Abstract
In order to study the mechanism of cadmium (Cd) uptake by the roots of Celosia argentea Linn. (Amaranthaceae), the effects of various inhibitors, ion channel blockers, and hydroponic conditions on Cd2+ fluxes in the roots were characterized using non-invasive micro-test technology (NMT). The net Cd2+ flux (72.5 pmol∙cm−2∙s−1) in roots that had been pretreated with Mn was significantly higher than that in non-pretreated roots (58.1 pmol∙cm−2∙s−1), indicating that Mn pretreatment enhanced Cd uptake by the roots. This finding may be explained by the fact that the addition of Mn significantly increased the expression of the transporter gene and thus promoted Cd uptake and transport. In addition, Mn pretreatment resulted in an increase in root growth, which may in turn promote root vigor. The uncoupler 2,4-dinitrophenol (DNP) caused a significant reduction in net Cd2+ fluxes in the roots, by 70.5% and 41.4% when exposed to Mn and Cd stress, respectively. In contrast, a P-type ATPase inhibitor (Na3VO4) had only a small effect on net Cd2+ fluxes to the plant roots, indicating that ATP has a relatively minor role in Cd uptake by roots. La3+ (a Ca channel inhibitor) had a more significant inhibitory effect on net Cd2+ fluxes than did TEA (a K channel inhibitor). Therefore, Cd uptake by plant roots may occur mainly through Ca channels rather than K channels. In summary, uptake of Cd by the roots of C. argentea appears to occur via several types of ion channels, and Mn can promote Cd uptake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the Ministry of Land and Resources Report, 16% of the soils surveyed in China are polluted by heavy metals (He et al. 2020). Cadmium is the main pollutant, and 7% of the soil samples exceeded the national limit for this non-essential element (Yu et al. 2020). Due to its high toxicity and bioavailability, Cd poses a major threat not only to the environment but also to human health (Sun et al. 2013), as it can cause a number of diseases, including itai-itai disease, breast cancer, and prostate cancer (Lan et al. 2020). Therefore, there is an urgent need to develop effective techniques for the remediation of Cd-polluted soils.
Phytoextraction is regarded as an effective method of extracting heavy metals from soils because it is cost-effective, environmentally friendly, and can be used for in situ bioremediation (Liu et al. 2011). The main pathway of heavy metal uptake by plants is via the roots. Cd, a non-essential element, is taken up and transported into the roots via essential macronutrient element transporters or channels. For example, Koren’kov et al. (2007) demonstrated that CAX2 and CAX4, which are members of the Ca2+/cation antiporter superfamily, can also selectively transport Cd. The latter enters the root system via Ca transporters or channels as these have similar physical and/or chemical properties (Liu et al. 2020a). In addition, K can alleviate Cd phytotoxicity and accumulation in plants due to the fact that K and Cd may share the same ion channels (Yang and Juang 2015; Li et al. 2017a). Furthermore, Liu et al. (2020b) found that exogenous application of Mn could alleviate Cd uptake and transport in plants grown under hydroponic conditions, as Cd and Mn compete with each other for the same root transporters. However, Mn addition increased Cd uptake by plants in a pot-culture experiment as Mn addition significantly increased the Cd concentration in the soil solution (Liu et al. 2020b; Ge et al. 2021).
Whether Cd uptake and transport in plants are influenced by a number of different channel blockers and culture conditions deserves further study.
Non-invasive micro-test technology (NMT) (YoungerUSA LLC, MA, USA) is a new approach for real-time and dynamic measurement of the net fluxes of ions and molecules in living samples. This technology has been successfully used to study the characteristics of Cd uptake and transport in Microsorum pteropus (Lan et al. 2020), Sedum alfredii Hance (Sun et al. 2013; Tao et al. 2020), Triticum arstivum Linn. (Li et al. 2017a), Brassica chinensis Linn. (Wu et al. 2019), and Typha latifolia Linn. (Li et al. 2017b), and has proved to be an ideal tool for measuring ion fluxes in plant roots in real time.
In the present study, the application of exogenous Mn decreased Cd uptake and accumulation under hydroponic conditions and increased these processes in pot-culture conditions in C. argentea. It is still unclear whether Mn pretreatment of C. argentea seedlings promotes or inhibits Cd uptake by the roots. In addition, there is little direct evidence that uptake of Cd by plants occurs via other ion channels. Therefore, the aims of this study were to determine the effect of metabolic inhibitors and ion channel blockers on the mechanism of Cd uptake by roots of C. argentea under different hydroponic conditions (half-strength Hoagland nutrient solution, and Mn and Cd stress), and NMT technology was used to measure the real-time Cd2+ fluxes at the root surface.
Materials and methods
Plant seedling culture
Seeds of C. argentea were collected from the heavy metal remediation center in Yangshuo County, Guangxi, China. The seeds were soaked overnight and were then surface sterilized with 10% hydrogen peroxide solution for 10 min. After they had been rinsed with deionized water, the seeds were sown in seedbeds filled with nutrient soil in a greenhouse. The greenhouse control conditions are as follows: temperature, 25℃/daytime, 18℃/night; relative humidity, around 75%; photoperiod, 14 h. Deionized water was added to the soil to maintain the soil moisture content at around 50% field capacity. After the seeds had germinated, seedlings 6–8 cm in height with two or three leaves were selected for hydroponic experiments 1 and 2.
Experiment 1
To assess the effect of different hydroponic conditions on net Cd2+ flux at the root surface, plants were cultured in half-strength Hoagland solution containing either 10 μM Mn (as MnCl2) or 5 μM Cd (as CdCl2) or without Mn/Cd (control group). The plants were cultured under hydroponic conditions for 7 days, and then they were used in the uptake experiments. The plants were then separated into roots, stems, and leaves. The roots, stems, and leaves were first washed with tap water and then rinsed with deionized water three times. Finally, the cleaned roots, stems, and leaves were dried in an oven at 65℃ until a constant weight was achieved, in order to determine the biomass (dry weight, DW).
Experiment 2
To investigate the effect of a metabolic inhibitor (NDP, Na3VO4), a Ca channel blocker (La3+), and a K channel blocker (TEA) on Cd accumulation, the plants were cultured in half-strength Hoagland solutions for 2 days. NDP (50 μM), Na3VO4 (500 μM), La3+ (50 μM), or TEA (100 μM) was then added to each solution. Plants were cultured with different inhibitors and each inhibitor had three repeats. Each inhibitor had two treatments times of 6 h and 12 h, respectively. The cultured solutions were replaced with Cd solution (5 μM) after the inhibitor treatments. The plants were harvested after they had been exposed to Cd stress (5 μM) for 7 days.
Analyses of plant samples
Harvested plants were cultured as described for pot experiment 2, and were separated into roots and shoots. Dry weights of samples were determined as described for hydroponic experiment 1. Samples (approximately 0.5 g) were digested with 12 mL of HCl: HNO3 (4:1, v/v). Plant Cd concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS) (PE-2000B, USA), and dry weight and Cd concentrations were then used to calculate Cd accumulation.
Measurement of net Cd2+ flux
Net Cd2+ flux at the plant root surface was measured by NMT for plants that were pretreated in hydroponic experiment 1. Tested roots were soaked in the test solution (100 μM CdCl2, 0.1 mM KCl, 0.3 mM MES, pH 5.8) for 10 min. The Cd concentration in high calibration solutions contained 200 μM CdCl2, 0.1 mM KCl, and 0.3 mM MES at pH 5.8, while the Cd concentration in low calibration solutions contained 20 μM CdCl2, 0.1 mM KCl, and 0.3 mM MES at pH 5.8. The high and low calibration solutions were used to carry out the calibration process of NMT. After the calibration process, the real-time Cd2+ fluxes to the plant roots that were along the root apex at 50 μm intervals from the root tip were measured. The DNP, La3+, Na3VO4, and TEA were added to the Cd2+ test solutions, respectively, to get the inhibitors. The test concentrations of DNP, La3+, Na3VO4, and TEA were 50 μM, 50 μM, 500 μM, and 100 μM, respectively. Six successive Cd2+ fluxes were measured for each treatment.
Statistical analysis
Microsoft Excel 2010 was used to calculate mean values ± standard deviation (SD). The data were analyzed by one-way analysis of variance (ANOVA) using SPSS 18.0 to determine statistical significance at p = 0.05. All of the figures were generated by Origin 2020b.
Results and discussion
Biomass
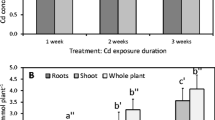
Compared with the control group, plants that were pretreated with Mn showed an increase in root, stem, and leaf biomass, whereas plants that were pretreated with Cd showed a reduction in root and leaf biomass (Table 1). The highest values of stem and root biomass (2.30 ± 0.10 g and 1.91 ± 0.09 g, respectively) were obtained in plants that had been pretreated with Mn. This finding indicated that Mn could promote the growth of C. argentea at the concentration that was used in the experiment. Some studies have demonstrated a positive effect of relatively low concentrations of Mn on plant growth (Shao et al. 2017; Liu et al. 2018); even Mn concentrations of 500 μM had no inhibitory effect on plant growth (Sasaki et al. 2011; Chen et al. 2013). Therefore, the concentration of Mn (10 μM) that was used in this study had a positive effect on the growth of C. argentea.
Cd2+ fluxes at different positions along the root apex
To identify the largest net Cd2+ fluxes at the surface of the root apex in C. argentea, the net Cd2+ fluxes to the root were measured at nine positions located 50 to 450 μm from the root tip. The largest net Cd2+ fluxes (57.4 pmol∙cm−2∙s−1) to the root surface were observed 250 μm from the root tip (Fig. 1). Net Cd2+ fluxes decreased with increasing distance beyond 300 μm; net Cd2+ fluxes to the root surface were 36.4, 17.3, 17.5, and 14.9 pmol∙cm−2∙s−1 at distances of 300, 350, 400, and 450 μm, respectively, from the root tip. Net Cd2+ fluxes to the root surface were 30.8 pmol∙cm−2∙s−1 at 50 μm and 29.5 pmol∙cm−2∙s−1 at 100 μm from the root tip. Li et al. (2017a) reported that the net Cd2+ flux to the roots of intact Triticum arstivum seedlings was highest (about 39 pmol∙cm−2∙s−1) 300 μm from the tip, and then gradually decreased along the root. However, Piñeros et al. (1998) and Farrell et al. (2005) found that the Cd2+ flux at the root surface of Triticum aestivum and Triticum turgidum L. var. durum was highest in the regions 0.6–1.2 mm (0.28–0.35 pmol∙Cd2+ cm−2∙s−1) and 0.5–1.5 mm (0.4–0.5 pmol∙Cd2+ cm−2∙s−1), respectively, from the root tip. This indicated that in the different varieties of wheat, the net Cd2+ fluxes to the root surface were influenced by the different Cd2+ uptake systems (Page and Feller 2005). Root morphology may be another factor that contributes to this difference (Fathi et al. 2016). For example, net Cd2+ fluxes that were detected with the same Cd2+-selective microelectrode showed different net Cd2+ flux characteristics at the root surface of Triticum aestivum varieties that differed in their root morphology (Farrell et al. 2005; Li et al. 2017a). Net Cd2+ fluxes to the root hairs in the non-hyperaccumulating and hyperaccumulating ecotypes of Sedum alfredii exhibited different responses to Cd in the region between 0 and 10.5 mm from the root tip (Tao et al. 2020). In summary, the differences in the results obtained for net Cd2+ flux in the present study compared with previous studies may be due to differences in the plant species and in their root morphology.
Effects of Cd/Mn pretreatment on Cd uptake
Mn pretreatment significantly increased net Cd2+ flux (72.5 pmol∙cm−2∙s−1) to the root surface compared with the control and Cd pretreatment groups (Fig. 2). There was no significant difference in net Cd2+ flux to the root surface between the control and Cd pretreatment groups (50.2 pmol∙cm−2∙s−1 and 58.1 pmol∙cm−2∙s−1, respectively). Therefore, the application of Mn could promote Cd uptake by plant roots.
Liu et al. (2020b) found that net Cd2+ fluxes were decreased by the exogenous application of Mn under hydroponic conditions. The mean net Cd2+ fluxes to the roots of C. argentea decreased by 10.5% and 56.9% in response to the application of Mn at concentrations of 0.01 mM and 0.5 mM, respectively, under these conditions. They may compete for the same root transporters, as a result of which the application of exogenous Mn reduces Cd uptake by the roots. In the present study, the plants were only pretreated with Mn. Therefore, there was no exogenous Mn in the test solution that could compete with Cd for the same ion transporter. In addition, we found that the addition of Mn led to an increase in expression of the transporter ZIP2 gene (unpublished results). The ZIP family of transporters has an important role in Mn and Cd transport in a range of plants (Xu et al. 2012; Socha and Guerinot 2014). Therefore, ZIP2 may also transport Mn and Cd in C. argentea, and seedlings that have been pretreated with Mn may promote Cd uptake by the roots. Some researchers have also demonstrated that heavy metals at low concentrations can promote an increase in root length and the uptake of heavy metals by roots (Xin et al. 2020; Rasafi et al. 2021). In the present study, the biomass of C. argentea roots was also increased by pretreatment with Mn (Table 1). Therefore, we speculated that plant root vigor was enhanced by Mn pretreatment and thus increased Cd uptake by the roots. Fu (2019) reported the same phenomenon in rice, whereby pretreatment with Mn promoted Cd uptake by the roots, although the underlying mechanism was not explored. Thus, there is a need for future studies to investigate the precise mechanism whereby Mn promotes Cd uptake.
Effects of DNP on Cd uptake
DNP treatment significantly decreased net Cd2+ fluxes to the root surface (Fig. 3). Plants were cultured with supplementary Mn, and the net Cd2+ flux at the root surface was found to be the lowest (21.4 pmol∙cm−2∙s−1) after the DNP treatment. However, the net Cd2+ fluxes at the root surface after DNP treatment showed no significant differences between the three hydroponic conditions. The net Cd2+ fluxes to the surface of roots that had been exposed to Cd and roots in the control group were 29.4 and 23.0 pmol∙cm−2∙s−1, respectively.
DNP uncouples oxidative phosphorylation by increasing the proton permeability of biomembranes (Kopec et al. 2018), which in turn inhibits the biosynthesis of ATP. The inhibitory effect of DNP on Cd uptake into the root suggests that the latter process requires metabolic energy. Cataldo et al. (1983) reported that the addition of DNP had a significant inhibitory effect on Cd uptake by Glycine max Linn. roots, and demonstrated that metabolic processes played an important role in the movement of Cd into root cells. Li et al. (2017a) also found that DNP significantly decreased Cd flux to the root surface in Triticum arstivum. In addition, the inhibitory effect of DNP on Cd uptake indicated that Cd entered the root via the symplastic pathway rather than the apoplastic pathway. Some earlier studies have also noted that the symplastic pathway is the main transport route from root to shoot in Triticum turgidum (Van der Vliet et al. 2007; Quinn et al. 2011).
Effects of Na3VO4 on Cd uptake
Na3VO4 treatment decreased net Cd2+ fluxes to the root surface (Fig. 4). After Na3VO4 treatment, net Cd2+ fluxes at the surface of roots in the Mn pretreatment, Cd pretreatment, and control groups were 56.2, 47.3, and 33.8 pmol∙cm−2∙s−1, respectively. Compared with the treatment without Na3VO4, the net Cd2+ fluxes to the root surfaces in the Mn pretreatment, Cd pretreatment, and control groups were reduced by 22.5%, 32.7%, and 18.6%, respectively. Na3VO4 could inhibit the P-type ATPase in all membranes. Thus, the results suggested that Cd uptake by the roots of C. argentea was not strongly linked to the plasma membrane P-type ATPase. However, Li et al. (2017a) reported that pretreatment of Triticum arstivum with Na3VO4 did not significantly affect the net Cd2+ flux to the root. This could possibly be explained by the low-affinity transport system having a more important role in the Cd uptake system than the high-affinity transport system (Pedas et al. 2005).
Cd2+ flux before and after Na3VO4 treatment: A, control group; B, Mn pretreatment group; C, Cd pretreatment group. Results are presented as mean values ± SD (n = 3). Different lowercase letters below the bars indicate that differences are statistically significant according to the LSD test (p < 0.05)
Effects of La3+ and TEA on Cd uptake
La3+ treatment significantly decreased net Cd2+ fluxes to the root surface (Fig. 5). After La3+ treatment, net Cd2+ fluxes at the surface of roots in the Mn pretreatment group, the Cd pretreatment group, and the control group were 31.1, 26.0, and 20.3 pmol∙cm−2∙s−1, respectively, representing decreases in net Cd2+ flux at the roots of 57.1%, 48.2%, and 65.1%, respectively.
The net Cd2+ flux showed a slight decrease at the roots of plants that had been exposed to Cd stress after the TEA treatment compared with those that had been exposed to Cd before the TEA treatment (Fig. 6). After the TEA treatment, the net Cd2+ flux in the roots that had been exposed to Mn was higher than that for the Cd treatment group, and the control group had the lowest net Cd2+ flux. The net Cd2+ fluxes in the roots of plants in the control group, the Mn pretreatment group, and the Cd pretreatment group were 35.4, 51.4, and 44.4 pmol∙cm−2∙s−1, respectively, representing decreases of 39.1%, 29.1%, and 11.6%, respectively.
The results shown in Fig. 5 and Fig. 6 suggest that Cd may use the same ion channels as Ca and K, although Ca had more significant effects than K on Cd uptake. Some studies have demonstrated that Ca and K can reduce Cd uptake (Lindberg et al. 2004; Yang and Juang 2015; Liu et al. 2020a). Lindberg et al. (2004) and Yang and Juang (2015) found that the addition of Ca and K inhibitors decreased Cd accumulation in Triticum aestivum. This also indicated that the uptake of Cd by plant roots is influenced by Ca and K. The Cd uptake by roots of Arabidopsis seedlings was inhibited when the seedlings were treated by Ca channel blockers. (Suzuki 2005). However, it is still unclear whether plant uptake of Cd occurs via K channels. In addition, the effects of K on Cd absorption by plants may vary according to the species. For example, K treatment has little effect on Cd absorption by Glycine max (Yang and Juang 2015).
Cd accumulation after treatment with inhibitors
Plants were pretreated with metabolic inhibitors—specifically, Ca or K ion channel inhibitors for 6 or 12 h. Then, they were replaced with Cd solution for 7 days, and the different harvested plants were measured for their Cd accumulation (Fig. 7). The results illustrated that there was no significant difference in Cd accumulation between the 6- and 12-h treatments. Cd accumulation decreased by 33.8%, 15.9%, 12.3%, and 30.9% after 12 h of treatment with LaCl3, Na3VO4, TEA, and DNP, respectively. Plants that were treated with LaCl3 and DNP showed more significant decreases than those treated with Na3VO4 and TEA. Cd transport may be largely dependent on the availability of metabolic energy and Ca ion channels. In the present discussion, we found that Cd uptake by roots of C. argentea depended mainly on Ca channels and metabolic energy. Therefore, the results of Cd accumulation in plants were consistent with the other experimental results in this study.
Conclusion
Net Cd2+ flux to the roots in C. argentea was significantly suppressed by a metabolic inhibitor compared with a P-type ATPase inhibitor, which indicated that metabolic energy played an important role in Cd uptake by C. argentea. Both Ca and K channel blockers decreased net Cd2+ fluxes, but the Ca channel blocker had a more significant inhibitory effect on Cd2+ flux to the roots than did the K channel blocker. This indicated that Cd uptake by the roots of C. argentea occurred mainly via Ca channels rather than K channels. Mn treatment significantly increased plant biomass and Cd uptake by the roots of C. argentea compared with either Cd treatment or control group, which demonstrated that Mn had a positive effect on plant growth and Cd uptake in C. argentea. This may be mainly due to the fact that Mn promoted the expression of the transport gene and increased root vigor, but further studies are needed to clarify the exact mechanisms involved.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cataldo DA, Garlnd TR, Wildung RE (1983) Cadmium uptake kinetics in intact soybean plants. Plant Physiol 73:844–848
Chen ZH, Yumi FJ, Naoki Y, Sakine M, Yuma T, Takehiro K, Yusufujiang Y, Kozo I, Shin-ichiro K, Masayoshi M, Feng MJ, Daisei U (2013) Mn tolerance in rice is mediated by MTP8.1, a member of the cation diffusion facilitator family. J Exp Bot 64(14):4357–4387
Farrell RE, McArthur DFE, Van Rees KCJ (2005) Net Cd2+ flux at the root surface of durum wheat (Triticum turgidum L. var. durum) cultivars in relation to cultivar differences in Cd accumulation. Can J Plant Sci 85:103–107
Fathi S, Sabet MS, Lohrasebi T, Razavi K, Karimzadeh G, Malekroudi MG (2016) Effect of root morphological traits on zinc efficiency in Iranian bread wheat genotypes. Acta Agr Scand B-S P 66(7):575–582
Fu JX (2019) Effects of Zinc/Manganese on cadmium uptake, transport and physico-biochemical properties of rice (Oryza sativa L.) under cadmium stress [D]. Taiyuan University of Technology
Ge J, Tian SK, Yu HY, Zhao JQ (2021) Exogenous application of Mn significantly increased Cd accumulation in the Cd/Zn hyperaccumulator Sedum alfredii. Environ Pollut 278:116837
He CQ, Zhao YP, Wang FF, Oh K, Zhao ZZ, Wu CL, Zhang XY, Chen XP, Liu XY (2020) Phytoremediation of soil heavy metals (Cd and Zn) by castor seedlings: tolerance, accumulation and subcellular distribution. Chemosphere 252:126471
Koren’kov V, Park S, Cheng NH, Sreevidya C, Lachmansingh J, Morris J, Hirschi K, Wagner GJ (2007) Enhanced Cd2+-selected root-tonoplast in tobaccos expressing Arabidopsis cation exchangers. Planta 225:403–411
Kopec KT, Friermuth C, Maynard S, Beuhler M (2018) Dinitrophenol (DNP) farality associated with a falsely elevated salicylate level: a case report with verification of laboratory cross reactivity. J Med Toxicol 14(4):323–326
Lan XY, He QS, Yang B, Yan YY, Li XY, Xu FL (2020) Influence of Cd exposure on H+ and Cd2+ fluxes in the leaf, stem and root of a novel aquatic hyperaccumulator- Microsorum pteropus. Chemosphere 249:126552
Li LZ, Yu SY, Peijnenburg WJGM, Luo YM (2017a) Determining the fluxes of ions (Pb2+, Cu2+ and Cd2+) at the root surface of wetland plants using the scanning ion-selective electrode technique. Plant Soil 414(1/2):1–12
Li ZZ, Tu C, Peijnenburg WJGM, Luo YM (2017b) Characteristics of cadmium uptake and membrane transport in roots of intact wheat (Triticum arstivum L.) seedlings. Environ Pollut 221:351–358
Lindberg S, Landberg T, Greger M (2004) A new method to detect cadmium uptake in protoplasts. Planta 219:526–532
Liu J, Duan CQ, Zhang XH, Zhu YN, Hu C (2011) Characteristics of chromium (III) uptake in hyperaccumulator Leersia hexandra Swartz. Environ Exp Bot 74:122–126
Liu J, Mo LY, Zhang XH, Yao SY, Wang YX (2018) Simultaneous hyperaccumulation of cadmium and manganese in Celosia argentea Linn. Int J Phytoremediat 20(11):1106–1112
Liu J, Yu G, Jiang PP, Zhang XF, Meng DJ, Chen Z, Baker AJM, Qiu RL (2020a) Interaction of Mn and Cd during their uptake in Celosia argentea differs between hydroponic and soil systems. Plant Soil 450:323–336
Liu YK, Yao Q, Guo XY, Luo JP, Li JX, Liang YC, Li TQ (2020) Low calcium-induced delay in development of root apoplastic barriers enhances Cd uptake and accumulation in Sedum alfredii. Sci Total Environ 723(25):137810
Page V, Feller U (2005) Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants. Ann Bot-London 96:25–434
Pedas P, Hebbern CA, Schjoerring JK, Holm PE, Husted S (2005) Differential capacity for high-affinity manganese uptake contributes to differences between barley genotypes in tolerance to low manganese availability. Plant Physiol 139:1411–1420
Piñeros MA, Shaff JE, Kochian LV (1998) Cadmium-selective microelectrode for the measurement of cadmium fluxes in roots of Thlaspi Species and wheat. Plant Physiol 116:1393–1401
Quinn CJ, Mohammad A, Macfie SM (2011) Accumulation of cadmium in near-isogenic lines of durum wheat (Triticum turgidum L. var durum): the role of transpiration. Physiol Mol Biol Pla 17(4):317–325
Rasafi TE, Bouda S, Hamdali H, Haddioui A (2021) Seed germination and early seedling growth of fenugreek (Trigonella foenum-gracium L.) under Cu, Ni and As stress. Acta Ecological Sinica 41(3):223–227
Sasaki A, Yamaji N, Xia JX, Ma JF (2011) OsYSL6 is involved in the detoxification of excess manganese in rice. Plant Physiol 157:1832–1840
Shao JF, Yamaji N, Shen RF, Ma JF (2017) The key to Mn homeostasis in plants: regulation of Mn transporters. Trends Plant Sci 22(3):215–224
Socha AL, Guerinot ML (2014) Mn-euvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Front Plant Sci 5:106
Sun J, Wang RG, Liu ZQ, Ding YZ, Li TQ (2013) Non-invasive microelectrode cadmium flux measurements reveal the spatial characteristics and real-time kinetics of cadmium transport in hyperaccumulator and nonhyperaccumulator ecotypes of Sedum alfredii. J Plant Physiol 170:355–359
Suzuki N (2005) Alleviation by calcium of cadmium-induced root growth inhibition in Arabidopsis seedlings. Plant Biotechnol-Nar 22(1):19–25
Tao Q, Liu YK, Li M, Li JX, Luo JP, Lux A, Kováč J, Yuan S, Li B, Li QQ, Li HX, Li TQ, Wang CQ (2020) Cd-induced difference in root characteristics along root apex contributes to variation in Cd uptake and accumulation between two contrasting ecotypes of Sedum alfredii. Chemosphere 243:125290
Van der Vliet L, Peterson C, Hale B (2007) Cd accumulation in roots and shoots of durum wheat: the roles of transpiration rate and apoplastic bypass. J Exp Bot 58(11):2939–2947
Wu SB, Shi KL, Hu CX, Guo JL, Tan QL, Sun XC (2019) Non-invasive microeletrode cadmium measurements reveal the decrease of cadmium uptake by zinc supply in pakchoi root (Brassica chinensis L.). Ecotox Environ Safe 168:363–368
Xin XP, Zhao FL, Rho JY, Goodirch SL, Sumerlin BS, He ZL (2020) Use of polymeric nanoparticles to improve seed germination and plant growth under copper stress. Sci Total Environ 745(25):141055
Xu J, Sun JH, Du LG, Liu XJ (2012) Comparative transcriptome analysis of cadmium responses in Solanum nigrum and Solanum torvum. New Phytol 196:110–124
Yu G, Jiang PP, Fu XF, Liu J, Sunahara IG, Chen Z, Xiao H, Lin FY, Wang XS (2020) Phytoextraction of cadmium-contaminated soil by Celosia argentea Linn: a long-term field study. Environ Pollut 266:155408
Yang CM, Juang KW (2015) Alleviation effects of calcium and potassium on cadmium rhizotoxicity and absorption by soybean and wheat roots. J Plant Nutr Soil Sci 178:748–754
Funding
This research was sponsored by the Natural Science Foundation of China (41867022), the Natural Science Foundation of Guangxi (2020GXNSFDA297018), the Special Funds of Guangxi Distinguished Experts, and the Program for High Level Innovation Team and Outstanding Scholar of Universities in Guangxi (GuiCaiJiaoHan[2018]319).
Author information
Authors and Affiliations
Contributions
P. Jiang and J. Liu: conceived the study.
Y. Zheng and P. Jiang: collected data and prepared the data for analysis.
G. Yu and F. Lin: performed statistical analyses and literature review.
P. Jiang: wrote the main manuscript text.
G. Yu and J. Liu: improved the draft.
All authors contributed to the interpretation of results and revised the manuscript critically.
All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, P., Zheng, Y., Liu, J. et al. Pathways of cadmium fluxes in the root of the hyperaccumulator Celosia argentea Linn.. Environ Sci Pollut Res 29, 44413–44421 (2022). https://doi.org/10.1007/s11356-021-17352-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17352-2