Abstract
Iron (Fe), an important micronutrient and a critical determinant of plant growth and human nutrition, is deficient in cultivable soils and limits crop productivity and nutritional quality of food grains. Plants tolerant to Fe deficiency reveal either or all of these i.e., a higher uptake of Fe, better root to shoot partitioning of Fe or a higher remobilization of relatively immobile Fe. The physiological and biochemical regulators of in-plant Fe-remobilization are not clearly understood. The present study was conducted to elucidate the effect of Fe and nitrogen (N) deficiency, either alone or in combination, on plant growth attributes, shoot, root Fe and N uptake and Fe remobilization from a fully developed 2nd older leaf (OL) to a younger developing 3rd leaf (YL) in bread and durum wheat. Dual nutrient deficiency of N and Fe induced senescence, measured in terms of reduced chlorophyll and higher expression of NAM-B1activity. High nitrogen availability reduced Fe translocation as evident from a higher Fe retention in OL under N sufficient treatments (N+Fe+ and N+Fe−) than the N deficient treatments (N−Fe+ and N−Fe−) and could be correlated with transcript level expression of the DMAS gene. The present study provides evidence for the N and Fe deficiency induced senescence as the key determinant of Fe-remobilization in wheat, facilitated by a hyped biosynthesis of phytosiderophore. The results indicate that any favourable manipulation or selection for higher Fe remobilization process could improve nutrient deficiency tolerance of wheat and aid in grain biofortification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat is one of the first domesticated food crop and a widely cultivated cereal crop. It occupies over 223 million hectare world area and plays a significant role in providing global food security. From the total cereal consumption, wheat contributes about 41% of the dietary calories and 50% of the protein (Shiferaw et al. 2013). The introduction of nutrient intensive wheat and rice cultivars no doubt ushered the green revolution in 1960s but their continuous cultivation has deteriorated our soils of essential micronutrients, especially that of iron and zinc which are also important for meeting the dietary requirement of humans (Cakmak et al. 2010). Half of the 1.62 billion cases of anaemia recorded across the globe are attributed to acute iron deficiency (De Benoist et al. 2008). Further, the poor micronutrient status of soil also inhibits the availability of micronutrients for plant uptake and their content in grains. Widespread micronutrient deficiencies have been reported in Indian soils. It is estimated that nearly half of the cultivable soil is deficient in zinc followed by boron (33%) and iron (15%) (Shukla et al. 2014). Thus, the improvement of iron and zinc content in cereal grains has become a pivotal research area for agriculture scientist.
Iron (Fe) is an essential element for plant growth and development. It is required for the life sustaining processes such as photosynthesis and respiration. Iron is involved in the biosynthesis of amino levulinic acid (ALA), the precursor for chlorophyll biosynthesis. Iron is also an important constituent of various heme and non-heme Fe–S proteins. Fe is a key determinant for plant productivity and plant product quality (Briat et al. 2015). Iron deficiency in plants causes inter-veinal chlorosis of young leaves. Plants need to maintain a Fe concentration of 10− 9 to 10− 4 M for optimal growth, but iron acquisition in soil is challenging due to its low solubility in soil solution (Kim and Guerinot 2007).
Poor solubility and mobility of Fe, respectively, in the soil and the plant system are a major production challenge under condition of Fe limitation and that its uptake and translocation is regulated by the availability of external and internal Fe chelators viz., citrate, nicotianamine (NA) and deoxymugineic acid (DMA). This complex formation avoids precipitation of iron at the alkaline pH and at high phosphorous concentration found in phloem sap. Both NA and DMA are involved in phloem loading of Fe (Grillet et al. 2014). Three DMAS1 genes have been recently identified in bread wheat which play critical role in Fe uptake and Fe homeostasis during the early seedling growth (Beasley et al. 2017). It is believed that phloem transport limits the Fe and Zn content in cereal grains (White and Broadley 2011).
Leaf senescence is a process of programmed cell death which constitutes the final stage of leaf development. Most agricultural crops such as cereals show monocarpic senescence, where the entire plant dies during senescence and only few specialized tissues of seeds remains viable. During vegetative growth, senescing organs translocate nutrients to younger plant parts (Gan and Amasino 1997; Shi et al. 2012).During the reproductive stage of monocarpic plants, all tissues and organs of parental plant die, and nutrients are remobilized to the developing grains (Davies and Gan 2012). Senescence induced nutrient remobilization was associated with the higher grain protein and micronutrient concentration such as Fe and Zn in wheat (Waters et al. 2009). Abiotic stresses including nutrient stress particularly deficiency of macronutrients such as nitrogen may hasten the process of senescence (Aguera et al. 2010) and influence nutrient remobilization. Sufficient nitrogen supply resulted in reduction iron export from source to sink leaves in barley plants grown under nutrient solution culture (Shi et al. 2012).
Molecular regulation of senescence has been studied and it was shown recently that NAC transcription factor family (NAM/ATAF/CUC) plays an important role in regulating the process of senescence and nutrient quality of grain (Uauy et al. 2006; Christiansen and Gregersen 2014). In wheat, NAM-B1 (Gpc-B1) gene encodes for a NAC transcription factor and plays important role in regulating senescence and improves grain protein, iron and zinc content (Waters et al. 2009). In the present study, we hypothesized that N and Fe deficiency either alone or in combination will induce variable senescence to affect the magnitude of Fe remobilization from the source to the sink leaves in both bread and durum wheat.
Materials and methods
Plant growth conditions
Seeds of bread (cv. HD-2967) and durum (cv. HI-8713) wheat cultivars were treated with 0.5% solution of sodium hypochlorite (NaOCl3). Treated seeds were sown in autoclaved sand in plastic trays. Trays were kept in dark at 25 °C for proper germination. Trays were watered when required during germination. After 3 days into dark, trays were transferred into light source to prevent etiolation. Healthy non-etiolated seedlings at 5 days of growth were transferred to nutrient solution culture (Zhang et al. 1991) in 10 L glass tanks which were darkened on all sides to prevent algal growth. Four nutrient combinations of nitrogen and iron were used in the present experiment which constituted the four treatments i.e., nitrogen sufficient and iron sufficient control N+Fe+, 2 mM Ca(NO3)2 and 100 µM Fe-III EDTA; nitrogen sufficient and iron deficient N+Fe−, 2 mM Ca(NO3)2 and 1 µM Fe-III EDTA; nitrogen deficient and iron sufficient culture (N−Fe+) consist of 0.5 mM Ca(NO3)2 and 100 µM Fe-III EDTA; and; nitrogen deficient and iron deficient culture (N−Fe−) consist of 0.5 mM Ca(NO3)2 and 1 µM Fe-III EDTA. The concentration of calcium (Ca) in nitrogen deficient treatment was supplemented as CaCl2. Plants were grown in a growth chamber under 300 µmol m− 2 s− 1 PAR at leaf level and 14 h/10 h day/night regime (temperature 27 °C diurnal; 20 °C nocturnal; relative humidity 70–80%). A total of 120 plants per container were maintained in three container replicates per treatment.
Chlorophyll estimation
Induction of senescence under individual and combined deficiency of N and Fe was measured in terms of chlorophyll content in the oldest leaves (OL, 2nd fully developed leaf) of bread and durum wheat at 9, 11, 13 and 16 days after transfer (DAT) to the respective nutrient solution, as per method described by (Hiscox and Israelstam 1979). Total chlorophyll content was estimated following Arnon (1949) and was expressed as mg g− 1 fw.
Iron estimation
Iron concentrations were determined as per the following methods at 9, 13 and 16 DAT. Plant samples for 9 and 13 DAT were divided into shoot and root. However, samples collected at 16 DAT were divided into various plant organs such as oldest leaf (OL, essentially older 2nd leaf), youngest leaf (YL, younger 3rd leaf), remaining shoot (RS) and root. For iron estimation in plant samples, di-acid digestion method was used. Dried plant materials were divided into parts namely, roots, shoots and different plant organs (OL, YL, RS and Root) and were grinded and weighted accurately (100–200 mg) into clean, dry digestion flasks (100 ml). Up to 10 ml concentrated nitric acid (HNO3) and 4 ml perchloric acid (HClO4) were added into digestion tubes and the samples were allowed to stand over-night for pre-digestion. The tubes were placed on an open air digester maintained at temperature of 80 °C for 1 h and then at 200 °C until white fumes start emanating from the vials indicating an end of digestion process. After digestion, the solution was cooled to room temperature, diluted to 25 ml with deionized water, and filtered using whatman filter paper into plastic bottles. The iron concentration in digested tissue samples was determined using Atomic Absorption Spectrophotometry (AAS, ECIL make, India). Translocation Index of Fe (YL/OL) was calculated following the method of (Rengel and Graham 1996). Translocation index of Fe (YL/OL) = Fe content in young leaf (YL)/Fe content in old leaf (OL).
Nitrogen estimation
Nitrogen concentrations were determined at 9 and 13 DAT. Plant samples were dried and separated into shoot and root. For the determination of total nitrogen in plant samples Kjeldahl method was used (Kjeldahl 1883). In this method, organic nitrogen in the plant samples were converted into NH4-N by digestion with concentrated H2SO4 containing a catalyst mixture (Na2SO4 and CuSO4). The NH4-N in the digest is determined from the amount of NH3 liberated by distillation of the digest with NaOH. Ammonia absorbed in boric acid containing mixed indicator is determined by titration with a standard sulphuric acid. The total nitrogen in plant samples was expressed as %. Tissue N (%) = (S × 100)/Tissue mass in (g) where, S is amount (g) of N in shoot/root tissue = (ml of acid used for tissue - ml of acid used for blank) × normality of acid × 14 × 10− 3.
Plant biomass
Shoot, root and total biomass of bread and durum wheat plants raised on individual and combined deficiency of N and Fe and under N and Fe sufficient condition in the nutrient solution culture was measured at the end of the experiment i.e., at 16 DAT (Days after transfer to nutrient solution culture) to determine the treatment effect on the dry matter accumulation capacity. Shoot and root tissues were collected, separated and dried in hot air oven at 80 °C for 4 h and then at 60 °C till constant weights were achieved and their dry weights were recorded.
Expression analysis by quantitative real-time PCR (qRT-PCR)
Transcript expression level of NAM-B1 and DMAS genes were studied in oldest leaves (OL, 2nd fully developed leaf) sampled at 16 DAT in nitrogen and iron sufficient/deficient nutrient solution culture. Total RNA was extracted using material of three biological replicates via Trizol method. cDNA synthesis was carried out using RevertAid H Minus First Strand cDNA synthesis kit (Thermo scientific, USA) as per the instructions of the manufacturer’s protocol. Quantitative RT-PCR analysis was performed using KAPA SYBR Green qPCR mix with 100 ng of amplified cDNA in each PCR. Using the NAM-B1 and DMAS specific primers: forward, 5′CAAGCAACACACACCAGACC3′ and reverse, 5′AGATTCGAGGAAGCCCTGTT3′; forward, 5′ACGTGGACCTGTACCTCGTC3′ and reverse, 5′CCAGCTTCTTGCAGGAGAAA3′, respectively, on a Bio-Rad CFX96 machine. Forward and reverse primers of the target genes NAM-B1 and DMAS were synthesized using Primer3 software. Wheat β-Actin gene was used as an internal control gene with forward, 5′AGCGAGTCTTCATAGGGCGATTGT3′ and reverse, 5′TAGCTCTGGGTTCGAGTGGCATTT3′ primers. Relative expression levels of other target genes were quantified using the 2− ΔΔCt method (Pfaffl 2004).
Statistical analysis
All analyses were carried out in triplicate. Results are presented as mean ± standard deviation (SD). Experimental data were evaluated using analysis of variance (three-way ANOVA) and significant differences among the means of three replicates (p < 0.05) were determined by post-hoc multiple mean comparison Tukey’s honestly significant difference (HSD) test using Statistical Analysis System (SAS) software version 9.22 (SAS Institute Inc., Cary, NC, USA).
Results
Effect of N and Fe deficiency on leaf chlorophyll
Mean leaf chlorophyll averaged over growth stages, decline significantly under nutrient (N and Fe) deficiency than the nutrient sufficient control (N+Fe+). Across days of observation average total leaf chlorophyll over nutrient treatments increased from 9 DAT to 11 DAT followed by a decline over 13 DAT to 16 DAT in nutrient (N and Fe) deficient treatments. Bread wheat was more responsive to nitrogen and iron availability than durum wheat as evident from a higher increase in leaf chlorophyll in nutrient sufficient control (N+Fe+) from 9 to 16 DAT. Bread wheat maintained a relatively higher chlorophyll level than durum wheat even under nitrogen and iron deficient conditions (Table 1).
Effect of N and Fe deficiency on plant Fe concentration
Shoot of bread wheat accumulated a higher iron concentration than durum wheat on both 9 DAT and 13 DAT, iron sufficient treatments (N+Fe+ and N−Fe+) exhibited a higher iron level than iron deficient treatments (N+Fe− and N−Fe−) across the experimental wheat cultivars (Table 2). Iron concentration of N−Fe+ shoots of both bread and durum wheat were significantly lower than that of N+Fe+ shoots with an average decline of about 25% and 45% for bread and durum wheat, respectively.
Root iron concentration, in general, was higher than shoot iron concentration (Table 2). Bread wheat showed (30–40) % higher mean root iron concentration than the durum wheat particularly under N+Fe+ condition. Durum root accumulated a significantly lower iron than the bread wheat under nutrient (N, Fe) deficiency/ sufficiency combinations.
Effect of N and Fe deficiency on plant N concentration
Shoot nitrogen concentration in general was higher than root nitrogen level across the nitrogen and iron sufficient/deficient nutrient treatment combinations and the wheat cultivars. Highest shoot nitrogen levels were recorded for N+Fe+ followed by N+Fe− for both experimental wheat cultivars (Table 3).Treatments exhibiting nitrogen deficiency with or without iron, measured almost half shoot nitrogen than the nitrogen sufficient treatments. Least shoot nitrogen levels was recorded for N−Fe− treatment for both bread and durum wheat. Iron availability did not significantly improve the accumulation of nitrogen in the shoot as observed under N−Fe+, particularly for the bread wheat.
Pattern of variation for root nitrogen across cultivars, days of observation and nutrient (N and Fe) treatments was more or less similar to that of shoot nitrogen with nitrogen sufficient treatment combinations possessing more root nitrogen than nitrogen deficient nutrient treatments. Presence of iron appears to improve the uptake nitrogen as evident from significantly higher root nitrogen uptake under N+Fe+ than N+Fe− conditions for both experimental wheat cultivars (Table 3).
Plant growth attributes under N and Fe deficiency
Mean shoot mass of bread wheat averaged over nutrient treatments and stage of sampling, in general was higher than durum wheat (Table 4). Iron deficiency irrespective of nitrogen availability significantly reduced shoot growth of both wheat cultivars. However, the decline in shoot mass over nutrient sufficient control (N+Fe+) was higher under dual nutrient deficiency condition (N−Fe−) for durum than bread wheat.
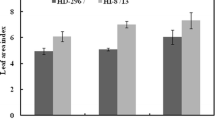
Root mass of both wheat cultivars increased with crop age and was higher under nutrient sufficient (N+Fe+) than under nutrient deficient (N−Fe−) condition (Table 4). The durum wheat produced more roots than bread wheat under combined deficiency of nitrogen and iron. Shoot to root ratio for biomass accumulation were also calculated (Fig. 1). Bread wheat cultivar, in general, showed higher S/R than the durum wheat under combined deficiency of N and Fe.
Shoot to root biomass ratio of bread (cv. HD-2967) and durum (cv. HI-8713) wheat averaged at 9 and 16 DAT, grown under individual and combined deficiency of nitrogen and iron in nutrient solution culture. Plants raised similarly in the presence of Fe and N served as nutrient sufficient control. Bars represent mean ± SD and different letters denote significant differences according to Tukey test at P < 0.05, n = 3
Effect of Fe and N deficiency induced senescence on retranslocation of Fe
A separate experiment was conducted where in iron concentration was measured in different in plant organs such as oldest leaf (OL, fully developed older 2nd leaf), younger leaf (YL, younger 3rd leaf), remaining shoot (RS) and roots of bread and durum seedlings raised on nitrogen and iron deficient and sufficient nutrient solution culture at 16 DAT. It is important to resubmit that at 16 DAT the leaf senescence has already set in for both experimental wheat cultivars under nitrogen and iron deficiency treatments as compared to the respective nutrient sufficient control (N+Fe+).
Plant organs, with an exception of older leaf possessed significantly higher iron concentrations under iron sufficient over iron deficient treatment combination across both the experimental cultivars of wheat (Fig. 2). Least iron concentration was measured under combined deficiency treatment of nitrogen and iron (N−Fe−) for all plant organs when compared with other nitrogen and iron treatments. A comparison of mean iron concentration between different plant organs reveals a higher accumulation of iron in the roots followed by remaining shoot (stem), older and younger leaf. Iron sufficient treatment irrespective of nitrogen availability showed a more iron accumulation in the older as well as the younger leaf. However, under iron deficiency and nitrogen sufficient treatment (N+Fe−) older leaf maintained a higher iron concentration than the nitrogen and iron deficient (N−Fe−) treatment combinations for wheat cultivars. Younger developing leaf of bread wheat accumulated a higher iron under combined deficiency of nitrogen and iron (N−Fe) than the deficiency of iron alone. Stem portion (RS) measured a least iron concentration under combined nutrient deficiency (N−Fe−) than the other nutrient treatments and when compared with iron accumulation in OL and YL of plants raised on (N−Fe−) treatments.
Tissue Fe concentration of bread (cv. HD-2967) and durum (cv. HI-8713) wheat in different plant organs at 16 DAT, grown under individual and combined deficiency of nitrogen and iron in nutrient solution culture. Plants raised similarly in the presence of Fe and N served as nutrient sufficient control. Bars represent mean ± SD and different letters denote significant differences according to Tukey test at P < 0.05, n = 3. Plant organs: OL: 2nd older leaf ; YL: 3rd younger leaf; RS: remaining shoot and Root tissue
Iron translocation index (YL Fe/OL Fe) was calculated by measuring the Fe content in young leaves to that of old leaves of bread and durum wheat cultivars growing under nitrogen and iron sufficiency or deficiency. Bread wheat, in general, measured two to three times higher iron translocation index than the durum wheat across different nutrient treatments. The YL Fe/OL Fe was highest in N−Fe+ treatment in both bread and durum wheat (Fig. 3).The lowest YL Fe/OL Fe was observed in case of N+Fe− treatment for both bread and durum wheat cultivars.
Relative translocation of Fe from older to younger leaf (Fe translocation index) of bread (cv. HD-2967) and durum (cv. HI-8713) wheat at 16 DAT, grown under individual and combined deficiency of nitrogen and iron in nutrient solution culture. Plants raised similarly in the presence of Fe and N served as nutrient sufficient control. Bars represent mean ± SD and different letters denote significant differences according to Tukey test at P < 0.05, n = 3
Effect of N and Fe deficiency on DMAS and NAM-B1 expression
DMAS gene involved in synthesis of DMA which chelates Fe and may determine the extent of Fe remobilization from the older leaves and was highly expressed in durum than bread wheat under Fe deficiency treatment with or without nitrogen. Relatively lower expression of DMAS was observed under iron sufficient treatments irrespective of nitrogen availability in both bread and durum wheat (Fig. 4). DMAS expression was almost two fold higher in durum than bread wheat under combined deficiency of nitrogen and iron. DMAS transcript level expression was induced the maximum under iron deficiency suggesting that DMAS activity is regulated by the iron availability.
Relative mRNA levels of DMAS in the 2nd oldest leaf (OL) of bread (HD-2967) and durum (HI-8713) wheat plants at 16 DAT, grown under individual and combined deficiency of nitrogen and iron in nutrient solution culture. Plants raised similarly in the presence of Fe and N served as nutrient sufficient control. Bars represent mean ± SD and different letters denote significant differences according to Tukey test at P < 0.05, n = 3
mRNA level of the NAM-B1 gene involved in senescence was induced under nitrogen and/or iron deficiency when compared with nitrogen and iron sufficient control in both bread and durum wheat cultivars. However, the induction of NAM-B1 was greater under nitrogen deficiency or under combined deficiency of nitrogen and iron when compared with NAM-B1 expression under nitrogen sufficient treatments (Fig. 5). Relative NAM-B1 expression in general and across nitrogen or iron deficiency treatments was higher for the bread than the durum wheat. Dual deficiency of N and Fe caused the highest expression of NAM-B1 across both the experimental wheat cultivars.
Relative mRNA levels of NAM-B1 in the 2nd oldest leaf (OL) of bread (HD-2967) and durum (HI-8713) wheat plants at 16 DAT, grown under individual and combined deficiency of nitrogen and iron in nutrient solution culture. Plants raised similarly in the presence of Fe and N served as nutrient sufficient control. Bars represent mean ± SD and different letters denote significant differences according to Tukey test at P < 0.05, n = 3
Discussion
Senescence reflects the initiation of final leg of growth and development of a plant tissue or organ and is marked by degradation of chlorophyll and numerous other molecular and biochemical changes that lead to widespread metabolic catalysis and loss of cellular integrity and cell function. Onset of senescence is also accompanied by remobilization of phloem-mobile nutrients from the senescing mature developed source to the developing sinks, such as mature leaf or seed. Process of senescence can come naturally to a plant at the final end of its life cycle or can get induced by different biotic or abiotic factors that adversely affect the plant growth. It sets into motion a chain of events that involve remobilization of plant mineral nutrients and breakdown of various nitrogen-containing macromolecules (proteins and nucleic acids), all of which are transported to the demanding and developing sink through phloem loading and transport.
Plants need to cope up with a highly fluctuating availability of soil nutrients which not only affects the direct uptake of nutrient in question but also the indirect availability and uptake of other interacting nutrient ions. Plants, however, possess several strategies to adapt under nutrient stress, which involves realigning the nutrient uptake, their translocation and efficiency of nutrient utilization in a manner sufficient to reduce the physiological and molecular aberrations aimed to achieve partial to optimum nutrient deficiency tolerance. The strategy on nutrient acquisition involves overexpression of genes encoding high affinity nutrient specific root transporters together with favorable root characteristics that allow exploration of larger soil volumes (Giehl and von Wirén 2014).
A less worked out mechanism operates through remobilization of nutrients from the storage organs or fully developed mature plant organs upon initiation of senescence into the developing reproductive sinks or to the other developing plant organs during vegetative growth. It may compensate for transient low soil availability and uptake of nutrients by roots as has been clearly shown for N and sulfur (Abdallah et al. 2010). Phloem mediated remobilization of nutrients has been evidenced as the major determinant ensuring nutrient availability to the younger growing plant organs and plant nutrient use efficiency (Avice and Etienne 2014). Thus, nutrient remobilization depends on the deficiency of the nutrient i.e., macro or micro nutrient and also on severity of deficiency which was assessed in terms of reduction in plant growth and on set of senescence related changes in plant metabolism. Loss of leaf chlorophyll was regarded as a simple measure of senescence at the plant level. Multiple deficiency of nutrients further hastened the rate of chlorophyll degradation (Khobra and Singh 2017). Leaf senescence is related to progressive degradation of its chlorophyll pigment. Significant decline in leaf chlorophyll was measured for both bread and durum wheat cultivars under N and Fe deficiency at 13 DAT and beyond, however, under N+Fe+ treatment leaf chlorophyll continued to rise during this period. Pattern of chlorophyll degradation was similar for N- and /or Fe− treatment. The results helped in marking the growth stage for the onset of leaf senescence at 13DAT.
Further, the bread and the durum wheat are known to differ in their iron deficiency tolerance (Aciksoz et al. 2011), however, their response under combined deficiency of N and Fe is far from clear. A measure of shoot and root Fe and N level under different N/Fe deficient and sufficient treatments shows the execution of flawless experimental setup, with a lower accumulation of plant tissue Fe under Fe deficiency than under Fe sufficiency. Similarly, an almost two fold higher mean N accumulation was measured in plant tissues of N+ than N− treatments. Least accumulation of Fe and N were measured under N−Fe− treatment for both bread and durum wheat, with shoot accumulating a higher amount of both Fe and N than the root tissues across nutrient treatments. The cultivar difference in Fe accumulation was particularly evident under nutrient deficiency than nutrient sufficient condition of growth. Positive interaction of N nutrition on Fe accumulation was evident, with observed 20–30 per cent reduction in both shoot and root iron concentration under N−Fe+ than N+Fe+ treatment. Earlier studies also indicate an increase in micronutrient acquisition at high N supplies (Kutman et al. 2012) and vice versa. This can be explained based on the fact that a higher biomass under N+ may simply generate a higher plant demand and acquisition of Fe than that originating from a low biomass under N- treatment. On the other hand N accumulation, in general was not affected by iron availability. High N supply is reported to improve not only the acquisition of micronutrients but also their translocation to the shoot (Kutman et al. 2012; Barunawati et al. 2013).
Results of the present study corroborate the above observation with bread wheat producing more shoot mass but lesser root mass than the durum wheat under N and Fe deficiency when compared to their respective N+Fe+ control treatment. Between the bread and durum wheat, a higher shoot mass of bread wheat indicated relative nutrient stress tolerance while, a higher root mass of durum wheat indicated relative nutrient stress susceptibility, as is also evident from the respective shoot to root ratio values for the bread and durum wheat cultivars. Another experiment conducted to assess the pattern of Fe retention in different plant organs such as older 2nd leaf (OL), younger 3rd leaf (YL), remaining shoot (RS) and root showed that the plant organs accumulated Fe in the following order root > OL ˃RS > YL, when averaged over the nutrient deficient/ sufficient treatments. Bread wheat, in general, accumulated more tissue Fe than the durum wheat. Significantly low Fe accumulation was measured in all plant organs except RS under Fe deficiency than under Fe sufficiency. Role of N in influencing Fe acquisition as well Fe partitioning was evident under both Fe sufficient and Fe deficient condition. A relatively lower Fe concentration of OL under nitrogen deficiency than under nitrogen sufficiency was measured and that the effect was independent of Fe availability. Opposing effect of N supply on Fe retranslocation from the source to the developing sink has been reported (Shi et al. 2012). Our findings reveal that iron export from the senescing leaf is inhibited under high N supply and that a low N availability facilitates the export/ retranslocation of iron from the older to the younger developing leaf. High YL/OL Fe under N−Fe+ than N+Fe+ and under N−Fe− than N+Fe− clearly evidences the involvement of N in influencing the partitioning of Fe at the plant level irrespective of the Fe availability for growth and development.
Further, involvement of DMA in Fe mobilization within senescing source leaf was also determined. In this regards, the graminaceous plants are known to adapt to iron deficiency through secretion of phytosiderophore “metallophore” of mugineic acid (MA) family which facilitate in Fe acquisition and homeostasis at the plant level. All MAs are synthesized from methionine to 2-deoxymugineic acid (DMA) via a common pathway involving reduction of 3′ keto intermediate to DMA by deoxymugineic acid synthase (DMAS) and is a major phytosiderophore in root exudates of wheat, rice and maize (Shojima et al. 1990). While working with winter wheat, Barunawati et al., (2013) have highlighted the importance of DMA for the transport and retranslocation of micronutrients to and from the flag leaf into the developing reproductive sink i.e., the grain. However, most Fe chelators including DMA also complex with other metal micronutrients. Present results report Fe deficiency induced upregulation of DMAS gene expression and that the expression is independent of N nutrition. Bashir et al., (2006) showed that under Fe sufficient condition, OsDMAS1 expression is observed only in the root portion unlike that of Fe deficient condition wherein a large activity of DMAS gene and that of DMA was found in the shoot, indicating the involvement of shoot synthesized DMA in Fe retranslocation and not in Fe acquisition from the soil. However, sources for Fe remobilization during leaf senescence are not clear. Ferritin-Fe or protein-Fe whether has ferrous or ferric forms. Although DMA has a higher affinity for ferric (FeIII) form, substantial amount of FeIII-DMA complex were not detected in senescing leaf (Koster et al. 2011).
A closely knitted network is postulated between onset of leaf senescence, nutrient remobilization, nutrient use efficiency and grain micronutrients (Distelfeld et al. 2014). Recent characterization of NAM-B1, a NAC transcription factor, as a regulator of plant stress response and that of macro and micronutrient translocation from the vegetative source to the reproductive sink makes it a key determinant of Fe-remobilization efficiency during senescence in wheat (Asplund et al. 2013). N and Fe deficiency in the present study significantly improved the transcript level expression of NAM-B1 in the following order of nutrient availability: N−Fe−> N−Fe+ > N+Fe−>N+Fe+ more so in bread than in durum wheat. Highest induction under combined deficiency of N and Fe not only signifies a relatively greater intensity/induction of senescence related changes but also suggests a greater efficiency for iron remobilization for this treatment compared to N+ or Fe+ treatment combinations. Induction of NAM-B1 is believed to increase grain protein and its manipulation could help breeders to improve grain N and grain micronutrient quality (Uauy et al. 2006; Pearce et al. 2014). However, lines with functional Gpc-B1 or NAM-B1 gene would also mean an early senescence which on one hand will improve grain quality but may also reduce the overall yield (Distelfeld et al. 2014), depending upon the growing environment and relative availability of plant acquired nutrients.
Conclusion
This study clearly shows that N management and/or N-nutritional status is a critical determinant of Fe-remobilization in a plant and regulates the translocation of Fe from the fully developed senescing leaf to the growing/ demanding sink i.e., leaf/grain. Deficiency of N and Fe alone or in combination induced senescence to better the Fe remobilization. Bread wheat in general possessed a higher nutrient deficiency tolerance than the durum wheat. The study indicates that the DMA production may hold the key for in-plant Fe mobility as evident from an increased relative expression of DMAS gene under Fe deficiency and that the relative expression of DMAS was higher in durum than bread wheat which is in line with the nutrient susceptibility nature of the later group. Results of the present study improve our understanding of the genetic and the physiological determinants of micronutrient remobilization during senescence and may help researchers working on biofortification of food crops.
Author contribution statement
SP performed the experimental work and wrote the draft manuscript, RRK helped with studies on expression analysis, AA helped with the physiological studies and BS supervised the research and finalized the manuscript.
References
Abdallah M, Dubousset L, Meuriot F, Etienne P, Avice JC, Ourry A (2010) Effect of mineral sulphur availability on nitrogen and sulphur uptake and remobilization during the vegetative growth of Brassica napus L. J Exp Bot 61(10):2635–2646
Aciksoz SB, Yazici A, Ozturk L, Cakmak I (2011) Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 349(1–2):215–225
Agüera E, Cabello P, De La Haba P (2010) Induction of leaf senescence by low nitrogen nutrition in sunflower (Helianthus annuus) plants. Physiol Plant 138(3):256–267
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1
Asplund L, Bergkvist G, Leino MW, Westerbergh A, Weih M (2013) Swedish spring wheat varieties with the rare high grain protein allele of NAM-B1 differ in leaf senescence and grain mineral content. PLOS One 8(3):59704
Avice JC, Etienne P (2014) Leaf senescence and nitrogen remobilization efficiency in oilseed rape (Brassica napus L.). J Exp Bot 65(14):3813–3824
Barunawati N, HettwerGiehl RF, Bauer B, Von Wirén N (2013) The influence of inorganic nitrogen fertilizer forms on micronutrient retranslocation and accumulation in grains of winter wheat. Front Plant Sci 4:320
Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 281(43):32395–32402
Beasley JT, Bonneau JP, Johnson AA (2017) Characterisation of the nicotianamine aminotransferase and deoxymugineic acid synthase genes essential to Strategy II iron uptake in bread wheat (Triticum aestivum L.). PLOS One 12(5)
Briat JF, Dubos C, Gaymard F (2015) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci 20(1):33–40
Cakmak I, Pfeiffer WH, McClafferty B (2010) Review: biofortification of durum wheat with zinc and iron. Cereal Chem 87(1):10–20
Christiansen MW, Gregersen PL (2014) Members of the barley NAC transcription factor gene family show differential co-regulation with senescence-associated genes during senescence of flag leaves. J Exp Bot 65:4009–4022
Davies PJ, Gan S (2012) Towards an integrated view of monocarpic plant senescence. Russ J Plant Physiol 59(4):467–478
De Benoist B, McLean E, Egli I, Cogswell M, Cogswell M (2008) WHO global database on anaemia. WHO, Geneva, pp 1993–2005
Distelfeld A, Avni R, Fischer AM (2014) Senescence, nutrient remobilization, and yield in wheat and barley. J Exp Bot 65(14):3783–3798
Gan S, Amasino RM (1997) Making sense of senescence (molecular genetic regulation and manipulation of leaf senescence). Plant Physiol 113(2):313
Giehl RF, von Wirén N (2014) Root nutrient foraging. Plant Physiol 166(2):509–517
Grillet L, Mari S, Schmidt W (2014) Iron in seeds—loading pathways and subcellular localization. Front Plant Sci 4:535
Hiscox JT, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57(12):1332–1334
Khobra R, Singh B (2017) Phytosiderophore release in relation to multiple micro nutrient metal deficiency in wheat. J Plant Nutr 41(6):679–688
Kim SA, Guerinot ML (2007) Mining iron: iron uptake and transport in plants. FEBS lett 581(12):2273–2280
Kjeldahl J (1883) A new method for the determination of nitrogen in organic matter. Am J Analyt Chem 22(1):366–382
Köster J, Shi R, Von Wiren N, Weber G (2011) Evaluation of different column types for the hydrophilic interaction chromatographic separation of iron-citrate and copper-histidine species from plants. J Chromatogr A 1218(30):4934–4943
Kutman UB, Kutman BY, Ceylan Y, Ova EA, Cakmak I (2012) Contributions of root uptake and remobilization to grain zinc accumulation in wheat depending on post-anthesis zinc availability and nitrogen nutrition. Plant Soil 361(1–2):177–187
Pearce S, Tabbita F, Cantu D, Buffalo V, Avni R, Vazquez-Gross H, Dubcovksy J (2014) Regulation of Zn and Fe transporters by the GPC1 gene during early wheat monocarpic senescence. BMC Plant Biol 14(1):1
Pfaffl MW (2004) Quantification strategies in real-time PCR. AZ Quant PCR 1:89–113
Rengel Z, Graham RD (1996) Uptake of zinc from chelate-buffered nutrient solutions by wheat genotypes differing in zinc efficiency. J Exp Bot 47(2):217–226
Shi R, Weber G, Köster J, Reza-Hajirezaei M, Zou C, Zhang F, von Wirén N (2012) Senescence-induced iron mobilization in source leaves of barley (Hordeumvulgare) plants. New Phytol 195(2):372–383
Shiferaw B, Smale M, Braun HJ, Duveiller E, Reynolds M, Muricho G (2013) Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur 5(3):291–317
Shojima S, Nishizawa NK, Fushiya S, Nozoe S, Irifune T, Mori S (1990) Biosynthesis of phytosiderophores in vitro biosynthesis of 2′-deoxymugineic acid from L-methionine and nicotianamine. Plant Physiol 93(4):1497–1503
Shukla AK, Tiwari PK, Prakash C (2014) Micronutrients deficiencies vis-a-vis food and nutritional security of India. Indian J Fertil 10(12):94–112
Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314(5803):1298–1301
Waters BM, Uauy C, Dubcovsky J, Grusak MA (2009) Wheat (Triticum aestivum) NAM proteins regulate the translocation of iron, zinc, and nitrogen compounds from vegetative tissues to grain. J Exp Bot 60(15):4263–4274
White PJ, Broadley MR (2011) Physiological limits to zinc biofortification of edible crops. Front Plant Sci 2:80
Zhang FS, Römheld V, Marschner H (1991) Diurnal rhythm of release of phytosiderophores and uptake rate of zinc in iron-deficient wheat. J Soil Sci Plant Nutr 37(4):671–678
Acknowledgements
SP acknowledges funding support in terms of ICAR scholarship for the Masters program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest.
Additional information
Communicated by G. Klobus.
Rights and permissions
About this article
Cite this article
Parveen, S., Ranjan, R.K., Anand, A. et al. Combined deficiency of nitrogen and iron increases senescence induced remobilization of plant immobile iron in wheat. Acta Physiol Plant 40, 211 (2018). https://doi.org/10.1007/s11738-018-2782-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2782-9