Abstract
Water flow from soil to plants depends on the properties of the soil next to roots, the rhizosphere. Although several studies showed that the rhizosphere has different properties than the bulk soil, effects of the rhizosphere on root water uptake are commonly neglected. To investigate the rhizosphere’s properties we used neutron radiography to image water content distributions in soil samples planted with lupins during drying and subsequent rewetting. During drying, the water content in the rhizosphere was 0.05 larger than in the bulk soil. Immediately after rewetting, the picture reversed and the rhizosphere remained markedly dry. During the following days the water content of the rhizosphere increased and after 60 h it exceeded that of the bulk soil. The rhizosphere’s thickness was approximately 1.5 mm. Based on the observed dynamics, we derived the distinct, hysteretic and time-dependent water retention curve of the rhizosphere. Our hypothesis is that the rhizosphere’s water retention curve was determined by mucilage exuded by roots. The rhizosphere properties reduce water depletion around roots and weaken the drop of water potential towards roots, therefore favoring water uptake under dry conditions, as demonstrated by means of analytical calculation of water flow to a single root.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
How water flows from soil to plants is a fundamental question in both plant and soil sciences. At the beginning of the last century, Richards (1928) gave an appropriate formulation of this problem by distinguishing two important aspects: the capacity of roots to adsorb water from the soil in their vicinity; and the rapidity with which soil water content redistributes to replace the water that has been taken up. These two aspects can be summarized in terms of root and soil conductivity. According to Richards and Wadleigh (1952) “not much progress has been made in separating these two aspects of water availability because of the difficulty of determining moisture gradients in the immediate vicinity of small roots”. Today, more than 50 years later, this statement is still valid.
A formulation of the water flow to a single root was proposed by Gardner (1960). He solved the Richards’ equation in cylindrical coordinates. Assuming that the soil hydraulic conductivity and the diffusivity do not vary along the radial distance to root, he calculated the water content distribution around a root for different time steps. His calculations showed uniform water contents towards the roots as long as soil conductivity was high enough to meet uptake rate, and small water depletion next to roots when soil dried and its conductivity decreased. Gardner’s work has been implemented in models with increasing complexity. The last generation of these models is now capable of coupling the microscopic “Gardner” flow to a single root with the water redistribution along the soil profile and the water potential inside the root (Roose and Fowler 2004; Doussan et al. 2006; Siqueira et al. 2008; Javaux et al. 2008; Schneider et al. 2009).
Most of these models assume that the soil around roots has the same properties as the bulk soil, neglecting the potential role of the soil region that is in contact with roots. This portion of soil is called rhizosphere and there is significant amount of literature showing that its physical and chemical properties markedly differ from those of the bulk soil (Lavelle 2002; Strayer et al. 2003; Gregory 2006; Watt et al. 2006; Hinsinger et al. 2009). For instance, Young (1995) measured higher water contents in the rhizosphere than in the bulk soil. He suggested that mucilage increased the water-holding capacity of the rhizosphere. His hypothesis was confirmed by Read et al. (1999), who measured the water retention curve of mucilage collected from the root tips of maizes and lupins. They measured a water content of 99% at a matric potential of − 50 kPa, demonstrating the high water holding capacity of mucilage. Despite the evidence of the rhizosphere’s specific behavior, most of the studies on root water uptake still neglect it. Probably, the main reason for this lack in understanding remains the difficulty in determining moisture gradients around roots in-situ. In other words, the statement by Richards and Wadleigh (1952) is still valid.
Today, advances in imaging techniques allow visualization of water distribution in situ at high spatial and temporal resolution. Hainsworth and Aylmore (1989) used computer tomography to investigate water content profile towards a single root of a transpiring radish. They observed water depletion next to the root at different depths. Segal et al. (2008) investigated the same problem by means of magnetic resonance imaging (MRI). They observed a decreasing MRI signal towards the roots and interpreted it as water depletion next to roots. Their interpretation was based on the assumption that rhizosphere and bulk soil had identical properties. Water depletion around roots of a lupin was also observed by Garrigues et al. (2006) by means of light transmission imaging of thin slabs. Small water content next to roots of lupins and maizes have been reported by Oswald et al. (2008) during infiltration experiments monitored with neutron radiography. The authors supposed that the observed patterns were caused by a quick water uptake by roots. However, there are also studies showing contrary results on moisture gradients next to roots: Pierret et al. (2003) used X-ray radiography to monitor water distributions around roots in two-dimensions. They found that the changes in water content near and far from the roots were identical. In other words, they did not observe water depletion next to roots. Nakanishi et al. (2005) showed an accumulation of water in the vicinity of soybean root by using neutron tomography. A similar result was obtained by Tumlinson et al. (2008) for a corn seed in sand. The authors concluded that water content gradients in the rhizosphere need further investigation.
In theory, when plants transpire, the water potential next to the roots decreases driving water from bulk soil towards roots. If the water retention curve of bulk soil and rhizosphere are identical, there will be a smaller water content next to roots. Else, if rhizosphere and bulk soil have different water retention curve, for instance if rhizosphere has a higher water holding capacity than bulk soil, then the rhizosphere may have a larger water content even during transpiration. In other words, moisture gradients next to roots may depend not only on transpiration rate and soil conductivity, but also on differences in water retention curve between bulk soil and rhizosphere. Effect of rhizosphere properties on root water uptake have not yet been studied.
To investigate whether rhizosphere affects moisture dynamics next to roots, we used neutron radiography to image water distribution next to the roots of transpiring plants. Neutron radiography is an optimal technique for imaging root and water distributions in thin samples (Menon et al. 2007; Oswald et al. 2008; Moradi et al. 2009). Compared to three-dimensional tomography, radiography allows investigation of quick processes such as water infiltration in the root zone. Additionally, radiography requires much shorter exposure time compared to tomography, allowing investigation of several replicates.
Based on the observed water content distributions, we intended to derive information on the water retention curve of the rhizosphere. In addition, we investigated whether the rhizosphere properties have an effect on root water uptake. To this end, we calculated the water content and the water potential towards a single root in two scenarios: 1) the soil around the root is homogeneous, as in Gardner’s model; and 2) the soil is composed of two regions, the rhizosphere and the bulk soil. The water flow to a single root was calculated extending the analytical approach of de Willigen and van Noordwijk (1987) to a medium composed of two domains.
Material and methods
Experimental set-up
A sandy soil was collected nearby the artificial catchment Hühnerwasser located in the Lusatian lignite-mining area close to Cottbus, Germany. The soil consisted of quartenary sand in the initial phase of soil development. The soil was composed of approximately 92% sand, 5% silt, 3% clay. It contained almost no organic matter. Previous experiments with X-ray tomography showed that this soil had negligible swelling/shrinking behavior (Carminati et al. 2009). The collected soil was sieved to 2 mm, in order to remove aggregates, and mixed with a nutrient solution.
The soil was exposed to ambient air for two days and then filled in eight rectangular containers of 15 cm height, 15 cm width and 1.5 cm thickness. This size fits well within the field of view of 27.9×27.9 cm of our neutron radiography set-up. The small thickness of the sample is a requirement of neutron radiography. Thicker sample could not be crossed by thermal neutrons, neutron scattering would increases, and quantification of water content by image analysis would become more uncertain. The containers had one side open, were placed horizontally, and were filled as homogeneously as possible in order to avoid formation of soil layers. One lupin seed (Lupinus albus) was gently placed in each container. The seeds were previously sterilized and then germinated for one day in a solution of CaSO4×2H2O.
After planting, the open side of the containers was closed, the sample was turned in vertical position, and it was gently shaken to achieve a stable packing. The resulting density was equal to 1.45 ±0.04 [g cm − 3]. In addition, the samples were covered with a 1 cm-thick layer of quartz gravel of 5 mm grain size to reduce evaporation.
The containers had a porous plate at the bottom connected with a water reservoir. After the filling procedure the connection to the water reservoir was opened and the samples were watered by setting the water potential at the bottom to h = − 20 cm, where h is the matric potential expressed in pressure heads. A pressure head of 1 cm corresponds to a pressure of 0.98 hPa. The plants were grown for 3 weeks with a daily photoperiod of 14 h, day temperature of 22°C, night temperature of 15°C, and relative humidity of about 60%. The water potential at the bottom of the sample was kept at h = − 20 cm, corresponding to an average volumetric water content of 0.18, as derived by weighing the sample.
Three weeks after planting, the connection to the water reservoir was closed and the top of the sample was covered with aluminum tape to minimize evaporation. All samples have been radiographed at 8:00, 14:00 and 20:00 hours, each day for 9 days . The samples were weighed before radiography in order to monitor the water loss by transpiration.
A picture of one of the samples before taking the radiography series is shown in Fig. 1.
On day 6, when the plants showed severe wilting symptoms, the samples were rewetted from the bottom by re-connecting the water reservoir and setting the water potential to h = − 20 cm. After 1 h the connection to the water reservoir was closed again and the radiography experiment continued for three more days.
One of the containers was not planted and was used to measure the hydraulic properties of the bulk soil. The water retention curve was measured by stepwise decreasing the water potential from h = 15 cm to h = − 150 cm and weighing the water outflow. The water retention curve was fitted according to van Genuchten (1980). The hydraulic conductivity at saturation was measured with the falling-head method. The water potential at the bottom was set to h = 15 cm, which resulted in a free water table of 3 cm height at the top of the sample. Then the water potential at the bottom was decreased to h = − 20 cm and the decrease of the free water table was imaged by neutron radiography. The saturated conductivity of the sample was then calculated using Darcy’s law.
Neutron radiography
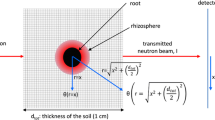
Neutron radiography was performed at NEUTRA, Paul Scherrer Institute, PSI, Switzerland. Neutron radiography is an imaging technique which is very sensitive to hydrous materials, making water and roots easily visible compared to other soil components (Pleinert and Lehmann 1997; Hassanein et al. 2005; Menon et al. 2007; Oswald et al. 2008). It consists of a collimated neutron beam guided through the sample. The neutron beam intensity behind the sample is detected by a scintillator plate and contains the information about the mass and thickness of the sample components. Effect of scattered neutrons is calculated with the Quantitative Neutron Imaging—QNI software (Hassanein et al. 2005). QNI is a Monte-Carlo based algorithm that subtracts from the radiograph the contribution of neutron scattering.
For radiography we used a field of view of 27.9×27.9 cm, obtaining an image of \(\text{1,024}\times\text{1,024}\) pixels with pixel side of 0.0272 cm. Exposure time of 22 s yielded an optimal dynamics of gray values in the radiograph. After correction for neutron scattering, the intensity of the transmitted neutron beam through the sample is described by the exponential law:
where (x,z) is the plane perpendicular to the neutron beam direction y, t is the time, I o and I are the in-coming and out-going beam intensities, L and Σ are the thickness in the beam direction and the linear attenuation coefficient of the sample components. The subscript Al refers to the aluminum walls of the container, soil to the dry soil (solid+air), and w to water. The latter component includes also the attenuation by roots.
All samples were radiographed in their initial dry condition just after planting. These radiographs yielded the contribution of the aluminum and dry soil. The effective attenuation coefficient of water was calculated by the QNI software. Then, rearranging Eq. 1, we calculated L w(x,z,t). The volumetric water content was defined as
where L tot(x,z) is the total thickness of the inner space of the container, which is equal to 1.5 cm. Equation 2 gives the sum of water in soil and roots along the beam direction. In the pixels containing no roots Eq. 2 gives the soil water content. Integration of Eq. 2 gives the volume of water in the sample.
To estimate the water distribution towards the roots, we calculated θ as a function of the distance to roots. Segmentation of the root network was performed according to Menon et al. (2007). The segmented root network was then used to evaluate the distance map from the root network and calculate the average soil water content as a function of distance to the roots.
Model of root water uptake
Root water uptake was modeled by means of the analytical approach proposed by de Willigen and van Noordwijk (1987). The model assumes a cylindrical water flow to a single root and steady-rate behavior, i.e. \(\left(\partial\theta/\partial t\right)=0\). First, the matric flux potential ϕ is defined as:
where h c is the current matric potential, h − ∞ is the lower integral boundary that we assume equal to –15,000 cm, and k [cm s − 1] is the soil hydraulic conductivity. Introduction of the matric flux potential helps to linearize the Richards’ equation. Under steady rate behavior, it yields:
where r is the distance to the root center, r root is the root radius, r out is the outer radius of the cylindrical domain, q root and q out [m s − 1] are the fluxes at the root surface and at outer radius of the domain, and ρ = r out/r root. Numerical inversion of Eq. 3 gives the water potential h towards the roots.
To implement this model for the case of a rhizosphere with distinct hydraulic properties, we divided the flow domain in two concentric cylinders, one external describing the bulk soil and one internal describing the rhizosphere. The flow equations in the two domains were calculated separately and then coupled imposing potential and flux continuity at the boundary between the two domains. As boundary conditions we imposed no flux at the outer radius of the bulk soil, and flux equal to the observed uptake rate per root surface area at the root-rhizosphere interface. The experimental uptake rate was calculated from the weighed water loss. To estimate the root surface, we calculated the root length from the skeletonized root network and we multiplied it by the average root circumference, obtained by applying the opening and closing operation to the segmented roots (Serra 1982). As initial condition, we imposed h = − 20 cm at the outer radius of the bulk soil. For the following time steps the water potential in the flow domain was calculated such that it was consistent with the imposed flow boundary conditions, i.e. the difference in the total water content between two times was equal to the flow rate multiplied by the time step and the root cross-section.
The water retention curve of the two domains were parameterized according to van Genuchten (1980):
where Θ = (θ − θ res) / (θ sat − θ res) is the water saturation, θ sat being the water content at saturation and θ res the residual water content. The hydraulic conductivity was calculated according to Mualem (1976):
where k sat being the conductivity at saturation and τ the tortuosity factor.
The hydraulic parameters of the bulk soil were obtained from the water retention curve and the saturated conductivity of the un-planted sample. The tortuosity factor was assumed equal to − 1, which is a realistic value for sandy soils (van Dam et al. 1992). The parameters of the rhizosphere were estimated based on the observed water dynamics next to the roots. Specifically, we made the assumption that the difference in water potential between rhizosphere and bulk soil was small during the experiment. This assumption is justified when the soil conductivity is much higher than the uptake rate, which is the case when soil is relatively wet (Gardner 1960). For the case of dry soil we leave justification of this assumption until later. The conductivity of the rhizosphere is discussed later.
Results
Observed water distribution
The average water loss in the samples during the first three days of measurements was 1.1 g per h during the photoperiod, and 0.25 g per h during night. After 4 days the water uptake during the photoperiod decreased to 0.4 g per h. After rewetting the water uptake during the photoperiod increased to 0.7 g per h. The sample that was not planted had a water loss of approximately 0.1 g per h, confirming that the water loss was primarily caused by root uptake. In Fig. 2 we plot the average water content over time for one sample, as obtained from neutron radiography and from weight measurements. The data matched well, validating the estimation of water content by image analysis. For brevity, we only show data of this sample. All the other samples showed a similar behavior.
Figure 3 shows the radiograph of the sample taken immediately after planting, i.e. 3 weeks before the start of the drying experiment. The image is a close-up of the original field of view (27.9×27.9 cm) and shows the water content distribution θ(x,z), when the sample was equilibrated at h = − 20 cm. The radiograph shows that θ(x,z) was quite uniform. In Fig. 4 we show the time-series of radiographs during the first 6 days of the drying experiments. The images show θ(x,z) at 14:00 during day 1 to 6. Note that the sample was rewetted between day 5 and 6. On day 1 θ(x,z) was patchy and non-uniformly distributed. We attribute the non-uniformity of θ(x,z) to roots activity. In fact, radiography of the sample immediately after planting showed uniform θ at h = − 20 cm, i.e. no initial soil heterogeneity (Fig. 3). On day 2 the patchiness increased and higher θ appeared next to some parts of the roots, in particular along the more distal parts of lateral roots (Fig. 5). On day 3, θ distribution became quite uniform, although regions of high θ were still visible in the more distal parts of lateral roots. On day 4 the average θ was 0.05. On day 5, θ was 0.01 in the lower 10 cm of the sample and 0.02–0.03 in the upper layer where no roots were present. At this stage the plant showed severe wilting symptoms. On day 6, the plant was rewetted by applying a water potential of h = − 20 cm at the bottom. Surprisingly, the radiograph taken 10 min after this event showed that parts of the rhizosphere remained markedly drier than the bulk soil. In the following days the rhizosphere was slowly rewetted and the dry regions disappeared.
Close-up of Fig. 4 showing the lower, left part of the sample at day 2 and day 6. At day 2 the soil next to roots was darker and probably wetter than the soil far from the roots. At day 6, just after rewetting, the region next to roots appeared bright, indicating that it was not rewetted
Digital root segmentation was performed on the radiograph taken at day 5, when the contrast between soil and roots was maximal. The original radiograph and the segmented one are shown in Fig. 6.
Left: radiograph of the sample at day 5, when the contrast between soil and root was the highest. Right: segmentation of the roots according to Menon et al. (2007)
Based on the segmented image, we calculated the distance map to the roots and the average water content as a function of distance to roots, θ(d) (Fig. 7). Figure 7 shows that the water content increased towards the roots during the all drying period. After rewetting the picture reversed, with θ decreasing towards the roots. In the following days (day 8 and 9), the water content near roots became larger than far from the root, and the water content profile became similar to that during the drying period. Figure 7 (right side) shows that the characteristic extension of the rhizosphere having distinct hydraulic properties is about 0.15–0.2 cm.
Since the radiographs resulted from neutron transmission across the sample, the calculated θ(d) is an average along the sample thickness (Moradi et al. 2009). Next to the roots θ(d) is the average of θ in the rhizosphere and in the portions of soil in front and behind the rhizosphere (Fig. 8). To correct for such averaging effect and calculate the actual water content in the rhizosphere, we assumed uniform water contents in the bulk soil, θ bulk = θ(d = 0.5 cm), and we calculated:
where 0.38 cm is the assumed thickness of the rhizosphere in the beam direction in the pixels adjacent to the roots, and 1.12 cm is the remaining thickness of the bulk soil. These values are derived from geometrical considerations assuming root radius of 0.05 cm and rhizosphere thickness of 0.15 cm (Fig. 8). The calculated θ bulk and θ rhizo versus time are plotted in Fig. 9. The data show that θ rhizo was about 0.05 larger than θ bulk during day 1–3. At day 4 and 5 both the rhizosphere and the bulk soil had a similar, very small water content. After rewetting the rhizosphere remained markedly drier than the bulk soil. In the following period, the rhizosphere was slowly rewetted and after two days became again wetter than the bulk soil.
Schematic cross-section of one root (black), rhizosphere (light gray), and bulk soil (dark gray). Neutron radiography yielded the water content averaged along the 1.5 cm sample thickness, θ(d). For d < 0.2 cm, θ is an average of the water content in the rhizosphere θ rhizo and in the bulk soil θ bulk
Water content θ in the rhizosphere and in the bulk soil during the drying period and after rewetting as derived from the radiographs and Eq. 7. The jump in water content corresponds to the rewetting event. The water content was larger in the rhizosphere then in the bulk soil during the initial drying. Immediately after rewetting the rhizosphere remained markedly drier than the bulk soil. During the following days the water content of the rhizosphere increased again exceeding that of the bulk soil
Rhizosphere hydraulic properties
Neutron radiography showed that water content dynamics in the rhizosphere were markedly different than in the bulk soil: during drying θ rhizo > θ bulk, after rewetting θ rhizo < θ bulk, and a few day after rewetting θ rhizo increased again to values larger than θ bulk. These results suggest that the rhizosphere had a different water retention curve compared to the bulk soil and that it changed during time.
To estimate the water retention curve of the rhizosphere we followed these steps: we measured the water retention curve of the bulk soil from the un-planted sample (Table 1). For the water retention curve of the rhizosphere we assumed that the differences in water potential between the rhizosphere and the bulk soil were small. The validity of this assumption will be re-examined later. Then, taking the observed water content in the bulk soil for each time (Fig. 9) and using the water retention curve of the bulk soil, we calculate the water potential in the sample. Now, assuming that the rhizosphere was at the same potential as the bulk soil, we obtain the values of water potential at each time step. Plotting these points versus the water content gives the water retention curve of the rhizosphere (Fig. 10). Figure 10 shows two different curves for the rhizosphere water retention. The points referring to the drying period (circles) shows that the water holding capacity of the rhizosphere was stronger than that of the bulk soil. The points referring to the period after rewetting fall on a different curve (squares) and show that the water holding capacity of the rhizosphere was initially small and then it increased over time. The data demonstrate the distinct, hysteretic and time-dependent water retention curve of the rhizosphere.
Water retention curve of the bulk soil (measured) and of the rhizosphere, as derived from Fig. 9. The water retention curve of the rhizosphere during drying (circles) and after rewetting (squares) markedly differ, with the latter suggesting a hysteretic and time-dependent wettability. The curves were fitted with the van Genuchten parameterization (Table 1). For the rewetting phase we took only the first point after rewetting
The water retention curve of the rhizosphere during drying was fitted with the van Genuchten parameterization (Table 1).
It has to be expected that the water potential in the rhizosphere was lower than in the bulk soil in order to allow water fluxes towards the roots, especially for low potentials. Therefore, the data of the water content in the rhizosphere should be slightly shifted to higher values. However, this error does not affect our conclusion that the rhizosphere had a higher water holding capacity than the bulk soil. Actually, the difference between rhizosphere and bulk soil would be even larger.
Now the question is: what determines the water retention curve of the rhizosphere? A good candidate to explain our results is mucilage exuded by roots. It is known that mucilage exuded by roots is primarily composed of polymeric substances and it is capable of holding large volumes of water at low water potentials: i.e. maize mucilage at −10 kPa can have a water content of 99.9% (Watt et al. 1994; Young 1995; McCully and Boyer 1997; Read and Gregory 1997; Read et al. 1999). Therefore, mucilage could explain the higher water content in the rhizosphere. Additionally, mucilage’s phospholipids decrease the soil wettability after a period of drying (Read et al. 2003), explaining the observed low wettability of the rhizosphere after rewettining. Finally, mucilage is able to rehydrate, swell and recover its high water holding capacity, which would explain the observed dynamics of the water retention curve of the rhizosphere. Based on these considerations, we conclude that mucilage exuded by roots is a fully reasonable hypothesis to explain the observed behavior of the rhizosphere.
Model of root water uptake
The water flow to a single root was calculated analytically by extending the approach of de Willigen and van Noordwijk (1987) to a medium composed of two concentric cylindrical domains, the bulk soil and the rhizosphere. We assumed that the root had a radius of 0.05 cm, the rhizosphere had an extension of 0.15 cm, i.e. it extended from d = 0.05 to 0.2 cm, and the bulk soil extended from d = 0.15 to 0.65 cm. We imposed a flux equal to the measured water uptake of 17.9 g d − 1 divided by the estimated root surface of 80 cm2, giving a flow of 0.22 cm d − 1.
As initial condition we imposed a water potential of h = − 20 cm at the outer radius of the bulk soil, i.e. at d = 0.65 cm. For the hydraulic properties of the bulk soil we used the measured water retention curve and saturated conductivity of the un-planted sample (Table 1). For the rhizosphere properties we used the data of the drying curve of the rhizosphere reported on Table 1. For the rhizosphere conductivity we refer to the review of Or et al. (2007) on unsaturated water flow in porous media containing extracellular polymeric substances (EPS). Since we hypothesize that the high water holding capacity of the rhizosphere is due root-exuded mucilage and since mucilage is mostly composed of polymeric substances, we expect that transport properties of soil with EPS mimic are similar to those of the rhizosphere. According to Or et al. (2007), EPS reduces the saturated soil conductivity by a few orders of magnitude and it increases the unsaturated conductivity. We assumed that the rhizosphere saturated conductivity was two orders of magnitude smaller than that of the bulk soil, and that the tortuosity factor was equal to τ = − 2. Note that in soil τ = − 1, and the smaller is the tortuosity, the lower is the decrease of conductivity with decreasing water potential.
The water potential towards the root for different time steps are plotted in Fig. 11. For comparison, we plotted the water potential in the two cases: (1) without rhizosphere, i.e. homogeneous soil having the bulk soil properties, and (2) with rhizosphere. For the first 72 h the water potential towards the root was almost horizontal, indicating that the uptake rate was much smaller that the soil conductivity. After 86 h in the case (1) the water potential started to decrease in the vicinity of the roots. In the case (2) the drop in water potential was smaller. After 87 h the decrease in water potential in the case (1) became dramatic, while in the case (2) it remained relatively small.
The water contents towards the root are plotted in Fig. 12. The water content distribution reflected the water retention curve of the two domains. At the same potential the rhizosphere held more water than the bulk soil (0 − 72 h). This was true also after 87 h, when the water potential in the rhizosphere became slightly lower than in the bulk soil.
It is worth noting that the calculations show that the decrease of water potential towards the root was small. This justifies the approximation of equal water potential in the bulk soil and rhizosphere that we used to derive the water retention curve of the rhizosphere (Fig. 10).
Discussion
Our measurements showed relatively high neutron attenuation close to roots during drying and low neutron attenuation after rewetting. We related the signal to water content, and based on this relation we derived the water retention curve of the rhizosphere. The assumption of relating the neutron attenuation to water content is justified by the high sensitivity of neutrons to hydrous materials compared to other soil components. In other words, neutrons see mainly water, while changes in soil density are almost invisible. The calculated water content could include water in root hairs. However, root hairs volume is not sufficient to justify the observed signal close to the roots. In fact, assuming root hairs diameter of 10 μm and number of root hairs per root surface of 100 per mm − 2, average data from literature (Foehse et al. 1991), then the water content produced by root hairs would be around 0.005–0.01, which is smaller than the observed difference between rhizosphere and bulk soil (Fig. 9). Moreover, root hairs should always lead to high neutron attenuation next to the roots and cannot explain the lower signal next to the roots after rewetting and its subsequent increase. By the same token, we also exclude the possibility that the high signal next to the roots was due to an error in the segmentation that underestimates the root diameter. If this was the case, we would have not observed less water near roots after rewetting. Based on these considerations, we believe that the particular behavior of water content derived from neutron radiography is something real and not an artefact.
We found that during drying the soil next to roots was wetter than the bulk soil. After rewetting the rhizosphere remained relatively dry. During the following days it became again wetter than the bulk soil. We explained these observations with the distinct, hysteretic, and time-dependent water retention curve of the rhizosphere. Our results explain some contradictions in literature. Young (1995) measured larger volumes of water in soil from rhizosphere than in bulk soil, and he argued that this was primarily caused by mucilage. Similarly, Roberson and Firestone (1992) and Chenu (1993) found that extracellular polysaccharides (main components of mucilage) increase the water holding capacity of soils. In contrast, Read et al. (2003) showed that mucilage may act as a surfactant reducing the soil wettability. Slight water repellency of the rhizosphere was measured by Hallett et al. (2003) by means of mini-infiltrometer. Similarly, Whalley et al. (2004) measured lower sorptivity in the rhizosphere than in the bulk soil.
Our data on the rhizosphere’s water retention curve solve the debate on whether mucilage increases or decreases the soil water holding capacity. It is a question of non-equilibrium dynamics between rhizosphere’s mucilage and bulk soil’s water content. During drying mucilage increases the soil water holding capacity. After dessication mucilage re-hydrates at a lower rate than the bulk soil, temporarily decreasing the wettability of the rhizosphere.
An additional aspect that is still not clear is the effect of mucilage and rhizosphere on water flow to roots. We started from the model of Gardner (1960), which describes the cylindrical water flow to a single root, and we calculated the evolution of water potential and water content towards the root. The calculations required the hydraulic properties of the rhizosphere. The water retention curve was calculated from the imaged water content distributions under the assumption that bulk soil and rhizosphere were in equilibrium. The calculations showed the importance of the rhizosphere for water flow to roots. The rhizosphere properties avoided the drop in water potential at the root surface, facilitating the roots’ work to take up water from drying soils. In this way, the rhizosphere acted as optimal hydraulic connector between soil and roots, while a homogeneous soil would not be able to sustain the same uptake rate. Additionally, the low wettability of rhizosphere after severe drought, prevents roots from being exposed to fast rewetting and consequent osmotic shock, which may have harmful consequences for root cell membranes. Also, mucilage exuded by roots may favor the contact between roots and soil, in particular as soils dry and roots shrink (Carminati et al. 2009). In conclusion, the rhizosphere acts as a buffer that softens the hydraulic stress experienced by roots in soils, and favors water availability to roots during drought.
Finally, we come to the question whether and when water depletion occurs near roots. Our results showed that there was no water depletion during the drying period. On the contrary, we observed an increase of water relative to the bulk soil. This was caused by the different hydraulic properties of rhizosphere and bulk soil. The capacity of the rhizosphere to retain more water than the bulk soil overcame the negative gradients in water potentials needed to drive water into roots.
In other experimental conditions, for instance with high transpiration rate and low soil conductivity, water depletion may occur. However, it will be moderated by the rhizosphere properties.
In our experiment, water depletion near roots was visible immediately after rewetting. Similar patterns were observed by Oswald et al. (2008) during infiltration in soils with maize and lupin. We believe that the observed patterns were not caused by a quick water uptake, but rather by the low wettability of the rhizosphere subsequent to drying.
In this discussion, we refer to water depletion as the small-scale moisture gradients in the few millimeters adjacent to the roots. At the scale of the root system, water depletion is controlled by distribution and conductivity of roots, as well as by the soil conductivity. We emphasize the need to distinguish between moisture dynamics at the rhizosphere scale, controlled by the rhizosphere properties, and at plant scale, controlled by root distribution and soil properties.
Conclusions
-
We used neutron radiography to image the water distribution in soils with plants during a drying period and rewetting. We observed that during drying the water content next to roots was higher than in the bulk soil, while after rewetting the soil next to roots remained markedly drier than the bulk soil.
-
The soil water content dynamics next to roots were explained by the specific, hysteretic and time-dependent properties of the rhizosphere. Based on the observed water distribution we estimated the water retention curve of the rhizosphere. The rhizosphere held more water than the bulk soil during drying, explaining why there was more water next to roots even during transpiration. After rewetting the rhizosphere became less wettable, but its water holding capacity recovered with time. Our hypothesis is that this rhizosphere behavior was caused by mucilage exuded by roots.
-
The significance of the rhizosphere properties for root water uptake was investigated by calculating the water potential towards a single root assuming a constant transpiration. The rhizosphere, with its high water holding capacity, avoided water depletion next to roots and significantly weakened the drop in water potential as the soil dried. Evidently, this favors water availability to plants during drought.
-
Overall, our work indicates that plants alter the soil properties in their vicinity. The resulting properties of the rhizosphere are of high relevance for root water uptake, especially in helping plants to overcome decreasing hydraulic conductivity during dry conditions. Since such an effect and the underlying mechanism are of high importance to plants, it should be further investigated by means of techniques from different disciplines.
References
Carminati A, Vetterlein D, Weller U, Vogel HJ, Oswald SE (2009) When roots lose contact. Vadose Zone J 8:805–809
Chenu C (1993) Clay- or sand-polysaccharide associations as models for the interface between micro-organisms and soil–water related properties and microstructure. Geoderma 56:143–156
de Willigen P, van Noordwijk M (1987) Roots, plant production, and nutrient use efficiency. Ph.D. thesis, Wageningen Agric. Univ., The Netherlands
Doussan C, Pierret A, Garrigues E, Pages L (2006) Water uptake by plant roots: II—modelling of water transfer in the soil root-system with explicit account of flow within the root system—comparison with experiments. Plant Soil 283:99–117
Foehse D, Claassen N, Jungk A (1991) Phosphorus efficiency of plants. II. Significance of root radius, root hairs and cation–anion balance for phosphorus influx in seven plant species. Plant Soil 132:261–272
Gardner WR (1960) Dynamic aspects of water availability to plants. Soil Sci 89:63–73
Garrigues E, Doussan C, Pierret A (2006) Water uptake by plant roots: I—formation and propagation of a water extraction front in mature root systems as evidenced by 2D light transmission imaging. Plant Soil 283:83–98
Gregory PJ (2006) Roots, rhizosphere and soil: the route to a better understanding of soil science? Eur J Soil Sci 57:2–12
Hainsworth JM, Aylmore LAG (1989) Non-uniform soil water extaction by plant roots. Plant Soil 113:121–124
Hallett PD, Gordon DC, Bengough AG (2003) Plant influence on rhizosphere hydraulic properties: direct measurements using a miniaturized infiltrometer. New Phytol 157:597–603
Hassanein R, Lehmann E, Vontobel P (2005) Methods of scattering corrections for quantitative neutron radiography. Nucl Instrum Methods Phys Res Sect A 542:392–398
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152. doi:10.1007/s11104-008-9885-9
Javaux M, Schroeder T, Vanderborght J, Vereecken H (2008) Use of a three-dimensional detailed modeling approach for predicting root water uptake. Vadose Zone J 7:1079–1088
Lavelle P (2002) Functional domains in soils. Ecol Res 17:441–450
McCully ME, Boyer JS (1997) The expansion of maize root-cap mucilage during hydration. 3: changes in water potential and water content. Phisiol Plantarum 99:169–177
Menon M, Robinson B, Oswald SE, Kaestner A, Abbaspour KC, Lehmann E, Schulin R (2007) Visualization of root growth in heterogeneously contaminated soil using neutron radiography. Eur J Soil Sci 58:802–810
Moradi AB, Conesa HM, Robinson B, Lehmann E, Kuehne G, Kaestner A, Oswald S, Schulin R (2009) Neutron radiography as a tool for revealing root development in soil: capabilities and limitations. Plant Soil 318:243–255
Mualem YA (1976) A new model for predicting the hydraulic conductivity of unsaturated porous media. Water Resour Res 12:513–522
Nakanishi TM, Okuni Y, Hayashi Y, Nishiyama H (2005) Water gradient profiles at bean plant roots determined by neutron beam analysis. J Radioanal Nucl Chem 264:313–317
Or D, Phutane S, Dechesne A (2007) Extracellular polymeric substances affecting pore-scale hydrologic conditions for bacterial activity in unsaturated soils. Vadose Zone J 6:298–305
Oswald SE, Menon M, Carminati A, Vontobel P, Lehmann E, Schulin R (2008) Quantitative imaging of infiltration, root growth, and root water uptake via neutron radiography. Vadose Zone J 7:1035–1047
Pierret A, Kirby M, Moran C (2003) Simultaneous X-ray imaging of plant root growth and water uptake in thin-slab systems. Plant Soil 255:361–373
Pleinert H, Lehmann E (1997) Determination of hydrogenous distributions by neutron trasmission analysis. Phys B 234:1030–1032
Read DB, Gregory PJ (1997) Surface tension and viscosity of axenic maize and lupin root mucilages. New Phytol 137:623–628
Read DB, Gregory PJ, Bell AE (1999) Physical properties of axenic maize root mucilage. Plant Soil 211:87–91
Read DB, Bengough AG, Gregory PJ, Crawford JW, Robinson D, Scrimgeour CM, Young IM, Zhang K, Zhang X (2003) Plant roots release phospholipid surfactants that modify the physical and chemical properties of soil. New Phytol 157:315–326
Richards LA (1928) The usefulness of capillary potential to soil-moisture and plant investigators. J Agric Res 37:719–742
Richards LA, Wadleigh CH (1952) Soil water and plant growth. In: Soil physical conditions and plant growth. Academic, New York, pp 73–251
Roberson EB, Firestone MK (1992) Relationship between dessication and exopolysaccharide production in a soil Pseudomonas. Appl Environ Microbiol 58:1284–1291
Roose T, Fowler AC (2004) A mathematical model for water and nutrient uptake by plant root systems. J Theor Biol 228:173–184
Schneider CL, Attinger S, Delfs JO, Hildebrandt A (2009) Implementing small scale processes at the soil–plant interface—the role of root architectures for calculating root water uptake profiles. Hydrol Earth Syst Sci Discuss 6(3):4233–4264. http://www.hydrol-earth-syst-sci-discuss.net/6/4233/2009/
Segal E, Kushnir T, Mualem Y, Shani U (2008) Microsensing of water dynamics and root distributions in sandy soils. Vadose Zone J 7:1018–1026
Serra J (1982) Image analysis and mathematical morphology. Academic, London
Siqueira M, Katul G, Porporato A (2008) Onset of water stress, hysteresis in plant conductance, and hydraulic lift: scaling soil water dynamics from millimeters to meters. Water Resour Res 44:1–14
Strayer DL, Power ME, Fagan WF, Pickett STA, Belnap J (2003) A classification of ecological boundaries. Bioscience 53:723–729
Tumlinson LG, Liu H, Silk WK, Hopmans JW (2008) Thermal neutron computed tomography of soil water and plant roots. Soil Sci Soc Am J 72:1234–1242
van Dam JC, Stricker JNM, Droogers P (1992) Inverse method for determining soil hydraulic functions from one-step outflow experiments. Soil Sci Soc Am J 56:1042–1050
van Genuchten MT (1980) A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci Soc Am J 44:892–898
Watt M, McCully ME, Canny MJ (1994) Formation and stabilization of rhizosheaths of Zea mays l. effect of soil water content. Plant Physiol 106:179–186
Watt M, Silk WK, Passioura JB (2006) Rates of root and organism growth, soil conditions, and temporal and spatial development of the rhizosphere. Ann Bot 97:839–855
Whalley WR, Leeds-Harrison PB, Leech PK, Riseley B, Bird NRA (2004) The hydraulic properties of the root–soil interface. Soil Sci 169:90–99
Young IM (1995) Variation in moisture contents between bulk soil and the rhizosheath of wheat Triticum-Aestivum l. cv. wembley. New Phytol 130:135–139
Acknowledgements
The authors acknowledge Dani Or for suggesting the role of mucilage. We thank A. Badorreck and W. Schaaf for providing the soil. The positions of A. Carminati and A. Moradi were funded by the EU-Marie Curie Project “Water Watch”, contract MTKKD-2006-042724.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter J. Gregory.
Rights and permissions
About this article
Cite this article
Carminati, A., Moradi, A.B., Vetterlein, D. et al. Dynamics of soil water content in the rhizosphere. Plant Soil 332, 163–176 (2010). https://doi.org/10.1007/s11104-010-0283-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-010-0283-8