Abstract
A soil column method was used to determine the effect of nitrification on leaching of nitrate and ammonium from three acid subtropical soils after application of ammonium bicarbonate. Three soils, designated QF, GB and SU, derived from Quaternary red earth, granite and tertiary red sandstone, were collected from forest land, brush land and upland field, ranged in nitrification potential and cation exchange capacity. The results indicated that nitrate leaching increased with the soil nitrification potential. The soils with higher nitrification potential had a higher nitrate peak concentration and required a shorter time to reach it. In soils QF and GB with low cation exchange capacity, and a low content of exchangeable base cations, there were not sufficient base cations to accompany the nitrate leached with the result that ammonium and hydrogen ions were leached from the soil, and pH changes occurred in different layers of the soil column.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The leaching of nitrate from agricultural land (Stewart et al. 1968; Ramos et al. 2002; Beaudoin et al. 2005) and natural ecosystems (Sophie et al. 2002; Herrmann et al. 2005) to groundwater supplies, and its effect on human health has concerned environmentalists, policy makers, medicos etc for several decades.
Nitrate leaching losses from different land-use systems can be influenced by several soil chemical, biological, physical, and hydrologic properties (Silva et al. 2000; Di and Cameron 2002), and climatic conditions (Di and Cameron 2002). Therefore, identifying sites that are prone to nitrate leaching is necessary so that they can be regularly monitored and appropriately managed. Up to now, several indicators have been proposed, such as total nitrogen concentration, carbon/nitrogen ratio, in situ nitrogen mineralization, and nitrification rate (Andersson et al. 2002; Christ et al. 2002; Bengtsson et al. 2003; Chen and Mulder 2007).
Nitrification, the biological conversion of ammonium to nitrate is a key process in the nitrogen cycle, influencing nitrogen losses through the production of the highly mobile nitrate form (Carnol and Ineson 1999). There is, however, little information on the effect of nitrification on leaching. Some reports (Williard et al. 1997; Christ et al. 2002; Chen and Mulder 2007) indicate that net nitrification potentials might account for the difference in nitrate leaching under field conditions. Nevertheless, nitrate leaching in the field is the resultant of the influence of climatic conditions, plant uptake and other factors, thus the differences in nitrate export cannot be attributed solely to differences in nitrification until the effects of variation in other factors are considered. In this respect, laboratory experiments are more suitable than field studies because there is more control of the various factors which influence leaching.
Our objective is to determine the effect of soil nitrification on nitrate leaching, and the accompanying changes such as leaching of base cations, and distribution of mineral nitrogen, which take place in soil, so that methods can be designed for the mitigation of nitrate leaching from agro- and natural ecosystems.
Materials and methods
The effect of nitrification potential and nitrogen addition on leaching of nitrogen from subtropical soils was studied in soil columns in the laboratory. The experiments were conducted with three soils with different nitrification potential and three levels of addition of ammonium bicarbonate (NH4HCO3).
Soils
The three soils used for this study were taken from the 0–20 cm soil layers in Yingtan, Jiangxi province, China. This region has a typical subtropical monsoon climate with an annual precipitation of 1,795 mm, annual evaporation of 1,318 mm, and a mean annual temperature of 17.6°C. The soils are Ultisols or Oxisols (US Soil Taxonomy) developed from Quaternary red earth (Q), granite (G), or Tertiary red sandstone (S) (Zhao et al. 1988). Currently the land from which the soils were taken is covered by forest (F), brush (B) or is used for upland crops (U). The soils were air-dried and passed through a 2-mm sieve before use. The nitrification potential of these three soils had been determined previously (Zhao et al. 2007) are given in Table 1. The Quaternary forest soil (QF) had a low nitrification potential, the granite brush soil (GB) was intermediate and the upland sandstone soil (SU) had a high nitrification potential. Nitrification in the SU soil was greatly stimulated by the N input, but addition of ammonium bicarbonate did not greatly stimulate nitrification in GB and QF soils (Table 1). Some other properties of the soils are given in Table 1.
Leaching and transformations
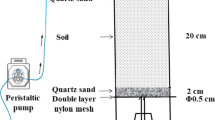
The leaching and transformation of nitrogen was carried out in columns constructed from polyvinyl chloride (PVC) tubing. Each column had a diameter of 5.0 cm, was 60 cm long, and had a porous baffle fixed at the bottom. After the baffle was covered with glass wool and quartz sand, each tube was filled with a homogeneous mixture of soil (950 g oven-dry basis) and quartz sand (475 g, ≤2 mm), to a height of ∼50 cm. The surface of the mixture was then covered with 1 cm of quartz sand so that the soil surface would not be disturbed during the addition of water. Deionised water was added to the soil columns to bring the moisture content to 65% water holding capacity (WHC). All columns were incubated at 25°C throughout the experiment.
After a preincubation of 7 days, three levels of NH4HCO3, 0, 150, and 300 mg N kg soil−1 (designated N0, N1 and N2) were added evenly over the surface with 100 ml deionised water, and the first leaching was executed following a 1 h equilibrium. Leachate (100 ml) was then collected from the bottom of each tube by the control of negative pressure for 24 h. Additional leachings with 100 ml deionised water were carried out during a 7 days interval. Between leachings the tops of the PVC tubes were covered with parafilm and the moisture content was maintained at ∼65% WHC. At the end of the experiment, subsamples were collected from the top 10 cm, the bottom 10 cm, and the remainder of the column (middle layer).
The leachate was analyzed for pH, nitrate, ammonium and base cations (Ca2+, K+, Mg2+, Na+), and the soil subsamples were analyzed for pH, nitrate and ammonium.
Analytical
The pH of soil subsamples was measured in a slurry (1 soil/2.5 water) using standard combination reference electrodes and a pH meter. Nitrate and ammonium in soils was extracted with 2.0 mol l−1 KCl solution and determined colorimetrically on a continuous flow analyzer (Skalar, Breda, Netherlands). The pH, and nitrate and ammonium concentrations of the leachate were determined as above, and the hydrogen leached was calculated from the pH changes in the leachate. Ca2+, K+, Mg2+, and Na+ in leachate were determined by inductively coupled plasma spectroscopy (IRIS Advantage, Thermo Electron, Boston, MS).
Results
Leaching of nitrate and ammonium
In each treatment the nitrate concentration in the leachate increased to a maximum and then declined (Fig. 1). The lowest peak nitrate concentration (∼77 mg N l−1) appeared in the leachate of the QF soil and the highest peak concentration (∼280 mg N l−1) occurred in the SU soil. The time of appearance of the peak concentration also varied with soil type, with the sequence being SU (∼21 days), GB (∼55 days) and QF (∼85 days). The time of appearance of the peak concentration seemed to be determined by the nitrification potential. Regardless of the soil type, the peak nitrate concentration occurred at the high level of nitrogen addition.
As far as ammonium concentrations are concerned, the results differed with nitrogen input level and soil type (Fig. 1). Very little ammonium was leached from the N0 treatments of the QF and SU soils during the period of the experiment. A little more ammonium (∼10 mg N l−1) was leached from the N0 treatment of the GB soil, but there was little variation in its concentration during the initial 8 weeks period; the concentration then decreased slowly to zero. For the N1 and N2 input levels, the ammonium concentrations in the leachates of the QF and GB soils changed in a similar fashion to the nitrate concentrations. More ammonium was leached from the N2 treatments than the N1 treatments and the time of appearance of the ammonium peak occurred earlier in the N2 treatments. Very little ammonium was leached from the N1 and N2 treatments of the SU soil.
Apart from soil SU where very little ammonium was leached, the total amount of nitrate and ammonium leached from the soil columns increased with the level of nitrogen addition (Fig. 2).
The effect of soil type on the leaching of added nitrogen was determined by subtracting the results for the control soils (N0) from those for the treated soils regardless of any priming effect of fertilizer. The results (not shown directly) indicated that at a particular nitrogen level more of the added nitrogen was leached as nitrate from soil SU and least was leached as nitrate from soil QF, in accord with the descending sequence of nitrification potential (Table 1). On the other hand, the maximum amount of added nitrogen leached as ammonium occurred in soil GB and there was essentially no added nitrogen leached as ammonium from soil SU.
More than 50% of the nitrogen added to the GB and SU soils was leached but only about one third of the added nitrogen was leached from the QF soil. The proportion leached decreased with increasing level of nitrogen addition (Fig. 2). This may have been due to increases in the magnitude of other loss processes, such as denitrification, at the higher rate of addition.
Leaching of hydrogen ion
In the QF soil there was little change in the pH of the leachate in the N0 treatment (∼7.2 to ∼6.7) over the period of the experiment. In the N1 and N2 treatments the pH of the leachate decreased sharply from ∼6.5 on ∼day 42 to a final value of ∼4.5 (Fig. 3). In soil GB at all nitrogen input levels the leachate pH decreased rapidly at about the same time as the nitrate concentration increased (Figs. 1 and 3). In the N0 treatment the pH decreased rapidly from ∼7.0 on day 56 to ∼4.0 on day 70. In treatments N1 and N2 the pH change occurred earlier at ∼day 28. The decrease in pH occurred slightly before the rapid change in nitrate concentration. With the SU soil all treatments behaved similarly and there was little change in the pH of the leachates.
The overall effect of nitrogen addition and leaching on acidification can be assessed more readily by considering the changes in hydrogen ion concentration [H+] (Table 2). More H+ was leached from the GB soil than the QF and SU soils in all nitrogen treatments. Addition of nitrogen increased the amount of H+ leached in all soils, but the effect in the SU soil was very small (0.2–0.7 μmol H+). In the GB soil significant H+ (16.4 μmol) was leached in the absence of added nitrogen.
In the control soils there was little difference in pH between the different layers of the columns at the end of experiment (Fig. 4). The pH of the top 10 cm of soil in columns GB and SU decreased with increasing nitrogen level, but in column QF the pH of the top layer increased with an increase in nitrogen addition.
In soils QF and GB the pH of the upper layer tended to be higher, sometimes significantly, than that of deeper layers, whereas in soil SU, the pH of the upper layer was lower than that of the deeper layers (Fig. 4).
Leaching of base cations
The cumulative amount of base cations leached from the soils increased with the level of nitrogen addition (Fig. 5). In line with the content of exchangeable base cations in soil (Table 1), leaching from soil SU was significantly greater than that from soil GB and QF at the same nitrogen level. There was a strong positive relationship between the amount of base cations and nitrate leached (r 2 = 0.74, P < 0.01).
The leaching of Ca2+ was significantly greater than that of the other three base cations (Table 3), probably because of its higher exchangeable content in soil (Table 1). Although the amount of Na+ leached was low, the percentage of exchangeable Na+ lost was greater than that for other cations. The level of nitrogen addition had no significant effect on Na+ leaching from any soil.
Mineral N concentration in soil columns at the end of incubation
After 112 days periodic leaching the columns that received the greatest amount of NH4HCO3 retained the most inorganic nitrogen (Fig. 6), although there was no significant difference between nitrogen levels for soil SU. Soil GB retained significantly more of the added nitrogen as nitrate than the other two soils, and soil QF retained significantly more of the added nitrogen as ammonium. Overall soil QF retained more of the added nitrogen in inorganic forms than the other two soils.
The amounts of nitrate and ammonium remaining in the soil column are presented in Fig. 7. The amounts of nitrate remaining in the mid-layers were higher significantly than those in the upper and bottom layers in soils GB and SU. The differences in the amounts of nitrate remaining in the different layers were not significant in soil QF. On the other hand, the amounts of ammonium remaining in the upper layers were significantly greater than those in the mid- and bottom layers in soil QF. For soil SU, there was no significant difference between the amounts of ammonium in the different layers.
The recovery of added nitrogen ranged from 61.9% to 91.8% and decreased with increasing input of nitrogen. The greatest recovery (91.7%) was obtained in the N1 treatment with soil GB and the worst recovery occurred in the N2 treatment of soil SU (Table 4).
The low recoveries of added nitrogen as nitrate and ammonium may have been due to immobilization of ammonium, ammonia volatilization or denitrification. In soil SU that had a higher pH and greater nitrification capacity the possibility of loss by ammonia volatilization and denitrification is greater than that from the other two soils that have weaker nitrification and a lower pH.
Discussion
The results given above show that nitrification potential is an important factor influencing nitrate leaching in soil. When the soil nitrification capacity was low, as in soil QF, inorganic nitrogen in the soil existed mainly as ammonium and was retained strongly by the soil for ∼50 days. The pattern obtained for the leaching of nitrate in Fig. 1 is similar to that obtained for the oxidation of ammonium in soil perfusion studies by Lees and Quastel (1946); there is a lag phase, followed by a period of logarithmic increase then a decline. The lag phase, (from ∼55 days in soil QF, to ∼20 days with soil GB and <10 days for soil SU), where microbial numbers are low and no nitrate was leached, was related to the nitrification potential (QF < GB < SU), as was the peak nitrate concentration and the total amount of nitate leached (Figs. 1 and 2).
Most soils in temperate regions, especially surface soils, have low ammonium concentrations and a net negative charge; consequently ammonium is usually adsorbed onto the soil exchange sites and not leached (Mengel 1985; Di and Cameron 2005). However, leaching of ammonium can occur in sandy soils with little or no cation exchange capacity, or when the adsorption capacity of the soil is overloaded by addition of large amounts of ammonium based fertilizer, when ammonium deposition from the atmosphere is large, or where nitrification is low due to unfavourable environmental conditions (Haynes 1985, 1986a, b; Deare et al. 1995; Wang and Bettany 1995; Li et al. 1997; Gundersen 1998; Wang and Zhang 2004; Herrmann et al. 2005). In addition in soils with low CEC, especially those with low content of exchangeable base cations, there may not be sufficient base cations to balance the charges on the nitrate leached, with the result that ammonium, hydrogen or aluminium ions would be leached to balance the charges (Reuss and Johnson 1986; Edwards and Helvey 1991). The results above (Fig. 2 and Table 3) showed that in soil SU, which is enriched with exchangeable Ca2+ and Mg2+ (Table 1), the leached cations were mainly base cations. However, in soils GB and QF, ammonium was leached in addition to base cations because there were insufficient base cations to leach and balance the charges on the nitrate leached.
Hydrogen ion, from acid deposition, nitrification or decomposition of organic matter will displace the exchangeable base cations and ammonium ions by ion exchange (Sun et al. 2006). In this study, when the nitrogen level increased from N0 to N1, hydrogen ion leaching also increased, but when the nitrogen level increased from N1 to N2, it decreased (Table 2). When the nitrogen level increased from N0 to N1, the total of leached base cations and ammonium ions was not sufficient to balance the charges on the nitrate leached. Consequently the leaching of hydrogen ions increased. However, when the nitrogen level increased from N1 to N2, the charges on the leached base cations and ammonium ions were more than sufficient to balance the charges on the leached nitrate, with the result that leaching of hydrogen ions was reduced.
Due to the higher mobility of nitrate in water, most of the added nitrogen was leached from the soil column in nitrate form. The nitrate not leached tended to accumulate in the middle layer that is larger than the top and bottom ones (Fig. 7). Because ammonium ions are strongly adsorbed by soil, ammonium concentrated mainly in upper layer and the amount adsorbed increased with increasing nitrogen level (Fig. 7). However, for soil SU, there was no significant difference between the amounts of ammonium adsorbed by the different soil layers. This appears to be due to the high nitrification capacity of this soil that resulted in the rapid transformation of ammonium to nitrate.
Conclusions
The results show that soil nitrification is an important factor controlling nitrate leaching. In soils with low cation exchange capacity, and insufficient base cations to accompany the nitrate leached, ammonium and hydrogen ions may be leached from the soil to balance the positive and negative charges.
References
Andersson P, Berggren D, Nilsson I (2002) Indices for nitrogen status and nitrate leaching from Norway spruce (Picea abies (L.) Karst.) stands in Sweden. Forest Ecol Manage 157:39–53
Beaudoin N, Saad JK, Van Laethem C, Machet JM, Maucorps J, Mary B (2005) Nitrate leaching in intensive agriculture in northern France: effect of farming practices, soils and crop rotations. Agric Ecosyst Environ 111:292–310
Bengtsson G, Bengtsson P, Mansson KF (2003) Gross nitrogen mineralization-, immobilization-, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol Biochem 35:143–154
Carnol M, Ineson P (1999) Environmental factors controlling \( {\text{NO}}^{ - }_{{\text{3}}} \) leaching, N2O emissions and numbers of \( {\text{NH}}^{ + }_{{\text{4}}} \) oxidisers in a coniferous forest soil. Soil Biol Biochem 31:979–990
Chen XY, Mulder J (2007) Indicators for nitrogen status and leaching in subtropical forest ecosystems, South China. Biogeochemistry 82:165–180
Christ MJ, Peterjohn WT, Cumming JR, Adams MB (2002) Nitrification potentials and landscape, soil and vegetation characteristics in two Central Appalachian watersheds differing in \( {\text{NO}}^{ - }_{3} \) export. Forest Ecol Manage 159:145–158
Deare FM, Ahmad N, Ferguson TU (1995) Downward movement of nitrate and ammonium nitrogen in a flatland Ultisol. Nutr Cycl Agroecosyst 42:175–184
Di HJ, Cameron KC (2002) Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutr Cycl Agroecosyst 64:237–256
Di HJ, Cameron KC (2005) Reducing environmental impacts of agriculture by using a fine particle suspension nitrification inhibitor to decrease nitrate leaching from grazed pastures. Agric Ecosyst Environ 109:202–212
Edwards PJ, Helvey JD (1991) Long-term ionic increases from a central Appalachian forested watershed. J Environ Qual 20:250–255
Gundersen P (1998) Effects of enhanced nitrogen deposition in a spruce forest at Klosterhede, Denmark, examined by moderate NH4NO3 addition. Forest Ecol Manage 101:251–268
Haynes RJ (1985) Principles of fertilizer use for trickle irrigation crops. Nutr Cycl Agroecosyst 6:235–255
Haynes RJ (1986a) Mineral Nitrogen in the Plant–Soil System. Academic Press, New York
Haynes RJ (1986b) Effects of soil acidification and subsequent leaching on levels of extractable nutrients in a soil. Plant Soil 95:327–336
Herrmann M, Pust J, Pott R (2005) Leaching of nitrate and ammonium in heathland and forest ecosystems in Northwest Germany under the influence of enhanced nitrogen deposition. Plant Soil 273:129–137
Lees H, Quastel JH (1946) Biochemistry of nitrification in soils 1. Kinetics of, and the effects of poisons on, soil nitrification, as studied by a soil perfusion technique. Biochem J 40:803–815
Li YC, Stoffella PI, Alva AK, Calvert DV, Graetz DA (1997) Leaching of nitrate, ammonium and phosphate from compost amended soil columns. Compost Sci Util 5:63–67
Mengel K (1985) Dynamics and availability of major nutrients in soils. Adv Soil Sci 2:65–131
Ramos C, Agut A, Lidón AL (2002) Nitrate leaching in important crops of the Valencian Community region (Spain). Environ Pollut 118:215–223
Reuss JO, Johnson DW (1986) Acid deposition and the acidification of soils and waters. Ecological Studies 59, Springer, New York
Silva RG, Cameron KC, Di HJ, Smith NP, Buchan GD (2000) Effect of macropore flow on the transport of surface applied cow urine through a soil profile. Aust J Soil Res 38:13–23
Sophie ZB, Maria H, Simone M, Robert J (2002) Nitrous oxide emissions and nitrate leaching in relation to microbial biomass dynamics in a beech forest soil. Soil Biol Biochem 34:823–832
Stewart BA, Viets FG Jr, Hutchinson GL (1968) Agriculture’s effect on nitrate pollution of groundwater. J Soil Water Conserv 23:13–15
Sun BH, Hu ZY, Lü JL, Zhou LN, Xu CK (2006) The leaching solution chemistry of a broad-leaved forest red soil under simulated N deposition in Southern China. Acta Ecologica Sinica 26:1872–1881
Wang FL, Bettany JR (1995) Carbon and nitrogen losses from undisturbed soil columns under short-term flooding conditions. Can J Soil Sci 75:333–341
Wang Y, Zhang YP (2004) \( {\text{NH}}^{ + }_{4} \) adsorption in a Eum-Orthic Anthrosol at different solution/soil ratios and temperatures. Pedosphere 14:253–257
Williard KWJ, DeWalle DR, Edwards PJ, Schnabel RR (1997) Indicators of nitrate export from forested watersheds of the mid-Appalachians, USA. Glob Biogeochem Cycles 11:649–656
Zhao QG, Xie WM, He XY, Wang MZ et al (1988) Red Soils of Jiangxi Province (In Chinese). Jiangxi Science and Technology Publishing House, Nanchang
Zhao W, Cai ZC, Xu ZH (2007) Does ammonium-based N addition influence nitrification and acidification in humid subtropical soils of China. Plant Soil. DOI 10.1007/s11104-007-9334-1
Acknowledgments
The project was financially supported by the National Natural Science Foundation of China (40471065) and CAS Research Program on Soil Biosystems and Agro-Product Safety (No. CXTD-Z2005-4). Deep appreciations go to the Center for Plant Analysis & Soil Test (ISSAS) for sample analyses and Yingtan Red Soil Ecological Experiment Station (ISSAS) for sampling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Dr. Nicolardot.
Rights and permissions
About this article
Cite this article
Qian, C., Cai, Z. Leaching of nitrogen from subtropical soils as affected by nitrification potential and base cations. Plant Soil 300, 197–205 (2007). https://doi.org/10.1007/s11104-007-9404-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9404-4