Abstract
Key message
(1) The fes1a bag6 double mutant shows an increased short term thermotolerance compared to fes1a. BAG6 is a suppressor of Fes1A; (2) IQ motif is essential to effective performance of BAG6. (3) Calmodulin was involved in signal transduction. (4) BAG6 is localized in the nucleus.
Abstract
HSP70s play an important role in the heat-induced stress tolerance of plants. However, effective HSP70 function requires the assistance of many co-chaperones. BAG6 and Fes1A are HSP70-binding proteins that are critical for Arabidopsis thaliana thermotolerance. Despite this importance, little is known about how these co-chaperones interact. In this study, we assessed the thermotolerance of a fes1a bag6 double mutant. We found that the fes1a bag6 double mutant shows an increased short-term thermotolerance compared to fes1a. However, calmodulin inhibitors diminished this enhanced thermotolerance in the fes1a bag6 double mutant. In addition, we found the IQ motif to be essential for effective BAG6 performance. Since BAG6 is localized in the nucleus, the signal transduction is likely to involve nuclear calcium signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have developed various strategies for high-temperature stress resistance. For example, brief exposure to sub-lethal temperatures in one study resulted in plants that were able to survive more severe heat stress (Vierling 1991). However, this feature (termed acquired thermotolerance) is only exhibited when heat shock proteins (HSPs) acclimate. For example, HSP70 is specifically able to disperse protein aggregates and to facilitate the re-folding of heat-denatured proteins. Overexpression of cytosolic AtHSC70-1 resulted in an increase in basal thermotolerance (Sung and Guy 2003). However, a decrease in the expression of cytosolic HSP70s in Arabidopsis thaliana led to a 2 °C reduction in the survival temperature of the antisense lines when compared to the wild type (WT) (Lee and Schöffl 1996). Furthermore, co-chaperones are essential to some HSP performance. For example, HSP70s, only in assistance of nucleotide exchange factors (NEFs) promote the re-folding of heat-denatured proteins and also facilitate the folding of newly synthesized proteins (Kityk et al. 2012; Mayer 2013).
HSP70s are composed of a highly conserved N-terminal nucleotide-binding domain (NBD) with ATPase activity and a substrate-binding domain (SBD). SBD substrates are processed with the energy generated by ATP hydrolyzation. Only when ADP is liberated from NBD does HSP70 release the substrate. After the substrate is released, the next catalytic cycle begins. In the functional cycle of HSP70, the disassociation of ADP with HSP70 is a rate-limiting step that is accelerated by NEFs (Shomura et al. 2005). In plants, the Fes1A protein is a NEF that increases molecular chaperone efficiency (Fu et al. 2015). Furthermore, the knockout of Fes1A results in HSP70 degradation and reduced thermotolerance (Zhang et al. 2010).
In addition to performing other diverse roles, plant BAG (Bcl-2-associated-athanogene) proteins are thought to function as NEFs (Doukhanina et al. 2006). Arabidopsis has seven BAG homologues; BAG1–4 are similar to animal BAGs, while BAG5–7 each have a calmodulin-binding domain (Doukhanina et al. 2006). In transgenic tobacco, Arabidopsis BAG4 confers tolerance to a wide range of abiotic stresses, such as UV light, cold, oxidants, and salt (Doukhanina et al. 2006). BAG6, the longest protein in the BAG family, is involved in programmed cell death (Kang et al. 2006), which aids in cell defense against pathogens (Kabbage and Dickman 2008; Li et al. 2017a). BAG6 also improves basal thermotolerance in Arabidopsis (Echevarría-Zomeño et al. 2016).
Using Saccharomyces cervisae, Alberti et al. (2004) proposed a model to describe how HSP70 co-chaperones cooperate and compete in interactions with HSP70 to affect the destiny of the HSP70 substrate. In the model, the carboxyl-terminus HSP70-interacting protein (CHIP) is responsible for directing the degradation of the HSP70-targeted protein. However, the initiation of this process is determined by competition between BAG1 and HSPBP1 (HSP70-binding protein one which is a homolog of Fes1) (Alberti et al. 2004). While BAG1 associates with the ATPase domain of HSC70, CHIP induces the degradation of the HSP70 client protein (Alberti et al. 2004). However, HSPBP1 antagonistically prevents the degradation of HSP70 by inhibiting CHIP ubiquitin ligase activity (Alberti et al. 2004). Despite these findings, the degree of cooperation and/or antagonism between these plant HSP70 co-chaperones remains largely unknown.
Heat stress can destroy membrane integrity and disturb intracellular Ca2+ levels; therefore, Ca2+ and Ca2+-regulated pathways are likely to be involved in thermotolerance (Dat et al. 1998; Larkindale and Knight 2002; Sangwan et al. 2002). AtCaM3 knockout mutants showed a clear reduction in thermotolerance and exhibited down-regulated accumulation of HSPs (Zhang et al. 2009). Thus, AtCaM3 is in the heat stress signal transduction pathway. In addition, the nuclear localized OsCaM1-1 mediates the signaling of acquisition thermotolerance in rice (Wu et al. 2012), and the nuclear calcium signaling might affect the thermotolerance. BAG6 contains an IQ domain and therefore may be capable of binding calmodulin (CaM) (Kang et al. 2006), but little is known about the potential connection between the function of BAG6 and CaM.
In this experiment, we first explored the subcellular location of BAG6. By evaluating alterations in acquired thermotolerance of a fes1a bag6 double mutant, we then determined the cooperative dynamics between BAG6 and Fes1A. Finally, the correlation between calmodulin and the performance of BAG6 in thermotolerance was examined.
Results
BAG6 is a heat-induced nuclear protein
Western blot indicated that Arabidopsis grown at 22 °C did not express BAG6 protein (Fig. 1). However, BAG6 molecules with a 180 kDa molecular weight began to markedly accumulate when Arabidopsis was exposed to high temperatures in the range of 36–38 °C (Fig. 1; Supplementary Fig. S1a). When heat-treated Arabidopsis was homogenized with 100 mM Tris-HCl (pH 8.0) and subjected to 10,000×g centrifugation for 30 min, no BAG6 protein was detected in the supernatant. Despite the fact that it was very abundant in the centrifugation pellet (Fig. 2a). We isolated the nucleus and the tonoplast membrane from Arabidopsis. Histone 3 (nuclei protein marker) and Vacuolar H+-pyrophosphatase (vacuolic marker protein) Western blot results showed that there was no contamination from vacuolic marker proteins in purified nuclei and no contamination from Rubisco (Fig. 2b). Histone 3 and BAG6 were concurrently concentrated in the purified nuclear fraction (Supplementary Fig. S1b). When Histone 3 was used as a loading control for Western blot, the ratio of BAG6 to Histone 3 in the total protein content almost equaled that in the purified nuclei fraction (Fig. 2b; Supplementary Fig. S1c). This suggests that BAG6 is a nucleus protein. In contrast, no BAG6 protein was detected in the purified fraction of tonoplast membrane (Supplementary Fig. S1d). Furthermore, GFP images of root cells (Fig. 2c; Supplementary Fig. S2a and b) and guard cells (Supplementary Fig. S2c) indicated that the BAG6–GFP fusion was located in the nucleus. This result was found for both the BAG6–GFP transgenic plants grow at room temperature and plants exposed to high temperature (Supplementary Fig. S2d). These findings are consistent with the conclusion from the determination of BAG6 in the isolated nucleus fraction.
Heat-induced expression of BAG6. The 10-day-old seedlings grown at 22 °C were exposed to a range of high temperatures for 2 h, and total protein was then extracted. BAG6 was immunodetected with the anti-BAG6 antibody. The bag6 mutant exposed to high temperature was used as a negative control. Actin functioned as a loading control
The enrichment of BAG6 in the purified nucleus fraction and the sub-cellular location of BAG6 as shown by confocal images of BAG6–GFP fusion. a BAG6 is evident in the pellet by differential centrifugation between 500 and 10,000×g, but it is not detected in the supernatant after 10,000×g centrifugation for 30 min. b The detection of BAG6 in the purified cell nucleus fraction. Nuclear and vacuolar marker proteins (i.e., Histone 3 and V-PPase) were determined. Histone 3 was used as a loading control. c Confocal images of BAG6–GFP fusion in root tip cells of a transgenic Arabidopsis. The green and blue light channels show BAG6–GFP fluorescence and DAPI fluorescence, respectively. The two light channel images are merged
BAG6 is a suppressor of the Fes1A mutant
Fes1A is a nucleus- or cytosolic-located protein. Therefore, only the nucleus- or cytosolic-located BAG proteins, BAG 1–4 and 6, were included in this study on the cooperation between Fes1A and BAG. PCR and sequencing results indicated that all bag1–4 and 6 T-DNA mutants were null mutants (Supplementary Fig. S3). Salk_017591C harbored a T-DNA insertion at the 5′-UTR of the BAG1 gene; salk_030295 had a T-DNA insertion at the fourth exon of the BAG2 gene; the T-DNA in salk_124153C was located in the first intron of the BAG3 gene; and the BAG4 gene was interrupted by a T-DNA insertion in the first exon (salk_052541) (Supplementary Fig. S4). Since BAG6 was the primary research focus for this study, we included and identified two independent bag6 mutants. One mutant has a T-DNA insertion in the second exon (salk_047959C), while another was interrupted by a T-DNA insertion in the third exon (salk_073331C) (Supplementary Fig. S4). Despite the knockout of these BAG genes, the growth of these single bag mutants was normal and all presented phenotypes that were the same as the WT (Supplementary Fig. S5).
Acquired thermotolerance evaluation (Fig. 3a) indicated that each of these bag mutants showed no change in acquired thermotolerance (Fig. 3b, c, f, g) when compared to the WT. This suggests that a single mutation of these BAG genes has no effect on their acquired thermotolerance.
Thermotolerance in fes1a or bag single mutants and in fes1a bag double mutants. a The 10-day-old seedlings were first acclimated at 38 °C for 2 h, followed by a lethal heat treatment at 45 °C for 1–3 h. Subsequently, the seedlings were transported to a growth chamber with normal conditions for recovery. The phenotypes were photographed after 10 days. b, c After exposure to the high temperature stress, bag1, bag2, bag3, and bag4 mutant phenotypes were not different from those of the WT. Likewise, there were no differences between the WT and the two bag6 mutants (f, g). d, e There was no change in thermotolerance in fes1a plus bag1, bag2, bag3, or bag4 double mutants. However, the two fes1a bag6 (fes1a bag6-1 and fes1a bag6-2) double mutants manifested improved thermotolerance compared to fes1a (f, g)
To assess the effect of the second mutation at BAGs in fes1a, we bred the fes1a bag series of double mutants. These double mutants grew normally at room temperature (Supplementary Fig. S5), but some of them exhibited differences in acquired thermotolerance. The results of thermotolerance evaluations for the fes1a bag series of double mutants revealed that the second mutation of BAG1, BAG2, BAG3, or BAG4 in fes1a did not alter the heat sensitivity in fes1a (Fig. 3d, e). However, knockout of BAG6 notably increased thermotolerance in fes1a (Fig. 3f, g). Nonetheless, the bag6 single mutant exhibited no change in acquired thermotolerance, and our second independent replicate of the fes1a bag6 mutant confirmed this pattern.
We selected HSP70 and HSP17.7 as representatives to determine HSP expressions in the fes1a bag6 mutants. At the heat acclimation stage, the fes1a, bag6, and fes1a bag6 mutants accumulated approximately equal amounts of HSP70 and HSP17.7, but the fes1a bag6 double mutant expressed more HSP at the recovery stage, especially HSP17.7 (Fig. 4). This suggests that BAG6 depressed HSP expression in fes1a.
Differential expression of HSPs among Arabidopsis fes1a and bag6 single mutants, fes1a bag6 double mutants, and WT plants. Ten-day-old seedlings were first acclimated at 38 °C for 2 h, followed by a sub-lethal heat treatment at 44 °C for 2 h. Subsequently, seedlings were transported to a growth chamber with normal conditions for recovery (Re). At the indicated times (shown at the top of this figure), total proteins were extracted for Western blot. Actin was used to confirm equal protein loading
BAG6 binds HSP70
BAG6 possesses a commonly conserved BAG domain, which is likely responsible for association with HSP70. Via co-immunoprecipitation, we investigated the possible binding between BAG6 and HSP70 in WT and fes1a mutant. BAG6, regardless of Fes1A knockout, was able to bind HSP70 in vivo (Fig. 5). Thus, the capacity of BAG6 or Fes1A to bind to HSP70 appears to be independent.
Binding of Arabidopsis BAG6 with HSP70. Ten-day-old plants were subjected to 2 h treatment at 38 °C and used for co-immunoprecipitation. HSP70 in the homogenate of the bag6-1, fes1a, or WT were trapped by BAG6–IgG/protein A agarose; resulting HSP70 was determined with Western blot. Inputs of proteins are indicated
Calmodulin is involved in fes1a bag6 thermotolerance
BAG6 contains a characteristic IQ motif capable of CaM (Kang et al. 2006). To determine the contribution of the IQ motif to BAG6 performance, we substituted three highly conserved residues (575I → A, 576Q → A, and 580R → A) in the IQ motif (this mutant is referred to hereafter as BAG6–mIQ). Subsequently, we studied the subcellular localization of BAG6–mIQ proteins. We saw instant expression of GFP fusion in relevant onion epidermis cells, which indicates that both fusion proteins (i.e., BAG6–GFP and BAG6–mIQ–GFP) were located in the nucleus (Fig. 6a). Utilizing a heat inducible promoter, we generated the BAG6–mIQ and BAG6 complementary lines with the genetic background of the fea1a bag6 double mutant (Fig. 6b; Supplementary Fig. S6a). Western blot of BAG6 in the isolated nucleus fraction indicated that the heat-induced complementary BAG6–mIQ and BAG6 proteins were intensely enriched in the nucleus material from BAG6–mIQ and BAG6 complementary lines (Supplementary Fig. S6b). This suggests that the complementary BAG6–mIQ and BAG6 proteins entered the nucleus and that mutation of the IQ motif did not change the subcellular localization of BAG6. Thermotolerance evaluation showed that overexpression of BAG6–mIQ had no effect on the thermotolerance of the fes1a bag6 double mutant (Fig. 6c), while the complementary expression of BAG6 proteins in the fes1a bag6 double mutant resulted in a decrease of thermotolerance in the fes1a bag6 (Fig. 6d). These results suggest that the IQ motif is an essential domain for BAG6.
The BAG6–mIQ (IQ mutated BAG6) and BAG6 were introduced to fes1a bag6 double mutants to complement the loss of BAG6, respectively. a A BAG6–mIQ–GFP fusion and a BAG6–GFP fusion were introduced into onion epidermal cells via a gene gun. Confocal images of both BAG6–mIQ–GFP and BAG6–GFP (control) fusions are shown. b The presence of BAG6–mIQ and BAG6 proteins in complementary lines was examined with Western blot. Rubisco was used as a loading control. c Acquired thermotolerance in two OE–BAG6–mIQ complementary lines (mIQ-1 and -2). d Acquired thermotolerance in two OE–BAG6 complementary lines (BAG6–OE-1 and -2). The temperature regime used for this trial is identical to that depicted in Fig. 3a
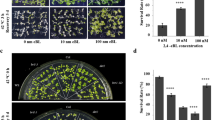
When 10 µM of trifluoperazine (TFP) and 60 µM of W7 were included in the culture medium, the acquired thermotolerance of the fes1a bag6 double mutant was identical to that of fes1a (Fig. 7). However, the fes1a bag6 double mutant manifested its thermotolerance better than the fes1a in Murashige–Skoog (MS) medium (Fig. 3f, g). In other words, the effect of BAG6 was diminished when the function of calmodulin was inhibited.
Effect of the calmodulin inhibitors TFP and W7 on fes1a thermotolerance-enhancing suppressor BAG6. Then 10 µM TFP (left) and 60 µM W7 (right) were included in the culture medium. After 10 days of growth, Arabidopsis seedlings were evaluated for acquired thermotolerance (as depicted in Fig. 3a)
Discussion
BAG6 is a nuclear-located protein
Homologs of the Arabidopsis BAG protein family are divided into groups and are identified by the numbers one through seven (Doukhanina et al. 2006). Some of their sub-cellular localizations have been determined. BAG7 is constitutively located in the ER, and it is involved in modulating cell death pathways as part of the unfolded protein response during heat stress (Williams et al. 2010). When plants are subjected to high temperatures, BAG7 undergoes SUMOylation, proteolytic cleavage, and translocation from the ER to the nucleus (Li et al. 2017b). Although BAG1–BAG4 contain ubiquitin-like and BAG domains, their sub-cellular localizations are different. BAG1–3 are located mainly in the cytosol, whereas BAG4 is both a cytosolic and nuclear protein (Lee et al. 2016). BAG1 acts as a cofactor in HSC70-mediated proteasomal degradation of unimported plastid proteins (Lee et al. 2016). Meanwhile, BAG5 is a mitochondrial protein and is involved in regulation of this organelle’s Ca2+ levels. Studies have found that BAG5 affects leaf senescence (Li et al. 2016; Fu et al. 2017).
At the beginning of our experiment, we performed Western blot on BAG6. We homogenized the heat-treated Arabidopsis sample with 100 mM Tris-HCl (pH8.0) and collected the supernatant after 10,000×g centrifugation (Fig. 2). No BAG6 was detected in the supernatant, but we did find vacuolar H+-pyrophosphatase in the supernatant. Instead, BAG6 was abundantly present in the pellet created by differential centrifugation (in the range of 500–10,000×g). In principle, a 10,000×g centrifugation force should only precipitate very large particles, for example, the cell nucleus. Thus, our result seems to disagree with Li and Dickman (2016) suggestion that BAG6 is a vacuolar protein.
BAG6 has possible nuclear targeting sequences. We used the online software PSORT (https://psort.hgc.jp/) to predict the location of BAG6. The PSORT prediction shows that BAG6 has nucleus targeted sequences and might localize with high confidence (certainty = 0.987) to the nucleus. In concordance with this prediction, we found the concentrated BAG6 in our purified nuclear fraction (Fig. 2; Supplementary Fig. S1b), but no BAG6 was detected in the purified tonoplast membrane fraction (Supplementary Fig. S1d). Furthermore, the green fluorescence distribution of the BAG6–GFP fusion we saw suggested that BAG6 accumulated in the nucleus (Fig. 2; Supplementary Fig. S2b–d). Our transformation work with Allium cepa epidermis cells provided additional evidence that BAG6 may localize to the nucleus (Fig. 6a). However, more work is needed to confirm these findings and to better understand BAG6 function.
BAG6 acts as a suppressor of Fes1A via a calmodulin-related pathway
BAG6 is a heat-induced protein and is involved in plant responses to both biotic and abiotic stresses (Kang et al. 2006; Kabbage and Dickman 2008; Li et al. 2017a). For example, the Arabidopsis bag6 mutant exhibits increased basic thermotolerance (Echevarría-Zomeño et al. 2016). In this experiment, we found that the bag6 single mutation had no effect on acquired thermotolerance (Fig. 3f, g). However, BAG6 acted as a suppressor of Fes1A (Fig. 3f, g). Furthermore, an additional knockout of BAG6 in fes1a increased HSP70 and small HSP 17.7 expression (Fig. 4). This result agrees with the observed improvement in theromtolerance of the fes1a bag6 double mutant. This concurs with previous work, which showed that loss of BAG6 enhances basic thermotolerance (Echevarría-Zomeño et al. 2016).
In co-immunoprecipitation assays, we detected that BAG6 associated with HSP70 (Fig. 5). In contrast, Kang et al. (2006) found no connection between BAG6 and HSP70 in S. cervisiae. In light of the fact that BAG6 is a nuclear protein and that HSP70s can move into the nucleus (Calikowski et al. 2003; Noel et al. 2007; Jungkunz et al. 2011; Burstenbinder et al. 2017), we suggest that the interaction of BAG6 and HSP70 likely takes place in the nucleus. Since Fes1A is a cytosolic and nuclear protein (Zhang et al. 2010; Inze et al. 2012), we also predict that both cooperative and antagonistic interactions between Fes1A and BAG6 are apt to take place in the nucleus.
Kang et al. (2006) found that the calmodulin-related pathway was involved in BAG6 function. In a related manner, we found that the mutation of the IQ motif in BAG6 inhibited the function of BAG6 as a suppressor of Fes1A (Fig. 6), and we found that application of calmodulin inhibitor TFP or W7 to the culture medium prevented the improvement of thermotolerance in the fes1a bag6 double mutant (Fig. 7). BAG6, when acting as a Fes1A suppressor, depends on calmodulin. Calmodulin and calmodulin-like proteins belong to a large protein family, and they have functions in various subcellular structures (Zeng et al. 2015; Mohanta et al. 2017). Some of these proteins can move into the nucleus (Liu et al. 2008; Wu et al. 2012; Charpentier and Oldroyd 2013) and regulate, together with a calcium/calmodulin dependent kinase, the nuclear calcium signaling that affects many downstream pathways (Charpentier and Oldroyd 2013). Therefore, knockout of BAG6 means that there may be a release of calmodulin or calmodulin-like proteins from the nucleus. This likely changes the dynamics of nuclear calcium signaling (Charpentier and Oldroyd 2013), further enhancing HSP expression in fes1a bag6 and improving acquired thermotolerance.
Materials and methods
Plant material and growth conditions
Arabidopsis thaliana mutants (Supplementary Table S1) were obtained from ABRC (http://abrc.osu.edu), and T-DNA insertions were confirmed using PCR and sequencing (Supplementary Table S2). Homozygous mutants bred from a twice-backcrossed individual were used for experiments. Arabidopsis (ecotype Col-0) and mutant lines were grown under controlled conditions (a 16-h light/8-h dark cycle with a 22 /18 °C temperature cycle) in a plant growth chamber.
Generation of transgenic Arabidopsis strains
We amplified the entire BAG6 cDNA sequence using RT-PCR and then inserted it into a pGEM-T vector, resulting in the plasmid BAG6–pGEM-T. To determine the importance of the IQ motif to thermotolerance in fes1a bag6 double mutants, we substituted three highly conserved residues in the IQ motif (we refer to this mutant hereafter as BAG6–mIQ). BAG6–mIQ was amplified from the plasmid BAG6–pGEM-T via splicing overlap extension PCR and was then inserted into a pGEM-T vector, generating the plasmid BAG6–mIQ–pGEM-T. Subsequently, we inserted BAG6–mIQ or BAG6 into the plasmid ProLeHSP23.8-pBI121. Accordingly, the expression of BAG6–mIQ or BAG6 was controlled by a strongly heat-inducible promoter of LeHSP23.8 (Yi et al. 2006). We then introduced the plasmids into Arabidopsis by floral-dip transformation. The transgenic lines were selected using kanamycin and further confirmed by PCR. All of the primers are listed in Supplementary Table S3.
Chemical and heat treatments of A. thaliana
To evaluate the role of Ca2+ in BAG6 suppression of thermo-sensitivity in fes1a, 10 µM TFP (Sigma-Aldrich) and 60 µM W7 (Sigma-Aldrich) were used to inhibit calmodulin in MS medium. After 10 days of growth, the seedlings were exposed to a 38 °C condition for 2 h which was followed by a 2 h lethal treatment at 45 °C. The challenged seedlings were then shifted to a growth chamber with normal growth conditions for seven days of recovery. Subsequent to recovery, the phenotypes were photographed.
Sub-cellular localization of BAG6
The BAG6 and the BAG6–mIQ cDNAs were amplified with the primers (BAG6–GFP-F and BAG6–GFP-R) from the plasmids BAG6–pGEM-T and BAG6–mIQ–pGEM-T, respectively. Subsequently, we inserted these cDNAs into the XhoI and EcoRI sites of the plasmid P35sCaMV–GFP–pRT101, producing the plasmid P35sCaMV–BAG6–GFP–pRT101 and P35sCaMV–BAG6–mIQ–GFP–pRT101, respectively. The construct of P35sCaMV–BAG6–GFP was cloned into the PstI site of the pCAMBI3301 vector, which was used for transforming Arabidopsis with use of the floral-dip method. The transgenic lines were then selected using the herbicide BASTA and were further confirmed by PCR (all of the primers are listed in Supplementary Table S4). Before observation of GFP, 0.1 µg/ml DAPI solution was used to stain the nucleus of the transgenic lines. A confocal laser scanning microscope (Leica TCS SP8 MP) and a fluorescent microscope (Carl Zeiss Axio Imager Z2) were used to determine the subcellular localization of the GFP fusion proteins. For instant expression analysis of GFP, the construct of P35sCaMV–BAG6–mIQ–GFP was introduced into onion epidermal cells using a Bio-Rad Biolistic PDS-1000/He Particle Delivery System (Bio-Rad Laboratories, Hercules, CA).
To purify the nucleus, the seedlings were treated at 38 °C for 2 h. Nuclei isolation was then performed via the CelLytica PN Plant Nuclei Isolation/Extraction Kit (Sigma-Aldrich). The purity of the isolated nuclear fraction was examined by Western blot with a few critical protein markers. The tonoplast membrane was isolated according to the method of Vera-Estrella et al. (2005).
Immunoblotting
Western blot was performed according to the method of Zhang et al. (2010). Two BAG6 rabbit polyclonal antibodies were generated by Abmart using the BAG6 amino acids 1–300 (N-terminal) and the amino acids 672–1043 (C-terminal) as antigens. The primary antibodies used were the anti-BAG6 polyclonal antibody, the anti-HSC70 monoclonal antibody (SPA-817; Stressgen, San Diego, CA, US), the anti-HSP17.7 antibody (AS07 255; Agrisera), the anti-Histone three antibody (AS10 710; Agrisera), the anti-vacuolar H+-pyrophosphatase (V-PPase) antibody (AS12 1849; Agrisera), and the Anti GFP antibody (AS15 2987; Agrisera).
In vivo co-immunoprecipitation assays
An immuno-precipitation assay was performed to examine possible protein interactions (Zhang et al. 2010). The plant sample was first homogenized in 50 mM Tristan-HCl. The homogenized sample was then sonicated for 30 min at 4 °C and centrifuged at 12,000×g. The supernatant from the protein extraction was subsequently mixed with prepared anti-BAG6 polyclonal antibody/protein-A SepharoseTM beads, and the mixture was then incubated at 4 °C for 2 h. After five washes, we analyzed the bound proteins with Western blot using the anti-HSC70 monoclonal antibody.
References
Alberti S, Böhse K, Arndt V, Schmitz A, Höhfeld J (2004) The cochaperone HspBP1 inhibits the CHIP ubiquitin ligase and stimulates the maturation of the cystic fibrosis transmembrane conductance regulator. Mol Biol Cell 15:4003
Burstenbinder K, Moller B, Plotner R, Stamm G, Hause G, Mitra D, Abel S (2017) The IQD family of calmodulin-binding proteins links calcium signaling to microtubules, membrane subdomains, and the nucleus. Plant Physiol 173:1692–1708
Calikowski TT, Meulia T, Meier I (2003) A proteomic study of the arabidopsis nuclear matrix. J Cell Biochem 90:361–378
Charpentier M, Oldroyd GE (2013) Nuclear calcium signaling in plants. Plant Physiol 163:496–503
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116:1351–1357
Doukhanina EV, Chen S, van der Zalm E, Godzik A, Reed J, Dickman MB (2006) Identification and functional characterization of the BAG protein family in Arabidopsis thaliana. J Biol Chem 281:18793–18801
Echevarría-Zomeño S, Fernández-Calvino L, Castro-Sanz AB, López JA, Vázquez J, Castellano MM (2016) Dissecting the proteome dynamics of the early heat stress response leading to plant survival or death in Arabidopsis. Plant Cell Environ 39:1264–1278
Fu C, Zhang J, Liu X, Yang W, Yu H, Liu J (2015) AtFes1A is essential for highly efficient molecular chaperone function in Arabidopsis. J Plant Biol 58:366–373
Fu S, Li L, Kang H, Yang X, Men S, Shen Y (2017) Chronic mitochondrial calcium elevation suppresses leaf senescence. Biochem Biophys Res Commun 487:672–677
Inze A, Vanderauwera S, Hoeberichts FA, Vandorpe M, Van Gaever T, Van Breusegem F (2012) A subcellular localization compendium of hydrogen peroxide-induced proteins. Plant Cell Environ 35:308–320
Jungkunz I, Link K, Vogel F, Voll LM, Sonnewald S, Sonnewald U (2011) AtHsp70-15-deficient Arabidopsis plants are characterized by reduced growth, a constitutive cytosolic protein response and enhanced resistance to TuMV. Plant J 66:983–995
Kabbage M, Dickman MB (2008) The BAG proteins: a ubiquitous family of chaperone regulators. Cell Mol Life Sci 65:1390–1402
Kang CH, Jung WY, Kang YH, Kim JY, Kim DG, Jeong JC, Baek DW, Jin JB, Lee JY, Kim MO, Chung WS, Mengiste T, Koiwa H, Kwak SS, Bahk JD, Lee SY, Nam JS, Yun DJ, Cho MJ (2006) AtBAG6, a novel calmodulin-binding protein, induces programmed cell death in yeast and plants. Cell Death Differ 13:84–95
Kityk R, Kopp J, Sinning I, Mayer MP (2012) Structure and dynamics of the ATP-bound open conformation of Hsp70 chaperones. Mol Cell 48:863–874
Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128:682–695
Lee JH, Schöffl F (1996) An Hsp70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF, and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol Gen Genet 252:11–19
Lee DW, Kim SJ, Oh YJ, Choi B, Lee J, Hwang I (2016) Arabidopsis BAG1 functions as a cofactor in Hsc70-mediated proteasomal degradation of unimported plastid proteins. Mol Plant 9:1428–1431
Li Y, Dickman M (2016) Processing of AtBAG6 triggers autophagy and fungal resistance. Plant Signal Behav 11:e1175699
Li L, Xing Y, Chang D, Fang S, Cui B, Li Q, Wang X, Guo S, Yang X, Men S, Shen Y (2016) CaM/BAG5/Hsc70 signaling complex dynamically regulates leaf senescence. Sci Rep 6:31889
Li Y, Kabbage M, Liu W, Dickman MB (2017a) Aspartyl protease-mediated cleavage of BAG6 is necessary for autophagy and fungal resistance in plants. Plant Cell 28:233–247
Li Y, Williams B, Dickman M (2017b) Arabidopsis B-cell lymphoma2 (Bcl-2)-associated athanogene 7 (BAG7)-mediated heat tolerance requires translocation, sumoylation and binding to WRKY29. New Phytol 214:695–705
Liu HT, Gao F, Li GL, Han JL, Liu DL, Sun DY, Zhou RG (2008) The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. Plant J 55:760–773
Mayer MP (2013) Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem Sci 38:507–514
Mohanta TK, Kumar P, Bae H (2017) Genomics and evolutionary aspect of calcium signaling event in calmodulin and calmodulin-like proteins in plants. BMC Plant Biol 17:38
Noel LD, Cagna G, Stuttmann J, Wirthmuller L, Betsuyaku S, Witte CP, Bhat R, Pochon N, Colby T, Parker JE (2007) Interaction between SGT1 and cytosolic/nuclear HSC70 chaperones regulates Arabidopsis immune responses. Plant Cell 19:4061–4076
Sangwan V, Orvar BL, Beyerly J, Hirt H, Dhindsa RS (2002) Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J 31:629–638
Shomura Y, Dragovic Z, Chang HC, Tzvetkov N, Young JC, Brodsky JL, Guerriero V, Hartl FU, Bracher A (2005) Regulation of Hsp70 function by HspBP1: structural analysis reveals an alternate mechanism for Hsp70 nucleotide exchange. Mol Cell 17:367–379
Sung DY, Guy CL (2003) Physiological and molecular assessment of altered expression of Hsc70-1 in Arabidopsis. Evidence for pleiotropic consequences. Plant Physiol 132:979–987
Vera-Estrella R, Barkla BJ, Liliana García-Ramírez L, Pantoja O (2005) Salt stress in Thellungiella halophila activates Na+ transport mechanisms required for salinity tolerance. Plant Physiol 139:1507–1517
Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42:579–620
Williams B, Kabbage M, Britt R, Dickman MB (2010) AtBAG7, an Arabidopsis Bcl-2-associated athanogene, resides in the endoplasmic reticulum and is involved in the unfolded protein response. Proc Natl Acad Sci USA 107:6088–6093
Wu HC, Luo DL, Vignols F, Jinn TL (2012) Heat shock-induced biphasic Ca(2+) signature and OsCaM1-1 nuclear localization mediate downstream signalling in acquisition of thermotolerance in rice (Oryza sativa L.). Plant Cell Environ 35:1543–1557
Yi SY, Sun AQ, Sun Y, Yang JY, Zhao CM, Liu J (2006) Differential regulation of Lehsp23.8 in tomato plants: analysis of a multiple stress-inducible promoter. Plant Sci 171:398–407
Zeng H, Xu L, Singh A, Wang H, Du L, Poovaiah BW (2015) Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front Plant Sci 6:600
Zhang W, Zhou RG, Gao YJ, Zheng SZ, Xu P, Zhang SQ, Sun DY (2009) Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol 149:1773–1784
Zhang JX, Wang C, Yang CY, Wang JY, Chen L, Bao XM, Zhao YX, Zhang H, Liu J (2010) The role of Arabidopsis AtFes1A in cytosolic Hsp70 stability and abiotic stress tolerance. Plant J 62:539–548
Acknowledgements
The National Natural Science Foundation of China (Grant No. 31270298) and Research Fundamental Capacity Improvement Project for Middle Age and Youth Teachers of Guangxi Universities (Grant No. 2019KY0517) supported this study.
Author information
Authors and Affiliations
Contributions
CF carried out the experiment with YH, LZ, XL, and PH, and wrote the main manuscript text. YH and JG supplemented the experiments that the reviewers suggested to improve the manuscript. JL designed and supervised the research and revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, C., Hou, Y., Ge, J. et al. Increased fes1a thermotolerance is induced by BAG6 knockout. Plant Mol Biol 100, 73–82 (2019). https://doi.org/10.1007/s11103-019-00844-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-019-00844-8