Abstract
The effects of nitrogen source on iron deficiency responses were investigated in two Vitis genotypes, one tolerant to limestone chlorosis Cabernet Sauvignon (Vitis vinifera cv.) and the other susceptible Gloire de Montpellier (Vitis riparia cv.). Plants were grown with or without Fe(III)-EDTA, and with NO −3 alone or a mixture of NO −3 and NH +4 . Changes in pH of the nutrient solution and root ferric chelate reductase (FC-R) activity were monitored over one week. We carried out quantitative metabolic profiling (1H-NMR) and determined the activity of enzymes involved in organic acid metabolism in root tips. In iron free-solutions, with NO −3 as the sole nitrogen source, the typical Fe-deficiency response reactions as acidification of the growth medium and enhanced FC-R activity in the roots were observed only in the tolerant genotype. Under the same nutritional conditions, organic acid accumulation (mainly citrate and malate) was found for both genotypes. In the presence of NH4+, the sensitive genotype displayed some decrease in pH of the growth medium and an increase in FC-R activity. For both genotypes, the presence of NH + 4 ions decreased significantly the organic acid content of roots. Both Vitis genotypes were able to take up NH +4 from the nutrient solution, regardless of their sensitivity to iron deficiency. The presence of N-NH +4 modified typical Fe stress responses in tolerant and sensitive Vitis genotypes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants differ in susceptibility to iron (Fe) deficiency. Several perennial crops, including grapevine, are particularly susceptible when grown on calcareous and alkaline soils. The development of chlorosis symptoms in orchards and vineyards may severely reduce fruit yield and quality (Álvarez-Fernández et al. 2006). Among many parameters, the form of N present in the soil was shown to impact Fe nutrition and the development of chlorotic symptoms (Korcak 1987; Lucena 2000; Marschner 1995).
The adaptive response of so-called Strategy I plants, including grapevine, involves morphological and physiological changes in the roots (Andaluz et al. 2002; Bavaresco et al. 1991; Römheld 1987; Rombolà et al. 2002). These typical responses include proton extrusion, release of reducing or chelating substances, and increase in membrane-bound ferric chelate reductase activity (FC-R) in the roots, to reduce FeIII before its uptake via an Fe (II) transporter (Briat and Lobréaux 1997). Proton extrusion and root FeIII reducing capacities have been demonstrated for several Vitis genotypes, and shown to be related to their adaptation to Fe deficiency (Bavaresco et al. 1991; Brancadoro et al. 1995; Mengel and Malissiovas 1982; Nikolic et al. 2000; Varanini and Maggioni 1982).
Proton extrusion has been attributed to activation of the root plasma membrane H+-ATPase, based on the activity and steady-state levels of the enzyme throughout the root (Dell’Orto et al. 2000b) and the co-localization of intense immunolabeling of the H+-ATPase protein and proton extrusion in the subapical root zones of Fe-deficient plants (Schmidt 2003). Acidification of the rhizosphere facilitates the mobilization of sparingly soluble ferric iron. Proton excretion has been shown to be associated with an increase in FeIII reduction (Toulon et al. 1992), although the induction of root FC-R activity can be uncoupled from the acidification (Yi and Guerinot 1996). FC-R is highly sensitive to pH and is inhibited at high pH (Kosegarten et al. 2004; Nikolic et al. 2000; Susin et al. 1996).
Several metabolic changes have also been described in Fe-deficient roots, including the accumulation of organic acids, shifts in the redox state of the cytoplasm, and increases in the activities of phosphoenolpyruvate carboxylase (PEPC) and several enzymes of the Krebs cycle and of the glycolytic pathway (Agnolon et al. 2001; López-Millán et al. 2000a; McCluskey et al. 2004; Rombolà et al. 2002). Citrate, which accumulates in large amounts in roots under Fe deficiency, is thought to be beneficial for Fe nutrition, and has been linked to Fe transport, proton extrusion and the capacity to produce reducing power in the form of NADPH (Bienfait 1996; Brown and Tiffin 1965; Landsberg 1981). Organic acid accumulation has been reported as part of the grapevine response to Fe deficiency and the presence of bicarbonates (Ollat et al. 2003).
Most plants can make use of either ammonium or nitrate ions. The uptake of these two forms of nitrogen (N) is controlled by genotype, plant development and physiological status, and also by soil properties such as texture, structure, water content and pH (Lea and Morot-Gaudry 2001; Loulakakis and Roubelakis-Angelakis 2001). Ammonium uptake results in strong acidification of the rhizosphere, due to the excretion of protons via the H+-ATPase. In contrast, nitrate uptake is associated with the proton consumptions via 2H+/NO −3 symport, leading to an increase in the pH of the outer solution (Mengel and Kirkby 2001). Ammonium assimilation requires carbon skeletons in the form of keto acids, mainly tricarboxylic acid cycle intermediates. Nitrate triggers a shift from starch biosynthesis to organic acid production (Foyer et al. 2003). The activity of key enzymes, such as PEPC, is modified by the form of nitrogen in the growth medium (Pasqualini et al. 2001). Little is known about the Vitis genotypes with respect to their different assimilation capacity for the forms of nitrogen. The expression of genes encoding various enzymes involved in ammonium assimilation, such as glutamate synthase, glutamine synthetase and glutamate dehydrogenase has been identified in grapevine roots (Loulakakis and Roubelakis-Angelakis 2001). Nitrate reductase activity has also been detected in roots and varied according to the seasonal root growth pattern (Hunter and Ruffner 1997).
Nitrogen is taken up almost exclusively as nitrate by the roots of plants growing in calcareous soil, in which ammonium is rapidly nitrified (Mengel 1994). The high nitrate levels of calcareous soils are thought to favor the development of Fe chlorosis (Korcak 1987; Tagliavini and Rombolà 2001). Several studies have demonstrated that nitrate can induce Fe-deficiency chlorosis in plants (Aktas and Van Egmond 1979; Kosegarten et al. 1998; Mengel et al. 1994; Smolders et al. 1997). The primary cause of Fe deficiency in NO −3 -fed plants is the high root apoplastic pH as a consequence of the removal of protons during H+/NO −3 cotransport, which impairs Fe uptake by the roots, most probably by inhibiting FeIII reduction (Kosegarten et al. 2004; Nikolic and Römheld 2003). Aktas and Van Egmond (1979) studied the effect of nitrogen fertilization on Glycine max L. genotypes with different susceptibilities to Fe chlorosis. They showed that increasing the amount of nitrate supplied to plants growing in calcareous soils worsened the symptoms of chlorosis in Fe-inefficient cultivars, but increased the growth of Fe-efficient cultivars. They also suggested that the Fe-inefficient cultivar was unable to balance the large increase in rhizosphere pH resulting from very active NO −3 uptake.
The aim of this work was to study the combined effects of Fe and the form of nitrogen on some Strategy I responses and root metabolism of two Vitis cultivars, one considered tolerant and the other susceptible to Fe deficiency.
Materials and methods
Plant material
Micropropagated plants of the Fe chlorosis tolerant genotype Vitis vinifera cv. Cabernet Sauvignon (CS) (Brancadoro et al. 1995; Dell’Orto et al. 2000a; Tagliavini and Rombolà 2001) and the Fe chlorosis-susceptible genotype Vitis riparia cv Gloire de Montpellier (RG) (Brancadoro et al. 1995; Nikolic et al. 2000) were acclimated in perlite for 3 weeks. The plants were then transferred to 10-l plastic containers filled with 8-l of a continuously aerated nutrient solution, with 20 plants per container. The growth chamber was programmed for a 16 h photoperiod (300 μmol photons m−2 s−1) at 25°C and 8 h of darkness at 20°C, with 75% relative humidity. The two genotypes were grown separately throughout the experiment. The nutrient solutions contained either nitrate as the only nitrogen source [NO3] or both ammonium and nitrate [NH4/NO3]. The macronutrient composition of the half-strength Hoagland solution [NO3] was 2.5 mM KNO3, 2.5 mM Ca(NO3)2·4H2O, 1 mM MgSO4·7H2O, 1 mM KH2PO4 and that of the [NH4/NO3] solution was 1 mM NH4NO3, 1 mM KNO3, 2.5 mM Ca(NO3)2·4H2O, 0.87 mM MgSO4·7H2O, 0.5 mM K2SO4, 1 mM KH2PO4. Nitrogen concentrations have been set according to Rodriguez-Lovelle and Gaudillère (2002), in order to maintain ion equilibrium in both conditions. The two solutions had identical micronutrient compositions: 9.1 μM MnCl2·4H2O, 46.3 μM H3BO3, 2.4 μM ZnSO4·H2O, 0.5 μM CuSO4, 0.013 μM (NH4)6Mo7O24·4H2O. Iron was supplied in the form of 90 μM Fe(III)-EDTA. After 4 days, two thirds of the plants for each genotype and nitrogen source were transferred to Fe-free solutions [−Fe]. The rest of the plants were maintained in the solution containing 90 μM Fe(III)-EDTA [+Fe]. There were therefore four different nutrient solutions for each genotype: [+FeNH4/NO3], [−FeNH4/NO3], [+FeNO3], [−FeNO3]. The day on which plants were transferred to Fe-free solution was counted as day 0 of the experiment. The plants were grown under these conditions for one week. The pH of the nutrient solutions was adjusted to 6 at day 0. The pH changes of the medium by roots was monitored daily.
In vivo root Fe(III)-EDTA reduction by intact plants
The root FC-R (EC 1.16.1.7) activity of whole plants was determined by monitoring the formation of the Fe(II)-disulfonic acid BPDS3 complex from Fe(III)-EDTA (Bienfait et al. 1983). Individual plants were transferred to 50-ml plastic beakers (covered with black tape to exclude light) containing 46 ml of 300 μM BPDS (Sigma), 10 mM MES, pH 6.0, as described by Gogorcena et al. (2000). Measurements were made in the growth chamber, under illumination (300 μmol photons m−2 s−1). The buffer solution was continually aerated by means of plastic tubing. Once the plants were placed in the beaker, 1 ml of Fe(III)-EDTA was added from a stock solution, to give a final concentration of 500 μM. After keeping the final solution for one hour under light exclusion, a 1 ml aliquot was removed from each beaker to measure the absorbance at 535 nm. An extinction coefficient of 22.14 mM−1 cm−1 was used to calculate the concentration of the Fe(II)-BPDS3 complex.
Determination of plant parameters and root sample collection
After FC-R determination, the plants were used to measure the length of the stem and the fresh weights of the stems and roots. The chlorophyll content of the leaves was determined, using a SPAD 502 chlorophyll meter (Minolta Co., Osaka Japan). Root tip samples (20–30 mm long) were taken from each plant assessed for FC-R activity, rinsed in deionized water, weighed, deep-frozen in liquid nitrogen, and kept at −80°C for metabolic profiling and enzyme activity determination.
Metabolic profiles obtained by 1H-NMR spectroscopy on root tips
Root tips were freeze-dried, weighed and crushed. Polar compounds were extracted by aqueous ethanol at 80°C, in three incubation steps, each lasting 20 min (step 1: 0.75 ml 80% ethanol; steps 2 and 3: 0.75 ml 50% ethanol) and then centrifuged for 10 min at 4,800g. Slurries were pooled. The ethanol was allowed to evaporate and the dry extracts were solubilized in 1 ml 200 mM oxalate buffer to maintain the pH of the extracts at 4.0. To improve spectrum resolution and to eliminate paramagnetic ions the extracts were further purified on 200 mg of Chelex 100 resin (BioRad, Marnes-la-Coquette) in oxalate buffer (pH = 4.0). The resin was rinsed three times with 1 ml double-distilled water. The pH of each extract was checked after this step. The extracts were lyophilized, solubilized in 500 μl D2O and lyophilized again to eliminate residual water. The dried extracts were stored in a dry atmosphere until 1H-NMR analysis.
Dried root extracts were solubilized in 500 μl D2O, followed by the addition of the sodium salt of (trimethyl) propionic-2,3,3,3-d4 acid (TSP) in D2O to a final concentration of 0.01%, for chemical shift calibration. The mixture was transferred to an NMR tube and 1H-NMR spectra were recorded as previously described (Moing et al. 2004) at 500.162 MHz and 300 K on a Bruker Avance spectrometer (Wissenbourg, France), using a 5-mm dual probe. We acquired 64 scans of 64 K data points with a spectral width of 6,000 Hz and an acquisition time of 2.73 s. The recycle delay was 15 s. The ERETIC method was used to determine absolute concentrations of metabolites (Akoka et al. 1999), using calibration curves for C1-H(α + β) glucose.
Enzyme extraction
The enzymes assayed included phosphoenolpyruvate carboxylase (EC 4.1.1.31), malate dehydrogenase (EC 1.1.1.37), citrate synthase (EC 4.1.3.7), and isocitrate dehydrogenase (EC 1.1.1.42).
Extracts for enzyme assays were prepared by grinding the root material in liquid nitrogen in a mortar with 50% PVPP (w/w FW) and 1 ml of extraction buffer containing 400 mM Tricine (pH 7.6), 5 mM MgSO4, 0.25 mM EDTA, 10% (v/v) glycerol, 0.5% (w/v) BSA, 5 mM NaHCO3, 2 mM PMSF, 10 mM sodium ascorbate and 1% (v/v) Triton. The slurry was filtered through glass wool and the filtrate was desalted on a Sephadex G-25 column (equilibrated with extraction buffer). The desalted extracts were centrifuged at 1,400g for 2 min (4°C) and used for enzyme activity assays directly, or after a short period of storage (less than 2 h) in liquid N2.
Enzyme assays
Phosphoenolpyruvate carboxylase was determined by coupling its activity to malate dehydrogenase-catalyzed NADH oxidation (Vance et al. 1983) with 25 μl of extract in 1 ml of 5 mM NaHCO3, 0.2 mM NADH, 2 mM DTT, 3 units ml−1 MDH (Sigma), 2.2 mM PEP, 2.5 mM MgSO4, 0.25 mM EDTA and 100 mM tricine, pH 8.1. Nicotinamide adenine dinucleotide (NADH) consumption was determined spectrophotometrically by monitoring the decrease in absorbance at 340 nm, at 25°C. Malate dehydrogenase activity was determined by monitoring the increase in absorbance at 340 nm, due to the enzymatic reduction of NAD+ at 25°C (Smith 1974). The reaction was carried out with 5 μl of extract in 1 ml of 3 mM NAD+, 29 mM malate, 5 mM MgCl2 and 100 mM DEA buffer, pH 9.2. Citrate synthase activity was assayed by monitoring the reduction of acetyl CoA to CoA with DTNB at 412 nm and 25°C (Srere 1967). The reaction mixture contained 25 μl of extract in 1 ml of 0.4 mM acetyl CoA, 0.1 mM DTNB, 0.5 mM oxalacetate and 200 mM Tris–HCl, pH 8.1. Isocitrate dehydrogenase was assayed spectrophotometrically, as described by Goldberg and Ellis (1974), by monitoring the reduction of NADP+ at 340 nm and 37°C. The reaction was carried out with 25 μl of extract in 1 ml of 3.9 mM MgCl2, 0.42 mM NADP+, 6.7 mM sodium isocitrate and 100 mM TEA buffer, pH 7.3.
Statistical analysis
Each set of data were analyzed by a two-way (Fe × N) analysis of variance (MANOVA) with SPSS software version 12.0 (SPSS, Inc., Chicago, USA). Analyses were performed on raw data or Ln-transformed data, to maximize variance homogeneity. If a significant result was obtained for the F-test, means were compared using the Newman and Keuls test (p ≤ 0.05).
Results
Plant growth and appearance of leaf chlorosis symptoms
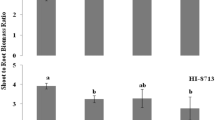
After one week of Fe deprivation, leaf chlorophyll content was significantly reduced in both genotypes, and plants displayed initial symptoms of Fe chlorosis (Table 1). At this stage, CS plants displayed significantly higher rates of root and shoot growth under [NH4/NO3] treatment than under [NO3] treatment. The shoot growth of CS plants was reduced by Fe deficiency if NO −3 was used as the sole nitrogen source. The root and shoot growth was unaffected by any treatment in RG plants.
Nutrient solution acidification
When CS plants were grown without Fe, the pH of the nutrient solutions quickly decreased (Fig. 1). The pH reached 3.5 after 5 days for [NH4/NO3] and after 7 days for [NO3]. In the presence of Fe, the pH of the [NH4/NO3] solution decreased slightly, whereas the [NO3] treatment had the opposite effect. For RG plants, the pH of the Fe-free [NH4/NO3] solution decreased to 4.9 by 7 days. In all other treatments, the pH remained close to 6.
Changes in the pH of the medium (8-l containers with 20 plants in each) for two grapevine genotypes, one chlorosis tolerant (CS) and the other chlorosis susceptible (RG), during the first week of growth in a nutrient solution containing 0 μM Fe(III)-EDTA [−Fe] or 90 μM Fe(III)-EDTA [+Fe] and with NO − 3 as the only source of nitrogen [NO3] or a mixed NH +4 /NO −3 supply [NH4/NO3]. The nutrient solution was not replenished during this monitoring
Root FC-R (ferric chelate reductase) activity
Root FC-R activities of CS and RG plants were measured after 3, 5 and 7 days (Fig. 2).
Time course of root ferric chelate reductase (FC-R, nmol Fe2+ g−1 FW min−1) activity for whole plants of two grapevine genotypes, one chlorosis tolerant (CS) and the other chlorosis susceptible (RG), plants growing in nutrient solution containing 0 μM Fe(III)-EDTA [−Fe] or 90 μM Fe(III)-EDTA [+Fe], and with NO −3 as the only source of nitrogen [NO3] or a mixed NH +4 /NO −3 supply [NH4/NO3]. Values were obtained after 3, 5, 7 days of Fe depletion. Data are means ± SE of three replicates
For CS plants, the FC-R activity of [+Fe] plants remained low and steady during the growing period whereas that of [−Fe] plants increased significantly by days 5 and 7, under both nitrogen treatments. The Fe effect was significant (p < 0.05) on day 5 and highly significant (p < 0.01) on day 7. At this stage, the presence of NH +4 in the nutrient solution significantly decreased FC-R activity and the interaction with Fe treatments was highly significant. For RG plants, the elimination of Fe from the growth medium had a significant positive effect on FC-R activity (p < 0.05) on day 7 only. The highest levels of activity were recorded for the [−FeNH4/NO3] treatment at this time point. FC-R activity for this treatment was two times higher than for the corresponding [+Fe] treatment. However, the interaction between nitrogen and Fe treatments was not significant.
1H-NMR metabolic profiling of root tips
1H-NMR metabolic profiles of root tip extracts were obtained after 6 days of Fe deficiency. We identified and quantified 17 compounds in root tip extracts, including two sugars, six organic acids and eight amino acids. The major compounds identified in root tip extracts were organic acids, primarily malate and citrate (Table 2). Glutamine and glutamate, which could not be quantified separately, were the main amino acids identified in the root tips (Table 3). Data for the rest of compounds are not shown.
MANOVA analysis of organic acid content showed that Fe treatment significantly increased the concentration of root organic acids other than fumarate for both genotypes (Table 2). Citrate predominated in [−Fe] CS roots, whereas malate was the main organic acid in RG roots. Nitrogen source had a significant effect on malate, citrate and succinate concentrations in CS roots, and on malate and fumarate in RG roots. The presence of NH +4 decreased organic acid concentration in the roots of both genotypes. The concentration of amino acids in the roots was influenced by the form of N, but not by Fe status (Table 3). With the exception of alanine in RG plants, the presence of NH +4 in the nutrient solution increased significantly amino acid levels.
Enzyme activities in root extracts
The activities of some enzymes involved in organic acid metabolism were measured in the root tips of the plants from all treatments after 6 days of Fe starvation (Table 4).
Fe deficiency induced significant increase in PEPC activity in both genotypes. In RG plants, MDH and citrate synthase activities were stimulated by Fe deficiency. Fe status did not affect these activities in CS plants. Regardless of the genotype, there was no nitrogen effect on these enzymatic activities. In RG plants, Fe deficiency significantly decreased NADP+-IDH activity in NH +4 -fed plants whereas it had an opposite effect in NO −3 -treated plants. No significant effect on NADP+-IDH activity was demonstrated for CS plants.
Discussion
After one week of Fe starvation, the chlorophyll content of the leaves, as indicated by SPAD readings, had significantly decreased for both genotypes. The shoot growth of the chlorosis tolerant genotype CS plants was significantly decreased by the lack of Fe, whereas root growth was not. Similar increase in root-to-shoot ratio has been previously reported for grapevine (Gruber and Kosegarten 2002) and peach (Shi et al. 1993) under conditions of Fe deficiency. Indeed, in grapevine, the shoots appear to be more sensitive to Fe deficiency regarding dry matter increment than the roots. However, some species, such as Beta vulgaris L., display the opposite pattern (Rombolà et al. 2005). Shoot and root growth of chlorosis-susceptible genotype did not respond to Fe deficiency nor to nitrogen source. The low growth rate registered for this genotype may partly explain this lack of response.
Acidification of the rhizosphere is known to be part of the mechanism by which some dicotyledonous plants respond to Fe starvation. This acidification has been attributed to activation of the root plasma membrane H+-ATPase (Dell’Orto et al. 2000b). Ammonium uptake also results in strong rhizosphere acidification whereas NO −3 uptake increases the pH of the outer solution (Mengel and Kirkby 2001). Under our growing conditions, the pH of the medium depended on both mechanisms: response to Fe deficiency and nitrogen uptake. The chlorosis-tolerant genotype plants displayed high capacity of acidification when grown without Fe, regardless of the nitrogen source, indicating a marked response to Fe deficiency. The pH decreased more rapidly if NH + 4 was present in the nutrient solution. A slight decrease in pH was also observed when plants were supplied with Fe, indicating that active NH +4 uptake occurred. For the chlorosis-susceptible genotype Riparia Gloire de Montpellier plants fed with NH +4 , the pH reached 4.9 after 7 days of Fe deficiency, whereas no decrease was observed for plants supplied with NO −3 . The changes in pH depended primarily on nitrogen source for this genotype.
As previously demonstrated (Brancadoro et al. 1995), medium acidification and root FeIII reducing capacities for the two Vitis genotypes studied here are related to their adaptation to Fe deficiency. However, when supplied with NH + 4 under Fe-deficiency conditions, both genotypes displayed unexpected responses. The root FC-R activity of the chlorosis-tolerant genotype was significantly reduced. On the opposite the susceptible one was able to acidify the nutrient solution and to increase FC-R activity. Kosegarten et al. (2004) and Nikolic and Römheld (2003) demonstrated close relationships between the form of nitrogen in the nutrient solution, root apoplast pH and FC-R activity in Helianthus annuus L.. Based on these relationships, the stimulation of FC-R activity observed for the susceptible genotype may be linked to the decrease in pH associated with NH +4 uptake. Nevertheless, our results for the chlorosis tolerant genotype are not in agreement with the expected effect of various nitrogen forms on FeIII reduction. The very low pH of the nutrient solution associated to NH4+ uptake may have increased the solubility of sparingly soluble Fe compounds in the root apoplast resulting in lower FC-R activity under these conditions (Gogorcena et al. 2001).
The known differences in susceptibility to Fe deficiency of the two Vitis genotypes studied here may therefore be related to their capacity to induce FC-R, and may also depend on the regulation of apoplastic pH, as suggested by Kosegarten et al. (2004). The transcriptional regulation of a specific isoform of H+-ATPase in response to Fe deficiency was recently demonstrated in Cucumis sativus L. (Santi et al. 2005). However, as root apoplastic pH was not determined in our work and using MES could have affected FC-R response to in vivo apoplastic pH, further work is required to investigate this relationship in different Vitis genotypes.
Among the 17 metabolites quantified simultaneously in the roots with 1H-NMR analysis, organic acid accumulated in larger amount in response to Fe deficiency, confirming previous results obtained with other methodologies for grapevine (Ollat et al. 2003), other woody species (Rombolà et al. 2002; Sun et al. 1987) and herbaceous plants (Abadía et al. 2002). Similar increase in malate concentrations was found in both genotypes. The chlorosis-tolerant genotype CS was characterized by a very high citrate concentration in the root tips and a ratio of citrate concentration under −Fe conditions to citrate concentration under +Fe conditions varying from 4 to 7. These data suggest that citrate could be used as biochemical marker of Fe chlorosis tolerance in some species, as suggested by Ollat et al. (2003) and Rombolà et al. (2002).
For both genotypes, the changes in root organic acid composition under Fe-deficiency conditions were associated with an increase in maximal PEPC activity. This difference was highly significant for the chlorosis tolerant one. Root tip citrate content and PEPC activity measured in vitro were significantly related (citrate content = 4.3 e 1.03PEPC activity, r 2 = 0.74, n = 8, p < 0.01). This stimulation of PEPC activity under Fe-deficiency conditions appears to be a general feature (Andaluz et al. 2002; Landsberg 1986; López-Millán et al. 2000b; Nisi and Zocchi 2000; Rombolà et al. 2005) consistent with the increase in CO2 fixation in the roots and the increase in organic acid concentration. In the absence of Fe, the chlorosis-susceptible genotype Riparia Gloire de Montpellier presented significantly higher MDH and citrate synthase activities. The stimulation of citrate synthase activity under Fe-deficiency conditions was not reported previously by McCluskey et al. (2004) and Rombolà et al. (2002).
These results suggest that PEPC activity may be one of the limiting steps for citrate accumulation. Other activities, such as MDH and citrate synthase activities, are probably in excess of the normal cellular requirements (Delhaize et al. 2003). Other factors, such as reducing power and compartmentalization of substrates and products, may control the activities of these enzymes in vivo. Export to the external medium and to the xylem sap must also be taken into account.
Organic acid and amino acid contents were significantly affected by nitrogen source. The effect on citrate content was significant for CS only, whereas the effect on malate content was significant for both genotypes. Citrate and malate contents were lower in the presence of NH +4 than in its absence. Amino acid content was also significantly higher in the presence of NH +4 . The roots of CS plants contained six times higher concentrations of glutamate and glutamine when NH +4 was added to the nutrient solution.
Amino acid biosynthesis requires the allocation of assimilated carbon—2-oxoglutarate (2-OG) in particular—for ammonia incorporation, and the production of C skeletons by the TCA cycle for amino acid synthesis downstream from the GS/GOGAT pathway (von Wirén et al. 2001). Ammonium assimilation may direct more organic acids to the GS/GOGAT pathway, resulting in lower levels of accumulation in the roots (Pasqualini et al. 2001) and an increase in carbon flow through the TCA cycle. In our study, the lower organic acid concentration in the root tips of NH +4 -fed plants probably resulted from the use of these compounds to produce 2-OG for NH +4 assimilation.
Although grapevine rootstocks are known to display different responses to N fertilization under field and pot trial conditions (Keller et al. 2001; Zerihun and Treeby 2002), their capacity to assimilate different forms of N forms has been little investigated. Our data suggest that both Vitis genotypes can take up NH +4 from the nutrient solution. Significantly higher growth rate of CS plants supplied with NH +4 , the slight acidification of the [+FeNH4/NO3] nutrient solution observed with this genotype, and high root amino acid concentration recorded for both genotypes may illustrate the ability to assimilate this form of nitrogen.
The interaction between Fe treatment and N source was highly significant for NADP+-IDH activity in Riparia Gloire de Montpellier. This enzyme has several roles in cells, depending on the particular isoform considered and its subcellular distribution (Hodges et al. 2003). This enzyme is determinant for production of the reducing power (Hodges et al. 2003; López-Millán et al. 2000b; McCluskey et al. 2004) required for FC-R activity, as previously suggested by Bienfait (1996). It is also thought to be involved in the production of 2-OG (Suárez et al. 2002). In our study, the interaction identified for NADP+-IDH activity may be the expression of these two roles.
Our results show that nitrogen uptake and metabolism interfere with Fe metabolism in Vitis roots in a complex manner. The chlorosis-tolerant genotype Vitis vinifera cv. CS showed medium acidification, the induction of root FC-R activity and citrate accumulation in the roots when subjected to Fe deficiency. The acidification of the nutrient solution resulting from the ammonium uptake may, at least partly, account for the unexpected stimulation of FC-R activity in the genotype Vitis riparia cv. Gloire de Montpellier, which is known to be chlorosis susceptible. Our results confirm that citrate is a biochemical marker of Fe deficiency and that PEPC activity limits the accumulation of this compound. However, the assimilation of NH +4 also interfered with organic acid accumulation in roots, particularly in CS plants, limiting citrate accumulation. Ammonium in the medium changes some typical biochemical responses to Fe deficiency for both tolerant and susceptible cultivars, and this cation should therefore be avoided in screening methods based on nutrient solution experiments.
Abbreviations
- 2OG:
-
2-oxoglutarate
- BPDS:
-
bathophenanthrolinedisulfonic acid disodium salt hydrate
- BSA:
-
bovine serum albumin
- CoA:
-
coenzyme A
- DEA:
-
diethanolamine
- DTNB:
-
5-5′-dithio-bis-2-nitrobenzoic acid
- DTT:
-
DL-dithiothreitol
- EDTA:
-
ethylenediaminetetracetic acid
- ERETIC:
-
electronic reference to access in vivo concentrations
- FW:
-
fresh weight
- GOGAT:
-
glutamate synthetase
- GS:
-
glutamine synthetase
- MDH:
-
malate dehydrogenase
- MES:
-
2-(N-morpholino)ethanesulfonic acid
- NMR:
-
nuclear magnetic resonance
- PEP:
-
phosphoenolpyruvate
- PEPC:
-
phosphoenolpyruvate carboxylase
- PMSF:
-
phenylmethylsulfonyl fluoride
- PVPP:
-
polyvinylpolypyrrolidone
- TCA:
-
tricarboxylic acid
- TEA:
-
triethylamine
- TSP:
-
(trimethyl) propionic-2,3,3,3-d4 acid sodium salt
References
Abadía J, López-Millán A-F, Rombolà A, Abadía A (2002) Organic acids and Fe deficiency: a review. Plant Soil 241:75–86
Agnolon F, Santi S, Varanini Z, Pinton R (2001) Enzymatic responses of cucumber roots to different levels of Fe supply. Plant Soil 241:35–41
Akoka S, Barantin L, Trierweiler M (1999) Concentration measurement by proton NMR using the ERETIC method. Anal Chem 71:2554–2557
Aktas M, Van Egmond F (1979) Effect of nitrate nutrition on iron utilization by an Fe-efficient and an Fe-inefficient soybean cultivar. Plant Soil 51:257–274
Álvarez-Fernández A, Abadía J, Abadía A (2006) Iron deficiency, fruit yield and fruit quality. In: Barton LL, Abadía J (eds) Iron nutrition in plants and rhizospheric microorganisms. Springer, Dordrecht, the Netherlands, pp 85–101
Andaluz S, López-Millán A-F, Peleato ML, Abadía J, Abadía A (2002) Increases in phosphoenolpyruvate carboxylase activity in iron-deficient beet roots: analysis of spatial localization and post-translational modification. Plant Soil 241:43–48
Bavaresco L, Fregoni M, Fraschini P (1991) Investigations on iron uptake and reduction by excised roots of different grapevine rootstocks and a V. vinifera cultivar. Plant Soil 130:109–113
Bienfait HF (1996) Is there a metabolic link between H+ excretion and ferric reduction by roots of Fe-deficient plants? A viewpoint. J Plant Nutr 19:1211–1222
Bienfait HF, Bino RJ, Bliek JF, Duivenvoorden JF, Fontaine JM (1983) Characterization of ferric reducing activity in roots of Fe-deficient Phaseolus vulgaris. Plant Physiol 59:196–202
Brancadoro L, Rabotti G, Scienza A, Zocchi G (1995) Mechanisms of Fe-efficiency in roots of Vitis spp. in response to iron deficiency stress. Plant Soil 171:229–234
Briat J-F, Lobréaux S (1997) Iron transport and storage in plants. Trends Plant Sci 2:187–193
Brown JC, Tiffin LO (1965) Iron stress as related to the iron and citrate occurring in stem exudate. Plant Physiol 40:395–400
Delhaize E, Ryan PR, Hocking PJ, Richardson AE (2003) Effects of altered citrate synthase and isocitrate dehydrogenase expression on internal citrate concentrations and citrate efflux from tobacco (Nicotiana tabacum L.) roots. Plant Soil 248:137–144
Dell’Orto M, Brancadoro L, Scienza A, Zocchi G (2000a) Use of biochemical parameters to select grapevine genotypes resistant to iron-chlorosis. J Plant Nutr 23:1767–1775
Dell’Orto M, Santi S, Nisi PD, Cesco S, Varanini Z, Zocchi G, Pinton R (2000b) Development of Fe-deficiency responses in cucumber (Cucumis sativus L.) roots: involvement of plasma membrane H+ ATPase activity. J Exp Bot 51:695–701
Foyer CH, Parry M, Noctor G (2003) Markers and signals associated with nitrogen assimilation in higher plants. J Exp Bot 54:585–593
Gogorcena Y, Abadía J, Abadía A (2000) Induction of in vivo root ferric chelate reductase activity in fruit tree rootstock. J Plant Nutr 23:9–21
Gogorcena Y, Molias N, Larbi A, Abadía J, Abadía A (2001) Characterization of the responses of cork oak (Quercus suber) to iron deficiency. Tree Physiol 21:1335–1340
Goldberg DM, Ellis G (1974) Isocitrate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie/Academic Press, New York, pp 183–189
Gruber B, Kosegarten H (2002) Depressed growth of non-chlorotic vine grown in calcareous soil is an iron deficiency symptom prior to leaf chlorosis. J Plant Nutr Soil Sci 165:111–117
Hodges M, Flesh V, Galvez S, Bismuth E (2003) Higher plant NADP+-dependent isocitrate dehydrogenases, ammonium assimilation and NADPH production. Plant Physiol Biochem 41:577–585
Hunter JJ, Ruffner HP (1997) Diurnal and seasonal changes in nitrate reductase activity and nitrogen content of grapevines: effect of canopy management. Vitis 36:1–6
Keller M, Kummer M, Carmo Vasconcelos M (2001) Soil nitrogen utilization for growth and gas exchange by grapevines in response to nitrogen supply and rootstock. Aust J Grape Wine Res 7:2–11
Korcak RF (1987) Iron deficiency chlorosis. In: Janick J (ed) Horticultural review. Van Nostrand Reinhold Company, New York, pp 133–186
Kosegarten H, Schwed U, Wilson G, Mengel K (1998) Comparative investigation on the susceptibility of faba bean (Vicia faba L.) and sunflower (Helianthus annuus L.) to iron chlorosis. J Plant Nutr 21:1511–1528
Kosegarten H, Hoffmann B, Rroco E, Grolig F, Glüsenkamp K-H, Mengel K (2004) Apoplastic pH and FeIII reduction in young sunflower (Helianthus annuus) roots. Physiol Plant 122:95–106
Landsberg EC (1981) Organic acid synthesis and release of hydrogen ions in response to Fe-deficiency stress of mono- and dicotyledonous plant species. J Plant Nutr 3:579–591
Landsberg EC (1986) Function of rhizodermal transfer cells in the Fe stress response mechanisms of Capsicum annuum L. Plant Physiol 82:511–517
Lea PJ, Morot-Gaudry J-F (2001) Plant nitrogen. Springer-Verlag, INRA Paris, Berlin, 407 p
López-Millán A-F, Morales F, Abadía A, Abadía J (2000a) Effects of iron deficiency on the composition of the leaf apoplastic fluid and xylem sap in sugar beet. Implications for iron and carbon transport. Plant Physiol 124:873–884
López-Millán A-F, Morales F, Andaluz S, Gogorcena Y, Abadía A, Abadía J (2000b) Responses of sugar beet roots to iron deficiency. Changes in carbon assimilation and oxygen use. Plant Physiol 124:885–897
Loulakakis KA, Roubelakis-Angelakis KA (2001) Nitrogen assimilation in grapevine. In: Roubelakis-Angelakis KA (ed) Molecular biology and biotechnology of the grapevine. Kluwer Academic Publishers, Dordrecht/Boston/London, pp 59–85
Lucena JJ (2000) Effects of bicarbonate, nitrate and other environmental factors on iron deficiency chlorosis. A review. J Plant Nutr 23:1591–1606
Marschner H (1995) Mineral nutrition of higher plants, II edn. Academic Press Limited, London, 889 p
McCluskey J, Herdman L, Skene KR (2004) Iron deficiency induces changes in metabolism of citrate in lateral roots and cluster roots of Lupinus albus. Physiol Plant 121:586–594
Mengel K (1994) Iron availability in plant tissues – iron chlorosis in calcareous soil. Plant Soil 165:275–283
Mengel K, Kirkby EA (2001) Principles of plant nutrition. Kluwer Academic Publishers, Dordrecht, The Netherlands
Mengel K, Malissiovas N (1982) Light-dependent proton excretion by roots of entire vine plants (Vitis vinifera L.). Z Pflanzenernaehr Bodenk 145:261–267
Mengel K, Planker R, Hoffmann B (1994) Relationship between leaf apoplast pH and iron chlorosis of sunflower (Helianthuus annuus L.). J Plant Nutr 17:1053–1065
Moing A, Maucourt M, Renaud C, Gaudillère M, Brouquisse R, Lebouteiller B, Gousset-Dupont A, Vidal J, Granot D, Denoyes-Rothan B, Lerceteau-Köhler E, Rolin D (2004) Quantitative metabolic profiling by 1-dimensional 1H-NMR analyses: application to plant genetics and functional genomics. Funct Plant Biol 31:889–902
Nikolic M, Römheld V (2003) Nitrate does not result in iron inactivation in the apoplast of sunflower leaves. Plant Physiol 132:1303–1314
Nikolic M, Römheld V, Merkt N (2000) Effect of bicarbonate on uptake and translocation of 59Fe in two grapevine rootstocks differing in their resistance of Fe deficiency chlorosis. Vitis 39:145–149
Nisi PD, Zocchi G (2000) Phosphoenolpyruvate carboxylase in cucumber (Cucumis sativus L.) roots under iron deficiency: activity and kinetic characterization. J Exp Bot 51:1903–1909
Ollat N, Laborde B, Neveux M, Diakou-Verdin P, Renaud C, Moing A (2003) Organic acid metabolism in roots of various grapevine (Vitis) rootstocks submitted to iron deficiency and bicarbonate nutrition. J Plant Nutr 26:2165–2176
Pasqualini S, Ederli L, Piccioni C, Batini P, Belluci M, Arcioni S, Antonielli M (2001) Metabolic regulation and gene expression of root phosphoenolpyruvate carboxylase by different nitrogen sources. Plant Cell Environ 24:
Rodriguez-Lovelle B, Gaudillère JP (2002) Carbon and nitrogen partitioning in either fruiting or non-fruiting grapevines: effects of nitrogen limitation before and after veraison. Aust J Grape Wine Res 8:86–94
Rombolà AD, Bruggemann W, López-Millán AF, Tagliavini M, Abadía J, Marangoni B, Moog PR (2002) Biochemical responses to iron deficiency in kiwifruit (Actinidia deliciosa). Tree Physiol 22:869–875
Rombolà AD, Gogorcena Y, Larbi A, Morales F, Baldi E, Marangoni B, Tagliavini M, Abadia J (2005) Iron deficiency-induced changes in carbon fixation and leaf elemental composition of sugar beet (Beta vulgaris) plants. Plant Soil 271:39–45
Römheld V (1987) Different strategies for iron acquisition in higher plants. Physiol Plant 70:231–234
Santi S, Cesco S, Varanini Z, Pinton R (2005) Two plasma membrane H+-ATPase genes differentially expressed in iron-deficient cucumber plants. Plant Physiol Biochem 43:287–292
Schmidt W (2003) Iron solutions: acquisition strategies and signalling pathways in plants. Trends Plant Sci 8:188–193
Shi Y, Byrne DH, Reed DW (1993) Iron chlorosis development and growth response of peach rootstocks to bicarbonate. J Plant Nutr 16:1039–1046
Smith F (1974) Malate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie/Academic Press, New York, pp 163–175
Smolders AJP, Hendriks RJJ, Campschreur HM, Roelofs JGM (1997) Nitrate induced iron deficiency iron deficiency chlorosis in Juncus acutiflorus. Plant Soil 196:37–45
Srere PA (1967) Citrate synthase. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic Press, New York, pp 3–11
Suárez MF, Avila C, Gallardo F, Canton FR, Garcia-Gutierrez A, Claros MG, Canovas FM (2002) Molecular and enzymatic analysis of ammonium assimilation in woody plants. J Exp Bot 53:891–904
Sun XP, Wang SY, Tong YA, Korcak RF, Faust M (1987) Metabolic changes in iron-deficient apple seedlings. J Plant Nutr 10:1021–1030
Susin S, Abadía A, Gonzalez-Reyes JA, Lucena JJ, Abadía J (1996) The pH requirement for in vivo activity of the iron-deficiency induced “turbo” ferric chelate reductase: a comparison of the iron-deficiency-induced iron reductase activities of intact plants and isolated plasma membrane fractions in sugar beet. Plant Physiol 110:111–123
Tagliavini M, Rombolà AD (2001) Iron deficiency and chlorosis in orchard and vineyard ecosystems. Eur J Agron 15:71–92
Toulon V, Sentenac H, Thibaud J-B, Davidian J-C, Moulineau C, Grignon C (1992) Role of apoplast acidification by the H+ pump. Effect on the sensitivity to pH and CO2 of iron reduction by roots of Brassica napus L. Planta 186:212–218
Vance CP, Stade S, Maxwell CA (1983) Alfalfa root nodule carbon dioxide fixation. I: association with nitrogen fixation and incorporation into amino acids. Plant Physiol 72:469–473
Varanini Z, Maggioni A (1982) Iron reduction and uptake by grapevine roots. J Plant Nut 5:521–529
von Wirén N, Gojon A, Chaillou S, Raper D (2001) Mechanisms and regulation of ammonium uptake in higher plants. In: Lea PJ, Morot-Gaudry J-F (eds) Plant nitrogen. Springer-Verlag, INRA éditions, Berlin, pp 61–77
Yi Y, Guerinot ML (1996) Genetic evidence that induction of root Fe(III) chelate reductase activity is necessary for iron uptake under iron deficiency. Plant J 10:835–844
Zerihun A, Treeby MT (2002) Biomass distribution and nitrate assimilation in response to N supply for Vitis vinifera L. cv. Cabernet Sauvignon on five Vitis rootstock genotypes. Aust J Grape Wine Res 8:157–162
Acknowledgments
This work was partly funded by the Franco-Spanish bilateral cooperation program (Picasso Program HF2003-273) and by Aquitaine-Aragón cooperation programs (Aq23, Aq25). Sergio Jiménez was supported by a I3P fellowship from CSIC/FSE (Consejo Superior de Investigaciones Científicas/Fondo Social Europeo) and a travel fellowship from DGA/CAI (CA 5/03). We would like to thank Catherine Deborde and Mickaël Maucourt for assistance with 1H-NMR analysis and data processing, Dr Annick Moing for critical reading of the manuscript, and Dr Tatiana Buhner for helpful English reading.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiménez, S., Gogorcena, Y., Hévin, C. et al. Nitrogen nutrition influences some biochemical responses to iron deficiency in tolerant and sensitive genotypes of Vitis . Plant Soil 290, 343–355 (2007). https://doi.org/10.1007/s11104-006-9166-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9166-4