Abstract
The effect of N form (NO3 − versus NH4 +) on growth and uptake of Cd and Zn by the hyperaccumulator Thlaspi caerulescens (Ganges ecotype) was investigated in hydroponic and rhizobox experiments. In the hydroponic experiments, NO3 − or NH4 + was supplied to plants with the pH of the nutrient solution being unbuffered or buffered at around 6.0. A moderately contaminated soil was used in the rhizobox experiment with or without additions of NO3 −, NH4 + or NH4 + + DCD (dicyanodiamide, a nitrification inhibitor). A higher biomass was obtained when N was supplied as NO3 − in both experiments, indicating that T. caerulescens prefers NO3 − over NH4 +. In the hydroponic experiments, supplying NO3 − resulted in a doubling of Cd concentration in the shoots compared with the NH4 + treatment, regardless whether solution pH was buffered or not. The form of N also had a noticeable effect on root Zn concentrations. In the rhizosphere box experiment, rhizosphere pH was markedly influenced by the N treatment. The acidification in the NH4 + and NH4 + + DCD treatments increased the concentrations of extractable Cd and Zn, both of which showed a considerable depletion in the rhizosphere. However, total uptake of Cd and Zn were highest in the NO3 − treatment, despite the fact that concentrations of extractable Cd and Zn in the rhizosphere were the lowest in this treatment. The results showed that supplying N as NO3 − promoted growth and phytoextraction of Cd and Zn by T. caerulescens compared with NH4 +.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thlaspi caerulescens J. & C. Presl is a well-known Zn hyperaccumulator, being able to accumulate more than 3% Zn in the shoot dry matter (Brooks 1998). Some populations of this species are also able to hyperaccumulate Cd or Ni (Assunção et al. 2008; Assunção et al. 2003). The ecotype from south France near Ganges is particularly efficient in Cd accumulation, with shoot Cd concentration reaching 0.3% in the field and 1% in hydroponic experiments (Lombi et al. 2000; Robinson et al. 1998; Roosens et al. 2003). Phytoremediation of soil moderately contaminated with Cd appears to be feasible with the Ganges ecotype of T. caerulescens (Chaney et al. 2007; Maxted et al. 2007; McGrath et al. 2006; Zhao et al. 2003).
The mechanisms responsible for Zn/Cd hyperaccumulation in T. caerulescens are not fully understood yet, but are likely to involve enhanced root uptake, efficient root to shoot translocation and cellular detoxification (Chaney et al. 2007; McGrath and Zhao 2003). The rate of Zn influx into root cells of T. caerulescens is markedly higher than that of the non-hyperaccumulator Thlaspi arvense (Lasat et al. 1996). Uptake and root to shoot translocation of Cd vary greatly among different populations of T. caerulescens, with the Ganges ecotype showing a much higher influx rate than other ecotypes (Lombi et al. 2001b; Xing et al. 2008; Zhao et al. 2002). A number of genes putatively involved in metal transport and detoxification are constitutively highly expressed in T. caerulescens compared with non-hyperaccumulator species (Hammond et al. 2006; Pence et al. 2000; van de Mortel et al. 2006).
The dynamics of metals in the rhizosphere influence their bioavailability to plants. The rhizosphere of T. caerulescens has been investigated in a number of studies. There is some evidence that the roots of T. caerulescens tend to proliferate in Zn/Cd-rich patches of soil, whereas non-hyperaccumulators tend to avoid metal-rich patches (Schwartz et al. 1999; Whiting et al. 2000). On the other hand, there is no evidence for a significant mobilisation of metals in soil by the root exudates of T. caerulescens (Zhao et al. 2001). Soil pH has a strong influence on metal bioavailability. It has been shown that decreasing soil pH increased Zn and Cd uptake by T. caerulescens, but only if the decrease in pH did not causes mobilisation of Al to which T. caerulescens appears to be rather sensitive (Brown et al. 1994; Wang et al. 2006; Yanai et al. 2006). The form of N supplied to plants affects rhizosphere pH greatly (Marschner 1995). It has been reported that supplying NH4 + to plants led to rhizosphere acidification and enhanced Zn and Cd uptake by the non-hyperaccumulators sunflower and tobacco (Loosemore et al. 2004; Zaccheo et al. 2006). Manipulation of rhizosphere pH through the use of NH4 + and a nitrification inhibitor has been suggested as an effective way to enhance phytoextraction of Cd and Zn from contaminated soil by sunflower (Zaccheo et al. 2006). However, it is not presently known whether the N form can influence Cd and Zn accumulation by T. caerulescens and, if so, whether the effect is due to the N form directly or to changes in pH. Answers to these questions would be useful for improving the phytoremediation efficiency of T. caerulescens.
The objective of the present study was to investigate the effect of supplying NO3 − or NH4 + on growth and the uptake of Cd and Zn by T. caerulescens. Experiments were carried out in both hydroponic culture with the pH of the nutrient solution being buffered or unbuffered, and in a rhizobox experiment with a moderately contaminated soil.
Materials and methods
Hydroponic experiments
Seeds of Thlaspi caerulescens (the Ganges ecotype) were sterilised in 10% H2O2 for 10 min and then germinated in a tray of vermiculite. Ten days after germination, seedlings were transferred to an aerated hydroponic solution containing 1 mM NH4NO3, 0.5 mM MgSO4, 0.25 mM K2HPO4, 1 mM K2SO4, 1 mM CaCl2, 10 μM H3BO3, 1.8 μM MnSO4, 0.2 μM NaMoO4, 0.31 μM CuSO4, 5 μM ZnSO4 and 50 μM Fe-EDDHA (pH 6.0), and grown for 8 days. Two hydroponic experiments were carried out to test the effect of NH4 + versus NO3 − on plant growth and the uptake of Cd and Zn, one with solution pH unbuffered and the other buffered with 5 mM MES (2-morpholinoethanesulphonic acid) to maintain the solution pH at around 6.0. The nutrient compositions of the different treatments are shown in Table 1. Four seedlings were grown in each of 1 l pot, and each treatment was replicated in four pots. The nutrient solutions were aerated continuously and renewed every other day. Solution pH was recorded every day at 9:00 am before nutrient solution renewal or pH adjustment. In the -MES experiment, solution pH was adjusted to the initial value of 6.0 with either 1 M HCl or NaOH every day. The -MES and + MES experiments were conducted separately, with experimental durations of 24 and 30 days, respectively. CdSO4 (10 μM) was added to the nutrient solutions during the last 2 and 3 weeks of the -MES and + MES experiments, respectively. Plants were grown inside a growth room with 14 h/10 h day/night, 350 μmol m−2 s−1 light intensity, and 25°C/18°C day/night temperature. At the end of the experiments, plants were rinsed with deionised water, separated into roots and shoots, and dried at 65°C for 48 h before dry weights were recorded. Samples were ground to <0.5 mm for analysis. Plant materials (0.25 g) were digested with 4 ml HNO3 and 1 ml HClO4, and the concentrations of Cd and Zn were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES, Perkin Elmer DV3300).
Speciation of Cd and Zn in the nutrient solutions was calculated using Geochem-PC (Parker et al. 1995).
Rhizobox experiment
Seedlings of T. caerulescens (Ganges) were pre-cultured in a nutrient solution for 10 days as described above. Two seedlings were transferred to a 12 × 10 × 11 cm (L × W × H) rectangular rhizobox, which was divided into two equal halves with two layers of nylon mesh (40 μm) installed in the middle. Roots were placed inside the two nylon layers, which prevented roots from growing outside the mesh. On each side of the nylon mesh was placed 500 g air-dried soil (<2 mm). The soil was moderately contaminated with Cd and Zn due to the irrigation with sewage effluent water in the past. Selected soil properties are shown in Table 2. There were four treatments: Control (without N addition), NH4 +, NH4 + + DCD (dicyanodiamide, a nitrification inhibitor) or NO3 −. Each treatment was replicated in three boxes. Nitrogen was added at a rate of 150 mg kg−1 and mixed thoroughly with the soil. DCD was added at a rate of 10% of the total N dose. A previous study has shown that DCD was effective at inhibiting nitrification in the first 30 days after addition (Ju et al. 2004). Other basal nutrients included 100 mg K kg−1 and 80 mg P kg −1 added as KH2PO4. Deionised water was added daily to maintain soil moisture content at 20–25% (w/w). The experiment was conducted in a glasshouse with natural sunlight supplemented with sodium vapour lamps to maintain a minimum light intensity of 350 μmol m−2 s−1 for 14 h per day. Temperature was maintained at 18–25°C. Plants were harvested 90 days after transfer to the rhizoboxes. Shoots and roots were separated, rinsed with deionised water, and dried at 65°C for 48 h before dry weights were determined. The soils in the two rhizobox halves were frozen at −20°C for 12 h, and then sectioned into 0–2, 2–4, 4–6, 6–8 and >8 mm distance from the root surface (nylon mesh). The same sections from the two rhizobox halves were combined. A portion of the soil was sieved to <5 mm and frozen at −20°C until analysis, and the remaining portion was air dried and sieved to <1 mm.
Plant samples were analysed for Cd and Zn concentrations using ICP-AES. Fresh soil was used for the measurement of pH, extractable Cd and Zn concentrations. pH was determined in a suspension of 1:2.5 (v/w) of soil to water. Soil (2 g) was extracted with 50 ml 1 M NH4NO3 (DIN 1995) and the concentrations of Cd and Zn in the extracts were determined by ICP-AES.
The significance of treatment effects was evaluated by analysis of variance (ANOVA). Treatment means were compared using least significant difference (LSD) at p < 0.05.
Results
In both hydroponic and rhizobox experiments, two ecotypes of T. caerulescens (Ganges and Prayon) were grown. The results were similar, except that Ganges accumulated much more Cd than Prayon, which was consistent with previous reports (Lombi et al. 2000; Lombi et al. 2001b; Zhao et al. 2002). Therefore, only the results of the Ganges ecotype are presented here.
Hydroponic experiments
Without the addition of the pH buffer MES, solution pH showed substantial daily changes from the initial value (6.0) (Fig. 1a). As expected, the NH4 + treatment led to acidification in the nutrient solution, by up to 1.8 pH units. In contrast, the NO3 − treatment led to increased pH (mostly within 0.5 unit). MES (5 mM) was effective in buffering pH in the nutrient solutions, with pH being maintained at ±0.25 units of the initial value (Fig. 1b).
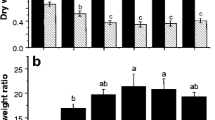
In the -MES experiment, the NO3 − treatment produced significantly higher shoot and root biomass than the NH4 + treatment (Fig. 2a), as well as a significantly higher root to shoot ratio (data not shown). In the + MES experiment, although plant growth was still better in the NO3 − treatment than in the NH4 + treatment, the difference was not significant (Fig. 2b).
Effects of N forms on the biomass of shoots and roots of Thlaspi caerulescens (a, b), concentrations of Cd (c, d) and Zn (e, f) in shoots and roots in the pH unbuffered (a, c, e) and pH buffered (b, d, f) hydroponic experiments. Error bars are SE (n = 4). * indicates a significant difference between the NH4 + and NO3 − treatment (p < 0.05)
In both -MES and + MES experiments, the concentration of Cd in roots was higher than that in shoots (Fig. 2c,d). In the absence of the MES buffer, both shoot and root Cd concentrations were markedly higher (2–2.2 fold, p < 0.001) in the NO3 − treatment than in the NH4 + treatment (Fig. 2c). When MES was used to buffer solution pH, the difference in root Cd concentration between the two N treatments became insignificant, but the shoot Cd concentration was 2.3 fold higher in the NO3 − treatment than in the NH4 + treatment (p < 0.001, Fig. 2d). Even though the -MES and + MES experiments were not strictly comparable because of different lengths of plant growth and Cd exposure, it is interesting to note that Cd concentrations in shoots were comparable in the same N treatment of the two experiments, whereas Cd concentrations in roots were comparable only in the NH4 + treatment.
In contrast to Cd, Zn concentrations in shoots were always higher than those in roots (Fig. 2e,f). In both -MES and + MES experiments, there were no significant differences in shoot Zn concentration between the NH4 + and NO3 − treatments, whereas root Zn concentration was significantly (p < 0.001) higher in the NO3 − treatment than in the NH4 + treatment. Zinc concentrations in roots were comparable in the same N treatment of the two experiments, whereas Zn concentrations in shoots were higher in the + MES than in the -MES experiment.
Calculations of metal speciation in the nutrient solutions showed that Cd was more likely to precipitate with phosphate than Zn within the pH range observed in the experiments. Precipitation of cadmium phosphate was predicted to occur at pH ≥ 6.1 under our experimental conditions; at pH 6.6 (the highest solution pH observed in the unbuffered NO3 − treatment) 76.4% of the Cd in the solution would precipitate with phosphate. In contrast, precipitation of zinc phosphate was not predicted to occur in all treatment solutions with pH ≤ 6.6.
Rhizobox experiment
At the end of the experiment, soil pH differed between different N treatments, and also varied spatially from the root surface to the bulk soil (Fig. 3a). Soil pH was the lowest in the NH4 + treatment, most probably as a result of nitrification. In both Control and NH4 + + DCD treatments, soil pH showed a small decrease (∼0.3 units) from the bulk soil toward the root surface, although pH was about 0.3 units higher in the Control than in the NH4 + + DCD treatment. In contrast, both NH4 + and NO3 − treatments resulted in an increasing pH gradient (∼0.5 unit) toward the root surface. Soil pH was approximately 0.6 units higher in the NO3 − treatment than in the NH4 + treatment along the rhizosphere gradient.
The concentrations of ammonium nitrate-extractable Cd and Zn were affected by different N treatments. The extractable concentrations were the highest in the NH4 + treatment and the lowest in the NO3 − treatment (Fig. 3b,c); this difference can be attributed to the difference in the pH of the rhizosphere soil. Both metals showed a similar pattern of depletion from the bulk soil toward the root surface; on average the depletion from the >8 mm section to the 0–2 mm section was 49% and 30% for Cd and Zn, respectively.
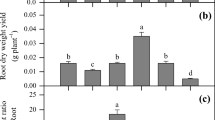
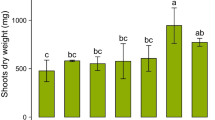
Shoot biomass was significantly (p < 0.001) affected by the treatments; the addition of NH4 + or NO3 − increased shoot biomass by 72 and 141%, respectively, over the control (Fig. 4a). The NH4 + + DCD treatment increased shoot biomass by 20% compared with the control, but this difference was not significant. The effects of treatments on root biomass were similar to those on shoot biomass, although the overall treatment effects were not significant (p = 0.065).
Effects of N forms on the biomass of shoots and roots of Thlaspi caerulescens (a), and the concentrations of Cd (b) and Zn (c) in shoots and roots in the rhizobox experiment. Different letters indicate significant difference at p < 0.05. There were no significant differences between treatments in root biomass. Error bars are ±SE (n = 3)
Shoot Cd concentration varied from 67 to 555 mg kg−1 dry weight (Fig. 4b). The bioconcentration factor for Cd (i.e. the ratio of shoot Cd to soil Cd concentrations) ranged from 25 to 202. Shoot Cd concentration was significantly (p < 0.001) affected by the additions of different N forms, and followed the order of Control > NO3 − > NH4 + > NH4 + + DCD (Fig. 4b). The total amount of Cd accumulated in the shoots accounted for 1–7.8% of the total Cd in the soil and followed the order of NO3 − > Control > NH4 + > NH4 + + DCD (data not shown). The total amount of Cd accumulated in the shoots in the NO3 − treatment was 2.6 and 8 fold higher than that in the NH4 + and NH4 + + DCD treatments, respectively. A dilution effect due to increased biomass probably accounted for the decreased Cd concentration in the shoots of the NO3 − treatment compared with the control. In contrast, the NH4 + and NH4 + + DCD treatments decreased shoot Cd concentration by mechanisms other than or in addition to the dilution effect. Root Cd concentrations were smaller than those in the shoots. The treatment effects on root Cd were only significant between the Control and the three + N treatments (Fig. 4b).
Shoot Zn concentration ranged from 630 to 4770 mg kg−1 dry weight (Fig. 4c), and were influenced by the treatments in a similar way as shoot Cd concentration. The addition of N, particularly NH4 + and NH4 + + DCD, significantly decreased shoot Zn concentration. The bioconcentration factor for Zn ranged from 3.6 to 27, and the Zn uptake by shoots accounted for 0.1–0.9% of the total Zn in the soil. The concentrations of Zn in roots were markedly smaller than those in shoots, but showed a similar trend among treatments as shoot Zn.
Discussion
Consistent with previous reports (Lombi et al. 2000, 2001a; Roosens et al. 2003; Zhao et al. 2002), the Ganges ecotype of T. caerulescens showed an exceptional ability to accumulate Cd in the shoots in both the hydroponic and the rhizobox experiments. Large values (>25) of the bioconcentration factor for Cd were obtained in the rhizobox experiment, even though the roots were confined to the central compartment inside two layers of nylon mesh without a direct contact with soil. A bioconcentration factor of >10 is considered to be necessary for an efficient phytoremediation of moderately contaminated soils within a reasonable time frame (McGrath and Zhao 2003; Zhao et al. 2003). Wang et al. (2006) reported that 36–40% of soil Cd was extracted by T. caerulescens shoots in a single planting in a pot experiment. Our results were smaller (1–7.8%), which can be attributed to the lack of a direct root-soil contact and a relatively short growth period. In contrast, T. caerulescens shoots removed <1% of the total soil Zn in the rhizobox experiment. This low rate of phytoextraction was because of the relatively high concentration of Zn in the soil (relative to Cd), and consequently the bioconcentration factor for Zn was substantially lower than that for Cd.
Growth of T. caerulescens was better when N was supplied as NO3 − than as NH4 + in both hydroponic and rhizobox experiments (Figs. 2 and 4). This difference could be due to a pH effect or an effect of N form, or both. As expected, N supply as NH4 + resulted in acidification in the nutrient solution in the unbuffered hydroponic experiment and in the soil of the rhizobox experiment, although the mechanisms of acidification were likely to be different. In the hydroponic experiment, acidification of the nutrient solution was a result of excess uptake of cations over anions (Marschner 1995). In the rhizobox experiment, a decrease in the bulk soil pH in the NH4 + treatment was caused by nitrification in soil, whereas rhizosphere acidification in the NH4 + + DCD treatment was a result of excess uptake of cations. The trend of increasing pH in the rhizosphere toward the root surface in the NO3 − and NH4 + treatments suggests that more anions than cations were taken up by roots, whereas opposite was true for the Control and NH4 + + DCD treatments. In soil experiments, acidification of some soils to a pH of <5 has been found to inhibit the growth of T. caerulescens mainly because of the mobilisation of toxic Al and Mn (Maxted et al. 2007; Wang et al. 2006; Yanai et al. 2006). In our hydroponic experiments, the difference in plant growth between the NO3 − and NH4 + treatments appeared to persist in both the pH unbuffered and buffered experiments, though more pronounced in the former than in the latter. In the rhizobox experiment, shoot growth showed significant differences among the three + N treatments in the order of NO3 − > NH4 + > NH4 + + DCD, even though the rhizosphere pHs were higher in the NH4 + + DCD treatment than in the NH4 + treatment. Also, soil pH was higher than 5.6 in all treatments of the rhizobox experiment; thus Al or Mn toxicity was unlikely. These results suggest that the main reason for the growth difference between different N treatments was the form of N supplied rather than pH, and that T. caerulescens preferred NO3 − to NH4 + as the N source.
In the hydroponic experiments, supplying N as NO3 − resulted in a doubling of Cd concentration in the shoots compared with the NH4 + treatment, regardless whether solution pH was buffered or not (Fig. 2). In contrast, the effect of N form on the Cd concentration in roots was dependent on solution pH; the NO3 − treatment significantly increased the concentration of Cd in roots compared with the NH4 + treatment only when solution pH was unbuffered. The form of N supplied to plants may influence Cd accumulation in plants through several possible mechanisms. First, precipitation of cadmium phosphate was predicted to occur at pH ≥ 6.1 under our experimental conditions, and the cadmium phosphate precipitate was likely to deposit on to the root surfaces (Küpper et al. 2000). This precipitation would largely explain why root Cd concentration in the unbuffered NO3 − treatment (solution pH varying from 6.0 to 6.7) was more than double of that in the unbuffered NH4 + treatment (solution pH ≤ 6.0), but not in the pH buffered experiment (solution pH around 6.0 in both N treatments). Zaccheo et al. (2006) also observed a much larger accumulation of Cd in sunflower roots in the NO3 − treatment than in the NH4 + treatment in a pH unbuffered hydroponic experiment. Although they did not attribute this difference to the possibility of cadmium phosphate precipitation onto the root surfaces, this was likely to occur. Second, a higher pH in the nutrition solution in the NO3 − treatment would increase the binding of metals to the root cell walls (Plette et al. 1999), which may in turn increase metal uptake into the root symplast (Marschner 1995). Third, uptake of NH4 + leads to a rapid depolarization of the membrane potential (Wang et al. 1994), which may decrease influx of metal ions to the root symplast (Marschner 1995). Fourth, assimilation of NO3 − in roots enhances synthesis of organic anions, which may increase uptake and xylem translocation of cations (Kirkby and Knight 1977). Results from the hydroponic experiments suggest that the effect of the N treatments on root Cd concentration can be attributed mainly to the difference in nutrient solution pH, but the effect on shoot Cd concentration was largely unrelated to solution pH, and possibly attributable to the third and fourth explanations given above. The exact mechanism for the NO3 − enhanced Cd accumulation in T. caerulescens remains to be investigated in the future.
In the hydroponic experiments N form had a significant effect on the concentration of Zn in the roots only, and this effect was independent of solution pH (Fig. 2). As precipitation of zinc phosphate was not predicted to occur in any of the treatment solutions, the increased Zn concentration in the roots of NO3 − treated plants may be a result of increased cell wall binding and/or increase uptake into the root cells. However, it appears that translocation of Zn to shoots, unlike that of Cd, was not increased by NO3 −.
The positive effect of NO3 − on Cd and Zn uptake and, particularly, Cd accumulation in the shoots was also apparent in the rhizobox experiment with a moderately contaminated soil (Fig. 4), even though the bioavailability of Cd and Zn, as indicated by the amounts of ammonium nitrate-extractable metals in the rhizosphere soil, was considerably higher in the NH4 + and NH4 + + DCD treatments than in the NO3 − treatment as a result of the pH difference (Fig. 3). These results indicate that, compared with NH4 +, NO3 − enhanced Cd and Zn uptake and/or Cd translocation from roots to shoots in T. caerulescens, and that this effect was substantially greater than the NH4 + -induced increase in the bioavailability of Cd and Zn in the rhizosphere soil. This result is opposite to that reported for the non-hyperaccumulator sunflower by Zaccheo et al. (2006), who found that supplying sunflower plants with NH4 + in combination with a nitrification inhibitor increased metal (Cd and Zn) uptake from contaminated soils. Our results show that rhizosphere acidification as a result of supplying NH4 + to plants would not enhance phytoextraction of Cd and Zn by T. caerulescens, because NH4 + suppresses growth and metal uptake in this plant species. In a pot study with the Ni hyperaccumulator Thlaspi goesingense, Puschenreiter et al. (2001) found little effect of the addition of ammonium sulphate and DCD on plant metal uptake, possibly because DCD was degraded and there was no acidification in the rhizosphere.
In the rhizobox experiment, the concentrations of ammonium nitrate-extractable Cd and Zn decreased considerably toward the root surface, indicating that a depletion zone developed in the rhizosphere for both metals as a result of fast uptake by T. caerulescens (Fig. 3). Depletion of both Cd and Zn in the rhizosphere occurred in all treatments, which differed in the rhizosphere pH and the levels of extractable metals. Similarly, Puschenreiter et al. (2005) found that ammonium nitrate-extractable Ni was depleted in the rhizosphere of T. goesingense. These results support the prediction by Whiting et al. (2003) using a solute transport model, who showed that a depletion of Zn in the rhizosphere of T. caerulescens is likely to occur in moderately contaminated soils and that the rate of Zn diffusion is crucial for maintaining sufficient Zn at the root surface. In contrast, mass flow delivers only a small percentage of Zn to the root surface of T. caerulescens (McGrath et al. 2001; Whiting et al. 2003). Methods that can enhance metal solubility and hence the rate of diffusion to the root surface may enhance the phytoremediation potential, but only if these methods do not suppress plant growth and interfere with the metal absorption processes in the roots. Field experiments showed that additions of the chelators EDTA and NTA did not enhance metal uptake by T. caerulescens (McGrath et al. 2006). Furthermore, use of chelators may result in unacceptable leaching of metals to subsoil or groundwater (Wenzel et al. 2003).
References
Assunção AGL, Bleeker P, ten Bookum WM, Vooijs R, Schat H (2008) Intraspecific variation of metal preference patterns for hyperaccumulation in Thlaspi caerulescens: evidence from binary metal exposures. Plant Soil 303:289–299. doi:10.1007/s11104-007-9508-x
Assunção AGL, Bookum WM, Nelissen HJM, Vooijs R, Schat H, Ernst WHO (2003) Differential metal-specific tolerance and accumulation patterns among Thlaspi caerulescens populations originating from different soil types. New Phytol 159:411–419. doi:10.1046/j.1469-8137.2003.00819.x
Brooks RR (1998) Plants that hyperaccumulate heavy metals. CAB International, Wallingford
Brown SL, Chaney RL, Angle JS, Baker AJM (1994) Phytoremediation potential of Thlaspi caerulescens and Bladder Campion for zinc- and cadmium-contaminated soil. J Environ Qual 23:1151–1157
Chaney RL, Angle JS, Broadhurst CL, Peters CA, Tappero RV, Sparks DL (2007) Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J Environ Qual 36:1429–1443. doi:10.2134/jeq2006.0514
Hammond JP, Bowen HC, White PJ, Mills V, Pyke KA, Baker AJM, Whiting SN, May ST, Broadley MR (2006) A comparison of the Thlaspi caerulescens and Thlaspi arvense shoot transcriptomes. New Phytol 170:239–260. doi:10.1111/j.1469-8137.2006.01662.x
Ju X, Liu X, Zhang F (2004) Nitrogen transformations in a Chinese Aquic Cambisol applied urea with dicyandiamide or plant residues. Commun Soil Sci Plant Anal 35:2397–2416. doi:10.1081/CSS-200030318
Kirkby EA, Knight AH (1977) Influence of level of nitrate nutrition on ion uptake and assimilation, organic-acid accumulation, and cation-anion balance in whole tomato plants. Plant Physiol 60:349–353
Küpper H, Lombi E, Zhao FJ, McGrath SP (2000) Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 212:75–84. doi:10.1007/s004250000366
Lasat MM, Baker AJM, Kochian LV (1996) Physiological characterization of root Zn2+ absorption and translocation to shoots in Zn hyperaccumulator and nonaccumulator species of Thlaspi. Plant Physiol 112:1715–1722
Lombi E, Zhao FJ, Dunham SJ, McGrath SP (2000) Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol 145:11–20. doi:10.1046/j.1469-8137.2000.00560.x
Lombi E, Zhao FJ, Dunham SJ, McGrath SP (2001a) Phytoremediation of heavy metal-contaminated soils: Natural hyperaccumulation versus chemically enhanced phytoextraction. J Environ Qual 30:1919–1926
Lombi E, Zhao FJ, McGrath SP, Young SD, Sacchi GA (2001b) Physiological evidence for a high-affinity cadmium transporter highly expressed in a Thlaspi caerulescens ecotype. New Phytol 149:53–60. doi:10.1046/j.1469-8137.2001.00003.x
Loosemore N, Straczek A, Hinsinger P, Jaillard B (2004) Zinc mobilisation from a contaminated soil by three genotypes of tobacco as affected by soil and rhizosphere pH. Plant Soil 260:19–32. doi:10.1023/B:PLSO.0000030173.71500.e1
Marschner H (1995) Mineral Nutrition of Higher Plants. Academic, London
Maxted AP, Black CR, West HM, Crout NMJ, McGrath SP, Young SD (2007) Phytoextraction of cadmium and zinc from arable soils amended with sewage sludge using Thlaspi caerulescens: development of a predictive model. Environ Pollut 150:363–372. doi:10.1016/j.envpol.2007.01.021
McGrath SP, Zhao FJ (2003) Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol 14:277–282. doi:10.1016/S0958-1669(03)00060-0
McGrath SP, Lombi E, Gray CW, Caille N, Dunham SJ, Zhao FJ (2006) Field evaluation of Cd and Zn phytoextraction potential by the hyperaccumulators Thlaspi caerulescens and Arabidopsis halleri. Environ Pollut 141:115–125. doi:10.1016/j.envpol.2005.08.022
McGrath SP, Zhao FJ, Lombi E (2001) Plant and rhizosphere processes involved in phytoremediation of metal-contaminated soils. Plant Soil 232:207–214. doi:10.1023/A:1010358708525
Parker DR, Norvell WA, Chaney RL (1995) GEOCHEM-PC - A chemical speciation program for IBM and compatible personal computers. In: Loeppert RH (ed) Chemical equilibrium and reaction models, soil science society of America. American Society of Agronomy, Madison, pp 253–269
Pence NS, Larsen PB, Ebbs SD, Letham DLD, Lasat MM, Garvin DF, Eide D, Kochian LV (2000) The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc Natl Acad Sci USA 97:4956–4960. doi:10.1073/pnas.97.9.4956
Plette ACC, Nederlof MM, Temminghoff EJM, van Riemsdijk WH (1999) Bioavailability of heavy metals in terrestrial and aquatic systems: a quantitative approach. Environ Toxicol Chem 18:1882–1890. doi:10.1897/1551-5028(1999)018<1882:BOHMIT>2.3.CO;2
Puschenreiter M, Stöger G, Lombi E, Horak O, Wenzel WW (2001) Phytoextraction of heavy metal contaminated soils with Thlaspi goesingense and Amaranthus hybridus: Rhizosphere manipulation using EDTA and ammonium sulfate. J Plant Nutr Soil Sci 164:615–621.
Puschenreiter M, Schnepf A, Millan IM, Fitz WJ, Horak O, Klepp J, Schrefl T, Lombi E, Wenzel WW (2005) Changes of Ni biogeochemistry in the rhizosphere of the hyperaccumulator Thlaspi goesingense. Plant Soil 271:205–218. doi:10.1007/s11104-004-2387-5
Robinson BH, Leblanc M, Petit D, Brooks RR, Kirkman JH, Gregg PEH (1998) The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant Soil 203:47–56. doi:10.1023/A:1004328816645
Roosens N, Verbruggen N, Meerts P, Ximenez-Embun P, Smith JAC (2003) Natural variation in cadmium tolerance and its relationship to metal hyperaccumulation for seven populations of Thlaspi caerulescens from western Europe. Plant Cell Environ 26:1657–1672. doi:10.1046/j.1365-3040.2003.01084.x
Schwartz C, Morel JL, Saumier S, Whiting SN, Baker AJM (1999) Root development of the Zinc-hyperaccumulator plant Thlaspi caerulescens as affected by metal origin, content and localization in soil. Plant Soil 208:103–115. doi:10.1023/A:1004519611152
van de Mortel JE, Villanueva LA, Schat H, Kwekkeboom J, Coughlan S, Moerland PD, van Themaat EVL, Koornneef M, Aarts MGM (2006) Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol 142:1127–1147. doi:10.1104/pp.106.082073
Wang AS, Angle JS, Chaney RL, Delorme TA, Reeves RD (2006) Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 281:325–337. doi:10.1007/s11104-005-4642-9
Wang MY, Glass ADM, Shaff JE, Kochian LV (1994) Ammonium uptake by rice roots. 3. Electrophysiology. Plant Physiol 104:899–906
Wenzel WW, Unterbrunner R, Sommer P, Sacco P (2003) Chelate-assisted phytoextraction using canola (Brassica napus L.) in outdoors pot and lysimeter experiments. Plant Soil 249:83–96. doi:10.1023/A:1022516929239
Whiting SN, Broadley MR, White PJ (2003) Applying a solute transfer model to phytoextraction: Zinc acquisition by Thlaspi caerulescens. Plant Soil 249:45–56. doi:10.1023/A:1022542725880
Whiting SN, Leake JR, McGrath SP, Baker AJM (2000) Positive responses to Zn and Cd by roots of the Zn and Cd hyperaccumulator Thlaspi caerulescens. New Phytol 145:199–210. doi:10.1046/j.1469-8137.2000.00570.x
Xing JP, Jiang RF, Ueno D, Ma JF, Schat H, McGrath SP, Zhao FJ (2008) Variation in root-to-shoot translocation of Cd and Zn among different accessions of the hyperaccumulators Thlaspi caerulescens and Thlaspi praecox. New Phytol 178:315–325. doi:10.1111/j.1469-8137.2008.02376.x
Yanai J, Zhao FJ, McGrath SP (2006) Effect of soil characteristics on Cd uptake by the hyperaccumulator Thlaspi caerulescens. Environ Pollut 139:167–175. doi:10.1016/j.envpol.2005.03.013
Zaccheo P, Crippa L, Pasta VD (2006) Ammonium nutrition as a strategy for cadmium mobilisation in the rhizosphere of sunflower. Plant Soil 283:43–56. doi:10.1007/s11104-005-4791-x
Zhao FJ, Hamon RE, Lombi E, McLaughlin MJ, McGrath SP (2002) Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. J Exp Bot 53:535–543. doi:10.1093/jexbot/53.368.535
Zhao FJ, Hamon RE, McLaughlin MJ (2001) Root exudates of the hyperaccumulator Thlaspi caerulescens do not enhance metal mobilization. New Phytol 151:613–620. doi:10.1046/j.0028-646x.2001.00213.x
Zhao FJ, Lombi E, McGrath SP (2003) Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant Soil 249:37–43. doi:10.1023/A:1022530217289
Acknowledgements
We thank the Changjiang Scholars Programme and the Innovative Research Team in the University Scheme (IRT0511) and the “863 Project” (2007AA061001) for financial support. Rothamsted Research receives grant-aided support from the UK Biotechnology and Biological Sciences Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Rights and permissions
About this article
Cite this article
Xie, H.L., Jiang, R.F., Zhang, F.S. et al. Effect of nitrogen form on the rhizosphere dynamics and uptake of cadmium and zinc by the hyperaccumulator Thlaspi caerulescens . Plant Soil 318, 205–215 (2009). https://doi.org/10.1007/s11104-008-9830-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9830-y