Abstract
To study the mechanism of chelant-metal complexes to be absorbed into plant roots in the presence of different concentration chelating agents, the sites, pathways, and mechanism of absorption of Cu-EDDS complex ([S, S’]-ethylene diamine disuccinic acid) in maize (Zea mays L.) primary roots were systematically studied. The results showed that, at low concentrations of the Cu-EDDS complex (<200 μmol L−1) in hydroponic culture, the complex was passively absorbed mainly from the apoplastic spaces where lateral roots penetrate the endodermis and the cortical region into the root xylem, the lateral root zone were the main absorption sites. At higher concentrations (<3,000 μmol L−1), under hydroponic culture and soil culture conditions, the passage cells, which form a physiological barrier controlling ion absorption, were either injured or killed, and the complex could enter the root xylem. Injury to the physiological barrier was a key factor in the complex being absorbed by roots in substantially larger quantities. In addition, the histochemical analysis of rubeanic acid can also be used for other researches involving Cu, and the negative–pressure measuring device provides a new research tool for studying the apoplastic absorption of other metal–chelating complexes, molecules, and ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic chelates have been used to supply plants with micronutrients in both soil culture and hydroponic culture for more than 50 years (Evangelou et al. 2007). Chelate-induced phytoextraction has received increasing attention in the last decade, which uses high-biomass plants with tolerant to metals to extract heavy metals from soil to decrease their concentration in the soil (Salt et al. 1995). Adding chelating agents such as ethylene diamine tetra acetic acid (EDTA) and [S, S’]-ethylene diamine disuccinic acid (EDDS) can significantly increase the accumulation of heavy metals (Pb, Cu, Zn, etc.) in the shoots in maize (Zea mays L.), mustard (Brassica juncea L.), and a few other plants (Vassil et al. 1998; Luo et al. 2005, 2006; Evangelou et al. 2007; Hernández-Allica et al. 2007). Metal-EDTA or metal-EDDS complexes have been detected in several species including mustard, bean (Phaseolus vulgaris L.), barley (Hordeum vulgare L.), and potato (Solanum tuberosum L.). These complexes can be absorbed by roots and translocated intact to shoots (Vassil et al. 1998; Sarret et al. 2001; Collins et al. 2002; Bell et al. 2003; Tandy et al. 2006). However, the mechanism through which such metal-chelating complexes are absorbed by the root system has not been fully understood so far. Several hypotheses have been proposed, which are summarized below. Tanton and Crowdy (1971, 1972) believe that the metal-chelating complex (Pb-EDTA) is absorbed from free space within cell walls whereas Bell et al. (2003), Luo et al. (2005, 2006), and Tandy et al. (2006) suggest that it is absorbed passively and non-selectively from the apoplastic pathway, entering the plant from the root tip without Casparian strips and/or the places where lateral roots penetrated the endodermis into the root xylem. Vassil et al. (1998) claim that the absorption follows damage to the trans-membrane ion transport mechanism due to the high concentrations of the chelate treatments, and that accumulation of Pb in shoots in significant quantities requires a certain minimum EDTA concentration. Collins et al. (2002) propose two absorption mechanisms for the complex (Zn-EDTA), namely that following physiological damage to the root system, as in mustard, and through the apoplastic pathway, as in barley and potato. However, these hypotheses, although based on experimental results, lack any support based on direct, systematic, and quantitative experiments, nor do they account for absorption of the Cu-EDDS complex.
Cu-EDDS complex, which has an UV absorption characteristics and with negative charge, can be determined by using an ion-exchange high-performance liquid chromatography (Collins et al. 2001; Adrian 2002; Knepper et al. 2005; Bakkaus et al. 2006; Niu and Shen 2009). Rubeanic acid (C2H4N2S2) (also known as Dithiooxamide) can react with divalent Cu2+ ions to form dark green Cu- rubeanic acid complex precipitation. It has been used for histochemical detection of the Wilson disease Cu deposition in liver tissue until now, has a higher sensitivity and specificity (Okinaka et al. 1954; Green 1955; Yang et al. 2006)
We investigated the sites, pathways, and the mechanism of absorption of Cu-EDDS complex in primary roots of maize (Zea mays L.) to provide experimental evidences for furthering the understanding of the role of chelating agents in enhancing absorption of micronutrients and in phytoremediation. The content of the Cu-EDDS complex and the rate at which it is absorbed in segments of primary roots were determined using anion chromatography and a negative pressure-type measuring device respectively. The absorption pathways and mechanism of Cu-EDDS complex were studied by using rubeanic acid histochemical analysis.
Material and methods
Hydroponic experiments
Seeds of maize (Zea mays L. ‘Zhengdan 958’) were soaked in deionized water (DIW) for 12 h and germinated on wet filter paper for 4 d in the dark. The seedlings were transferred to 3.5 L plastic boxes filled with the nutrient solution. Copper was excluded from its composition to avoid the effect of its background values on the experimental results. The nutrient solution contained (a) macronutrients (in mmol L−1) 0.54 KH2PO4, 2.50 KNO3, 2.50 Ca (NO3)2, 1.00 MgSO4, and 0.02 FeSO4 and (b) micronutrients (in μmol L−1) 46.02 H3BO3, 9.81 MnSO4, 0.76 ZnSO4, and 0.11 H2MoO4, pH 5.6. The solution was renewed every 2 d. The seedlings were grown in a growth chamber with a 14 h (30°C)/10 h (25°C) cycle, relative humidity of 60%, and light intensity of 300 μmol s−1 m−2. After 10–12 d, the seedlings were used for the Cu-EDDS absorption experiments.
To test and compare the absorption by the plants for Cu2+ ions at low and high concentrations in the Cu-EDDS complex, experiments were set up 100 μmol L−1 Cu (as CuSO4), 200 μmol L−1 and 3,000 μmol L−1 Cu-EDDS complex three treatments. Experimental solution was prepared with the Cu-free nutrient solution, composition of nutrient solution as above, pH 5.6. EDDS (C10H13N2Na3O8, Fluka, CAS 178949-82-1) molar concentrations in the solution were 10% more than those of CuSO4 to reduce the effect of Cu2+ absorption on the impact of Cu-EDDS complex absorption.
Pot experiments
For pot culture, the soil and seedlings were prepared by following the methods described by Luo et al. (2006). Air-dried soil was supplemented with Cu, as CuCO3, at 500 mg kg−1 soil. The experiments were conducted in a greenhouse. The temperature was about 20°C at night and 25°C at day. After 3 w, EDDS was applied to the soil surface as a solution (50 ml for each pot, or 3,000 μmol kg−1 of soil), the EDDS solution was prepared with DIW, pH 7.5. Pots watered with DIW served as a control.
Analysis of Cu and Cu-EDDS complex contents in root segments
The seedlings were harvested 2 d after they had been treated with the Cu-EDDS complex. Primary roots, after removing secondary roots, were rinsed in DIW and cut into six segments the lengths of which, in centimetres, measured from the root tips, were as follows: root tips, 0–0.5; mature zone, 0.5–2.5; lateral root formation zone, 2.5–5.0; lateral root zone 1, 5.0–7.5; lateral root zone 2, 7.5–10.0; and lateral root zone 3, 10.0–12.5. The root segments were ground to a slurry using a glass grinder, centrifuged at 10 000 g for 20 min, the supernatant filtered through a 0.45 μm membrane, and stored in a refrigerator at −40°C.
Cu-EDDS complex content of the root segments was determined by using a Finnigan LCQ DecaXP MAX high-performance liquid chromatography mass spectrometer (Niu and Shen 2009). The Cu-EDDS complex separation were carried out on a Dionex Ion Pac AS11-HC column (250 × 4.2 mm, 5 μm) with pH 6.5, 0.05 mol L−1 NH4NO3 as mobile phase at a flow rate of 1.2 mL min−1, and detected at 260 nm. The limit of detection was 0.18 μm L−1. The recovery was 97.9–103.2%. The Cu content was determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) (Perkin-Elmer Optima 2100DV).
Determination of absorption rates of Cu-EDDS complex in different root segments
The rate at which Cu-EDDS complex was absorbed in different root regions was determined using a negative-pressure measuring device. The device uses the negative pressure generated by a vacuum pump to simulate the pull exerted by transpiration. The device (Fig. 1) comprises a multi-compartment root segment absorption box and a negative-pressure control-xylem sap collection unit. The absorption box was made, using PVC, along the lines described by Kawasaki and Moritsugu (1987) and the collection unit was made using plexiglass, which is divided into upper and lower two parts.
Seedlings that were 10–12 d old were exposed to solutions of Cu-EDDS complex at 200 μmol L−1 and 3,000 μmol L−1 for 48 h. Primary roots, 16–20 cm in length and about 0.1 cm in diameter, and secondary lateral roots 0.2–0.5 cm in length, were used for determining the rate at which Cu-EDDS complex is absorbed by different root segments. The measurement procedure was as follows. (1) Primary roots (eight primary roots) were excised from the kernel while under water. (2) Basal parts of primary roots were fixed in the negative pressure room with white petroleum jelly. (3) Segments of the roots corresponding to the apical zone, mature zone, and lateral root zone were fixed in their respective absorption compartments. (4) The four screws of the negative pressure room were tightened. (5) 200 μmol L−1 Cu-EDDS complex solution was added to each of the three compartments and 3 ml DIW was added in the negative pressure room. (6) The vacuum pump was started; the screw clip on the negative pressure room was adjusted to maintain the negative pressure in the chamber at −0.03 MPa; and the pumping was continued for 15–20 min. (7) The screw clip was released and the solution in the negative pressure chamber was sucked out with a syringe.
The Cu-EDDS complex solution in the three compartments corresponding to the apical, mature, and lateral root zones was replaced by DIW, taking each compartment in turn, to collect the xylem sap absorbed from all the three zones together (total primary root), only from the mature and lateral roots zones, or only from the lateral root zone. Cu concentrations of the solutions were determined by using an ICP-AES and the rate at which Cu-EDDS complex was absorbed from the different zones was calculated.
Surface area of the root segments was determined using the root analysis software WinRHIZO Pro (Regent Instruments, Inc., Canada).
Histochemical analysis of Cu-EDDS complex absorption pathways
Primary roots of hydroponic seedlings exposed to 3,000 μmol L−1 Cu-EDDS complex for 6 d and soil-grown seedlings exposed to 3,000 μmol L−1 EDDS for 2 w were used for histochemical analysis of rubeanic acid, and primary roots of 14–16-d-old hydroponic seedlings without EDDS or Cu-EDDS complex treatments were used for root microstructure observations. Cryostat sections were prepared as described by Liu et al. (2006). Fluorescence staining of sections was based on methods described by Steudle and Peterson (1998) and Xu et al. (2004). Rubeanic acid staining of root sections followed the method of Yang et al. (2006) and Pen and Li (2001). Rubeanic acid (0.05 g) was first dissolved with 5 mL anhydrous ethanol, diluted to 100 mL with 10% (w/v) sodium acetate solution. Cryostat sections in Petri dish were stained with a 0.05% rubeanic acid (C2H4N2S2, Fluka, CAS: 79-40-3) in an incubator at 37°C for 24 h. The sections were transferred to slides, wash 3 times with DIW and mounted in 50% (v/v) glycerol. The samples were observed and photographed using a multi-function microscope (Nikon 80i).
Statistical analysis
The data reported in this paper are means of three replicates and were analysed using SPSS 13.0.
Results
Observations of the microstructure of primary roots
Cross-sections of primary roots stained with berberine—aniline blue are shown in Fig. 2.
Microstructure of maize primary roots grown hydroponically for 12–14 d. Transverse sections were photographed under UV/violet light (wavelength of 330–380 nm) after staining with the berberine—aniline blue. a root tip (up to 2 mm from apex), b mature zone 5 mm from the apex, c mature zone 20 mm from apex, d mature zone 40 mm from apex, e lateral root zone 70 mm from apex, and f lateral root zone beyond 70 mm from apex. Co: cortex, En: endodermis, Ep: epidermis, Ex: exodermis, Em: early metaxylem, Lm: secondary xylem, Lr: lateral root, P: passage cells. Bar = 100 μm
As can be seen in Fig. 2, cells in the root tip were packed closely together (Fig. 2a, b) and the epidermal and endodermal layers were well grown in the mature zone (Fig. 2c, d). Lateral roots originated from division of pericycle cells, and cracks (or breaks) are clearly visible in the figure at points where lateral roots have penetrated the endodermis and the cortex. The cracks probably served as a channel for large molecules such as those of the Cu-EDDS complex to enter the root xylem (Fig. 2e). The endodermis of the lateral root zone had some cells that were yet to form suberized lamellae and cellulosic walls (passage cells) (Fig. 2f). Typical Casparian strips were also seen in the exodermis (70 mm from the apex). Root segments in the region beyond 70 mm from the apex had the same microstructure as that of root segments 50–70 mm from the apex, but the aerenchyma were more developed in the latter region.
Contents of Cu and Cu-EDDS complex in different root segments
The contents of Cu and Cu-EDDS complex in different root segments that had been exposed to Cu-EDDS complex solutions of different strengths (100 μmol L−1 Cu, 200 μmol L−1 or 3,000 μmol L−1) for 48 h are shown in Fig. 3.
The change of Cu, Cu-EDDS complex and chelated Cu contents in different segments of primary roots. Seedlings were exposed to 100 μmol L−1 Cu or 200–3,000 μmol L−1 Cu-EDDS complex solutions for 48 h. a Cu in root segments from the 100 μmol L−1 Cu treatment, b Cu-EDDS complex in root segments from the 200 μmol L−1 Cu-EDDS and the 3,000 μmol L−1 Cu-EDDS treatments, c total and chelated Cu (as Cu-EDDS complex) in root segments from the 200 μmol L−1 Cu-EDDS complex treatment, d total and chelated Cu in root segments from the 3,000 μmol L−1 Cu-EDDS complex treatment. Values are means ± SD (n = 3). Primary root segments (in centimetres, measured from the root tips): root tips, 0–0.5; mature zone, 0.5–2.5; lateral root formation zone, 2.5–5.0; lateral root zone 1, 5.0–7.5 (fresh lateral root zone); lateral root zone 2, 7.5–10.0; and lateral root zone 3, 10.0–12.5
Cu content of roots from the 100 μmol L−1 Cu treatment decreased gradually from the mature zone to the lateral root zone, there being a significant difference in Cu content of different root segments (p < 0.001) (Fig. 3a). Cu-EDDS complex contents of root segments from the 200 μmol L−1 and 3,000 μmol L−1 Cu-EDDS complex treatments followed a similar tendency. The complex content was the highest (7.38–129.32 μg g−1) in the lateral root zone, followed by the apical part (6.25–58.44 μg g−1) and the lowest (2.50–46.30 μg g−1) in the mature zone. The content was higher in the zone with fresh lateral roots than that in the zone with mature lateral roots. The difference in Cu-EDDS complex contents of different root segments was significant (p < 0.001) (Fig. 3b). Total Cu in root segments from the 200 μmol L−1 Cu-EDDS complex treatment was significantly greater than chelated Cu (as Cu-EDDS complex) from the apical zone to the lateral roots formation zone (Fig. 3c) whereas the difference between the two forms in different root zones was not significant in the 3,000 μmol L−1 Cu-EDDS complex treatment (Fig. 3d).
Absorption of Cu-EDDS complex from different zones
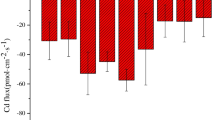
Differences in the ability to absorb Cu-EDDS complex of different root zones can be assessed using both the rate of absorption (in micrograms per square centimetre of surface area per hour) and the capacity (in micrograms for each root segment per hour). Both the measurements are shown in Fig. 4.
The rate of absorption of Cu-EDDS complex by different zones of the roots showed a similar tendency for both concentrations of Cu-EDDS complex. Absorption from the lateral root zone was the fastest (22.11 – 12.40 μg cm−2.h−1), followed by that from the apical zone (9.66 – 9.64 μg cm−2.h−1) and the mature zone (6.61 – 5.79 μg cm−2.h−1) in that order (Fig. 4a). The surface areas of laterals root zone, mature zone, and apical zone accounted for 72.51–83.09%, 22.16 – 10.70%, and 5.34–6.22% of the total surface area of primary roots, respectively. The tendency was the same for absorption capacity: the lateral root zone was the highest (it accounted for 89.00–90.35% of the total mass absorbed by the roots), followed by the mature zone (8.13 – 5.43%) and apical zone (2.86–5.26%) in that order (Fig. 4b).
Histochemical analysis of absorption sites of Cu and Cu-EDDS complex
Rubeanic acid can react with Cu-EDDS complex in transverse sections of the roots to form a dark green precipitate of Cu-rubeanic acid complex. Sections of primary root segments stained with 0.05% rubeanic acid are shown in Fig. 5.
Transverse sections of primary roots stained with 0.05% (w/v) rubeanic acid. Rubeanic acid reacted with Cu-EDDS complex in the sections to form a dark green precipitate of Cu-rubeanic acid complex, which was used for tracking the route of Cu-EDDS complex along the root xylem. a – e: sections of primary roots from the 3,000 μmol L−1 Cu-EDDS hydroponic treatment. a root tip (up to 2 mm from apex), b mature zone (10 mm from apex), c lateral root zone (70 mm from apex), d lateral root zone (beyond 70 mm from apex). e –f: sections of lateral root zone (70 mm from apex) from the 3,000 μmol L−1 Cu-EDDS soil culture treatment. g-h Cryostat sections of lateral root zone with 100 μmol L−1 Cu hydroponic treatment (70 mm from the apex) Co: cortex, Ep: epidermis, En: endodermis, Em: primary xylem, Lm: secondary xylem, Lr: lateral root, S: stele. Arrows show the sites of precipitation. Bar = 100 μm
Histochemical analysis using rubeanic acid showed that the different zones differed significantly in their capacity to absorb Cu-EDDS complex. Cells in the root tips were plasmolysed and the plasmalemmata were stained light green by rubeanic acid (Fig. 5a). The mature zone (5–25 mm from the apex) showed the precipitation at a few points, and the number of such sites reduced gradually from the epidermis to the inside of the cortex (Fig. 5b). In the lateral root zone (50–100 mm from the apex), lateral roots emerging from the pericycle created a discontinuity (crack) from the endodermis to the exodermis (dark green precipitates of Cu-rubeanic acid complex can be seen in the region in Fig. 5c and d). Besides these sites, in the 3,000 μmol L−1 Cu-EDDS complex treatment in pot culture, the precipitates were formed in passage cells and adjacent to early metaxylem (Fig. 5e), and the precipitates were more than those at the same Cu-EDDS complex concentration in the hydroponics treatment (Fig. 5f).
In addition, the root absorption sites of Cu2+ ions were also observed. At lateral root zone, some endodermis cells and the passage cells were stained dark-green, but the places where lateral root penetrated from the endodermis and cortex were stained light green (Fig. 5g, h). The results indicated the places from the apex to the lateral root formation zone were main absorption regions for Cu2 + ions, Casparian strips were the main barriers of apoplastic Cu2 + ions flow, high concentrations of Cu2 + ions could lead to cell death or injury and thus to enter the xylem.
Discussion
Histochemical analysis using rubeanic acid and measurement of the rate of absorption of Cu-EDDS complex
Under heating condition, rubeanic acid can slowly react with Cu-EDDS complex to form a dark green precipitate and thus can be used for histochemical analysis of Cu-EDDS complex at the sites of its absorption. To minimize or eliminate the interference of background Cu, We adopted two measures in the experiment. (1) To select “seedlings grown in Cu-free nutrient solution” as experimental material, Cu content in primary roots was below 20 μg g−1 DW. (2) To increase the ratio of EDDS and CuSO4 in treatment solutions, the Cu2+ ion activity in Cu-EDDS complex solutions was below 1E-17.
The main advantages of the negative-pressure measuring device for measuring absorption are that the device is simple and can determine absorption rates from different parts of the roots. Therefore, it can reduce the bias caused by material difference. For accurate results, it is necessary to select intact and representative roots and to repeat the experiment 3–5 times. In addition, to eliminate the effect of absorption by secondary lateral roots on apoplastic absorption in the lateral root zone, we selected 12–14-d-old primary roots and the secondary lateral roots shorter than 0.5 cm as the experimental material. Because soil culture maize primary roots’ tip zone, mature zone are short and there are a large number of lateral roots, only hydroponic maize root segments’ absorption rate for Cu-EDDS complex was measured using the negative pressure measuring device in this experiment.
The main absorption sites of Cu-EDDS complex in primary roots
The results obtained from Cu-EDDS content of root segments provided direct evidence that the lateral root zone was the main site of absorption of Cu-EDDS complex. At Cu-EDDS complex concentrations of 200 μmol L−1 and 3,000 μmol L−1, the lateral root zone recorded the highest content of the Cu-EDDS complex, followed, in that order, by root tips and the mature zone. The Cu-EDDS complex content in the zone of fresh lateral roots was higher than that in the zone of old lateral roots because as lateral roots mature, cracks in the endodermis and Casparian strips heal and are no longer open (Peterson et al. 1981; Ranathunge et al. 2005). The different root segments differed significantly in their Cu-EDDS complex contents. These results indicate that the lateral root zone and the apical zone are the main sites of absorption of Cu-EDDS complex. In addition, the higher content of total Cu compared to that of chelated Cu from the apical zone to the lateral root formation zone (Fig. 3c) was due to the absorption of free Cu2+ ions present in traces in the solution. These Cu2+ ions were produced by competitive binding of Fe3+ ions with EDDS of the Cu-EDDS complex (log K 18.4 for Cu2+; log K 22.0 for Fe3+) (Orama et al. 2002).
The rate of absorption provided another direct evidence for the lateral root zone being the main site of absorption of Cu-EDDS complex. Ranking the segments by the rate at which they absorbed Cu-EDDS complex yielded the same order as that of the Cu-EDDS content of roots, with the lateral root zone being the fastest, followed by the apical zone and the mature zone in that order. Surface area of the lateral root zone accounted for 72.51–83.09% of the total surface area of the roots, making it the main site of absorption (89.00–90.35% of the total quantity absorbed). At higher concentration (3,000 μmol L−1 as against 200 μmol L−1), due to lower permeability of the cell membrane, absorption was slower in all the zones, although, in relative terms, it was faster in the apical zone because of destruction of the apical organization. These rates of absorption of Cu-EDDS complex matched its content in different parts of the primary roots, confirming that the lateral root zone was the main site of absorption.
The main absorption pathways and mechanism of Cu-EDDS complex of primary roots
Many researchers believe that Pb-EDTA or Cu-EDDS complexes are absorbed by diffusion across the apoplastic spaces in the apical zone and the lateral root zone into the root xylem (Bell et al. 2003; Luo et al. 2005, 2006; Tandy et al. 2006), and the results of rubeanic acid histochemical analysis that we obtained provided direct confirmatory evidence. The apical cells were plasmolysed, and the condensed cytoplasm was stained light green with rubeanic acid, suggesting that Cu-EDDS complex can enter the apical parts and cause cell injury. Substantial quantities of the dark green precipitate of Cu-EDDS complex accumulated in the cracks where lateral roots penetrate the endodermis and the cortex, pointing to the cracks as major parts of entry to the root xylem for Cu-EDDS complex. On the other hand, the precipitate accumulated only to a limited extent in the mature zone, the quantity accumulated reducing gradually from the epidermis to the inside of the cortical region. Also, deposits of the precipitate were significantly greater along cell walls and in the intercellular region than in the cytoplasm, which showed that Cu-EDDS complex had entered the cortical region through gaps between epidermal cells. These results confirmed the hypothesis that Pb-EDTA or Cu-EDDS complex enters the root xylem by diffusion across the apoplastic spaces in lateral roots (Bell et al. 2003; Luo et al. 2005, 2006; Tandy et al. 2006). Because surface area of the apical zone was only 5.34–6.22% of the total surface area of the primary roots, apoplastic spaces of the lateral root zones were the main absorption pathway of Cu-EDDS complex into the root xylem.
The apoplastic spaces, however, are not the only pathway. In the process of chelate-induced accumulation, heavy metal contents in shoots increase slowly at first along with the concentration of the chelating agent; when the concentration exceeds a certain threshold, heavy metal contents increase more rapidly with the concentration, a phenomenon referred to as biphasic absorption (Vassil et al. 1998; Epstein et al. 1999; Luo et al. 2005, 2006; Schaider et al. 2006). The apoplastic bypass cannot explain the phenomenon nor can it account for the existence of a threshold value. However, both can be explained by the hypothesis of trans-membrane ion transport mechanism damage (Vassil et al. 1998). High EDDS concentrations result in injury to passage cells and the sites of injury serve as points of entry to the root xylem for Cu-EDDS complex (Fig. 5f).
The main absorption sites, pathways, and the mechanism of absorption of Cu-EDDS complex are ultimately determined by chemical properties of Cu-EDDS complex and the root structure. Because most metal-chelate complexes are large, carry charges, and have no known specific transporters, it was thought unlikely that they could pass through the cell membrane to enter the root xylem (Tandy et al. 2006). Although there is no Casparian strips in the apical zone, cells in the zone are tightly packed, and the maturation zone possesses a fully developed epidermis, cortex, and endodermis—therefore, neither of the zones contains significant apoplastic spaces. Only the contiguous cracks formed by lateral roots may provide the required apoplastic spaces for Cu-EDDS complex to enter the root xylem, considering that the lateral root zone accounted for 72.51–83.09% of the total surface area of primary roots. Therefore, the lateral root zone was the main absorption site and the apoplastic spaces of lateral zone were the main channels for the entry of Cu-EDDS complex into the root xylem. At the high concentrations of the Cu-EDDS complex, the injured passage cells may have served as potential channels.
For primary roots of maize, absorption of Cu-EDDS complex depends on the concentration of EDDS or Cu-EDDS complex. The mechanism of absorption of the metal-chelate complex may vary with the plant species, however, and the absorption mechanism in other species requires further research.
The absorption characteristics of Cu2+ ions were obviously different from Cu-EDDS complex. The primary root parts from the apex to the lateral root formation zones were main absorption regions for Cu2+ ions, Casparian strips were the main barriers of Cu2+ apoplastic pathways, high concentrations of Cu2 + ions could lead to passage cell death or injury and thus to enter the xylem.
Conclusions
From the above results, we can draw the following conclusions. Cu-EDDS complex enters the root xylem passively, mainly through apoplastic spaces in lateral roots, which serve as the main site of absorption. The absorption pathway depends on the concentration of EDDS or Cu-EDDS complex. At low concentrations (<200 μmol L−1), the absorption is mainly by diffusion across the root apoplastic spaces into the root xylem, and the places where lateral roots penetrate the endodermis and the cortex are the main pathways of entry, whereas Casparian strips form the main physical barrier. At high concentrations (<3,000 μmol L−1), whether under hydroponic culture or in soil, passage cells—physiological barriers that control ion absorption—are injured or killed, and, along with the adjacent early metaxylem vessels, offer additional channels of entry to the root xylem for the complex.
References
Adrian AA (2002) Determination of strong binding chelators and their metal complexes by anion-exchange chromatography and inductively coupled plasma mass spectrometry. J Chromatogr A 947:205–216
Bakkaus E, Collins RN, Morel JL, Gouget B (2006) Anion exchange liquid chromatography–inductively coupled plasma-mass spectrometry detection of the Co2+, Cu2+, Fe3+ and Ni2+ complexes of mugineic and deoxymugineic acid. J Chromatogr A 1129:208–215
Bell PF, McLaughlin MJ, Cozens G, Stevens DP, Owens G, South H (2003) Plant uptake of 14C-EDTA, 14C-Citrate, and 14C-Histidine from chelator-buffered and conventional hydroponic solutions. Plant Soil 253:311–319
Collins RN, Onisko BC, McLaughlin MJ, Merrington G (2001) Determination of metal–EDTA complexes in soil solution and plant xylem by ion chromatography electrospray mass spectrometry. Environ Sci Technol 35:2589–2593
Collins RN, Merrington G, McLaughlin MJ, Knudsen C (2002) Uptake of intact zinc-ethylenediamine tetraacetic acid from soil is dependent on plant species and complex concentration. Environ Toxicol Chem 21:1940–1945
Epstein AL, Gussman CD, Blaylock MJ, Yermiyahu U, Huang JW, Kapulnik Y, Orser CS (1999) EDTA and Pb-EDTA accumulation in Brassica juncea grown in Pb–amended soil. Plant Soil 208:87–94
Evangelou MWH, Ebel M, Schaeffer A (2007) Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 68:989–1003
Green CL (1955) Histochemical demonstration of copper in a case of hepatolenticular degeneration. Am J Pathol 31:545–553
Hernández-Allica J, Garbisu C, Barrutia O, Becerril JM (2007) EDTA-induced heavy metal accumulation and phytotoxicity in cardoon plants. Environ Exp Bot 60:26–32
Kawasaki T, Moritsugu M (1987) Effect of calcium on the absorption and translocation of heavy metals in excised barley roots: multi-compartment transport box experiment. Plant Soil 100:21–34
Knepper TP, Werner A, Bogenschűtz G (2005) Determination of synthetic chelating agents in surface and waste water by ion chromatography–mass spectrometry. J Chromatogr A 1085:240–246
Liu JF, Cheng YQ, Yan XF, Zhou G, Wang SF (2006) Improvement of plant cryo-sectioning technique. J Nanjing Forest Univ 30:128–130
Luo CL, Shen ZG, Li XD (2005) Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere 59:1–11
Luo CL, Shen ZG, Li XD, Baker AJM (2006) Enhanced phytoextraction of Pb and other metals from artificially contaminated soils through the combined application of EDTA and EDDS. Chemosphere 63:1773–1784
Niu LY, Shen ZG (2009) Analysis of metal-complexes of ethylenediamine disuccinic acid in plant and soil by ion chromatography and mass spectrometry. Chin J Chromatogr 27:829–834
Okinaka S, Yoshikawa M, Toyoda M, Mozai T, Toyokura Y, Kameyama M (1954) Pathogenesis of hepatocerebral disease. II. Histochemical study of copper of liver and brain in Wilson’s disease. Arch Neurol Psychiatr (Chicago) 72:573–578
Orama M, Hyvonen H, Saarinen H, Aksela R (2002) Complexation of [S, S] and mixed stereoisomers of N, N-ethylenediaminedisuccinic acid (EDDS) with Fe(III), Cu(II), Zn(II) and Mn (II) ions in aqueous solution. J Chem Soc Dalton Trans 24:4644–4648
Pen CE, Li SG (2001) Histochemistry, Beijing, People’s Health Publishing Press, pp 200–205
Peterson CA, Emanuel ME, Humphreys GB (1981) Pathway of movement of apoplastic fluorescent dye tracers through the endodermis at the site of secondary root formation in corn (Zea mays) and broad bean (Vicia faba). Can J Bot 59:618–625
Ranathunge K, Steudle E, Lafitte R (2005) A new precipitation technique provides evidence for the permeability of Casparian bands to ions in young roots of corn (Zea mays L.) and rice (Oryza sativa L.). Plant. Cell Environ 28:1450–1462
Salt DE, Blaylock MJ, Kumar NPBA, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment. Biotechnology 13:468–474
Sarret G, Vangronsveld J, Manceau A, Musso M, D’Haen J, Menthonnex JJ, Hazemann JL (2001) Accumulation forms of Zn and Pb in Phaseolus vulgaris in the presence and absence of EDTA. Environ Sci Technol 35:2854–2859
Schaider LA, Parker DR, Sedlak RL (2006) Uptake of EDTA-complexed Pb, Cd and Fe by solution and sand-cultured Brassica juncea. Plant Soil 286:377–391
Steudle E, Peterson CA (1998) How does water get through roots? J Exp Bot 322:775–788
Tandy S, Schulin R, Nowack B (2006) The influence of EDDS on the uptake of heavy metals in hydroponically grown sunflowers. Chemosphere 62:1454–1463
Tanton TW, Crowdy SH (1971) The distribution of lead chelate in the transpiration stream of higher plants. Pestic Sci 2:211–213
Tanton TW, Crowdy SH (1972) Water pathways in higher plants: II. Water pathways in roots. J Exp Bot 23:600–618
Vassil AD, Kapulnik Y, Raskin I, Salt DE (1998) The role of EDTA in lead transport and accumulation by Indian mustard. Plant Physiol 117:447–453
Xu JH, Tao Z, Huang Y, Sun JL (2004) A berberin-aniline blue counterstaining method for dying Caspary Band in root endodermis. Plant Physiol Commun 40:479–482
Yang JF, Pan D, Zhao CL, Sun YL, Zhao JM (2006) Express microwave copper staining at wilson disease transhepatic pathological diagnosis. Med J Chin People’s Liberation Army 31:735
Acknowledgments
We thank Li-Yang for the help in metal-EDDS complex analysis and elemental analysis, and Professor Honglan Lu for revising the manuscript. We also thank reviewers for his/her comments and suggestions on our manuscript. These comments and suggestions were highly insightful and enabled us to greatly improve the quality of our manuscript.This work was supported by the Natural Science Foundation of China (No: 20677029).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Rights and permissions
About this article
Cite this article
Niu, L., Shen, Z. & Wang, C. Sites, pathways, and mechanism of absorption of Cu-EDDS complex in primary roots of maize (Zea Mays L.): anatomical, chemical and histochemical analysis. Plant Soil 343, 303–312 (2011). https://doi.org/10.1007/s11104-011-0719-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0719-9