the percentage of mycorrhizal root infection increased with SB application, as has been shown in the above mentioned studies, but lettuce plants amended with SB grew less than non amended plants. The exact reason for this effect is not known but it is clear that most of the plants previously tested were woody shrubs and lettuce is an annual horticultural crop that may result negatively affected by some of the components of the composted SB waste. In fact, the use of other agro wastes such as DOC have been seen to have a detrimental effect on seed germination, plant growth and microbial activity in soil due to its phenol, organic acid and fatty acid content (Linares et al. 2003). Undesirable constituents potentially associated with agro wastes also include elevated levels of heavy metals and xenobiotics (Caravaca et al. 2006). Thus the composted SB waste may have a phytotoxic effect on lettuce plants, while on woody shrubs this effect was not evident. For instance, the release of lignin, as a consequence of mineralization of SB (Rodriguez et al. 1999), could have had adverse effects on the development of lettuce plants.
To counter with drought stress, many plants increase the osmotic potential of their cells by synthesizing and accumulating compatible osmolytes such as proline that participates in the osmotic adjustment (Morgan 1984; Kishor et al. 1995). However, proline performs also an important function as a protective compatible osmolyte in scavenging of free radicals and facilitating a correction of altered redox potential by replenishment of the NADP + supply (Hare et al. 1999; Hasegawa et al. 2000). The analysis of lsp5cs gene expression showed that, in general, that gene responded to drought and was up-regulated in drought stressed treatments, in parallel with the levels of proline accumulated in the plant tissues. This suggests that proline is important for the plant response against stresses involving water deficit (Kishor et al. 1995; Yoshiba et al. 1997; Hare et al. 2003). In any case, the induction of lsp5cs gene was lower in AM than in nonAM plants (mainly in absence of SB), as we already found in previous studies with soybean and lettuce plants (Porcel et al. 2004). The levels of proline accumulation followed also the same pattern in shoot tissues, but in root tissues they were higher in AM plants subjected to drought than in nonAM plants. This suggest that in root tissues AM plants accumulate more proline in order to cope with the low water potential of drying soil and to keep a water potential gradient favourable to water entrance into the roots, as was observed in soybean plants (Porcel and Ruiz-Lozano 2004). Thus, the AM plants would have a better water status than non AM plants and their shoots would be less strained by drought stress. By that reason shoots of AM plants would need to accumulate less proline, as shown in Fig. 2 and the gene lsp5cs is less expressed in the shoots of these plants (Fig. 3).
Many of the plant responses to soil drying occur via chemical signals such as the phytohormone ABA (Wilkinson and Davies 2002). In this study we investigated the expression of a nced gene, encoding for the key enzyme for the biosynthesis of ABA (Schwartz et al. 2003). In our study, when detected, the expression of Lsnced gene was higher in nonAM than in AM plants. This disagrees with previous results by Aroca et al. (2008a,b) in lettuce and tomato plants subjected to drought stress, where AM plants showed higher levels of nced gene expression. However, recently Fiorilli et al. (2009) have detected expression of a tomato nced gene only in cortical cells of nonAM roots, while in the cortical or arbusculated cells of AM tomato roots, the expression was not detected. The induction of nced genes by drought stress has been observed previously in a variety of plants (Iuchi et al. 2000; Tan et al. 2003; Rodrigo et al. 2006; Wan and Li 2006). In contrast, in our study, Lsnced gene expression could not be detected in plants cultivated in presence of SB waste, even under drought stress. Cheng et al. (2002) demonstrated in Arabidopsis that a minimum level of ABA is required in plant tissues for full induction of a nced gene since ABA-deficient mutants accumulated less mRNAs for this gene in response to drought and salt stress treatments. Thus, it is possible that in SB-treated plants the level of ABA could be low, even in those subjected to drought, and this avoided induction of Lsnced gene expression. Low ABA levels may also have avoided the induction of other genes (Bray 2002) and this contributed to the low performance of SB-treated plants. Another possible explanation for the lack of lsnced gene expression is that the SB ammendment could contain some specific inhibitor of the expression of this gene. However, this hypothesis needs further studies.
Several studies suggested that AM symbiosis helps plants to alleviate osmotic stress by enhancing the antioxidant plant defenses (Ruiz-Lozano et al. 2001; Alguacil et al. 2003; Ruiz-Sánchez et al. 2010). Drought stress-induced exacerbated ROS generation is well-recognized at the cellular level and is tightly controlled at both the production and consumption levels in vivo, through increased antioxidant systems (Moran et al. 1994; Mittler 2002; Reddy et al. 2004). Superoxide radicals (O .-2 ) and hydrogen peroxide (H2O2) are synthesized at very high rates even under optimal conditions (Noctor and Foyer 1998). The most important aspect of O .-2 and H2O2 toxicity is thought to be their ability to initiate cascade reactions that result in the production of hydroxyl radicals capable of causing lipid peroxidation, protein denaturation and DNA mutations (Bowler et al. 1992). However, plant cells contain an array of protective and repair systems that minimize the occurrence of oxidative damage. The effective destruction of O .-2 and H2O2 requires the synchronous action of several antioxidant enzymes. Superoxide is rapidly converted to H2O2 by SOD activity (Bowler et al. 1992). CATs convert H2O2 to water and molecular oxygen in peroxisomes (Noctor and Foyer 1998). An alternative mode of H2O2 destruction is via peroxidases, which are found throughout the cell and have a much greater affinity to H2O2 than CAT (Jiménez et al. 1997). The enzymes in the ascorbate-glutathione cycle, where H2O2 is scavenged, are highly active. In this cycle, APX catalyzes the reduction of H2O2 to water by ascorbate, and the resulting dehydroascorbate is reduced back to ascorbate with the help of GR (Iturbe-Ormaetxe et al. 2001).
In our study the accumulation of hydrogen peroxide, the oxidative damage to lipids and the activity of the four antioxidant enzymes measured varied considerably depending on the plant tissue considered, the presence or absence of SB waste and the inoculation or not with the AM fungus G. intraradices. Moreover, in some cases the patterns are almost the opposite in root and shoot tissues (i. e. glutathione reductase activity or oxidative damage to lipids). Thus, it is difficult to see a clear correlation between hydrogen peroxide accumulation and oxidative damage to lipids or among these two parameters and antioxidant enzyme activities, and none of the activities can be specifically correlated with the performance of lettuce plants under the different treatments assayed in this study. It has been proposed that host plants possess higher antioxidant enzyme activities as a result of mycorrhizal colonization but the response of the individual enzymes varies with respect to the host plant and the fungal species (Alguacil et al. 2003). In a previous study we also observed that the effects of SB amendment on antioxidant enzyme activities actually differed between the enzymes analyzed (Azcón et al. 2009). This variation may also depend on the micronutrients available to some of the enzymes, e.g. CAT, APX and SOD are metalloenzymes and their activity can be determined by the availability of the metals they utilize (Alguacil et al. 2003). Thus, both excess and deficiency of micronutrients can modulate the activity of these metalloenzymes.
Conclusions
In previous studies it has been shown that the combined application of treated SB waste as amendment and AM fungi can be beneficial for plant performance under a variety of environmental constraints (heavy metal pollution, degradation of soil properties, erosion, etc.). However, though generally regarded as beneficial, the activity of AM fungi in agroecosystems is neither easily predictable nor always beneficial (Gosling et al. 2006). In fact, the positive effect described above seems to depend on the plant characteristics. For woody shrub plants like Juniperus oxicedrus, Cistus albidus or Dorycnium penthaphyllum the positive effect is evident, but for an horticultural food crop such as Lactuca sativa this is not so. Indeed, the application of treated SB waste resulted negative for AM and nonAM plants development as compared to unamended plants (both under well watered and under drought stress conditions). This effect can not be ascribed to the impairment of specific plant antioxidant defenses. In contrast, a lack of induction of a gene from the ABA biosynthetic pathway was observed in SB-treated plants, which could have contributed to the low performance of these plants. Thus, before starting a program aimed at the utilization of different amendments based on transformed wastes, basic studies on functional and physiological compatibility between the plant and the amendment are necessary.
References
Aebi H (1984) Catalase in vitro. In: Packer L (ed) Methods in enzymology. Oxygen radicals in biological systems. Vol 105. Academic-Press, London, pp 121–126
Alguacil MM, Caravaca F, Azcón R, Pera J, Díaz G, Roldán A (2003) Improvements in soil quality and performance of mycorrhizal Cistus albidus L. seedlings resulting from addition of microbially treated sugar beet residue to a degraded semiarid Mediterranean soil. Soil Use Manage 19:277–283
Amako K, Chen GX, Asada K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35:497–504
Aral B, Kamoun P (1997) The proline biosynthesis in living organisms. Amino Acids 13:189–217
Aroca R, Irigoyen JJ, Sánchez-Díaz M (2003) Drought enhances maize chilling tolerance. II. Photosynthetic traits and protective mechanisms against oxidative stress. Physiol Plant 117:540–549
Aroca R, Aguacil MM, Vernieri P, Ruiz-Lozano JM (2008a) Plant responses to drought stress and exogenous ABA application are differently modulated by mycorrhization in tomato and an ABA-deficient mutant (sitiens). Microb Ecol 56:704–719
Aroca R, Vernieri P, Ruiz-Lozano JM (2008b) Mycorrhizal and non mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J Exp Bot 59:2029–2041
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Augé RM (2004) Arbuscular mycorrhizae and soil/plant water relations. Can J Soil Sci 84:373–381
Augé RM, Foster JG, Loescher WH, Stodola AW (1992) Symplastic sugar and free amino acid molality of Rosa roots with regard to mycorrhizal colonization and drought. Symbiosis 12:1–17
Augé RM, Stodola AJW, Tims JE, Saxton AM (2001) Moisture retention properties of a mycorrhizal soil. Plant Soil 230:87–97
Azcón R, Perálvarez MC, Biró B, Roldán A, Ruiz-Lozano JM (2009) Antioxidant activities and metal acquisition in mycorrhizal plants growing in a heavy-metal multicontaminated soil amended with treated lignocellulosic agrowaste. Appl Soil Ecol 41:168–177
Barea JM, Jeffries P (1995) Arbuscular mycorrhizas in sustainable soil plant systems. In: Varma A, Hock B (eds) Mycorrhiza: structure, function, molecular biology and biotechnology. Springer-Verla, Heidelberg, pp 521–559
Barea JM, Azcón R, Azcón-Aguilar C (2002) Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie van Leeuwenkoek 81:343–351
Basu S, Roychoudhury A, Saha PP, Sengupta D (2010) Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul 60:51–59
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Blig EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bowen GD, Rovira AD (1999) The rhizosphere and its management to improve plant growth. Adv Agron 66:1–102
Bowler C, Vanmontagu M, Inze D (1992) Superoxide-dismutase and stress tolerance. Ann Rev Plant Physiol Plant Mol Biol 43:83–116
Bray EA (2002) Abscisic acid regulation of gene expression during water-deficit stress in the era of the Arabidopsis genome. Plant Cell Environ 25:153–161
Bray EA (2004) Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. J Exp Bot 55:2331–2341
Caravaca F, Alguacil MM, Azcón R, Diaz G, Roldán A (2004) Comparing the effectiveness of mycorrhizal inoculation and amendment with sugar beet, rock phosphate and Aspergillus niger to enhance field performance of the leguminous shrub Dorycnium pentaphyllum L. Appl Soil Ecol 25:169–180
Caravaca F, Alguacil MM, Azcón R, Parladé J, Torres P, Roldán A (2005) Establishment of two ectomycorrhizal shrub species in a semiarid site after in situ amendment with sugar beet, rock phosphate and Aspergillus niger. Microb Ecol 49:73–82
Caravaca F, Alguacil MM, Azcón R, Roldán A (2006) Formation of stable aggregates in rhizosphere soil of Juniperus oxycedrus: effect of AM fungi and organic amendments. Appl Soil Ecol 33:30–38
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–489
Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, Leun P, Nambara E, Asami T, Seo M (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signalling and abscisic acid biosynthesis and functions. Plant Cell 14:2723–2743
Duncan DB (1955) Multiple range and multiple F-tests. Biometrics 11:1–42
Fiorilli V, Catoni M, Miozzi L, Novero M, Accotto GP, Lanfranco L (2009) Global and cell-type gene expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus. New Phytol 184:975–987
Giovannetti M, Mosse B (1980) Evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Gosling P, Hodge A, Goodlass G, Bending GD (2006) Arbuscular mycorrhizal fungi and organic farming. Agric Ecosyst Environ 113:17–35
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine, 2nd edn. Clarendon, Oxford
Hardie K (1985) The effect of removal of extraradical hyphae on water uptake by vesicular-arbuscular mycorrhizal plants. New Phytol 101:677–684
Hare PD, Cress WA, van Staden J (1999) Proline synthesis and degradation: a model system for elucidating stress-related signal transduction. J Exp Bot 50:413–434
Hare PD, Cress WA, van Staden J (2003) A regulatory role for proline metabolism in stimulating Arabidopsis thaliana seed germination. Plant Growth Regul 39:41–50
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. An Rev Plant Physiol Plant Mol Biol 51:463–499
Iturbe-Ormaetxe I, Matamoros MA, Rubio MC, Dalton DA, Becana M (2001) The antioxidants of legume nodule mitochondria. Mol Plant-Microbe Interact 14:1189–1196
Iuchi S, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2000) A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol 123:553–562
Jahromi F, Aroca R, Porcel R, Ruiz-Lozano JM (2008) Influence of salinity on the in vitro development of Glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microb Ecol 55:45–53
Jiménez A, Hernández JA, del Rio LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114:275–284
Kay R, Chau A, Daly M (1987) Duplication of CaMV 35S promoter sequences creates a strong enhancer for plants genes. Science 236:1299–1302
Kishor PB, Hong Z, Miao GH, Hu CA, Verma DPS (1995) Overexpression of Δ1-pyrroline-5-carboxilate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–1394
Kubikova E, Moore JL, Ownlew BH, Mullen MD, Augé RM (2001) Mycorrhizal impact on osmotic adjustment in Ocimum basilicum during a lethal drying episode. J Plant Physiol 158:1227–1230
Linares A, Caba JM, Ligero F, de la Rubia T, Martínez J (2003) Detoxification of semisolid olive-mill wastes and pine-chip mixtures using Phanerochaete flavido-alba. Chemosphere 51:887–891
Medina A, Vassilev N, Alguacil MM, Roldán A, Azcón R (2004a) Increased plant growth, nutrient uptake and soil enzymatic activities in a desertified Mediterranean soil amended with treated residues and inoculated with native AM fungi and a plant growth promoting yeast. Soil Sci 169:260–270
Medina A, Vassileva M, Caravaca F, Roldán A, Azcón R (2004b) Improvement of soil characteristics and growth of Dorycnium pentaphyllum by amendment with agrowastes and inoculation with AM fungi and/or the yeast Yarowia lipolytica. Chemosphere 56:449–456
Medina A, Vassilev N, Barea JM, Azcón R (2005) Application of Aspergillus niger-treated agrowaste residue and Glomus mosseae for improving growth and nutrition of Trifolium repens in a Cd-contaminated soil. J Biotechnol 116:369–378
Medina A, Vassileva M, Barea JM, Azcón R (2006) The growth-enhancement of clover by Aspergillus-treated sugar beet waste and Glomus mosseae inoculation in Zn contaminated soil. Appl Soil Ecol 33:87–98
Medina A, Jakobsen I, Vassilev N, Azcón R, Larsen J (2007) Fermentation of sugar beet waste by Aspergillus niger facilitates growth and P uptake of external mycelium of mixed populations of arbuscular mycorrhizal fungi. Soil Biol Biochem 39:485–492
Minotti G, Aust D (1987) The requirement for iron (III) in the initiation of lipid peroxidation by iron (II) and hydrogen peroxide. J Biol Chem 262:1098–1104
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Moran JF, Becana M, Iturbe-Ormaetxe I, Frechilla S, Klucas RV, Aparicio-Tejo P (1994) Drought induces oxidative stress in pea plants. Planta 194:346–352
Morgan JM (1984) Osmoregulation and water stress in higher plants. Annu Rev Plant Physiol 33:299–319
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol 49:249–279
Patterson BD, MacRae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139:487–492
Phillips JM, Hayman DS (1970) Improved procedure of clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Brit Mycol Soc 55:159–161
Porcel R, Ruiz-Lozano JM (2004) Arbuscular mycorrhizal influence on leaf water potential, solute accumulation and oxidative stress in soybean plants subjected to drought stress. J Exp Bot 55:1743–1750
Porcel R, Barea JM, Ruiz-Lozano JM (2003) Antioxidant activities in mycorrhizal soybean plants under drought stress and their possible relationship to the process of nodule senescence. New Phytol 157:135–143
Porcel R, Azcón R, Ruiz-Lozano JM (2004) Evaluation of the role of genes encoding for Δ1-pyrroline-5-carboxylate synthetase (P5CS) during drought stress in arbuscular mycorrhizal Glycine max and Lactuca sativa plants. Physiol Mol Plant Pathol 65:211–221
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Rodrigo MJ, Alquezar B, Zacarías L (2006) Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J Exp Bot 57:633–643
Rodriguez R, Vassilev N, Azcon R (1999) Increases in growth and nutrient uptake of alfalfa grown in soil amended with microbially treated sugar beet waste. Appl Soil Ecol 11:9–15
Ruiz-Lozano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13:309–317
Ruiz-Lozano JM, Azcón R (1995) Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol Plant 95:427–478
Ruiz-Lozano JM, Azcón R, Gómez M (1995a) Effects of arbuscular mycorrhizal Glomus species on drought tolerance: physiological and nutritional plant responses. Appl Environ Microbiol 61:456–460
Ruiz-Lozano JM, Gómez M, Azcón R (1995b) Influence of different Glomus species on the time-course of physiological plant responses of lettuce to progressive drought stress periods. Plant Sci 110:37–44
Ruiz-Lozano JM, Collados C, Barea JM, Azcón R (2001) Arbuscular mycorrhizal simbiosis can alleviate drought-induced nodule senescence in soybean plants. New Phytol 151:493–502
Ruiz-Lozano JM, Porcel R, Aroca R (2006) Does the enhanced tolerance of arbuscular mycorrhizal plants to water deficit involve modulation of drought-induced plant genes? New Phytol 171:693–698
Ruiz-Lozano JM, Porcel R, Aroca R (2008) Evaluation of the possible participation of drought-induced genes in the enhanced tolerance of arbuscular mycorrhizal plants to water deficit. In: Varma A (ed) Mycorrhiza: state of the Art, genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics, 3rd edn. Springer, Germany, pp 185–205
Ruiz-Sánchez M, Aroca R, Muñoz Y, Polón R, Ruiz-Lozano JM (2010) The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J Plant Physiol 167:862–869
Sambrook J, Fritsch EF, Maniatis TA (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, NY
Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Schwartz SH, Qin X, Zeevaart JAD (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol 131:1591–1601
Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis-epoxycarotenoid dioxygenase gene family. Plant J 35:44–56
Vassilev N, Vassileva M, Ganchev I (1986) Citric acid production by Aspergillus niger on starch hydrolysate media. Acta Microbiol Bulg 19:62–67
Vassilev N, Franco I, Vassileva M, Azcón R (1996) Improved plant growth with rock phosphate solubilized by Aspergillus niger grown on sugarbeet waste. Biores Technol 55:237–241
Vivas A, Vörös A, Biró B, Barea JM, Ruíz-Lozano JM, Azcón R (2003) Beneficial effects of indigenous Cd-tolerant and Cd-sensitive Glomus mosseae associated with a Cd-adapted strain of Brevibacillus sp in improving plant tolerance to Cd contamination. Appl Soil Ecol 24:177–186
Wan X, Li L (2006) Regulation of ABA level and water-stress tolerance of Arabidopsis by ectopic expression of a peanut 9-cis-epoxycarotenoid dioxygenase gene. Biochem Biophys Res Com 347:1030–1038
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25:195–210
Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozaki K, Sinozaki K (1997) Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol 18:1095–1102
Zhang J, Jia W, Yang J, Ismail AM (2006) Role of ABA in integrating plant responses to drought and salt stresses. Field Crop Res 97:111–119
Acknowledgements
This study was supported by “Plan Nacional I + D” Spain (Project AGL2009-12530-C02-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katharina Pawlowski.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1
Significance of the sources of variation after a three-way analysis of variance for SDW and accumulation of proline, hydrogen peroxide and MD (oxidative damage to lipids). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001, ns, significant (JPEG 63 kb)
Table 2
Significance of the sources of variation after a three-way analysis of variance for SOD, APX, GR and CAT activities. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001, ns, not significant. (JPEG 60 kb)
Rights and permissions
About this article
Cite this article
Ruíz-Lozano, J.M., del Carmen Perálvarez, M., Aroca, R. et al. The application of a treated sugar beet waste residue to soil modifies the responses of mycorrhizal and non mycorrhizal lettuce plants to drought stress. Plant Soil 346, 153–166 (2011). https://doi.org/10.1007/s11104-011-0805-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0805-z