Abstract
Nuclear ribosomal sequences and Cd, Zn, Pb and Fe accumulation of different populations of the recently discovered Cd/Zn-hyperaccumulating species Thlaspi praecox Wulfen (Noccaea) were studied to reveal their relationships to other representatives of the genus and especially to the well known hyperaccumulator T. caerulescens; comparisons of their accumulating properties were also made. Internal transcribed spacer (ITS) rDNA sequences from eight T. praecox populations from Slovenia showed 99% similarity and formed a sister group to T. caerulescens. Divergence estimates from the ITS rDNA support the origins of T. praecox in the Early Pleistocene, with further fragmentation of T. praecox populations in Slovenia since the Middle Pleistocene. Cd-hyperaccumulating features (>100 mg Cd kg−1 in the above-ground biomass) of T. praecox were seen for two populations collected at polluted sites (Žerjav and Mežica) and one population collected at a non-polluted site (Lokovec). The variability of the Cd concentrations in shoots was almost completely explained by the soil Cd concentrations, and were positively correlated with shoot Zn and Pb concentrations. The results from this molecular and metal accumulation characterisation of T. praecox populations provide new insights into the taxonomic affinities and accumulation potential of this hyperaccumulating species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increased interest has been shown in plants with the unusual potential for accumulation of more than 10,000 mg kg−1 Zn and Mn, 1,000 mg kg−1 Al, As, Se, Ni, Co, Cr, Cu and Pb, and 100 mg kg−1 Cd in their above-ground biomass (Reeves and Brooks 1983; Reeves 1988). This has been referred to as hyperaccumulation (Brooks et al. 1977), with the interest arising from their potential use in the cleaning of soils contaminated with metal(loid)s (Pollard et al. 2002). Several hyperaccumulating plants have been described in the Brassicaceae family, and particularly in the genus Thlaspi (Peer et al. 2003), with the Cd-, Zn- and Ni-hyperaccumulating Thlaspi caerulescens as the most studied plant species of this genus (Assunção et al. 2003a, b). Another species, namely T. praecox, has been reported to accumulate up to 2.1% Zn (Brooks et al. 1998), and more recently, up to 1.5% Zn, 0.6% Cd and 0.4% Pb when collected at a heavy-metal polluted site in northern Slovenia (Vogel-Mikuš et al. 2005).
In Europe, T. caerulescens is naturally distributed on soils with different heavy-metal compositions and it shows a variable capacity for hyperaccumulation (Baker et al. 1994; Schat et al. 2000; Roosens et al. 2003; Keller et al. 2006; Peer et al. 2006). Hyperaccumulation of Zn appears to be a constitutive feature of this species (Escarré et al. 2000; Reeves et al. 2001), whereas hyperaccumulation of Ni and Cd is more variable (Reeves et al. 2001). Within the species, T. caerulescens from Southern France (Ganges accession) was shown to have a superior ability to hyperaccumulate Cd (Robinson et al. 1998; Lombi et al. 2000) and this ability was matched by T. praecox in a pot experiment (Pongrac et al. 2009). High variability in metal accumulation potential was also demonstrated in the two populations of T. praecox from Slovenia that have been studied in detail to date (Vogel-Mikuš et al. 2005).
Based on seed morphology (Meyer 1973, 1979) and ribulose-1,5-bisphosphate carboxylase/oxygenase, ITS nuclear ribosomal DNA, and chloroplast DNA restriction-site variation (Mummenhoff and Zunk 1991; Mummenhoff and Koch 1994; Zunk et al. 1996; Mummenhoff et al. 1997), the genus Thlaspi has been divided into several genera/clades. In the process of the reorganisation of this genus, many of the metal hyperaccumulating species (including T. caerulescens and T. goesingense) have been moved into the Noccaea genus (see Koch and Mummenhoff 2001, for a complete list). Still, the phylogenetic position of T. praecox as a species separate from T. caerulescens remains to be examined. Thus, the present study was designed to: i) evaluate the taxonomic and phylogenetic positions of the T. praecox species through molecular characterization of nuclear ribosomal internal transcribed spacers (ITS); and ii) determine the (hyper)accumulation ability of different populations of T. praecox across Slovenia.
Material and methods
Sample collection
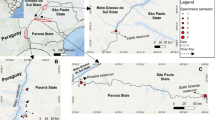
Five specimens of T. praecox Wulfen were collected from eight populations across Slovenia (Table 1): three populations were sampled in northern Slovenia, and five populations in south-western Slovenia (Fig. 1). Two populations (Žerjav and Mežica) from northern Slovenia were collected at a heavy-metal-polluted site. The choice of materials was dictated by the intention to cover the molecular variability of the species and to include polluted and non-polluted sites. As plant development has a significant impact on element uptake (Pongrac et al. 2007), all of the specimens were collected in their flowering phase.
Geographical locations of the eight populations of Thlaspi praecox included in this study for the analysis of ITS rDNA regions and metal-accumulation properties. The numbers correspond to the location numbers in Table 1. SLO, Slovenia; AUT, Austria; CRO, Croatia; I, Italy
Molecular analyses
Freeze-dried shoots of the collected plant materials were ground to a fine powder in liquid nitrogen, and the DNA was isolated using the GenElute® Plant Genomic DNA miniprep kit (Sigma), following the manufacturer instructions. All of the PCR reactions were carried out with an MJ Research thermal cycler, using Taq DNA polymerase (Promega). The 25 μl reaction mixtures contained: 2.5 μl 10× PCR buffer, 2.5 mM MgCl2, 200 μM of each nucleotide, 500 nM of each primer, 0.75 U DNA polymerase, and 12.5 μl of a 100-fold diluted DNA extract. The PCR conditions for amplification with the ITS1 and ITS4 primer pair were (White et al. 1990): 1 min at 94°C, followed by 35 cycles of 35 s denaturation at 94°C, followed by 53 s annealing at 55°C, and 30 s of elongation at 72°C. The time of the elongation step was increased for 5 s each cycle. A final elongation was performed at 72°C for 10 min.
The PCR products were cleaned and ligated into the pGEMT-Easy vector (Promega, Madison, WI, USA). Competent Escherichia coli JM109 cells were used for the transformation with recombinant vectors, as recommended by the manufacturer. The transformants were screened using blue/white selection on Luria-Bertani (LB) agar containing X-Gal/isopropyl beta-D-1-thiogalactopyranoside (IPTG) and 50 μl ml-1 ampicilin (Sigma). For confirmation of fragment insertion colonies, PCR was performed with the T7 and SP6 primer pair. Cycle-sequencing reactions were performed on three colonies per population (double stranded sequencing) with the T7 and SP6 primer pair using a BigDye™ terminator Ready Reaction Cycle Sequencing kit on an ABI 3730xl DNA Analyser (Applied Biosystems), as provided by the Macrogen Company (Korea). To double check that cloning did not incorporate any Taq polymerase mistakes in the sequences, the PCR products were also sequenced directly (three sequences per population, both DNA strands). Obtained sequences were confirmed to be identical to the sequences from the cloned products.
Sequence analyses
The sequence data have been submitted to the GenBank database under accession numbers FJ808507 to FJ808514. The sequences were subjected to a GenBank search to evaluate the taxonomic affinities of each of the ITS sequences, using the default option of gapped-BLAST (Altschul et al. 1997). The sequence alignments were carried out by ClustalX (Larkin et al. 2007), and refined by eye. The dataset that was subjected to phylogenetic analyses was composed of the T. praecox sequences obtained and 24 additional ITS1-5.8S-ITS2 sequences from Noccaea and Raparia from GenBank. The sequences of Thlaspi perfoliatum (Microthlaspi) and Thlaspi arvense (Thlaspi s. str.) were used as an out-group.
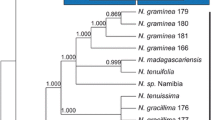
Neighbour-joining (NJ), maximum parsimony (MP), maximum likelihood (ML), and Bayesian analysis (posterior probabilities; PP) were used to analyse the aligned sequences. NJ, MP and ML were performed in PAUP* (version 4.0b8a; Swofford 2003). The MP and ML trees were constructed using heuristic searches with tree bisection–reconnection (TBR). In both the MP and NJ analyses, the evolutionary model K80+Γ (Kimura 1980) of Modeltest 3.7 (Posada and Crandall 1998) was used, selected by hierarchical likelihood ratio tests (hLRTs) and Bayesian information criterion (BIC). Bayesian analysis was carried out in MrBayes 3 (Ronquist and Huelsenbeck 2003; 5,000,000 generations, sample frequency: every 100th generation, four chains; burn-in determined according to the “sump” plot). Bootstrap values were obtained by 200 subsamples for maximum likelihood (ML), 500 subsamples for maximum parsimony (MP) and 1,000 subsamples for neighbour joining (NJ) and are given in Fig. 2.
Maximum clade credibility tree of Thlaspi praecox populations from Slovenia displayed as a chronogram from the BEAST analysis of the ITS rDNA alignments. All of the lineages were evolved according to a strict clock and the K80 + Γ model of evolution. For the analysis, the root node was given a normal age prior distribution with mean = 15.1. MCMC searches were run for 10,000,000 generations, with the first 2,000,000 discarded as burn-in. T. arvense (Thlaspi s. str.) and T. perfoliatum (Microthlaspi) were used for the calibration of the tree. The scale is represented as millions of years ago (Mya). Node bars illustrate the width of the 95% highest posterior density (HPD). For the specified nodes (n1–n10), support values in the order from left to right: neighbour-joining (NJ)/ maximum parsimony (MP)/ maximum likelihood (ML)/ posterior probabilities (PP), mean (Mya) and 95% CI for the estimated age (Mya) are given
Divergence dating
Prior to divergence dating of T. praecox and T. caerulescens, the null hypothesis of a molecular clock was evaluated following the test statistics: \( - 2\left( {\log \,{L_{\text{clock}}} - \log \,{L_{{\text{no}}\,{\text{clock}}}}} \right) \). This should be distributed as χ2 with (N–2) degrees of freedom, where N is the number of sequences in the tree (Felsenstein 1988; Sanderson 1998). The dates of divergence were inferred using the Bayesian strict-clock approach, implemented in BEAST v1.4.8 (Drummond and Rambaut 2007), with the Yule process for the tree prior. For the analysis, the root node was given a normal age prior distribution, with mean = 15.1. Markov chain Monte Carlo (MCMC) searches were run for 10,000,000 generations, with the first 2,000,000 discarded as burn-in. The searches achieved adequate mixing, as assessed by the high effective sample size (ESS) values for all of the parameters, the plateaus for divergence-time estimates over generations after the burn-in, and the repeatability of the results over multiple independent runs.
Soil and plant metal analyses
Soil samples were taken from the rhizosphere of the individual plants. The plants were carefully dug from the substrate and the majority of the bulk soil was manually removed from the roots (Vogel-Mikuš et al. 2005). Only the substrate closely attached to the root system was analysed. After drying at 30°C for 1 week, the soil samples were sieved (<2 mm) and homogenized (n = 5 from each site). To determine the metal availability, an extraction method with 1 M ammonium acetate (Baker et al. 1994) was used. The extract was filtered through 0.4 μm membrane filters (Milipore) and analysed by atomic absorption spectrometry (AAS; Perkin Elmer AAnalyst 100).
The shoots and roots of the T. praecox specimens were separated and carefully washed with tap and then distilled water, to remove any surface soil or dust deposits. The plant materials were frozen in liquid N2 and then freeze-dried for 1 week. Cd, Zn, Pb and Fe concentrations in the plant materials were analysed by AAS after wet digestion, as previously described by Vogel-Mikuš et al. (2005).
Statistical analyses
Translocation factors \( \left( {{\text{TF}} = {{{{\text{C}}_{\text{shoot}}}} \mathord{\left/{\vphantom {{{{\text{C}}_{\text{shoot}}}} {{{\text{C}}_{\text{root}}}}}} \right.} {{{\text{C}}_{\text{root}}}}}} \right) \) were calculated to quantify the root to shoot translocation (Pongrac et al. 2007) in particular populations, and bioaccumulation factors \( \left( {{\text{BAF}} = {{{{\text{C}}_{\text{shoot}}}} \mathord{\left/{\vphantom {{{{\text{C}}_{\text{shoot}}}} {{{\text{C}}_{\text{soil}}}}}} \right.} {{{\text{C}}_{\text{soil}}}}}} \right) \) were calculated to quantify accumulation (Baker et al. 1994; Vogel-Mikuš et al. 2005; Pongrac et al. 2007) of the individual metals, relative to the ammonium acetate extractable metal soil fraction.
Stepwise multiple regression analysis was carried out using extractable soil Cd, Zn and Pb concentrations as independent variables and shoot Cd, Zn, Pb and Fe concentration as the dependent variable. One-way ANOVA was applied to test the overall effects of population on the parameters studied, and when significant, Holm-Sidak post-hoc analyses were used to determine the significance of differences between populations at p < 0.05. Pearson′s correlation coefficients (R) were used when calculating correlations between metal concentrations in plant tissues and translocation factors. A test of normal distribution and homogeneity of variance was performed prior to the use of parametric tests. The statistical tests were performed using SigmaStat (SPSS, Inc.) software.
Results
Phylogenetic analysis of ITS sequences
The ITS rDNA sequences obtained from the T. praecox specimens collected were submitted to the GenBank database and can be retrieved using the accession numbers indicated in Table 1. Sequence alignments of the ITS rDNA resulted in a total of 501 characters, of which 389 were constant, 86 parsimony uninformative, and 26 parsimony informative. This data matrix required gaps at 16 nucleotide sites (3%), of which most were located in the ITS1 rDNA region. The sequences of the T. praecox populations studied showed 99% similarity, with only five variable sites (1% of total). Three nucleotide positions specific for sequences of T. praecox species were found when they were aligned with sequences of other Thlaspi s. l. species available in GenBank: C instead of T at bp 59, A instead of C or T at bp 162, and occasional replacements of A with T at bp 213 and of G with A at bp 252. Additionally, the same nucleotide replacements were seen for the two sequences from GenBank (DQ337369 and DQ337370) stored under T. caerulescens name, which were actually collected at locations in Mežica (Peer et al. 2006).
NJ, MP, ML and Bayesian analyses of the ITS sequences obtained provided similar topologies, with T. praecox positioned close to the T. caerulescens group (Fig. 2). The entity of T. praecox was separated from T. caerulescens by moderate bootstrap values: 65% for NJ (1,000 replicates), 43% for MP (500 replicates), 58% for ML (100 replicates), and a PP of 1.0 for Bayesian analysis (5,000,000 generations). The maximum parsimony TBR search recovered 112 equally most parsimonious trees, with lengths of 124 steps, a CI of 0.94, RI of 0.91, and a rescaled CI (RCI) of 0.86.
For the test of the molecular clock hypothesis, non-clock (unconstrained) and clock (constrained) searches for ML trees were performed. While the non-clock search resulted in a single tree (logL = −1280.58), the clock search recovered two equally likely trees of essentially identical topology (logL = −1292.04). For the constrained/ unconstrained ML trees, the test statistics of the rate inconstancy were not significant (χ2 = 22.92, with df = 29, p = 0.78), and hence compatible with a molecular clock hypothesis. Using the equation H = μT, where H is the node height derived from the constrained ML tree, and μ is the substitution rate, μ was estimated to be 1.1 × 10−8 substitutions per site per year, when the divergence time (T) of the species pair T. arvense and T. perfoliatum was set to 15.1 million years ago (Mya) (Koch and Al-Shehbaz 2004). The posterior mean of the divergence time was calculated by BEAST, and between T. caerulescens and T. praecox it was estimated at 1.2 Mya, with a 95% confidence interval of 0.7–1.7 Mya (Fig. 3).
Metal concentrations in the soil and plants
The concentrations of the ammonium-acetate-extractable metals in the soil ranged from 0.3–38 mg Cd kg−1, 3.0–293 mg Zn kg−1, and 33–9,078 mg Pb kg−1 (Table 1). Ammonium-acetate-extractable Fe concentrations in the soil were not measured, since they do not provide information on either Fe availability or plant accumulated Fe concentrations (Marschner 1995; Molitor et al. 2005). The highest Cd, Zn and Pb concentrations in T. praecox roots and shoots were seen in both of the populations from the polluted sites, while the highest Fe concentrations were measured in the Mežica population (Fig. 3). Beside the populations from polluted site (Žerjav and Mežica), Cd shoot concentrations exceeded the hyperaccumulating criteria also in Lokovec population from non-polluted site.
Shoot Cd concentrations of studied populations were almost completely explained by the ammonium-acetate-extractable soil Cd concentrations (R2 = 0.87, p < 0.01). Forward stepwise regression analysis additionally added soil Zn (R2 = 0.05, p < 0.001) and soil Pb (R2 = 0.01, p < 0.05) to the model. The final model explained a total of 94% of the variance. Shoot Zn concentrations were dependent mainly on ammonium-acetate-extractable soil Zn (R2 = 0.75, p < 0.001) and soil Pb (R2 = 0.04, p < 0.001) and shoot Fe concentrations (R2 = 0.04, p < 0.01), while shoot Pb concentrations were explained by a combination of ammonium-acetate-extractable soil concentrations: Pb (R2 = 0.90, p < 0.001), Cd (R2 = 0.06, p < 0.001) and Zn (R2 = 0.01, p < 0.001). The final models explained a total of 83% and 97% of the variance for Zn and Pb, respectively.
Cd translocation factors (TFCd) were significantly higher in plants sampled at the polluted sites, when compared to those from non-polluted sites (Table 2). No differences between populations were observed for TFZn and TFPb, whereas the highest TFFe was observed in Lokovec population. In addition, positive correlations between Cd, Zn and Fe translocation factors were obtained (Table 3). The highest Cd bioaccumulation factors (BAFCd) were measured in Mežica and Lokovec populations (Table 2). BAFZn were higher in the populations from non-polluted than from polluted sites, with the highest BAFZn seen in the Črnivec and Lozice population. BAFPb were in all cases below 1, with the exception of the Mežica population.
Discussion
Investigations into natural population systems are of great importance for our understanding of the genetic basis of the hyperaccumulation trait and the selective pressures that underlie it (Pollard et al. 2002). This paper reports on phylogenetic relationship of T. praecox to the well known hyperaccumulator T. caerulescens and on the Cd, Zn, Pb and Fe accumulating properties of different populations collected across Slovenia. The T. praecox ITS rDNA sequences grouped together in the phylogenetic trees, forming a sister group to the T. caerulescens sequences. In all of the analyses, two T. caerulescens sequences from GenBank (DQ337369 and DQ337370), obtained from material collected in Mežica in northern Slovenia (Peer et al. 2006), were positioned inside the T. praecox clade. As T. caerulescens is not native to Slovenia (Martinčič et al. 2007; Wraber 2005) the grouping of these sequences inside the T. praecox clade suggests that these sequences belong to misidentified T. praecox representatives.
The estimated substitution rate for our sequences was calculated to be 1.1 × 10−8 substitutions/site/year. Although molecular clock hypotheses are still under debate, for ITS a substitution rate of approximately 0.5% to 2.5% nucleotide divergence per 1 million years can be assumed (Koch et al. 2003). Similar ITS substitution rates have also been estimated for other members of Brassicaceae (Kropf et al. 2003; Koch and Al-Shehbaz 2004). Divergence dating showed that the species T. praecox and T. caerulescens diverged from the common ancestor around 1.2 Mya with further separation within the T. praecox, as northern populations (except sequence DQ337369) formed a sister group to the south-western Slovenian populations. The particularly strong climate oscillations in the northern hemisphere during this epoch (Bennett 1997; Rutherford and D’Hondt 2000) probably altered the distribution ranges of the species. Such conditions would increase the likelihood of diminished gene flow and population isolation, leading to the establishing of new plant species (Taberlet et al. 1998; Hewitt 2000). However, further examinations of intraspecific differences using molecular techniques like amplified fragment length polymorphism (AFLP), and other types of markers like SSR and/or cpDNA loci (Jimenez-Ambriz et al. 2007; Besnard et al. 2009) are needed to be able to draw any firm conclusions on any potential speciation events inside T. praecox.
The studied T. praecox populations accumulated a wide range of Cd, Zn and Pb concentrations, which was expected as they were collected from sites with prominent differences in soil metal concentrations. Cd, Zn and Pb (hyper)accumulation in T. praecox shoots mainly depended on the metal concentrations in the soil as revealed by the stepwise regression analyses. Cd concentrations in shoots exceeding the hyperaccumulation threshold (>100 mg Cd kg−1; Reeves and Baker 2000) were seen for two populations from the metal-polluted sites (Žerjav and Mežica) and in one population from the non-polluted site (Lokovec), indicating that as with T. caerulescens, Cd hyperaccumulation is not a constitutive trait in T. praecox, but rather specific for particular metalliferous populations. Evolutionary development of extraordinary Cd hyperaccumulation abilities in particular T. praecox populations may be closely related to the levels of this non-essential element in the soil. Similarly studies of T. caerulescens, which showed that ecotypes growing naturally in low Cd-containing soils have much lower hyperaccumulation capacity compared to the ecotypes growing in high Cd-containing soils (e.g. Ganges) (Basic et al. 2006a, b).
Only one of the collected specimens from the polluted site (from Mežica population) accumulated Zn above the criteria for Zn hyperaccumulation (>10,000 mg Zn kg−1; Reeves and Baker 2000), with 16,500 mg Zn kg−1 in the aboveground biomass. Otherwise shoot concentrations of up to 8,200 mg Zn kg−1 were typically measured in the populations from the polluted sites, and up to 4,300 mg Zn kg−1 in the populations from the non-polluted sites. In studied T. caerulescens populations, the Zn-(hyper)accumulation was found to be a constitutive trait but with high intraspecific variations present between and within different populations (Escarré et al. 2000). Significant differences in Zn shoot concentrations between the T. praecox populations from the polluted and non-polluted sites observed in this study and in our previous work (Vogel-Mikuš et al. 2005) suggest similar intraspecific variability in T. praecox.
The Pb hyperaccumulation threshold (>1,000 mg Pb kg−1; Reeves and Baker 2000) was exceeded in only the two populations from the polluted sites. The studies of tolerance to and accumulation of Pb have been mainly put aside in Thlaspi species. The only known Pb hyperaccumulator from this genus is the dwarfish plant that is typical of the Zn-mining region near Arnoldstein (Austria) and the Cave del Predil (Italy), Thlaspi rotundifolium ssp. cepaeifolium, with the highest ever measured Pb concentrations of 8,200 mg kg−1 in shoots (Reeves and Brooks 1983. It is, however, not clear whether this concentration was a consequence of air-borne pollution or root-to-shoot transfer, and therefore reports of high Pb leaf concentrations should be interpreted with caution. In T. praecox plants collected at a metal-polluted site, Pb concentrations seldom exceeded the Pb hyperaccumulation criteria (1,000 mg kg−1) (Vogel-Mikuš et al. 2005), although in controlled pot experiments, where air-borne pollution can be neglected, a relatively high concentration of Pb was found in shoots (up to 950 mg kg−1) (Vogel-Mikuš et al. 2006). Element localization studies of T. praecox leaf cross-sections using micro-proton induced X-ray emission have shown that Pb tissue localization patterns resemble those of Cd (Vogel-Mikuš et al. 2008a, b). As such, similarities in the mechanisms of transport and tissue partitioning of both metals could explain the higher Pb accumulation capacity seen in the higher Cd-accumulating T. praecox populations.
Significantly higher TFCd were seen in the two populations collected at the polluted sites, when compared to those collected at the non-polluted site, indicating more efficient translocation of Cd from root to shoot in metalliferous than non-metalliferous populations. In addition, there was a significant positive correlation between Cd, Zn and Pb TFs, indicating the possibility of common transport mechanisms from roots to shoots for measured metals. HMA4, a metal-transporting P1B-type ATPase, has been shown to have a key role in the root-to-shoot transport of Zn and Cd in A. thaliana, probably by acting as an efflux pump located on the plasma membrane of xylem parenchyma cells and delivering Zn and Cd to the xylem vessels (Mills et al. 2003, 2005). However, no differences were seen in TcHMA4 expression levels in the differentially Zn- and Cd-translocating T. caerulescens accessions (Xing et al. 2008). Since proportionally more Cd was stored in vacuoles of low Cd-translocating accessions of T. caerulescens, a difference in the levels of vacuolar sequestration was proposed as the key mechanism accounting for the differences in metal translocation between different Thlaspi populations (Xing et al. 2008).
In conclusion, we have shown that T. praecox is a closely related species to T. caerulescens, with the split from the common ancestor occurring around 1.2 Mya, and as such, both species share the constitutive Zn-(hyper)accumulation trait. The Cd hyperaccumulation in these T. praecox populations depends mainly on the soil Cd concentrations, and is closely correlated to the soil Zn and Pb concentrations. Differences in Cd (hyper)accumulation between populations from non-polluted and polluted sites were seen, as has also been reported for T. caerulescens.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Assunção AGL, Schat H, Aarts MG (2003a) Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol 159:351–360
Assunção AGL, Bookum WM, Nelissen HJM, Vooijs R, Schat H, Ernst WHO (2003b) Differential metal-specific tolerance and accumulation patterns among Thlaspi caerulescens populations originating from different soil types. New Phytol 159:411–419
Basic N, Keller C, Fontanillas P, Vittoz P, Besnard G, Galland N (2006a) Cadmium hyperaccumulation and reproductive traits in natural Thlaspi caerulescens populations. Plant Biol 8:64–72
Basic N, Salamin N, Keller C, Galland N, Besnard G (2006b) Cadmium hyperaccumulation and genetic differentiation of Thlaspi caerulescens populations. Biochem Sys Ecol 34:667–677
Baker AJM, Reeves RD, Hajar ASM (1994) Heavy metal accumulation and tolerance in British population of the metalophyte Thlaspi caerulenscens J. & C. Presl (Brassicaceae). New Phytol 127:61–68
Bennett KD (1997) Evolution and ecology: the pace of life. Cambridge University Press, Cambridge
Besnard G, Rubio R, Christin P-A, Vargas P (2009) Phylogenetics of Olea (Oleaceae) based on plastid and nuclear ribosomal DNA sequences: tertiary climatic shifts and lineage differentiation times. Ann Bot 104(1):143–160
Brooks RR, Lee J, Reeves RD, Jaffrè T (1977) Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J Geochemical Explorer 7:49–57
Brooks RR, Chambers MF, Nicks LJ, Robinson BH (1998) Phytomining. Trends Plant Sci 3:359–362
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolut Biol 7:214
Escarré J, Lefèbvre C, Gruber W, Leblanc M, Lepart J, Rivière Y, Delay B (2000) Zinc and cadmium hyperaccumulation by Thlaspi caerulescens from metalliferous and nonmetalliferous sites in the Mediterranean area: implication for phytoremediation. New Phytol 145:429–437
Felsenstein J (1988) Phylogenies from molecular sequences: inference and reliability. Ann Rev Genet 22:521–565
Hewitt G (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913
Jimenez-Ambriz G, Petit C, Bourrie I, Dubois S, Olivieri I, Rouce O (2007) Life history variation in the heavy metal tolerant plant Thlaspi caerulescens growing in a network of contaminated and noncontaminated sites in southern France: role of gene flow, selection and phenotypic plasticity. New Phytol 173:199–215
Keller C, Diallo S, Cosio C, Basic N, Galland N (2006) Cadmium tolerance and hyperaccumulation by Thlaspi caerulescens populations grown in hydroponics are related to plant uptake characteristics in the field. Funct Plant Biol 33:673–684
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Molec Evol 16:111–120
Koch M, Mummenhoff K (2001) Thlaspi s.str. (Brassicaceae) versus Thlaspi s.l.: morphological and anatomical characters in the light of ITS nrDNA sequence data. Plant Syst Evol 227:209–225
Koch M, Al-Shehbaz IA (2004) Taxonomic and phylogenetic evaluation of the American “Thlaspi” species: identity and relationship to the Eurasian genus Noccaea (Brassicaceae). Syst Botany 29:375–384
Koch M, Dobeš C, Mitchell-Olds T (2003) Multiple hybrid formation in natural populations: concerted evolution of the internal transcribed spacer of nuclear ribosomal DNA (ITS) in North American Arabis divaricarpa (Brassicaceae). Mol Biol Evol 20:338–350
Kropf M, Kadereit JW, Comes HP (2003) Differential cycles of range contraction and expansion in European high mountain plants during the Late Quaternary: insights from Pritzelago alpina (L.) O. Kuntze (Brassicaceae). Mol Ecol 12:931–949
Lombi E, Zhao FJ, Dunham SJ, McGrath SP (2000) Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol 145:11–20
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Martinčič A, Wraber T, Jogan N, Podobnik A, Turk B, Vreš B, Ravnik V, Frajman B, Strgulc Krajšek S, Trčak B, Bačič T, Fischer MA, Eler K, Surina B (2007) Mala flora Slovenije. Tehniška založba Slovenije, Ljubljana
Meyer FK (1973) Conspectus der “Thlaspi” Arten Europas, Afrikas und Vorderasien. Feddes Repertorium 84:449–470
Meyer FK (1979) Kritische Revison der “Thlaspi” Arten Europas, Afrikas und Vorderasien. Feddes Repertorium 90:129–154
Mills RF, Krijger GC, Baccarini PJ, Hall JL, Williams LE (2003) Functional expression of AtHMA4, a P1B-type ATPase of the Zn/Co/Cd/Pb subclass. Plant J 35:164–176
Mills RF, Franci A, Ferreira da Rocha PS, Baccarini PJ, Aylett M, Krijger GC, Williams LE (2005) The plant P1B-type ATPase AtHMA4 transports Zn and Cd and plays a role in detoxification of transition metals supplied at elevated levels. FEBS Lett 579:783–791
Molitor M, Dechamps C, Gruber W, Meerts P (2005) Thlaspi caerulescens on nonmetalliferous soil in Luxembourg: ecological niche and genetic variation in the mineral element composition. New Phytol 165:503–512
Mummenhoff K, Zunk K (1991) Should Thlaspi (Brassicaceae) be split? Preliminary evidence from isoelectric focusing analysis of Rubisco. Taxon 40:427–434
Mummenhoff K, Koch M (1994) Chloroplast DNA restriction site variation and phylogenetic relationships in the genus Thlaspi sensu lato (Brassicaceae). Syst Botany 19:73–88
Mummenhoff K, Franzke A, Koch M (1997) Molecular phyogenetics of Thlaspi s.l. (Brassicaceae) based on chloroplast DNA restriction site variation and sequences of the internal transcribed spacers of nuclear ribosomal DNA. Can J Bot 75:469–482
Peer WA, Mamoudian M, Lahner B, Reeves RD, Murphy AS, Salt DE (2003) Identifying model metal hyperaccumulating plants: germplasm analysis of 20 Brassicaceae accessions from a wide geographical area. New Phytol 159:421–430
Peer WA, Mahmoudian M, Freeman JL, Lahner B, Richards EL, Reeves RD, Murphy AS, Salt DE (2006) Assessment of plants from the Brassicaceae family as a model for the study nickel and zinc hyperaccumulation. New Phytol 172:248–260
Pollard AJ, Powell KD, Harper FA, Andrew J, Smith C (2002) The genetic basis of metal hyperaccumulation in plants. Crit Rev Plant Sci. 21:539–566
Pongrac P, Vogel-Mikuš K, Kump P, Nečemer M, Tolrà R, Poschenrieder C, Barceló J, Regvar M (2007) Changes in elemental uptake and arbuscular mycorrhizal colonisation during the life cycle of Thlaspi praecox Wulfen. Chemosphere 69:1602–1609
Pongrac P, Zhao FJ, Razinger J, Zrimec A, Regvar M (2009) Physiological responses to Cd and Zn in two Cd/Zn hyperaccumulating Thlaspi species. Environ Exp Bot 66:479–486
Posada D, Crandall KA (1998) Modeltesst: testing the model of DNA substitution. Bioinformatics 14:817–818
Reeves RD (1988) Nickel and zinc accumulation by species of Thlaspi L., Cochlearia L., and other genera of Brassicaceae. Taxon 37:309–318
Reeves RD, Brooks RR (1983) Hyperaccumulation of lead and zinc by two metallophytes from mining areas of central Europe. Environ Pollut A 31:277–285
Reeves RD, Baker AJM (2000) Metal accumulating plants. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York, pp 193–229
Reeves RD, Schwartz C, Morel JL, Edmonds J (2001) Distribution and metal-accumulating behaviour of Thlaspi caerulescens and associated metallophytes in France. Inter J Phytoremediation 3:145–172
Robinson BH, Leblanc M, Petit D, Brooks RR, Kirkman JH, Gregg PEH (1998) The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant Soil 203:47–56
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Roosens N, Verbruggen N, Meerts P, Ximénez-Embún P, Smith JAC (2003) Natural variation in cadmium tolerance and its relationship to metal hyperaccumulation for seven populations of Thlaspi caerulescens from Western Europe. Plant Cell Environ 26:1657–1672
Rutherford S, D’Hondt S (2000) Early onset and tropical forcing of 100 000-year Pleistocene glacial cycles. Nature 408:72–75
Sanderson MJ (1998) Estimating rate and time in molecular phylogenies: beyond the molecular clock? In: Soltis DE, Soltis PS, Doyle JJ (eds) Molecular systematics of plants II: DNA sequencing. Kluwer, Dordrecht, pp 242–264
Schat H, Llugany M, Bernhard R (2000) Metal specific patterns of tolerance, uptake, and transport of heavy metals in hyperaccumulating and nonhyperaccumulating metallophytes. In: Terry N, Bañuelos GS (eds) Phytoremediation of contaminated soil and water. Lewis, Boca Raton, pp 171–188
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland
Taberlet P, Fumagalli L, Wust-Saucy A-G, Cosson J-F (1998) Comparative phylogeography and postglacial colonization routes in Europe. Molec Ecol 7:453–464
Vogel-Mikuš K, Drobne D, Regvar M (2005) Zn, Cd and Pb accumulation and arbuscular mycorrhizal colonisation of pennycress Thlaspi praecox Wulf. (Brassicaceae) from the vicinity of a lead mine. Environ Pollut 133:233–242
Vogel-Mikuš K, Pongrac P, Kump P, Nečemer M, Regvar M (2006) Colonisation of a Zn, Cd and Pb hyperaccumulator Thlaspi praecox Wulfen with indigenous arbuscular mycorrhizal fungal mixture induces changes in heavy metal and nutrient uptake. Environ Pollut 139:362–371
Vogel-Mikuš K, Regvar M, Mesjasz-Przybyłowicz J, Przybyłowicz WJ, Simčič J, Pelicon P, Budnar M (2008a) Spatial distribution of cadmium in leaves of metal hyperaccumulating Thlaspi praecox using micro-PIXE. New Phytol 179:712–721
Vogel-Mikuš K, Simčič J, Pelicon P, Budnar M, Kump P, Nečemer M, Mesjasz-Przybyłowicz J, Przybyłowicz WJ, Regvar M (2008b) Comparison of essential and non-essential element distribution in leaves of the Cd/Zn hyperaccumulator Thlaspi praecox as revealed by micro-PIXE. Plant Cell Environ 31:1484–1496
Xing JP, Jiang RF, Ueno D, Ma JF, Schat H, McGrath SP, Zhao FJ (2008) Variation in root-to-shoot translocation of cadmium and zinc among different accessions of the hyperaccumulators Thlaspi caerulescens and Thlaspi praecox. New Phytol 178:315–325
Wraber T (2005) O verjetni nesamoniklosti nekaterih semenk, primerov za florulo castrensis, v flori Sovenije (On the probable non-native occurrence of some spermatophytes, examples of the florula castrensis, in the flora Slovenia). Hladnikia 18:3–10
White TJ, Bruns T, Lee S, Taylor J (1990) Amplication and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR—protocols and applications—a laboratory manual. Academic, London, pp 315–322
Zunk K, Mummenhoff K, Koch M, Hurka H (1996) Phytogenetic relationships of Thlaspi s.l. (subtribe Thlaspidinae, Lepidieae) and allied genera based on chloroplast DNA restriction-site variation. Theor Appl Genet 92:375–381
Acknowledgements
The authors are indebted to Assoc. Prof. Dr. Damjana Drobne for access to the AAS for the element analysis. The work was supported by the following projects: MSZS P1-0212 Biology of Plants Research Programme, “Young researchers” and EU COST 859. A scholarship from the World Federation of Scientists and a National Fellowship awarded by L’OREAL-UNESCO-The Slovenian Science Foundation to P. Pongrac are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Henk Schat.
Rights and permissions
About this article
Cite this article
Likar, M., Pongrac, P., Vogel-Mikuš, K. et al. Molecular diversity and metal accumulation of different Thlaspi praecox populations from Slovenia. Plant Soil 330, 195–205 (2010). https://doi.org/10.1007/s11104-009-0192-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-009-0192-x