Abstract
In this study we evaluated the ability of two wild strains of Azospirillum, A. lipoferum AZm5 and A. brasilense VS9, to produce ACC deaminase. We tested the effects of a deficiency and medium doses of nitrogenous fertilizers on the growth and physiology of tomato plants (Lycopersicon esculentum Mill cv. ACE VF55) inoculated with both Azospirillum strains independently. Tomato plants were evaluated by root elongation assay and grown in pot soil culture with different nitrogen levels (0 kg N ha–1 and 170 kg N ha–1). The root:shoot ratio (R:S) and some ecophysiological traits were determined after 42 days of plant growth. Results showed very different physiological characteristics in both strains. We found three relevant aspects related to the AZm5 strain: it produces high amounts of cytokinins, it contains the gene acdS, which encodes ACC deaminase, and it promotes plant growth. We conclude that AZm5 maybe useful to increase N uptake in N-deficient soil by production of cytokinins and the promotion of ACC deaminase activity, which favored leaf expansion and higher leaf N investment. Therefore, for tomato culture, a simultaneous biofertilization with AZm5 and a relatively low fertilization with N (170 kg N ha–1) to promote AZm5 activity could be advantageous.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Azospirillum is one of the best-known plant-growth-promoting rhizobacteria (PGPR) genera (Steenhoudt and Vanderleyden 2000). Suitable for use as a biofertilizer, this PGPR is capable of influencing the growth and yield of numerous plants species, many of which have agronomic and ecological significance (Bashan et al. 2004; Hartmann and Bashan 2009). The stimulatory effect of Azospirillum has been attributed to several mechanisms that eventually increase plant yield, including its ability to produce various phytohormones (indole-3-acetic acid, zeatin, gibberellic acid, abscisic acid, ethylene) and important bioactive molecules (putrescine, spermine, spermidine, cadaverine), to fix biological nitrogen, and to enhance water and mineral uptake by plants (Dobbelaere et al. 2001; Perrig et al. 2007). Azospirillum inoculation improves plant growth in the presence of optimum and medium doses of nitrogenous fertilizers (Kapulnik et al. 1981; Bhattarai and Hess 1998; Gadagi et al. 2004). In tomato (Lycopersicon esculentum L.) plants inoculated with Azospirillum, increases in length, volume and biomass of root, number of leaves, stem diameter, shoot height and plant biomass have been documented (Bashan 1989; Terry et al. 2005; Ribaudo et al. 2006). However, the mechanisms of Azospirillum contribution to plant growth promotion are still poorly understood.
In 1998 it was suggested that PGPR promote the growth of plants and lower ethylene levels by the action of the 1-amino-cyclopropane-1-carboxylic acid (ACC) deaminase enzyme, which can cleave ACC, the immediate precursor of ethylene (Glick et al. 1998). Ethylene is important for normal development in plants as well as for their response to stress, for example, extreme temperatures, high salinity, drought, amelioration and pathogen infection (Glick et al. 2007). Also, PGPR containing ACC deaminase may enhance seedling survival, protecting plants against the inhibitory effects of high or low concentrations of nutrients (Abeles et al. 1992; Lynch and Brown 1997; Shaharoona et al. 2008).
PGPR that contain ACC deaminase play a significant role in phytoremediation of contaminated soils (Glick 2003). It has also been documented that, even in the presence of optimum or appropriate doses of nitrogenous fertilizers, inoculation with PGPR containing ACC deaminase improves the growth and yield of inoculated plants (Tahir et al. 2006; Shaharoona et al. 2006, 2008). An important effect of PGPR inoculation is a reduction in inorganic N application needed in agricultural systems.
The enzyme ACC deaminase has been found in a wide range of PGPR, such as the genera Achromobacter, Acidovorax, Alcaligenes, Bacillus, Enterobacter, Klebsiella, Methylobacterium, Pseudomonas, Rhizobium, and Variovorax. In these bacteria, the acdS gene has been associated with the presence of the enzyme ACC deaminase (Glick et al. 1995; Belimov et al. 2001; Penrose and Glick 2001; Babalola et al. 2003; Ma et al. 2003; Mayak et al. 2004; Belimov et al. 2005; Madhaiyan et al. 2006). It has been previously documented that some members of Azospirillum sp. do not produce ACC deaminase gene (Kende 1993; Holguin and Glick 2001). However, Blaha et al. (2006) reported the presence of the ACC deaminase gene (acdS) in wild strains of A. lipoferum isolated from soil in Pakistan. In addition, our studies with soil of crops from the Mezquital Valley in Mexico detected ACC deaminase activity in A. lipoferum strains isolated from the rhizosphere (Esquivel-Cote 2002); however, presence of the acdS gene was not evaluated.
In this study we evaluated the presence of the ACC deaminase gene (acdS) in a wild strain of A. lipoferum, AZm5, isolated from a N-enriched crop soil, and we tested the ability of this strain to produce ACC deaminase. We also tested the effects of a deficiency of and medium doses of nitrogenous fertilizers on plant growth and physiology in tomato plants inoculated with A. lipoferum AZm5.
We hypothesized that A. lipoferum AZm5 containing ACC deaminase activity would improve the growth and physiology of tomato (Lycopersicon esculentum Mill.) plants under a deficiency of and medium doses of nitrogenous fertilizers. This improvement is relevant for the production of tomato, which is one of the most popular and widely grown vegetables in the world (USDA 2008) and one of the most economically important horticultural vegetables in Mexico (SIAP 2009). Tomato plants require the addition of high doses of nitrogenous fertilizers (150–450 kg ha−1), which may have toxic effects in soil and water.
Material and methods
Bacterial strains
Some of the Azospirillum strains were initially isolated from the rhizosphere of several crops growing under different environmental conditions in Mexico. The A. lipoferum strain AZm5 was isolated from maize (Zea mays L.) plants cultivated in soils contaminated by irrigation with wastewater enriched with N fertilizers in Valle del Mezquital, State of Hidalgo. The A. brasilense strain VS9 was isolated from sorghum (Sorghum sp.) plants cultivated in non-contaminated soils in Valle de Santiago, State of Guanajuato (Esquivel-Cote 2002). After isolation, the bacteria were grown in 100 ml NFB liquid medium (Döbereiner et al. 1976) supplemented with NH4Cl (1 g l−1). The flasks were incubated on a rotary shaker at 150 rpm at 34 ± 2°C for 48 h.
Physiological characteristics of Azospirillum strains
Production of indole acetic acid (IAA), indole butyric acid (IBA), giberellic acid (GA), trans-zeatin (t-Z) and trans-zeatin riboside (t-ZR) was determined after bacteria were incubated for 48 h, according to the protocol described by Tien et al. (1979). IAA, IBA, GA, t-Z and t-ZR production was determined by HPLC as follows: IAA, IBA, GA, t-Z and t-ZR were separated on a Zorbax 300 C18 reverse phase column of 4.6 mm × 15 cm (Agilent Technologies, Santa Clara, CA, USA) using a liquid chromatograph (Waters 600E system, Milford, MA, USA). Samples were analyzed under isocratic conditions using methanol:formic acid (35:65 ν/ν) as separation solvent, at a flow rate of 1 ml min–1. Eluates were detected by spectrophotometry at 274 nm (Waters 996, Milford, MA, USA). IAA, IBA, GA, t-Z and t-ZR were quantified by reference to the peak area obtained for the IAA standard (100 μg ml–1) (Sigma, St. Louis, MO, USA), IBA standard (100 μg ml–1; Sigma), GA standard (100 μg ml–1; Sigma), t-Z standard (40 μg ml–1; Sigma) and t-ZR standard (40 μg ml–1; Sigma), respectively. All standards were dissolved in methanol (HPLC grade). Nitrogenase activity was quantified using the acetylene-reduction assay. The bacterial suspension was inoculated into 5 ml of semisolid NFB medium contained in 10-ml sterile glass vials. Control vials were maintained without bacterial samples. Once visible growth was observed as a film on the surface of the medium, the cotton plugs, which allowed air exchange, were replaced with rubber stoppers. From these bottles, 10% of the gaseous phase was removed with an insulin syringe, and the same volume of acetylene gas was injected. After 12 h of incubation, the concentration of ethylene produced was analyzed by gas chromatography (3300 Varian, Inc, Palo Alto, CA, USA) with a flame ionizing detector. A stainless steel column containing Porapak-T column 3 and 3.1-mm (80–100 mesh; Waters and Associates, Inc., Milford, MA, USA) was used at an ignition temperature of 105°C. Each strain was analyzed in triplicate.
Bacterial ethylene production was determined as described by Arshad and Frankenberger (1989). One milliliter of bacterial suspension grown in NFB liquid medium supplemented with L-methionine (1 g l–1) as a sole source of nitrogen was inoculated into each of three sterile glass vials (50 ml) containing 20 ml of the same medium, covered with septum rubber and incubated on a rotary shaker at 150 rpm at 34 ± 2°C for 48 h. Control vials were maintained without the addition of bacteria. After incubation, the ethylene concentration was determined by gas chromatograph (see above).
The ACC deaminase activity was determined in cell-free extracts by estimating the production of α keto-butyrate generated by the enzymatic hydrolysis of ACC following the method of Honma and Shimomura (1978). The protein concentration in cell suspensions and in cells labilized by toluene (5% v/v) was determined by the method described by Bradford (1976).
Inoculum
Bacteria were incubated in a nutritive broth medium (Difco Laboratories, Detroit, MI, USA) on a rotary shaker at 150 rpm at 34 ± 2°C for 24 h. Cultures were harvested, and cell pellets were washed twice with a sterile isotonic saline solution (0.85% NaCl). Cell pellets were resuspended in NFB liquid medium supplemented with 3.0 mM ACC (Calbiochem-Novobiochem Corp., La Jolla, CA, USA) or 3.0 mM NH4Cl, pH 6.8. Bacteria cells were incubated on a rotary shaker at 150 rpm at 34 ± 2°C for 20 h. Produced bacterial cells were used to determine ACC deaminase activity, gnotobiotic root elongation and pot experiment with soil culture (see below).
PCR amplification of acdS (encoding ACC deaminase) genes
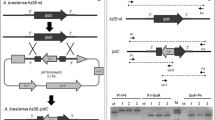
DNA was extracted following the manufacturer’s instructions (AquaPure Genomic DNA Kit BIO-RAD®) from cells cultured on nutritive agar medium. Polymerase chain reaction (PCR) was carried out (95°C 5 min; 35 cycles of 95°C 30 s, 50°C 30 s, 72°C 1 min; and 72°C 5 min) using the primers F1937(F) / F1938(R) (Blaha et al. 2006). PCR products were electrophoresed in 1% agarose containing TBE buffer in 10 μg ml–1 ethidium bromide. The correct-size band was purified with the High Pure Plasmid Isolation Kit® (Roche Applied Science 2004).
The gel-purified PCR products were cloned into the vector pCR2.1 3.9 kb (TA Cloning® Kit Invitrogen Ver.V 2004). Escherichia coli DH5α cells were transformed with the ligation mixture and cultured on solid medium containing isopropyl-β-D-thiogalactoside (IPTG) and 5-bromo-4-chloro-3-indole-β-D-galactopyranoside (X-Gal). Plasmid was isolated from white colonies and then digested with Ncol and SalI to release the insert. The acdS gene sequences were determined at Centro de Ciencias Genómicas (CCG), UNAM (Mexico) and compared with similar sequences in the databases using the BLAST analysis at NCBI.
The tree topology was inferred by the neighbor-joining method, and distance matrix analyses were performed according to Jukes and Cantor, using the program MEGA Ver. 4 (Kumar et al. 2008). The acdS gene sequences of the AZm5 strain have been submitted to the GenBank databases under the accession number GU727865.
Root elongation assay on tomato plants
The root elongation assay was conducted using a modification of the method described by Penrose and Glick (2003). The ACC utilizing bacterial cell pellet (see above) was resuspended in 0.5 ml of sterile 300 mM MgSO4 and placed on ice. A 0.5 ml sample was removed from the cell suspension and diluted in 300 mM MgSO4. The bacterial suspension of A. brasilense VS9 (the non-ACC-utilizing strain) was cultivated on salt medium with NH4Cl (1 g l–1) as sole nitrogen source. Tomato seeds (Lycopersicon esculentum Mill cv. ACE VF55 from Westar Seeds International Inc., CA, USA) were sterilized by soaking for 3 min in 70% ethanol and 5 min in 3% sodium hypochlorite and then rinsed thoroughly with sterile distilled water. The disinfected seeds were incubated for 1 h at room temperature, either in a bacterial suspension in 300 mM MgSO4, approximately 108 colony forming units (cfu) per ml, or in sterile 300 mM MgSO4 as a control. Seed germination and seedling growth were conducted in seed-pack growth pouches (15 × 20 cm, Mega International CYG, MN, USA). The pouches were arranged upright in a plastic tray and filled with a sufficient amount of water to cover the bottom and maintain the humidity. The trays were covered with transparent Saran Wrap®.
In one gnotobiotic experiment, the effect of Azospirillum strains on the elongation of tomato roots was evaluated. Twelve tomato seeds were placed in one growth pouch (previously autoclaved at 121°C for 20 min) and then moistened with 10 ml of distilled water and incubated at 25°C for 6 days. There were six replicates (pouches) per treatment with three treatments, for a total of 18 pouches. In another gnotobiotic experiment, the effect of the Azospirillum strains on the elongation of tomato root and shoot under different nitrogen levels was assessed. Each of eight tomato seeds was placed in a previously autoclaved seed-pack growth pouch and moistened with 10 ml of the nutrient solution recommended by Hazera Seeds, Inc. (FL, USA). Seeds were incubated for 10 days. Three treatments with different levels of nitrogen in the nutrient solution were applied (0 kg N ha–1, 170 kg N ha–1 and 340 kg N ha–1). The experiment contained six replicates (pouches) per treatment with three treatments and three levels of nitrogen, for a total of 54 pouches incubated in a growth chamber (Conviron E15, Winnipeg, Canada with Sylvania F72T12/CW/VHO/LT fluorescent lamps) a 12-h photoperiod, a photon flux of 1.2 μmol m−2 s−1 in the first experiment and 450 μmol m–2 s–1 in the second experiment, and a 25:20°C light/dark temperature cycle. Following incubation, the pouches were opened and seedling root and shoot length were measured.
Pot experiment with soil culture under different levels of nitrogen
A pot experiment was performed to test the effect of ACC deaminase on plant growth under two nitrogen levels. Plants were grown in plastic bags (one seedling per bag; seven bags per treatment) containing 320 g of non-sterile soil:agrolite mixture (1:5 v/v). The soil had the following characteristics: pH 6.5; organic matter 6.27%; cation exchange capacity 21.2 cmol kg–1. The following nutrients (in mg kg–1 per soil): total N (2900); available P (1.62); Ca (1447.33). Bags filled with non-sterile soil:agrolite mixture were moistened up to 60% of the field capacity through the addition of 90 ml nutrient solution. Two treatments with different levels of nitrogen in the nutrient solution were applied (0 kg N ha–1 and 170 kg N ha–1).
Seeds were surface sterilized and inoculated as described above, sown in pots with previously washed, dried, and autoclaved (121°C for 60 min) quartz sand. After 5 days, the germinated seeds were inoculated with 1 ml of bacterial suspension (approximately 108 cfu ml–1). Control seeds were treated with sterile 300 mM MgSO4. Ten days after inoculation, plants of uniform size were transplanted and placed in bags (one plant per bag). Bags were incubated for 27 days in a growth chamber (Conviron E15, Winnipeg, Canada with Sylvania F72T12/CW/VHO/LT fluorescent lamps) a 12-h photoperiod, a photon flux of 450 μmol m−2 s−1 and a 25:20°C light/dark temperature cycle. Plants were harvested two times (t) when seedlings were 15 days old (1, at transplanting moment) and 42 days old (2, after transplanting). There were six replicates (pots) per treatment with three treatments and two levels of nitrogen, for a total of 36 pots.
At each harvest time, the shoot was separated into leaves and stem. Leaf area was measured using a Delta-T Area Meter videocamera (Delta-T Devices Ltd., Cambridge, England) with a JVC (TK5310EG). Seedlings were dried at 60°C in an oven. The dry weight (DW) of the different parts of the seedlings (leaves, stem and roots) was determined to calculate total biomass, root:shoot (R:S) ratio, leaf:mass ratio (LMR = leaf DW / total DW), stem:mass ratio (SMR = steam DW / total DW), root:mass ratio (RMR = root DW / total DW), specific leaf area (SLA = leaf area / leaf mass), leaf:mass ratio (LMR = leaf mass / total mass), leaf area ratio (LAR = SLA × LMR), net assimilation rate (NAR = (DW2 - DW1 / t2 - t1) (lnLA2 - lnLA1 / LA2 - LA1)), and relative growth rate (RGR = LAR × NAR). The percentage of nitrogen in the leaves (LN%) was determined using an elemental analyser (NA 2500, Thermo Quest S.P.A., Rosanao, Milan, Italy). The equipment was calibrated with a standard curve obtained with sulphanilamide (C, 41.84%; H, 4.68%; N, 16.27%; O, 18.58% and S, 18.62%) (CE Elantech, Inc., Lakewood, N.J., USA). There were three replicates per analysis and per species.
Statistical analysis
Data were analyzed by analysis of variance (ANOVA/MANOVA) using the statistical analysis software StatGraphics Centurion XV (2006, Statpoint Technologies Inc., VA, USA). Means were compared using the Fisher’s least significant difference (LSD) procedure. Significance levels were within confidence limits of 0.05.
Results
Physiological characteristics
There were no significant differences in nitrogenase activity between AZm5 and VS9 and the control (F1,4 = 4.33; P = 0.1058). AZm5 induced significant differences in other physiological traits (Table 1). A. lipoferum strain, AZm5, showed ACC deaminase enzyme activity, produced a higher amount of zeatin than the VS9 strain (F1,4 = 788.91; P = 0.00001), and did not synthesize IAA in presence of 10 mg L-tryptophan 100 ml−1 in NFB liquid medium. In contrast, A. brasilense, VS9, produced high amounts of the phytohormones IAA (F1,4 = 226.99; P = 0.0001) and GA (F1,4 = 126.09; P = 0.00036), but it did not synthesize cytokinins (neither zeatin nor zeatin riboside). Neither strain produced ethylene from L-methionine.
PCR amplification of acdS (encoding ACC deaminase) genes
Amplification of the ACC deaminase (acdS) gene was performed using a set of degenerative primers (F1937(F) / F1938(R)); the 780-bp DNA product was cloned and sequenced (Fig. 1). The BLASTN search performed for the nucleotide sequence showed a 639-bp fragment with 100% homology to the complete sequence ACC-deaminase (acdS) gene of A. lipoferum strain 4B (DQ125242) (Blaha et al. 2006; Prigent-Combaret et al. 2008).
Agarose gel of genomic DNA of wild type Azospirillum strain AZm5 and VS9 was probed with the acdS gene using the primer 1937(F)/1938(R) (Blaha et al. 2006). Lane M, molecular weight marker (1 kb); Lane 2, Strain VS9 Azospirillum brasilense; Lane 3, Strain AZm5 Azospirillum lipoferum; Lane 4, negative control (C-)
Root elongation assay on tomato plants
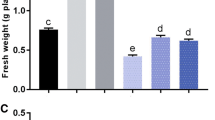
In the first gnotobiotic assay, A. lipoferum AZm5 significantly promoted the root elongation of 6-day-old tomato cv. ACE VF55 seedlings grown in absence N and under 1.2 μmol m–2 s–1 of photon flux. Root length increased 38.77% more than the control and 55.61% more than seedlings inoculated with A. brasilense VS9 (F2, 60 = 31.229; P = 0.0001; Fig. 2).
Root length of tomato cv. ACE VF55 seedlings (6 days-old) inoculated with Azospirillum strains in absence N and exposed to light intensity of 1.2 μmol m–2 s–1 (the average data of three experiments performed on gnotobiotic culture). Vertical lines denote standard errors. Letters indicate a statistically significant difference between inoculated samples and uninoculated control, according to Fisher’s least significant difference (LSD) procedure (P ≤ 0.05)
In the second gnotobiotic assay, inoculation with Azospirillum by itself did not produce significant differences in the root:shoot ratio in 10-day-old tomato cv. ACE VF55 seedlings (Table 2). The addition of N significantly reduced the root:shoot ratio. In the control, and in interaction with A. brasilense VS9, 340 kg N ha–1 showed the lowest root:shoot ratio.
Effect of different levels of nitrogen on seedlings growing in pots
After transplantation to pots, in 42-day-old seedlings 170 kg N ha–1 reduced the root:shoot ratio only in the control treatment (Table 3). The addition of 170 kg N ha–1 also resulted in significant differences in all other evaluated variables, except specific leaf area and net assimilation rate, and the interaction between factors, except in leaf:mass ratio, where the interaction was marginal. In the control treatments, the addition of N led an increase in leaf:mass ratio, stem:mass ratio, leaf:area ratio, relative growth rate and LN%; no effect on specific leaf area and net assimilation ratio; and a significant reduction in root:mass ratio. In the seedlings inoculated with A. lipoferum AZm5, the addition of 170 kg N ha–1 produced an increase in leaf:mass ratio, relative growth rate and LN%, but it had no effect on any other evaluated traits. Inoculation with A. brasilense VS9 increased specific leaf area, leaf area ratio, relative growth rate and LN%, decreased net assimilation rate NAR and had no significant effect on any other traits.
Comparing all treatments, control plants fertilized with N had the highest stem:mass ratio and non fertilized plants the lowest LN% values. In similar way, the inoculation of tomato seedlings with A. lipoferum AZm5 and without nitrogen presented the highest relative growth rate, specific leaf area and leaf:area ratio values but resulted in the lowest net assimilation rate value. In contrast, inoculation with A. brasilense VS9 resulted in the highest leaf:mass ratio and net assimilation rate values; however, inoculation with A. brasilense VS9 resulted in the lowest values for specific leaf area and leaf:area ratio.
Discussion
In this work we found three relevant characteristics of the strain AZm5: it produces high amounts of cytokinins, it contains the gene acdS, which encodes ACC deaminase, and it promotes plant growth. Azospirillum species are known for their ability to produce plant hormones, mainly IAA, and gibberellins, which play a major role in the promotion of plant growth (Bashan et al. 2004). In comparison with A. brasilense VS9, A. lipoferum AZm5 produced a high level of cytokinins, but it did not produce IAA. The production of cytokinins by A. brasilense and other PGPRs is common (Tien et al. 1979; Greene 1980; Horemans et al. 1986; Cacciari et al. 1989; Taller and Wong 1989; Timmusk et al. 1999; García de Salamone et al. 2001) and this phenomenon is less frequently reported for A. lipoferum (Bashan et al. 2004). However, although we tested for it twice, we did not observe the production of cytokinins in VS9. On the other hand, A. lipoferum AZm5 is capable of producing others substances like AIA (Esquivel-Cote 2002) but it did not produce specifically AIA and IBA. Therefore, the production of indole pyruvic acid, indole lactic acid, indole acetamide, indole acetaldehyde, indole ethanol and indole methanol, which are similar to IAA (Crozier et al. 1988), needs to be tested.
AZm5 produced high concentrations of ACC deaminase 2.68 ± 0.16 (in μmol α keto-butyrate mg protein per h), in relation to those produced by Enterobacter cloacae UW4 (2.43 ± 0.04), Escherichia coli pRKACC (1.74 ± 0.06), Rhizobium leguminosarum 128C53K Biovar viciae (1.06 ± 0.17) and Acidovorax facilis AY197008 (0.7 ± 0.1) (Holguin and Glick 2001; Ma et al. 2003, Belimov et al. 2005). This finding coincides with the presence of the acdS gene, which encodes ACC deaminase, in AZm5 and also with the root elongation observed in tomato seedlings grown under 1.2 μmol m−2 s−1 of light; root elongation is promoted by the interruption of ethylene synthesis from ACC (Glick et al. 1998). It was previously reported that tomato seedling root elongation is promoted by ACC deaminase (Grichko and Glick 2001). We also found a 639-bp DNA fragment in AZm5 which corresponds to the acdS gene. Has been proposed that more than one type of ACC deaminase gene may exist (Shah et al. 1998), based on the fact that nonspecific acdS bands whose nucleotide sequences did not match with the sequences found for other acdS-positive isolates have been reported (Shah et al. 1998; Blaha et al. 2006; Govindasamy et al. 2008).
This results, clearly confirmed the presence of ACC deaminase enzyme in bacteria from, soils polluted with sewage sludge in the Mezquital Valley, Mexico, at least as result of the presence of A. lipoferum AZm5. The presence of PGPR containing ACC deaminase has also been reported in bacteria from soils long-standing sewage sludge contaminated with heavy metals in Russia (Belimov et al. 2001), and other areas with similar contamination (Arshad et al. 2007; Rodríguez et al. 2008; Sheng et al. 2008), which suggests the presence of bacteria with a similar activity to AZm5.
It is known that biofertilization with Azospirillum strains promotes plant growth (Dobbelaere et al. 2001). However, the effect of this treatment on the variables of plant growth may suggest its functional effect on plants, has not been reported. In tomato plants grown in low N concentrations, AZm5 promoted RGR and an increase in specific leaf area and leaf:area ratio, which resulted in an increase in photosynthetic surface. However, net assimilation rate did not increase, indicating a relatively low photosynthetic efficiency due to the low LN% found in these plants, as occurs in other plants (Lambers et al. 1998); this result did not occur when soil inoculated with AZm5 was also fertilized with nitrates and ammonium (N 170). The effect of this combination match with the increasing the investment of N in leaves found in the tomato seedlings. In contrast, treatment with VS9 had a positive effect on net assimilation ratio in fertilized and unfertilized soils, but in N-fertilized soils, biofertilization with AZm5 was more advantageous for LN% than using VS9, which coincide with previous investigations where the following were reported: 1) the combination of Azospirillum strains and medium fertilizer N dose improved N uptake by plants (Bhattarai and Hess 1998; Gadagi et al. 2004) and 2) under low fertilizer application, the ACC deaminase activity of PGPR improved the effectiveness of nitrogen organic fertilization in tomato plants (Tahir et al. 2006; Shaharoona et al. 2008).
According to the hypothetical model proposed by Van der Werf and Nagel (1996), low availability of nitrogen induces low root cytokinin production; a reduction in the production of cytokinins leads to a reduction in their translocation to leaves, thereby diminishing the photosynthetic capacity and rate of leaf expansion. The high production of cytokinins by AZm5 may explain the increase in leaf expansion in inoculated plants compared with controls or VS9-inoculated plants.
In conclusion, our results suggest that AZm5 may be useful to increase N uptake by the production of cytokinins and promotion of ACC deaminase activity; both favored leaf expansion and leaf N investment, which might promote NAR in the subsequent steps of growth under AZm5 inoculation and low N fertilization (170 kg N ha–1). Inoculation with AZm5 alone or in combination did not modify the root:shoot ratio, suggesting that seedlings only increased photosynthetic surface to increase the capture of resources. These results indicate the plasticity of tomato plants in capturing nutrients by their roots. In consequence, to increase crop yield, simultaneous biofertilization with AZm5 and a relatively low concentration of N in comparison to the concentrations recommended for tomato culture (150–400 kg ha–1, SAGARPA-INIFAP 2009) may be recommended.
References
Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in plant biology. Academic, New York
Arshad M, Frankenberger WT Jr (1989) Biosynthesis of ethylene by Acremonium falciforme. Soil Biol Biochem 21:633–638
Arshad M, Saleem M, Hussain S (2007) Perspectives of bacterial ACC deaminase in phytoremediation. Trends Biotechnol 25:356–362. doi:10.1016/j.tibtech.2007.05.005
Babalola OO, Osir EO, Sanni AI, Odhiambo GD, Bulimo WD (2003) Amplification of 1-amino-cyclopropane-1-carboxylic (ACC) deaminase from plant growth promoting rhizobacteria in Striga-infested soil. Afri J Biotechnol 2:157–160
Bashan Y (1989) Nonespecific responses in plant growth yield and root colonization of noncereal crops plants to inoculation with Azospirillum brasilense Cd. Can J Bot 67:1317–1324
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003). Can J Microbiol 50:521–577. doi:10.1139/W04-035
Belimov AA, Safronova VI, Sergeyeva TA, Ergorova TN, Matveyeva VA, Tsyganov VE, Borisov AY, Tikhonovich IA, Kluge C, Preisfeld A, Dietz KJ, Stepanok VV (2001) Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylic acid deaminase. Can J Microbiol 47:642–652. doi:10.1139/cjm-47-7-642
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR (2005) Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol Biochem 37:241–250. doi:10.1016/j.soilbio.2004.07.033
Bhattarai T, Hess D (1998) Growth and yield responses of a Nepalese spring wheat cultivar to the inoculation with Nepalese Azospirillum spp at various levels of nitrogen fertilization. Biol Fertil Soils 26:72–77
Blaha D, Prigent-Combaret C, Sajjad MM, Moënne-Loccoz Y (2006) Phylogeny of the 1-aminocyclopropane-1-carboxylic acid deaminase-encoding gene acdS in phytobeneficial and pathogenic Proteobacteria and relation with strain biogeography. FEMS Microbiol Ecol 56:455–470. doi:10.1111/j.1574-6941.2006.00082.x
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dry binding. Anal Biochem 72:248–254
Cacciari I, Lippi D, Pietrosanti T (1989) Phytohormone-like substances produced by single and mixed diazotrophic cultures of Azospirillum pp. and Arthrobacter. Plant Soil 115:151–153. doi:10.1007/BF02220706
Crozier A, Arruda P, Jasmim JM, Monteiro AM, Sandberg G (1988) Analysis of indole-3-acetic acid and related indoles in culture medium from Azospirillum lipoferum and Azospirillum brasilense. Appl Environ Microbiol 54:2833–2837
Dobbelaere S, Croonenborghs A, Thys A, Ptacek D, Vanderleyden J, Dutto P, Labandera-González C, Caballero-Mellado J, Aguirre JF, Kapulnik Y, Brener S, Burdman S, Kadouri D, Sarig S, Okon Y (2001) Responses of agronomically important crops to inoculation with Azospirillum. Aust J Plant Physiol 28:871–879
Döbereiner J, Marriel IE, Nery M (1976) Ecological distribution of Spirillum lipoferum Beijerinck. Can J Microbiol 22:1464–1473
Esquivel-Cote R (2002) Selección e identificación de rizobacterias promotoras del desarrollo vegetal aisladas de cultivos regados con aguas residuales y su efecto sobre el desarrollo del jitomate (Lycopersicon esculentum). Tesis de Maestría. Facultad de Ciencias, Universidad Nacional Autónoma de México
Gadagi RS, Krishnaraj PU, Kulkarni JH, Sa T (2004) The effect of combined Azospirillum inoculation and nitrogen fertilizer on plant growth promotion and yield response of the blanket flower Gaillardia pulchella. Sci Hort 100:323–332. doi:10.1016/j.scienta.2003.10.002
García de Salamone IE, Hynes RK, Nelson LM (2001) Cytokinin production by plant growth promoting rhizobacteria and selected mutants. Can J Microbiol 47:404–411. doi:10.1139/cjm-47-5-404
Glick BR (2003) Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv 21:383–393. doi:10.1016/S0734-9750(03)00055-7
Glick BR, Karaturovic DM, Newell PC (1995) A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can J Microbiol 41:533–536
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190:63–68
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26:227–242. doi:10.1080/07352680701572966
Govindasamy V, Senthilkumar M, Gaikwad K, Annapurna K (2008) Isolation and characterization of ACC deaminase gene from two plant growth-promoting rhizobacteria. Curr Microbiol 57:312–317. doi:10.1007/s00284-008-9195-8
Greene EM (1980) Cytokinin production by microorganisms. Bot Rev 46:25–74. doi:10.1007/BF02860866
Grichko VP, Glick BR (2001) Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem 39:11–17. doi:10.1016/S0981-9428(00)01212-2
Hartmann A, Bashan Y (2009) Ecology and application of Azospirillum and other plant growth-promoting bacteria (PGPB) - special issue. Eur J Soil Biol 45:1–2. doi:10.1016/j.ejsobi.2008.11.004
Holguin G, Glick BR (2001) Expression of the ACC deaminase gene from Enterobacter cloacae UW4 in Azospirillum brasilense. Microb Ecol 41:281–288. doi:10.1007/s002480000040
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem 42:1825–1831
Horemans S, de Koninck K, Neuray J, Hermanss R, Vlassak K (1986) Production of plant growth substances by Azospirillum sp. and other rhizosphere bacteria. Symbiosis 2:341–346
Kapulnik Y, Kigel J, Okon Y, Nur I, Henis Y (1981) Effect of Azospirillum inoculums on sme growth parameters and N-content of wheat, sorghum and panicum. Plant Soil 61:65–67
Kende H (1993) Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 44:283–307
Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9:299–306. doi:10.1093/bib/bbn017
Lambers H, Chapin FS III, Pons TL (1998) Plant physiology ecology. Springer-Verlag, New York
Lynch J, Brown KM (1997) Ethylene and plant responses to nutritional stress. Physiol Plant 100:613–619
Ma W, Sebestianova S, Sebestian J, Burd GI, Guinel F, Glick BR (2003) Prevalence of 1-aminocyclopropane-1-carboxylate deaminase in Rhizobia spp. Ant Leew 83:285–291. doi:10.1023/A:1023360919140
Madhaiyan M, Poonguzhali S, Ryu J, Sa TM (2006) Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase-containing Methylobacterium fujisawaense. Planta 224:268–278. doi:10.1007/s00425-005-0211-y
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572. doi:10.1016/j.plaphy.2004.05.009
Penrose DM, Glick BR (2001) Levels of ACC and related compounds in exudates and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria. Can J Microbiol 47:368–372. doi:10.1139/cjm-47-4-368
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15
Perrig D, Boiero ML, Masciarelli OA, Penna C, Ruiz OA, Cassán FD, Luna MV (2007) Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl Microbiol Biotechnol 75:1143–1150. doi:10.1007/s00253-007-0909-9
Prigent-Combaret C, Blaha D, Pothier JF, Vial L, Poirier MA, Wisniewski-Dye F, Moëne-Loccoz Y (2008) Physical organization and phylogenetic analysis of acdR as leucine-responsive regulator of the 1-aminocyclopropane-1-carboxylate deaminase gene acdS in phytobeneficial Azospirillum lipoferum 4B and other Proteobacteria. FEMS Microbiol Ecol 65:202–219. doi:10.1111/j.1574-6941.2008.00474.x
Ribaudo CM, Krumpholz EM, Cassán FD, Bottini R, Cantore ML, Curá JA (2006) Azospirillum sp. promotes root hair development in tomato plants through a mechanism that involves ethylene. J Plant Growth Regul 24:175–185. doi:10.1007/s00344-005-0128-5
Rodríguez H, Vessely S, Shah S, Glick BR (2008) Effect of a nickel-tolerant ACC deaminase-producing Pseudomonas stain on growth of nontransformed and transgenic canola plants. Curr Microbiol 57:170–174. doi:10.1007/s00284-008-9181-1
SAGARPA-INIFAP (2009) http://www.inifap.gob.mx. Accessed 17 July 2009
Shah S, Li J, Moffatt BA, Glick BR (1998) Isolation and characterization of ACC deaminase genes from two different plant growth-promoting rhizobacteria. Can J Microbiol 44:833–843
Shaharoona B, Arshad M, Zahir ZA, Khalid A (2006) Performance of Pseudomonas spp. containing ACC-deaminase for improving growth and yield of maize (Zea mays L.) in the presence of nitrogenous fertilizer. Soil Biol Biochem 38:2971–2975. doi:10.1016/j.soilbio.2006.03.024
Shaharoona B, Naveed M, Arshad M, Zahir ZA (2008) Fertilizer-dependent efficiency of pseudomonads for improving growth, yield, and nutrient use efficiency of wheat (Triticum aestivum L.). Appl Microbiol Biotechnol 79:147–155. doi:10.1007/s00253-008-1419-0
Sheng XF, Xia JJ, Jiang CY, He LY, Qian M (2008) Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ Pollut 156:1164–1170. doi:10.1016/j.envpol.2008.04.007
SIAP-SAGARPA (2009) http://www.siap.sagarpa.gob.mx. Accessed 27 October 2009
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free-living bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Tahir M, Arshad M, Naveed M, Zahir ZA, Shaharoona B, Ahmad R (2006) Enrichment of recycled organic waste with N fertilizer and PGPR containing ACC-deaminase for improving growth and yield of tomato. Soil Environ 25:105–112
Taller BJ, Wong TY (1989) Cytokinins in Azotobacter vinelandii culture medium. Appl Environ Microbiol 55:266–267
Terry E, Leyva A, Hernández A (2005) Microorganismos benéficos como biofertilizantes eficientes para el cultivo del tomate (Lycopersicon esculentum, Mill). Rev Colomb Biotecnol 7:47–54
Tien TM, Gaskin MH, Hubbel DH (1979) Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Appl Environ Microbiol 37:1016–1024
Timmusk S, Nicander B, Granhall U, Tillberg E (1999) Cytokinin production by Paenibacillus polymyxa. Soil Biol Biochem 31:1847–1852. doi:10.1016/S0038-0717(99)00113-3
USDA (2008) Economic research service. http://www.ers.usda.gov/Briefing/Vegetables/tomatoes.htm. Accessed 19 November 2008
Van der Werf A, Nagel OW (1996) Carbon allocation to shoots and roots in relation to nitrogen supply is mediated by cytokinins and sucrose. Plant Soil 185:21–32
Acknowledgements
Our special thanks to Dr. E. Martínez, Dr. J. Caballero-Mellado and Dr. A. Rogel (CCG-UNAM) for technical assistance, and Diana Soriano and Irma Acosta for technical support. We also thanks to Dr. A. Gamboa deBuen for the critical review of the manuscript. R. Esquivel-Cote thanks to Posgrado en Ciencias Biológicas, UNAM and Consejo Nacional de Ciencia y Tecnología (CONACyT-181624) for scholarship. This work was supported by Dirección General de Asuntos del Personal Académico (DGAPA)-UNAM IN232802-3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter A.H. Bakker.
Rights and permissions
About this article
Cite this article
Esquivel-Cote, R., Ramírez-Gama, R.M., Tsuzuki-Reyes, G. et al. Azospirillum lipoferum strain AZm5 containing 1-aminocyclopropane-1-carboxylic acid deaminase improves early growth of tomato seedlings under nitrogen deficiency. Plant Soil 337, 65–75 (2010). https://doi.org/10.1007/s11104-010-0499-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-010-0499-7