Abstract
Background and aims
Water is often heterogeneously distributed in soils. Understanding roots’ responses to this soil-water heterogeneity is a key parameter defining plant survival in dry climates. To determine local root water uptake for partly dry conditions in a plant’s root system, we prepared soil patches with different water contents, then used neutron radiography to monitor daily changes in root structure and water uptake.
Methods
Lupin plants were grown in 30 × 25 × 1 cm3 aluminum containers filled with sandy soil. In two partitioning set-ups, the soil-root zone was divided into either two or nine hydraulically-isolated soil compartments. This was done by packing layers of coarse sand as capillary barriers, by which vertical and/or horizontal soil water heterogeneity as well as homogeneous well-watered treatments were applied for control. Daily changes in soil water content in each compartment, water uptake and root growth were monitored non-invasively and quantified by neutron radiography during a period of 15 consecutive days.
Results
In optimal homogeneously-wet soil, lateral roots in the top 10 cm of the root system showed the highest water uptake rate, up to around 10 mg/(mm. root. day), which on average was twice as much as that for younger lateral roots in lower position and taproot. In heterogeneous treatments, root water uptake declined strongly in compartments with the soil water content below 0.13–0.10 cm3/cm3 while in parallel an enhanced uptake rate, rising by up to 100 %, was observed for the roots in wet compartments, presumably to compensate for roots in dry compartments and, therefore, sustain the total transpiration. Also, our observations showed that in the drying compartment a reduction of soil water content to 0.10–0.15 triggered local cluster root formation.
Conclusions
With the experimental set-up presented the pattern of water uptake across a lupin root system can be quantified and normalized to root length. Water uptake was shown to be highly variable in different parts of the root system. A threshold for water stress to cause cessation of local water uptake was identified, and the considerable amount of compensation by water uptake in other parts identified. The dynamic trade-off among different parts of the root system seems to regulate total root uptake also during water stress to sustain the daily transpirational demand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatiotemporal heterogeneity of water availability around roots of plants is a general phenomenon, occurring widely in natural environments. The heterogeneity in soil water availability may restrict growth of roots and plant’s water and nutrient supply (Lawlor 1973; Maurel et al. 2010; McLean et al. 2011). There are two main strategies that plants use in general for acquisition of resources in patchy soils: 1) root proliferation within the localized resources, e.g. of nutrients or water, to occupy more of the favorable soil volume (structural response); and 2) increasing root activity and uptake rate in the resource-rich patches already intercepted by roots, resulting in increased and faster utilization of the resources (functional adjustment). Although many studies have been done on plant’s responses to heterogeneous nutrients distribution (Hodge 2010; Garcia-Palacios et al. 2012; Mommer et al. 2012) and above-ground responses to partial root-zone drying, there are only limited direct observations of root behavior in response to soil water heterogeneity. Studies on heterogeneous salinity distribution in the root zone and studies on partial root-zone drying have shown that plants take up the most easily accessible water that is in good contact with the roots, particularly from the parts of the root-zone having higher water potential (Bazihizina et al. 2012). For most plants where a part of the root system was in dry soil, a compensatory water uptake by the other part of the root system in wet soil has been reported, if soil conductance and water content around the wet root portion were not limiting (McLean et al. 2011).
Root water uptake in soil is modulated and determined at a range of spatial and temporal scales: at the scale of millimeters and centimeters, uptake rates are determined by local soil and root hydraulic properties, whereas at the plant scale processes are interacting within the root system and are integrated through the hydraulic architecture of the root system and plant transpiration (Doussan et al. 2006). For the latter, plant water uptake is proportional to the water potential gradient across the atmosphere, shoot, root and soil path, but is also controlled by the hydraulic conductance in each component of the soil-plant continuum (North and Nobel 1991; North and Nobel 1995). Another important parameter affecting water uptake is the root hydraulic conductivity, radial and axial, both varying along the length of the root system (Zarebanadkouki et al. 2013). Root hydraulic conductivity can change due to root development and also may vary in response to external (e.g. drought or salinity) or internal factors (such as the nutritional state and water status of the plant or the demand for water from the shoot caused by transpiration (Steudle 2000).
For a heterogeneous water distribution, particularly in studies with partial root zone drying, a wide range of plant’s responses in terms of root and stomata conductance, leaf water potential and water uptake rate have been reported. In some plants leaf water potential and transpiration remained constant when only a portion of the root system had access to high potential water showing enhanced root water uptake rate in the wet soil compartments (Lawlor 1973; Liu et al. 2008). Existing studies suggests different patterns of water uptake and growth response exists under localized soil water availability in deep layers, e.g. for cultivated and wild lettuce plants (Gallardo et al. 1996a). High water uptake was observed only for the roots of cultivated lettuce in the top layer, but for wild lettuce water uptake was spread across the root system including deep roots (Gallardo et al. 1996b). In other experiments, a decrease in transpiration was observed with or without decline in leaf water potential (Neales et al. 1989; Collins et al. 2010).
McLean et al. (2011), showed that draining an aquatic compartment holding a third of the roots of Melaleuca argentea, which beforehand took up about 60 % of the plant’s water use, caused an increase in water uptake rate in the remaining root parts in the wet sand. After two days, root water uptake rate was on average more than threefold greater in the sand compartment than for the control plants. They reported that this higher uptake rate was initially due to a threefold increase in hydraulic root conductance (Lp) and a change in the aquaporin expression.
Overall, for a long lasting water stress, root hydraulic conductance may change because of physiological adjustment as a response to partial root-zone drying. These changes can even vary within an individual plant root system and within different response periods. Increased root Lp and also fine-root growth in wet zones may be important means by which species adapt to soil moisture heterogeneity to compensate for partial drying events (Gallardo et al. 1994). But, increasing water uptake from intact roots may be also due to an increase in water potential gradients across the plant-soil system. Anyway, root hydraulic conductance may even greatly exceed requirements for sufficient shoot hydration (Black et al. 2011).
Nevertheless, there is still a lack of studies of living roots in soil quantitatively identifying which parts of the root system are taking up more water than others. This is mainly due to technical difficulties of quantifying local water fluxes into different parts of roots or root zones. In recent years some imaging techniques have been introduced and tested for plant-soil studies, providing the possibility of measuring specific properties of the plant-soil-water system. Among the few available non-destructive methods, Neutron Radiography (NR) has increasingly allowed researchers to simultaneously image living roots and quantify soil water distribution in soil in situ (e.g., Furukawa et al. 1999; Menon et al. 2007; Oswald et al. 2008; Tumlinson et al. 2008; Moradi et al. 2011; Zarebanadkouki et al. 2012; Moradi et al. 2013). Roots can be distinguished in neutron radiographs from soil, and soil water changes can be quantified with high spatial and temporal resolution (Moradi et al. 2009; Esser et al. 2010). Heavy water (D2O) has recently been used as a neutron contrast agent to follow the water uptake of single roots and water fluxes inside the roots, though there are discussions about the interpretability of resulting signal (Carminati and Zarebanadkouki 2013; Warren et al. 2013b). Warren et al. (2013a) used neutron imaging to show that individual root water uptake is autonomous to some degree - with differences in timing and rates of relative water uptake by individual roots. Zarebanadkouki et al. (2013) used neutron imaging to show that root water uptake varied along the length of an individual root, and roots in different soil layers had different uptake rates. More studies are needed to understand the dynamics of these variations in water uptake rate in various parts of the root system. Exposing various parts of the root system to different water availability can reveal some of these seemingly interrelated differences in water uptake rates along the root system.
Our objective is to quantitatively observe water content changes induced by individual parts of the root system for partial root-zone drying situations. For these parts of the root system we also want to derive the water uptake and relate this to the transpiration of a whole plant. The underlying hypothesis is that during partial root-zone drying plant water uptake can be maintained for a substantial amount of time before root growth structurally changes the root system. Relating the water uptake in parts of the root system to its root length should contribute to sharpening the analysis.
We have combined neutron radiography with a root-zone partitioning set-up that allows for separating water availability to different parts of a root system and for setting different levels of soil moisture in those parts. Thus, we can measure and quantify roots, soil water, and water uptake within each root-soil compartment over time, all being part of a single root system. Comparison between different compartments then could reveal compensating mechanisms during localized water stress in the root system. Moreover, we could quantify the amount of roots in each compartment based on the root length. Combining this information, with this novel set-up we could even go one step further and determine for each compartment the water uptake rate per root length, as being affected by water availability in this compartment and other parts of the root system during partial root zone drying.
Materials and methods
Experimental set-up
The approach of neutron imaging requires using either thin-slab containers with lateral extension of up to a few decimeters or cylinders not exceeding a few centimeters in diameter. We chose to use soil-filled thin-slabs of a relatively large size, to allow for a long undisturbed root growth in soil, in respect to root length and duration of the experiment, and topographical differences in the root system, i.e. tap root vs lateral roots, and upper vs lower roots. And the target quantities, soil water content and total root length in separated compartments, could be observed well also in this thin-slabs. A higher temporal resolution could be obtained in the two-dimensional radiographs, instead of tomographies, because it allowed for more frequent imaging of samples during the same experimental period.
Soil sample design and preparation
Natural sandy soil from Chicken Creek artificial catchment, Cottbus, Germany, was air dried and passed through a 2 mm sieve. It is quaternary sand in an initial stage of soil development, with a few percent each of silt and clay and low organic carbon content (Carminati et al. 2010). For more soil physical and chemical properties see Moradi et al. (2012). Soil material was not sterilized for the experiment or treated otherwise. Thin rectangular cubic aluminum containers (250 mm by 300 mm front side with 10 mm inner width) were prepared to be suited for 2-D neutron radiography (Fig. 1a). To avoid local soil micro heterogeneity from layering or compaction, all samples were filled with the sieved soil material horizontally, by opening the 250x300mm front-side wall. To create and keep soil water heterogeneity, in six containers (used for control and horizontal heterogeneity) the soil was partitioned into nine compartments separated by two intersecting vertical and horizontal capillary barriers made from round-shaped sand with 0.5 to 1.0 mm grain size (Fig. 1b). These containers will, henceforth, be named “C9” and “H9” for control and heterogeneous treatments with nine compartments, respectively. For other containers, one horizontal capillary barrier divided the root zone into two equal top and bottom compartments (to apply vertical heterogeneity). Those containers will henceforth be named “H2”, for heterogeneous treatment with two compartments (Fig. 1c and d).
Lupin planted in 30 × 25 × 1 cm3 aluminum container, (a), soil compartments partitioned with 1-cm-thick layers of course sand as capillary barriers after filling was completed (b), neutron radiography of a horizontal heterogeneity “H9” sample, equal to control samples “C9”, (c), and neutron radiography of container with vertical heterogeneity “H2” (d)
The capillary barriers had been designed and tested in a preliminary experiment to make sure that they prevent water redistribution between the compartments and also allow roots to easily penetrate through them with minimal disturbance. Prepared in that way, each compartment, hydraulically isolated by the capillary barriers, physically separates the part of the root system enclosed in its soil volume from other parts of the root system (Fig. 2a).
Unequal water content of different compartments in a horizontal heterogeneous ‘H9’ sample 15 h after water injection to the right side compartments: water content distribution in the individual compartments (a), and the relative change in soil water content of the compartments as obtained by the difference between two consecutive images, as water content ratio (b). Figure 2b also allows for identification of the compartments where most of the water was removed by roots. The two dark rectangles at the bottom of the container are parts of the polyethylene stand needed to fix the container in front of the neutron detector during imaging
Plant growth conditions
A germinated Lupin (Lupinus albus L) seed was sown on top of the soil in each container in a way that the seminal root tip was located right at the center of the middle container and pointing downwards. Then all containers were fully irrigated with nutrient solution and then placed in a walk-in plant growth chamber (Vötsch, Balingen, Germany) until the end of the experiment. The environmental conditions in the growth chamber were set as 10 h of dark, 14 h photoperiod with 8 h of constant, maximum light radiation (about 300 μmol m−2 s−1) and linear transition before and after, a day/night temperature of 25/19 °C and constant relative humidity of 50 % for the whole experimental period. For the first 10 days of growth, all containers were irrigated regularly to keep the soil moisture suited for plant growth and support uniform root distribution in the containers.
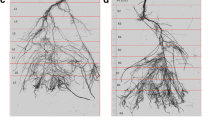
The root system showed a strong taproot, which had grown straight down to the bottom directly through the three central compartments (see for example supplementary material). A large number of laterals branched out of the tap root to both sides without being affected by vertical or horizontal barriers. Some laterals showed visible formation of dense clusters of short lateral rootlets called cluster roots or proteoid roots (Shane and Lambers 2005). Water was injected into each compartment with a syringe through an array of 72 small holes (1 mm in diameter and placed on a 3 × 3 cm grid) that were drilled on one of the container walls. This allowed for precise localized watering of each compartment as well as accurate quantification of local root water uptake. After closing the container, all seams and the top were covered by aluminum tape to prevent any water loss by evaporation. As it is shown in Fig. 2, water actually was kept in the irrigated compartments and did not pass through the capillary barriers.
Water treatment of samples
Three soil water treatments were applied, each with three replicates: I) Equally moist compartments for the whole root-zone; these were the control samples “C9” (Fig. 1c), II) Horizontal heterogeneity by irrigating the left-hand side, that is slightly less than half of the whole root-zone, while withholding water from the middle (taproot) and right hand-side “H9 (Fig. 1c), and III) keeping the lower half of the root zone wet creating a vertical heterogeneity in containers named “H2” (Figs. 1d and 2). To have equally grown root systems as starting point for different water treatments, for all samples the initial condition at the beginning of the imaging period was wet soil in all compartments. Then selected compartments were not irrigated further during the next days. This allowed monitoring spatiotemporal variations in water uptake in different parts of the root system, particularly to quantify shifting of uptake from drying parts to moist zones.
Neutron radiography and image analysis
Thermal neutron radiography was done at Paul Scherrer Institute (PSI), in Switzerland, during a 2-week period. Each sample was radiographed twice a day; once in the early morning before transpiration started (as the initial water status for the day), and once in the evening when active transpiration stopped. This allowed quantification of the daily water uptake in each compartment for each sample, based on observed changes in water content, as well as daily root growth. The field of view for the neutron beam was 185*185 mm with an image resolution of about 200 μm. To cover the whole sample (with size of 250 * 300 mm), four individual radiographs with slight overlaps were taken and then stitched together, similar to Rees et al. (2012) by ImageJ plugin ‘Mosaic’ (http://mosaic.mpi-cbg.de/?q=downloads/imageJ).
All raw neutron radiographs were first corrected for open beam, background intensity, speckling noises as basic image processing. Then also advanced image corrections, i.e. for beam curvature, black body background and secondary neutron scattering, were applied via the software QNI (Hassanein et al. 2005). This multi-step image correction and quantification was necessary to achieve the high accuracy required to detect small changes of water content in different parts of the images and for consecutive images.
Soil water content and root water uptake rate
Based on the high correlation between neutron attenuation coefficient from a soil-water sample and the amount of hydrogen atoms in the sample (usually from water molecules), volumetric water content was directly calculated from neutron radiographs for each pixel (Oswald et al. 2008; Moradi et al. 2009). Having the volume of each compartment and the daily changes in its volumetric water content (difference between morning and next morning radiographs), the water volume removed by root water uptake could be calculated on daily basis. The night time transpiration rates were also calculated in the same way and were shown to be small. The daily transpiration for the whole plant was measured gravimetrically and via radiographies. Due to experimental limitations, especially periods of neutron beam failure at the facility, it was not always possible to evaluate the daily differences directly, but only for longer or shorter periods, e.g. evening before to morning after. This was then modified to represent a day period. If it was not possible to include the daytime period (morning – evening) this value was omitted in the record.
Image analysis for root length calculation
To calculate root length, in each neutron radiograph image the whole root system was segmented from the background soil using the Roottracker-2D software (Menon et al. 2007) (Fig. 3a and b). For low soil moisture, the image segmentation identified almost all of the root system correctly. However, for wet soil conditions, very thin roots (such as cluster roots) could not be segmented completely. To avoid a possible bias of length quantification of fine roots by this slight dependency on soil water content, we included tap root, first order and second order lateral roots in the root length calculation, but no roots smaller than that. Instead, the cluster roots were removed from the segmented images manually before root length calculation.
Then, the binary images of the root system were processed in MATLAB software by skeletonizing the root in the image. The skeletonizing algorithm removes root pixels from the outward surface inwards until a continuous one-pixel line remains (Fig. 3c). Then, the number of pixels in the skeleton root system was counted and multiplied by the pixel size to obtain root length. This is an approximation only since roots growing diagonally through the quadratic pixel will not be attributed their full length. Though this could underestimate real root length by a factor of √2 at most, the error is actually much less because of the different root orientations without strict diagonal preference.
Water uptake rate per root length
Putting together information on both water uptake rate and root length, we were able to retrieve for each compartment a daily water uptake rate normalized to the root length in that compartment for that day. We called this the normalized root water uptake rate. Thus our approach allowed for quantifying the key property that is hard to obtain otherwise. By that we can distinguish compartments that sustain a net water uptake rate by a particular amount of roots, identified by their length, from another compartment achieving the same net water uptake with a smaller amount of roots.
Results
Whole-plant transpiration pattern
figure 4a shows average daily water content of the well-watered control samples “C9”, one “H9” horizontal heterogeneous treatment and the 2-layer vertical heterogeneous samples “H2” from day 10 to day 23 after transplanting (DAT). The “H9” samples are only shown exemplarily because some of them were treated differently, e.g. in the last 2 days of the experiment water supply was administered to formerly dry compartments. The corresponding daily transpiration of C9, H2 and H9, acquired from gravimetric measurements of the thin-slab systems, is shown in Fig. 4b.
Average daily water content of well-watered control (C9), 2-layer vertical heterogeneous (H2) treatment, and 9-compartment horizontal heterogeneous (H9) treatment (a). Their corresponding total daily transpiration is shown also (b). Soil water content values marked with * indicate a foregoing irrigation of the entire container in case of the C9 and H9 samples, but of only the lower compartment in case of the H2 samples; the latter with the exception that before the last day (23 DAT) the entire container was irrigated
The soil water content and daily transpiration values are for the entire container regardless of the spatial distribution of water content or uptake. In fact, water content in different compartments/layers of H9 and H2 containers varied considerably, which will be discussed later. During the measurement period, reproducible results were observed among the replicates of each treatment. Well-watered plants (C9) showed a constant increase of transpiration rate while the soil was continuously kept wet in a relatively narrow range of about 19–25 %. For the H2 samples, despite higher transpiration in the first days of measurement, transpiration pattern increased less than the control in the second week of imaging. On day 22 when the average water content decreased to 0.08 cm3/cm3, transpiration in H2 decreased for the first time. It partly recovered on day 23 after the entire container (including the dry upper layer) had been fully irrigated. Transpiration of H9 sample was quite similar to the control until day 21 when it started to decrease as average water content reduced to values below 0.13 cm3/cm3, then continued to decline for the next 2 days. On day 23, with average soil water content of below 0.10 cm3/cm3, the transpiration rate of the H9 sample reduced to almost only 55 % of the control.
Water uptake distribution in different parts of the root system
In control containers, C9, in which all 9 compartments were well-watered, a part of the root system showed significant differences in water uptake rate. Figure 5 details the water uptake rate in various parts of the root system in a C9 sample on the 17th day after transplanting (DAT). Although soil water content was relatively high in all compartments (soil water content mostly ranged from 0.15 to 0.30 during the measurement period), the lateral roots in the top right and top left compartments showed the highest water uptake rate per unit root length, compared to roots in other compartments. These laterals on average removed about twice as much water as the deeper lateral roots or the roots in the middle compartments (including taproot and associated lateral roots). Pooling the measurements of all C9 containers into a top compartment water uptake (left and right as equal representations of a top compartment) a Welch’s t-test showed that the higher uptake in the top compartment is statistically very significant (p < 0.001, n = 6). This was true for comparing laterals in top compartment water uptake with middle compartments, with bottom compartments and also with the central compartments containing the taproot. However, the same test gave no evidence for significant differences between the other compartments.
The spatial pattern of root water uptake in all compartments of an H9 sample on day 17, 20 and 23 after transplanting is shown in Fig. 6. Initially and similar to C9 samples, water uptake rate in H9 containers was dominated by the top right and top left lateral roots throughout the first week of measurement, when most of the compartments were still wet (Fig. 6a). Withholding irrigation to selected compartments in the second and third weeks in combination with different root density in compartments caused a patchy soil moisture in the compartments exposing various parts of the root system to different degrees of soil dryness. Consequently, the pattern of root water uptake was found to be dynamic and moderated by daily local soil moisture in each compartment.
On day 20, uptake rate in the top right and left compartments decreased as the soil water content in these compartments decreased. Roots in the top center compartment even stopped extracting water on day 20 as the soil water content in this compartment dropped below 0.06. At the same time, water uptake rate (per unit length of roots) from the wet lower compartments increased. Lateral roots in the middle left and right and in the bottom left and right compartments showed an average 50 % increase in water uptake per unit length of root. On day 23, most of the compartments were dry or relatively dry (soil water content ranged from 0.05 to 0.12). Therefore, local water uptake from dry compartments as well as total transpiration was severely reduced. Water uptake from the top left and top right compartments, which showed soil water contents of smaller than 0.06, was almost zero. These compartments initially had contributed the most to total transpiration on day 17 when they were wet. The largest increase in water uptake was observed in the bottom right compartment (by 100 % compared to day 20), which had the highest soil water content (i.e. 0.18).
In Fig. 7 changes in soil water content and root water uptake rate as well as root uptake, as fraction of the total of each compartment, are compared in three graphs. This relates details on soil water changes in various compartments of the sample to the contribution of compartments to the total transpiration; that is from day 20 to 21, when transpiration started to decrease due to decreasing soil moisture (cf. Fig. 4). The latter we define as the contribution of each compartment to the total daily transpiration of the plant. Average daily soil water content in all compartments showed 2–5 units (cm3 water/cm3 soil) decrease from day 20 to day 21, except the top center, right, and left compartments which were already dry (Fig. 7a and b). Despite enhanced root water uptake rate in wet middle and lower compartments, they could not fully compensate for the water uptake reduction in the top left and right compartments. This illustrates the importance of the top compartments in total water uptake, possibly due to the combination of higher root length and proximity to the collar (Fig. 7c and d).
Compensatory water uptake from wet compartments
Daily total transpiration of vertically heterogeneous containers, H2, as well as daily water content in both top and bottom compartments are shown in Fig. 8a. While the lower compartment was kept wet throughout the neutron radiography measurement, the upper compartment was left drying to the residual water content a week into the experiment. This exposed the roots in the upper compartment, which accounted for more than 60 % of the total roots, to dry soil conditions. Despite this decrease in available water for the plant, the transpiration rate increased continuously (although with lower rate than the control) over the next days as long as the soil water content in the lower compartment stayed above 0.15. Then soil water content in the lower, wetter compartment decreased to 0.1 on day 22. This then resulted in a decrease in transpiration. However, this was only temporary, and apparently without permanent damage to the root performance. After watering both compartments on day 23, the transpiration increased again in both and continued with the same trend as in the first week.
Furthermore we can include the information on the root length development. The top compartment roots continuously grew in the first week (Fig. 8b). Roots in the bottom compartment started with smaller root length, roots grew with similar rate, and the difference to the top compartment stayed constant for about a week. But from about 18 days after transplanting (DAT) root growth in the top compartment slowed down and almost stopped, coinciding with a complete stop in water uptake there when soil became very dry (Fig. 8a). In this phase roots in the bottom compartment increased further in length while maintaining the water uptake rate per length.
Figure 8b shows the root growth and the water uptake rate per unit length of the root in each compartment. When the top compartment was still relatively wet in the first days, similar root water uptake rates were calculated for both compartments. On day 22, water uptake from the upper compartment decreased as the soil water content decreased to below 0.1. The water uptake stopped when the soil water content dropped to 0.05 on day 8. As the top compartment dried, water uptake from the wet lower compartment increased as a compensatory response to the partial soil dryness in the upper compartment. Although less than 40 % of the roots were present in the wet lower compartment, it supplied almost all of the transpiration demand from day 17 onwards. This allowed even for an increase in total transpiration. After re-wetting the whole root system on day 23, water uptake rate in the top compartment increased substantially, whereas the water uptake rate in the bottom compartment reduced and then was almost equal in both compartments.
Cluster root formation
In the root length quantification cluster roots were excluded from the analysis, because a reliable automated length determination for them was not possible. Nevertheless, the presence and location of cluster roots was well visible and interesting information on cluster root formation could be derived from image quantification and analysis. Figure 9a exemplifies cluster root formation along the root system for an H9 sample at the end of the experiment. Figure 9b shows the temporal pattern of root growth and cluster root formation in three compartments of one H9 sample. Cluster roots appeared first in the middle compartment 12–14 days after transplanting when water content decreased from 0.15 to 0.05. Five days later, cluster roots formed in the right compartment as the soil there dried below 0.15. At this time, the roots with the same age and length properties in the wet left compartment had not formed any cluster roots. Only when the soil water content had decreased to around 0.10 five days later formation of cluster roots in the left compartment started. Our observation showed that consistently a soil water content reduction to 0.10–0.15 triggered local cluster root formation.
Discussion
Due to low root density in some compartments in the horizontally heterogeneous treatment, water extraction by roots was lower and drying in those compartments was slower. But also their water content reached dry levels towards the end of the experiment. Therefore, root systems were exposed to a (monotonic) transition through different soil water contents rather than cyclic changes. This situation provided a good framework for investigating how actual root water uptake was distributed over different parts of the root system, which was dominated by local soil water status over time.
Spatial pattern of root water uptake
We observed a significantly higher water uptake rate by lateral roots of lupin plants in the upper layer compared to other parts of the root system including lateral roots in lower layers. This confirms the findings of Zarebanadkouki et al. (2013) that root water uptake was different along the root length. We speculate that this may be due to the shorter distance of axial flow in the tap root to the shoot, and therefore smaller net root resistance. Further studies should focus on better understanding of these differences in water uptake rate along the root system. As for the roots in the top center compartment, including taproot and the older parts of the lateral roots, water uptake rate is lower. Notably water uptake is reduced not only in absolute terms but also if normalized to root length in the compartment, though axial root resistance is expected to be less than that of the lateral roots in the top left and right compartments (Zarehbanadkouki et al. 2013). Increasing radial root resistance due to root suberization in old parts as well as differences in root dimension and surface area could be responsible for lower root permeability. However, this pattern of uptake rate along the root system may differ across other plants and could be a compensation strategy depending on the spatiotemporal variation of water content in the soil profile in natural ecosystems.
Our results show dynamic interrelations between topological root properties, surrounding soil water status, total transpiration demand, and partial root water uptake. It has been suggested that plants grown in soil with heterogeneously-distributed water can maintain optimal transpiration, or be close to it, as long as other parts of the root system have access to easily-available water with enough roots (Gallardo et al. 1994). When there is no or low resistance for water movement from soil to the root system (in relatively wet soil), compensatory water uptake is controlled via enhanced uptake in wetter parts of the root system by root topology and properties such as age, type or aquaporin expression. In our experiment, for 4-week old lupin growing in sandy soil, the capacity of the root system for compensatory water uptake was calculated to be around 80 %. This means that 80 % of the roots needed to be in wet soil to meet the total transpirational demand. If smaller percentage of roots were in the wet soil, the compensatory water uptake might not be sufficient to maintain the transpiration demand. As for the soil water condition, there seemed to be a critical point that affects compensation capability of plant roots. Defining this critical soil water content might prove useful for water management purposes, although this might be difficult to define as it depends on soil properties, particularly hydraulic conductivity and spatial water distribution, as well as plant properties such as root density and transpiration rate. For the sandy soil we used in this experiment, this critical point was shown to be between 0.10 and 0.13 volumetric water content.
Compensatory water uptake
Water uptake calculated for various parts of the root system was highly variable and correlated with surrounding soil water content as well as with root properties such as type, age and position along the root system. Due to spatially-variable soil water availability in the root zone, created by soil partitioning, relative water uptake in each root segments varied from time to time depending on the soil water tension in corresponding compartment and water availability to other parts of the root system. Therefore each root segment showed a range of water uptake rates, varying from no uptake (in very dry compartments) to high compensatory uptake (in wet compartments when other compartments were dry).
Our results showed an almost fully compensatory water uptake by the upper half of the lupin root system. As long as enough easily-available water existed in soil for that part of the root system, it could just meet the transpiration demand. Without having investigated the mechanism behind, our results indicate the plasticity and the dynamics of roots in acquiring water from various root segments growing in soil with heterogeneously-distributed water.
Cluster root formation
Formation of cluster roots is known as a strategy in response to deficiency of plant nutrients, in particular phosphorous and sometimes other elements such as iron. It is well documented that cluster root formation by white lupin is controlled by internal P status (Shu et. al 2007). Some studies have shown that it also can be regulated by local P availability in the soil (reviewed by Shane and Lambers 2005). In our experiment, however, we found also a clear correlation between soil drying and cluster root formation. But there is no indication that after the observed appearance of cluster roots the water uptake rate could have been enhanced, and it also has not been reported in the literature that this should be expected. Thus, cluster root formation during drying conditions seems not to be a measure to sustain water uptake. A possible explanation for the cluster roots appearing is that soil dryness physically causes a lower mobility of P, and other nutrients, towards the roots and therefore could cause P deficiency in the shoot or roots which should stimulate cluster root formation. In any case, the induced unequal availability of water in the experiment has demonstrated that the root system was able to adjust to local conditions in response to water insufficiency or its consequences, and that seems to include this local stimulation of cluster root formation.
Conclusions
Using a combination of neutron radiography and well-defined hydraulically-separated soil compartments, we successfully quantified local water uptake for different root segments of white lupin in situ. We observed a spatially-distributed uptake pattern across the root system which was dominated by root distribution, but also root location. For a heterogeneous water distribution, the root uptake pattern was moderated by local soil water status in each part of the root system (particularly in relatively dry zones) and also was influenced by the overall water distribution in the root zone. In such a situation with unequal water distribution, root water uptake in dryer parts became limited below a critical soil water content level (in our experiment between 0.13 and 0.10 cm3/cm3). When this happened, water uptake consequently was increased in the rest of the root system in wetter zones, as we could determine quantitatively. This compensatory water uptake showed a dynamic soil-plant dependent pattern. The portion of the root system that has access to high potential soil water and also the type and topology of the roots in wet zones determine the compensation rate and therefore the possible transpiration deficit for water-restricted conditions.
From a practical point of view, the detected spatial and temporal differences in root water uptake in addition to the observed critical soil water status that initiates relocation of water uptake between root compartments could be used for operation of partial root-zone drying systems in croplands. In those only a part of the root system is irrigated, by which water use efficiency increases and irrigation water can be saved. Also extracting more water with the same amount of root length, instead of producing new roots, could partially save the plant some carbon. However, long term physiological and morphological adjustments and also nutritional effects of unequal water availability need to be investigated and then be considered in practices of partial irrigation.
References
Bazihizina N, Barrett-Lennard EG, Colmer TD (2012) Plant responses to heterogeneous salinity: growth of the halophyte Atriplex nummularia is determined by the root-weighted mean salinity of the root zone. J Exp Bot 63:6347–6358
Black MZ, Patterson KJ, Minchin PEH, Gould KS, Clearwater MJ, Meinzer F (2011) Hydraulic responses of whole vines and individual roots of kiwifruit (Actinidia chinensis) following root severance. Tree Physiol 31:508–518
Carminati A, Zarebanadkouki M (2013) Comment on: “neutron imaging reveals internal plant water dynamics”. Plant Soil 369:25–27
Carminati A, Moradi AB, Vetterlein D, Vontobel P, Lehmann E, Weller U, Vogel H, Oswald SE (2010) Dynamics of soil water content in the rhizosphere. Plant Soil 332:163–176
Collins MJ, Fuentes S, Barlow EWR (2010) Partial rootzone drying and deficit irrigation increase stomatal sensitivity to vapour pressure deficit in anisohydric grapevines. Funct Plant Biol 37:128–138
Doussan C, Pierret A, Garrigues E, Pages L (2006) Water uptake by plant roots: II - Modelling of water transfer in the soil root-system with explicit account of flow within the root system - Comparison with experiments. Plant Soil 283:99–117
Esser HG, Carminati A, Vontobel P, Lehmann EH, Oswald SE (2010) Neutron radiography and tomography of water distribution in the root zone. J Plant Nutr Soil Sci 173(5):757–764
Furukawa J, Nakanishi TM, Matsubayashi H (1999) Neutron radiography of a root growing in soil with vanadium. Nucl Inst Methods Phys Res Section a - Accelerators Spectrometers Detectors Assoc Equip 424:116–121
Gallardo M, Turner NC, Ludwig C (1994) Water relations, gas exchange and abscisic acid content of Lupinus cosentini leaves in response to drying different proportions of the root system. J Exp Bot 45:909–918
Gallardo M, Jackson LE, Thompson RB (1996a) Shoot and root physiological responses to localized zones of soil moisture in cultivated and wild lettuce (Lactuca spp). Plant Cell Environ 19:1169–1178
Gallardo M, Jackson LE, Schulbach K, Snyder RL, Thompson RB, Wyland LJ (1996b) Production and water use in lettuces under variable water supply. Irrig Sci 16:125–137
Garcia-Palacios P, Maestre FT, Bardgett RD, de Kroon H (2012) Plant responses to soil heterogeneity and global environmental change. J Ecol 100:1303–1314
Hassanein R, Lehmann E, Vontobel P (2005) Methods of scattering corrections for quantitative neutron radiography. Nucl Inst Methods Phys Res Sect A 542:353–360
Hodge A (2010) Roots: the acquisition of water and nutrients from the heterogeneous soil environment. Progress in Botany 71, 307–337. Eds. U Luttge, W Beyschlag, B Budel and D Francis
Lawlor DW (1973) Growth and water absorption of wheat with parts of the roots at different water potentials. New Phytol 72:297–305
Liu F, Song R, Zhang X, Shahnazari A, Andersen MN, Plauborg F, Jacobsen S-E, Jensen CR (2008) Measurement and modelling of ABA signalling in potato (Solanum tuberosum L.) during partial root-zone drying. Environ Exp Bot 63:385–391
Maurel C, Simonneau T, Sutka M (2010) The significance of roots as hydraulic rheostats. J Exp Bot 61:3191–3198
McLean EH, Ludwig M, Grierson PF (2011) Root hydraulic conductance and aquaporin abundance respond rapidly to partial root-zone drying events in a riparian Melaleuca species. New Phytol 192:664–675
Menon M, Robinson B, Oswald SE, Kaestner A, Abbaspour KC, Lehmann E, Schulin R (2007) Visualization of root growth in heterogeneously contaminated soil using neutron radiography. Eur J Soil Sci 58:802–810
Mommer L, van Ruijven J, Jansen C, van de Steeg HM, de Kroon H (2012) Interactive effects of nutrient heterogeneity and competition: implications for root foraging theory? Funct Ecol 26:66–73
Moradi AB, Conesa HM, Robinson BH, Lehmann E, Kühne G, Kaestner A, Schulin R (2009) Neutron radiography as a tool for revealing root development in soil: capabilities and limitations. Plant Soil 318:243–255
Moradi A, Carminati A, Vetterlein D, Vontobel P, Lehmann E, Weller U, Hopmans JW, Vogel HJ, Oswald SE (2011) Three-dimensional visualization and quantification of water content in the rhizosphere. New Phytol 192(3):653–663
Moradi AB, Carminati A, Lamparter A, Woche S, Bachmann J, Vetterlein D, Vogel HJ, Oswald SE (2012) Is the rhizosphere temporarily water repellent? Vadose Zone J 11:3
Moradi AB, Oswald SE, Menon M, Carminati A, Lehmann E, Hopmans JW (2013) Applications of neutron imaging in soil-water-root systems, in “Tomography of Soil-Water-Root Processes, 2nd edition”, edited by S H Anderson and J W. Hopmans, SSSA Special Publication 61:113–136. doi:10.2136/sssaspecpub61.c6
Neales TF, Masia A, Zhang J, Davies WJ (1989) The effects of partially drying part of the root system of helianthus annuus on the abscisic acid content of the roots. Xylem Sap Leaves J Exp Bot 40:1113–1120
North GB, Nobel PS (1991) Changes in hydraulic conductivity and anatomy caused by drying and rewetting roots of agave deserti (Agavaceae). Am J Bot 78:906–915
North GB, Nobel PS (1995) Hydraulic conductivity of concentric root tissues of Agave deserti Engelm. under wet and drying conditions. New Phytol 130:47–57
Oswald SE, Menon M, Robinson B, Carminati A, Vontobel P, Lehmann E, Schulin R (2008) Quantitative imaging of infiltration, root growth, and root water uptake via neutron radiography. Vadose Zone J 7:1035–1047
Rees R, Robinson BH, Evangelou MWH, Lehmann E, Schulin R (2012) Response of Populus tremula to heterogeneous B distributions in soil. Plant Soil 358:403–415
Shane MW, Lambers H (2005) Cluster roots: a curiosity in context. Plant Soil 274:101–125
Shu L, Shen J, Rengel Z, Tang C, Zhang F, Cawthray GR (2007) Formation of cluster roots and citrate exudation by Lupinus albus in response to localized application of different phosphorus sources. Plant Sci 172:1017–1024
Steudle E (2000) Water uptake by plant roots: an integration of views. Plant Soil 226:45–56
Tumlinson LG, Liu H, Silk WK, HopmansJ W (2008) Thermal neutron computed tomography of soil water and plant roots. Soil Sci Soc Am J 72:1234–1242
Warren JM, Bilheux H, Kang M, Voisin S, Cheng C, Horita J, Perfect E (2013a) Neutron imaging reveals internal plant water dynamics. Plant Soil 366:683–693
Warren JM, Bilheux H, Cheng C, Perfect E (2013b) Reply to: comment on “Neutron imaging reveals internal plant water dynamics”. Plant Soil 371:15–17
Zarebanadkouki M, Kim YX, Moradi AB, Vogel H -J, Kaestner A and Carminati A (2012) Quantification and modeling of local root water uptake using neutron radiography and deuterated water. Vadose Zone J 11(3)
Zarebanadkouki M, Kim YX, Carminati A (2013) Where do roots take up water? Neutron radiography of water flow into the roots of transpiring plants growing in soil. New Phytol 199:1034–1044
Acknowledgments
This work was supported by a PhD scholarship grant, stipend no. IPS 09/27P2, from the IPSWaT program of the German Federal Ministry of Education and Research (BMBF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Rafael S. Oliveira.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 697 kb)
Rights and permissions
About this article
Cite this article
Dara, A., Moradi, B.A., Vontobel, P. et al. Mapping compensating root water uptake in heterogeneous soil conditions via neutron radiography. Plant Soil 397, 273–287 (2015). https://doi.org/10.1007/s11104-015-2613-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2613-3