Abstract
Key message
UBIQUITIN-SPECIFIC PROTEASES play important roles in plant development and stress responses.
Abstract
Protein ubiquitination and deubiquitination are reversible processes, which can modulate the stability, activity as well as subcellular localization of the substrate proteins. UBIQUITIN-SPECIFIC PROTEASE (UBP) protein family participates in protein deubiquitination. Members of UBP family are involved in a variety of physiological processes in plants, as evidenced by their functional characterization in model plant Arabidopsis and other plants. UBPs are conserved in plants and distinct UBPs function in different regulatory processes, although functional redundancies exist between some members. Here we briefly reviewed recent advances in understanding the biological functions of UBP protein family in Arabidopsis, particularly the molecular mechanisms by which UBPs regulate plant development and stress responses. We believe that elucidation of UBPs function and regulation in Arabidopsis will provide new insights about protein deubiquitination and might shed light on the understanding of the mechanistic roles of UBPs in general, which will definitely contribute to crop improvement in agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ubiquitination/dequbiquitination and UBPs in plants
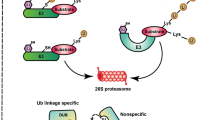
Ubiquitin (Ub) is a small peptide with 76 amino acids that could be covalently attached to the target proteins via ubiquitination (Hershko and Ciechanover 1998; Pickart and Eddins 2004). Protein ubiquitination is catalyzed by three sequential steps performed by E1 (Ub-activating enzyme), E2 (Ub-conjugating enzyme), and E3 (Ub ligase) respectively (Pickart 2003; Pickart and Eddins 2004). In this way, an isopeptide bond between the C terminus of Ub (Glycine76, G76) and the e-amino group of a target Lysine (K) residue is formed. Since there are seven K residues (K6, K11, K27, K39, K33, K48 and K63) in each Ub, additional isopeptide bonds might be created between G76 in one Ub and any K in a second Ub, resulting in different types of Ub polymers. The peptide bond between Ub G76 and the N terminus of Ub also exists, which is used to generate linear Ub chain (Pickart 2003). Ubiquitination participates in critical cellular processes, and dysfunction of ubiquitination machinery leads to detrimental impacts (Pickart 2003; Zhang 2003).
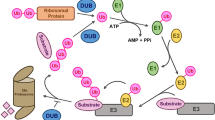
Monoubiquitination mainly modulates protein activity and subcellular location or is involved in establishing “histone code” which plays an important role in transcriptional regulation (Glickman and Ciechanover 2002; Zhang 2003). A recent example for monoubiquitination is IRON-REGULATED TRANSPORTER1 (IRT1), a high-affinity iron transporter functions in iron uptake from the soil at the root surface. IRT1 is monoubiquitinated and this monoubiquitination regulates IRT1 for proper iron uptake to prevent iron toxicity (Barberon et al. 2011). Most polyubiquitinated proteins (K48-linked) are subjected to 26S proteasome-mediated degradation, while under certain circumstance, however, the polyubiquitinated proteins are targeted to non-proteolytic pathways, determined by different types of Ub chains (Hochstrasser 1996; Glickman and Ciechanover 2002; Smalle and Viestra 2004; Chen and Sun 2009; Lim and Lim 2011). K63-linked polyubiquitination has been implicated in endocytosis, DNA damage repair as well as signaling transduction (Lim and Lim 2011; Ulrich 2012; Piper et al. 2014). For example, PIN2 is required for directional cellular efflux of auxin, and RGLG E3 ligase participates in K63-kinked polyubiquitination of PIN2. PIN2 vacuolar sorting and endocytosis depends on K63-linked polyubiquitination, resulting in adaptive growth in plants (Leitner et al. 2012). Conversely, the deubiquitinating enzymes (DUBs) cleave the conjugated Ub from its substrates and deubiquitination mediated by DUBs has been shown to modulate the stability, activity or destiny of their target proteins (Wilkinson 2000; Fischer 2003; Isono and Nagel 2014). Generally, there exist two major types of DUBs including cysteine proteases and metalloproteases (Verma et al. 2002; Nijman et al. 2005). They mainly possess two basic biochemical activities, one is to generate mature Ub from its precursors which are translated as polyproteins composed of Ub moieties or fusion with ribosomal proteins, and another is to cleave the covalently attached Ub chain from its substrates as well as to hydrolyze the Ub chain into free Ub (Fig. 1; Amerik and Hochstrasser 2004; Nijman et al. 2005).
Basic biochemical activities of UBPs. Ub is translated as its precursor with an extension at the C-terminus in plants. UBPs function in generating mature Ub by processing the peptide bond between Ub and the fused extension, or function in cleaving the covalently attached Ub chain from Ub-conjugated substrates as well as hydrolyzing Ub chain into free Ubs
UBIQUITIN-SPECIFIC PROTEASES (UBPs) belongs to the cysteine proteases DUBs and possess two conserved catalytic motifs, Cys- and His-box (Fig. 2; Hu et al. 2002; Reyesturcu et al. 2009). There are 27 UBPs in Arabidopsis and 21 putative UBP family members in the rice (Yan et al. 2000; Liu et al. 2008; Moon et al. 2009). Phylogenetic analysis revealed that AtUBPs can be further grouped into 14 subfamilies, which function in different regulatory processes including plant growth, development as well as the stress responses (Table 1; Yan et al. 2000; Liu et al. 2008; Isono and Nagel 2014). Here we reviewed the advances in understanding the biological functions of UBPs in Arabidopsis, aiming to comprehensively dissect the mechanisms by which AtUBPs modulate the plant development and stress responses.
Schematic representation of the protein structure of UBPs. UBPs belong to cysteine proteases DUBs and possess a signature catalytic Cys- and His-box. The Cys boxes of the UBPs show high conservation both in sequence and length (18 amino acids) while the His boxes are more variable. UBPs might also contain several less conserved motifs including Gln (Q), Gly (G), Leu (L), and Phe (F) boxes as well as other additional motifs, indicating their functional diversity of UBPs in plants
Structure, organization and specificity of UBPs in Arabidopsis
The capacity of UBP like-DUBs to hydrolyze the attached Ub via a peptide or isopeptide bond was first discovered in plants using a combination of Ub covalent affinity chromatography and anion exchange HPLC (Sullivan et al. 1990). Further analysis revealed that the DUB activity can be performed devoid of ATPs (Sullivan et al. 1990). Ultimately, the enzymes responsible for the UBP-like DUB activity were identified as a protein family consisting of 27 members in Arabidopsis (Wilkinson 1997). These AtUBPs typically possess a Cys-box and a His-box. The Cys boxes of the AtUBPs are more conserved in both sequence and length while the His boxes are more variable (Fig. 2; Yan et al. 2000; Liu et al. 2008). Positionally conserved Cys and His residues are essential for the DUB activities and mutations of these amino acids could substantially abolish the DUB activity of AtUBPs (Doelling et al. 2007; Liu et al. 2008; Zhou et al. 2012; Zhao et al. 2016). Furthermore, AtUBPs might also contain several less conserved motifs such as Phe, Gly, Leu, and Gln boxes as well as additional motifs, including zinc fingers (ZnFs), MATH, Ub-like (UBL) domains, and Ub associated (UBA) domains (Yan et al. 2000; Doelling et al. 2007; Liu et al. 2008). The biological functions of these motifs in AtUBPs have not been well understood. However, they might enable AtUBPs to participate in a broad range of physiological processes. For instance, ZnFs in some AtUBPs might mediate the protein–protein interactions following binding a zinc ion by the conserved Cys or His residues (Bonnet et al. 2008). Outside the conserved motifs, low sequence similarity was observed between different AtUBPs, indicating their functional diversity of AtUBPs (Yan et al. 2000; Liu et al. 2008).
The organization of the AtUBP protein family and the relationship between each member were determined with respect to the conserved domains, length as well as sequence similarity (Yan et al. 2000; Liu et al. 2008; Isono and Nagel 2014). These AtUBPs proteins were further grouped into 14 subfamilies and members of each subfamily share conserved motifs, which however might be less conserved among the whole AtUBP protein family (Yan et al. 2000; Liu et al. 2008). In fact, the aforementioned additional motifs such as ZnFs and UBA domains could also serve as signatures in specific subfamily of AtUBPs (Bonnet et al. 2008). Microarray analysis found that AtUBPs exhibit non-identical expression profiles in Arabidopsis. Furthermore, expression of each AtUBP displays various patterns in different organs, suggesting their potential regulatory function in specific organs (Liu et al. 2008).
UBPs contribute to Ub pool homeostasis regulation by cleaving Ub from substrates or its precursors (Isono and Nagel 2014). People have identified several AtUBPs targets, which will promote understanding the specificity and mechanistic roles of UBPs in Arabidopsis. Substrate recognition, Ub chain types preference and even their positioning on the target Ub chain might determine the specificity of UBPs. However, the detailed information about the substrate specificity of AtUBPs is limited. Most of human UBPs (which named USPs) are nonspecific and will cleave nearly any Ub chain type, while some members also display distinct specificities (Mcgouran et al. 2013). Similarly, AtUBPs might cleave various types of Ub chain, but the preference to targets and cleaving efficiency should be different due to particular structure of each type of Ub chain and/or the substrate itself. At present, most AtUBPs function on linear or K48-linked polyubiquitin chains, but no direct evidence has been found that UBPs also work on K63-linked polyubiquitin chains in plants. For example, AtUBP3, 4 and 5 exhibit preference for different linear Ub chain with specific Ub–Ub linkage (Chandler et al. 1997; Raonaik et al. 2000), while AtUBP12 and 13 show activity towards K48-linked diubiquitin (Ub2) (Ewan et al. 2011). Since the number of UBPs (even DUBs) is much smaller than that of E3 ligases in plants, each UBP might have more than one substrates (Moon et al. 2004; Nijman et al. 2005). To deal with a broader range of Ub-conjugated proteins, UBPs may not interact with the target proteins directly but interact with the attached Ub chain. In this way, Ub chain type and structure might be critical for UBPs specificity. Taken together, the overall picture of UBPs substrate specificity in Arabidopsis remains incomplete.
Accumulating evidences have revealed that UBPs function in plant development and stress responses via genetic or epigenetic regulation. However, the in vivo substrates and interacting partners as well as the physiological roles of most UBPs are still poorly defined. Therefore, the molecular functions of UBPs are far from being understood in plants. Here we will briefly review the biological roles of UBPs in various physiological processes in model plant Arabidopsis.
UBPs function in plant development
During the life cycle of flowering plants, their growth and development are tightly controlled through the integration of the physiological status of the seedling and environmental cues. Data have shown that UBPs and deubiquitination might function in regulating plant development in Arabidopsis. Targets of several AtUBPs have been characterized, and the detailed molecular mechanisms of AtUBPs involved in plant development now begin to be uncovered.
Embryo development in higher plant seeds is a highly orchestrated process including cell division and differentiation as well as pattern determination (Meinke 2003). During this process, a large number of proteins are implicated to ensure the single-celled zygote to develop into an organized multicellular embryo, which could produce a whole viable seedling (Meinke 2003). AtUBP14 has been identified as a regulator of embryo development in Arabidopsis and displays DUB activity on K48-linked or some linear Ub chains (Doelling et al. 2001). Data showed that growth of the ubp14 mutant was arrested at embryo stage and accumulated high amount of ubiquitinated proteins, which was consistent to the DUB activity of AtUBP14. In this way, Ub/26S proteasome pathway and AtUBP14 together plays an important role in plant embryogenesis, however, the molecular mechanisms by which AtUBP14 modulates embryo development is elusive (Doelling et al. 2001). In addition, the ubp19 mutant was also defective in embryogenesis, indicating that AtUBP19 might participate in embryo development in Arabidopsis (Liu et al. 2008).
In plant seeds, the development of the embryo and endosperm is highly coordinated (Berger et al. 2006; Lafonplacette and Kohler 2014). The FIS genes encode Polycomb group (PcG) proteins and mediate H3K27me3 modification of PHERES1 (PHE1), a regulator involved in seed development (Makarevich et al. 2006). AtUBP26 loss-of-function mutant displayed a weak phenotype of autonomous endosperm and arrested seed development (Luo et al. 2008). PHE1 was upregulated in the ubp26 ovules while the H3K27me3 level at the PHE1 locus decreased, suggesting that AtUBP26 is required for addition of H3K27me3 to PHE1 (Luo et al. 2008). AtUBP26 might be necessary for the FIS PcG function in seeds, however, we cannot exclude the possibility that AtUBP26 regulates the H3K27me3 modulation of PHE1 locus through a FIS-independent manner. Since AtUBP26 can reverse monoubiquitiantion of H2B (H2Bub1) and ubp26 mutant accumulates high level H2Bub1 (Sridhar et al. 2007), we propose that H2B deubiquitination at PHE locus mediated by AtUBP26 functions in FIS PcG-dependent histone H3 methylation and transcriptional regulation. Plants with AtUBP12 and 13 loss-of-functions also developed autonomous endosperm in the absence of fertilization (Derkacheva et al. 2016). AtUBP12 and 13 function as H2A deubiquitinases in plants, and AtUBP12-overexpressing transgenic plants accumulates lower level of H2Aub1, consistent to its DUB activity towards H2A. AtUBP12 and 13 work together with LHP1, a component of plant specific PcG proteins system, in regulating PcG target gene expression. AtUBP12 could bind to PcG target chromatin and H2K27me3 level decreased in ubp12 ubp13 mutant (Derkacheva et al. 2016).
Seed size is another important morphological character of plants and contributes to plant evolutionary fitness and stress responses (Leishman and Westoby 1994; Gegas et al. 2010; Linkies et al. 2010).The Arabidopsis da1-1 mutant was isolated with increased seed and organ size (Li et al. 2008), which possesses a mutation in the Ub receptor DA1 protein, which acts maternally to affect seed size by restricting cell proliferation in the integuments (Li et al. 2008; Xia et al. 2013). SUPPRESSOR2 OF DA1(SOD2)/AtUBP15 functions as a positive regulator of seed size (Du et al. 2014). sod2/ubp15 mutant produced small seeds compared to wild type, while overexpression of AtUBP15 resulted in large seeds. AtUBP15 promotes cell proliferation in maternal integuments of ovules as well as in developing seeds. AtUBP15 contains a MYND-type zinc finger domain, which might contribute to protein–protein interaction between AtUBP15 and its partner(s). DA1 interact with and targets AtUBP15 to degradation by the Ub/26S proteasome system (Du et al. 2014). Future work is required to identify the possible E3 ligase that responsible for AtUBP15 ubiquitination. The mechanistic role of AtUBP15 in organ size regulation still needs to be studied.
Leaf cell size is positively correlated with endopolyploidy level in plants (Melaragno et al. 1993; Gonzalez et al. 2010). The mitotic cyclins and cyclin-dependent kinase (CDK) complexes function as essential negative regulators of endocycle (Nowack et al. 2012; Edgar et al. 2014). The anaphase promoting complex/cyclosome (APC/C) selectively ubiquitinates mitotic cyclins, targeting them to degradation via the Ub/26S proteasome, then affect endocycle (Capron et al. 2003; Zielke et al. 2008; Heyman and Veylder 2012). AtUBP14 physically interacts with UV-B-INSENSITIVE4 (UVI4), an inhibitor of APC/C (Hase et al. 2006), and functions antagonistically with CELL CYCLE SWITCH52 A1 (CCS52A1), an activator of APC/C (Larson-Rabin et al. 2009). In this way, APC/C activity might be repressed by AtUBP14, leading to impaired endoreduplication (Xu et al. 2016). AtUBP14 loss-of-function resulted in decreased abundance of cyclin A2;3 (CYCA2;3) and CDKB1;1. Consistently, ubp14 displays large cotyledon and leaf with higher ploidy level (Xu et al. 2016). The authors reported that UVI4 could not be deubiquitinated by AtUBP14 and the mechansitic link between AtUBP14 and UVI4 is not clear. It is interesting to confirm whether AtUBP14 regulates the ubiquitination status of CYCA2;3 or CDKB1;1 directly. In summary, AtUBP14 functions in regulating endoreduplication and leaf size.
Plant floral transition depends on exact perceptions of both internal and environmental cues at optimal time during their life cycle (Searle 2003; Jung and Muller 2009). The circadian oscillators participate in regulating the expression of CONSTANS (CO), which subsequently upregulates FLOWERING LOCUS T (FT) to promote flowering in response to proper day length (Suarezlopez et al. 2001; Turck et al. 2008; Harmer 2009). FLOWERING LOCUS C (FLC), a MADS-box transcription factor that represses the expression of multiple genes related to flowering, is also involved in the floral transition and functions as a negative regulator of flowering in plants (Pien et al. 2008; Xu et al. 2008; Michaels and Amasino 1999; Sheldon et al. 1999). AtUBP12 and 13 are involved in regulating circadian clock and photoperiodic flowering. The ubp12 ubp13 double mutant displays phenotype of early flowering and short periodicity of circadian rhythms. AtUBP12 and 13 regulate photoperiodic flowering possibly through a CO-dependent pathway (Cui et al. 2013). Histone H2B ubiquitination status at FLC locus has been implicated in its transcriptional regulation (Cao et al. 2008; Schmitz et al. 2009). AtUBP26 loss-of-function resulted in an early-flowering phenotype. FLC and its related family members were downregulated in ubp26 mutant. H2Bub1 level markedly accumulated while H3K36me3 and H3K27me3 level decreased at FLC locus in ubp26 compared to that in wile type (Schmitz et al. 2009). Therefore, AtUBP26 is required for transcriptional activation of FLC by deubiquitinating H2Bub1 to maintain the H3K36me3 status, which in turn regulate plant response to internal or environmental signals (Sridhar et al. 2007). It is proposed that the H2B ubiquitination status has essential impacts on the recruitment of histone methytrsnsferase to corresponding chromatin region, resulting in altered histone H3 methylation. Please refer to the Zhang’s review paper for more insights in the possible mechanism (Zhang 2003). Interestingly, AtUBP15 has also been reported to be involved in plant flowering time control, however, the mechanisms is elusive (Liu et al. 2008).
Plant sexual reproduction following flowering depends on delivery of the sperm to the egg in the ovary via the long and polarized pollen tube (Higashiyama and Takeuchi 2015). Pollen tube growth and development are coordinately modulated by a series of regulatory processes (Taylor and Hepler 2003; Hepler et al. 2012; Steinhorst and Kudla 2013; Qu et al. 2015). Molecular cues produced by pollen tube itself or by the female tissues could serve as signals for pollen tube attraction and transmission (Hepler et al. 2001; Kanaoka and Higashiyama 2015). Studies revealed that the homologous AtUBP3 and 4 might function in pollen development and/or transmission (Doelling et al. 2007). The ubp3 ubp4 pollen often fails to undergo mitosis II and possesses defects in vacuole and endomembrane organization, resulting in failure in fertilization and lethality. In addition, this mutant is also defective in gametogenesis and displays pollen germination defects (Doelling et al. 2007). These results suggested that AtUBP3 and 4 is implicated in pollen tube development, however, the underlying mechanism still needs to be characterized.
Mitochondria are house-keeping organelles with dynamic morphology and function (Friedman and Nunnari 2014). In Arabidopsis, the homologous dynamin-related proteins (DRPs), DRP3A and DRP3B, are large GTPases function as the main mitochondrial fission factors and participate in mitochondria morphogenesis (Aung and Hu 2012). Phosphorylation and ubiquitination of DRPs have been reported to function in mitochondrial morphology modulation during mitosis (Kerscher et al. 2006; Wang et al. 2012). AtUBP27 is a mitochondrial-located DUB which might play a role in its morphogenesis or function (Pan et al. 2014). AtUBP27 over-expression results in altered mitochondrial morphology although its loss-of-function mutants do not display obvious phenotypes. AtUBP27 reduces the association of DRP3 proteins with mitochondria, possibly facilitating the recycling of DRP3 proteins from mitochondria to the cytosol (Pan et al. 2014). Since the direct interaction between AtUBP27 and DRP3 was not observed, it is hard to conclude that DRP3 is a target of AtUBP27. In addition, there is no report about ubiquitination of DRP3, which further supports AtUPP27 regulates DRP3 possibly in an indirect manner. It might be interesting to determine whether the respiration in ubp27 is changed due to altered mitochondrial morphogenesis.
UBPs function in plant stress responses
As sessile organisms, plants must cope with multiple environmental abiotic or biotic stresses during their life cycle. Ub/26S proteasome system has been implicated in plant stress tolerance regulation, possibly through affecting the stability, activity or subcellular localization of their substrates (Vierstra 1996; Haglund and Dikic 2005; Dreher and Callis 2007). Studies have revealed that UBPs participate in plant salt tolerance, ABA signaling, drought tolerance, plant nutrients deficiency response, toxic amino acid analog resistance as well as plant immunity regulation in Arabidopsis (Fig. 3; Yan et al. 2000; Li et al. 2010; Ewan et al. 2011; Zhou et al. 2012; Zhao et al. 2016). With the discovery of specific substrates or target proteins, the regulatory roles of UBPs in plant stress responses will be better understood in the future.
Soil salinity is an important and constantly increasing abiotic stress, which reduces the crop productivity significantly worldwide (Zhu 2001; Hasegawa 2013). Plant cellular ion and redox homeostasis are always disrupted under salt stress condition (Hasegawa 2013; Paul 2003; De Gara et al. 2010). Furthermore, disordered/unfolded proteins which induce endoplasmic reticulum (ER) stress in plants accumulate during salt stress (Urade 2009; Liu et al. 2011). Salt-Overly-Sensitive (SOS) pathway is specifically activated during salt stress to maintain ion homeostasis (Shi et al. 2000; Qiu et al. 2002; Quan et al. 2007; Zhou et al. 2012). It has been reported that mutation of SHM1, the major serine hydroxymethyltransferase isozyme in Arabidopsis, leads to ROS over accumulation and the mutant is sensitive to salt stress and pathogens (Somerville and Ogren 1981; McClung et al. 2000; Moreno et al. 2005; Voll et al. 2006). SHM1 functions in photorespiratory regulation (Jamai et al. 2009). AtUBP16 has been reported to be required for plant salt tolerance by regulating PM Na+/H+ antiport activity and SHM1 activity (Zhou et al. 2012). AtUBP16 interacts with SHM1 and modulates deubiquitination of SHM1 in planta, which regulates its stability and activity (Zhou et al. 2012). The degradation of SHM1 was enhanced in ubp16 mutant compared with the wild type, implying that AtUBP16 stabilizes SHM1 by removing the conjugated Ub. ubp16 mutant accumulates lower level of SHM1, but not SHM4 in contrast to wild type. In addition, AtUBP16 affect the proper subcellular localization of SHM1. Ser hydroxymethyltransferase activity is much lower in the ubp16 mutant than that in the wild-type. DUB activity of AtUBP16 is required for its function in salt tolerance regulation. These results suggest that deubiquitination of specific Ub-conjugated components of the photorespiratory pathway is critical for maintenance of ion homeostasis and cell death repression during salt stress (Zhou et al. 2012).
Plant hormone abscisic acid (ABA) plays an important role in many physiological processes, including seed maturation, germination and plant stress responses (Cutler et al. 2010). The ABA signaling pathway and its major components have been well characterized (Ma et al. 2009; Park et al. 2009; Raghavendra et al. 2010; Miyakawa et al. 2013). Ubiquitination also functions in ABA signaling and Ub E3 ligases involved in ABA signaling pathway have been identified such as AIP2, DDA1 and KEG (Zhang et al. 2005; Liu et al. 2011; Luisa et al. 2014). These E3 ligases could ubiquitinate different components of ABA signaling pathway and regulate their stability or activity, resulting in altered plant ABA signal transduction. In contrast, deubiquitination is also involved in ABA response and AtUBP24 is a negative regulator of the ABA signaling in Arabidopsis (Zhao et al. 2016). Loss-of-function mutant ubp24 was hypersensitive to ABA during seedling growth. However, the ubp24 mutant exhibited reduced ABA sensitivity in the guard cells, which resulted in rapid water loss and elevated sensitivity to drought stress than the wild type. These findings suggest that AtUBP24 might have different targets in ABA signaling pathway. AtUBP24-mediated DUB activity is essential for plant ABA response. Genetic analysis revealed that ABI2 was downstream of AtUBP24. Interestingly, AtUBP24 also participates in salt tolerance in plant, however, the underlying mechanism is still not clear (Zhao et al. 2016).
Plant root hairs mediate the uptake of water and provide access to immobile nutrients (Gilroy and Jones 2000; Lopez-Bucio et al. 2003). The differentiation of root hair depends on the position with regard to the underlying cortical cells and controlled by a coordinated regulatory circuit (Bernhardt et al. 2005; Kwak and Schiefelbein 2007; Ishida et al. 2008; Schiefelbein et al. 2009). Root epidermal pattern is plastic, especially under challenging environment such as immobile nutrients deficiency (Salazarhenao et al. 2016). Upon phosphate (Pi) deficiency, root epidermal cell elongation is reduced, while the frequency of root hairs per unit of root length increases significantly (Sanchezcalderon et al. 2005; Peret et al. 2011). Pi starvation responses is regulated by a conserved Myb transcription factor PHR1 and the Pi deficiency-specific root hairs development might be controlled by the WER cascade (Rubio et al. 2001; Schachtman and Shin 2007). A weak allele of AtUBP14 mutant, per1, displays a Pi-specific defect in root hair elongation and epidermal cell differentiation (Li et al. 2010). AtUBP14 is involved in regulating expression of genes that are important for the signaling of Pi deficiency. Interestingly, phenotype of per1 might not be caused by Pi deficiency, but is rather caused by disturbed Pi signaling. Together, AtUBP14 functions in the adaptive root hair development to Pi deficiency or even general nutrient availability in the environment (Li et al. 2010). However, the relationship between AtUBP14 and WER cascade still needs to be determined in the future.
Canavanine (CAN) is produced as an anti-herbivore compound in certain legumes. Its toxicity depends on the ability to substitute for Arg during translation (Racioppi et al. 1981). CAN can substantially alter the charge and structure of the proteins (Rosenthal 1991; Baetz and Martinoia 2014). Plants with AtUBP1 or 2 loss-of-function mutation were severely stunted with short roots and chlorotic leaves in the presence of CAN in the medium (Yan et al. 2000). It’s believed that deubiquitination of specific factors by AtUBP1 and 2 could save them from degradation by the Ub/26S proteasome system, resulting in increased level of the substrates and CAN resistance. The underlying mechanisms and biological functions of AtUBP1 and 2 in CAN resistance need to be elucidated. AtUBP6 has also been reported to be able to restore the CAN resistance of the yeast Δubp6 mutant (Moon et al. 2004). AtUBP6 contains a UBL domain and a Ca2+-dependent CaM-binding domain, which indicates that Ca2+ signal might play a role in UBPs-mediated CAN resistance in plants (Moon et al. 2004).
Plants also suffer pathogen invasion and efficient defense mechanisms have been evolved in plants which encompass both basal immunity initiated by the recognition of conserved pathogen-associated molecular patterns (PAMPs), named PTI, and pathogen-specific responses triggered via pathogen effectors and plant-specific recognition events, named ETI (Dangl and Jones 2001; Zipfel 2008; Thomma et al. 2011). The hypersensitive response (HR) with a highly localized programmed cell death (PCD) in the infected region is induced during plant defense response, and restricts pathogen spread (Heath 2000). Many components involved in plant immunity including receptor-like kinases such as pattern recognition receptors (PRRs) and transcription factors as well as specific plant hormones have been well characterized (Zipfel 2008). PRRs might be ubiquitinated and targeted to degradation via the Ub/26S proteasome system as a suppression strategy of plant defense by pathogens, indicating that protein ubiquitination and the Ub/26S proteasome system participates in plant immunity regulation (Lu et al. 2011). Two UBPs, AtUBP12 and 13, are functionally redundant and participate in regulating plant immunity against virulent Pseudomonas syringae pv tomato (Pst DC3000) in Arabidopsis. AtUBP12 and its Solanaceous orthologue NtUBP12 were both identified as negative regulators of the Cf-9-dependent HR. The salicylic acid (SA) signaling pathway is involved in AtUBP12- and 13-mediated plant immunity regulation. AtUBP12/13 and NtUBP12 are functional DUBs and their DUB activity is required for plant immunity and defense response (Ewan et al. 2011). In this way, AtUBP12- and NtUBP12-mediated deubiquitination of specific substrates play important role in plant disease resistance.
Concluding remarks and perspectives
In plants, ubiquitination functions as a central signaling mechanism for development and stress responses (Moon et al. 2004; Bartel and Cytovsky 2012; Lyzenga and Stone 2012; Marino et al. 2012). In contrast, plant UBPs and their DUB activity also participate in determining the destiny and activity of Ub-tagged substrates by cleaving Ub tags from target proteins (Isono and Nagel 2014). UBPs, together with other DUBs in plants, contribute to modulate the homeostasis of Ub pool during challenging environment, resulting in elevated plant stress tolerance (Fischer 2003). Increasing evidences demonstrated that UBPs function in plant development and stresses responses (Fig. 3). It should be noted that UBPs regulate their substrates not only by determining their stability. As it was shown for AtUBP26, which modulates histone H2B ubiquitination status, affects histone methylation and thus controls gene expression in the corresponding chromatin region (Sridhar et al. 2007). Another example is AtUBP16, which is involved in plant salt tolerance, deubiquitinates SHM1 and regulates its stability as well as subcellular localization (Zhou et al. 2012).
Substrates of most AtUBPs have not been found, thus precluding in-depth mechanistic understanding of UBPs in Arabidopsis. To elucidate the biological functions of AtUBPs, the identification of the targets of specific AtUBP is required. Structural studies proved that UBPs often interact with the Ub chain rather than their target proteins directly (Hu et al. 2002). In this way, the identification of AtUBP targets is difficult. Methods combined with interacting protein screening, proteomics investigation as well as plant mutant identification might be used for UBPs substrates determination in the future. Intact multivesicular body (MVB) and its functional component ESCRT are necessary for plasma membrane (PM)-bound cargo proteins transport and autophagosomal degradation (Filimonenko et al. 2007; Rusten et al. 2007; Lee et al. 2009; Zelazny et al. 2011). Two metalloprotease type DUBs, AtAMSH1 and 3, interact with ESCRT-III subunit and function in regulating endocytosis and autophagosomal degradation, then play a critical role in autophagy-mediated plant development and stress response (Katsiarimpa et al. 2013). Although AtUBPs and AtAMSHs belongs to different DUB subfamilies, we cannot exclude the possibility that some AtUBPs could also associate with MVBs (possibly through ESCRT) and participate in endocytosis as well as autophagic degradation regulation. To address this hypothesis will provide new evidences for understanding the biological functions of AtUBPs.
The other important question needs to be resolved is how the function of UBPs to be regulated. To address this question, UBP-interacting partners screening and determination of possible post-translational modifications (PTMs) on UBPs should be performed. DUBs can be regulated both at transcriptional and posttranslational level and UBPs might be regulated in a similar way (Huang and Cochran 2013). For example, circadian control of the expression of AtUBP12 and 13 has been observed (Cui et al. 2013). In addition, future studies should focus on the balance between the two opposite processes, ubiquitination and deubiquitination, in plants and the resulted substrates fate and activity regulation. Elucidation of the biological functions of UBPs in plants will definitely shed light on understanding plant adaption towards changing environment and crop improvement in agriculture.
References
Amerik AY, Hochstrasser M (2004) Mechanism and function of deubiquitinating enzymes. BBA 1695:189–207
Aung K, Hu J (2012) Differential roles of Arabidopsis dynamin-related proteins DRP3A, DRP3B, and DRP5B in organelle division. J Integr Plant Biol 54:921–931
Baetz U, Martinoia E (2014) Root exudates: the hidden part of plant defense. Trends Plant Sci 19:90–98
Barberon M, Zelazny E, Robert S et al (2011) Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci USA 108:E450–E458
Bartel B, Citovsky V (2012) Focus on ubiquitin in plant biology. Plant Physiol 160:1
Berger F, Grini PE, Schnittger A (2006) Endosperm: an integrator of seed growth and development. Curr Opin Plant Biol 9:664–670
Bernhardt C, Zhao M, Gonzalez A et al (2005) The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development 132(2):291–298
Bonnet JP, Romier C, Tora L et al (2008) Zinc-finger UBPs: regulators of deubiquitylation. Trend Biochem Sci 33:369–375
Cao Y, Dai Y, Cui S et al (2008) Histone H2B monoubiquitination in the chromatin of FLOWERING LOCUS C regulates flowering time in Arabidopsis. Plant Cell 20:2586–2602
Capron A, Okresz L, Genschik P (2003) First glance at the plant APC/C, a highly conserved ubiquitin-protein ligase. Trends Plant Sci 8:83–89
Chandler J, Mcardle B, Callis J (1997) AtUBP3 and AtUBP4 are two closely related Arabidopsis thaliana ubiquitin-specific proteases present in the nucleus. Mol Genet Genomics 255:302–310
Chen ZJ, Sun LJ (2009) Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell 33:275–286
Cui X, Lu F, Li Y et al (2013) Ubiquitin-specific proteases UBP12 and UBP13 act in circadian clock and photoperiodic flowering regulation in Arabidopsis. Plant Physiol 162:897–906
Cutler SR, Rodriguez PL, Finkelstein RR et al (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
Dangl J, Jones J (2001) Plant pathogens and integrated defence responses to infection. Nature 411:826–833
Edgar B, Zielke N, Gutierrez C (2014) Endocycles: a recurrent evolutionary innovation for post-mitotic cell growth. Nat Rev Mol Cell Biol 15:197–210
De Gara L, Locato V, Dipierro S et al (2010) Redox homeostasis in plants. The challenge of living with endogenous oxygen production. Respir Physiol Neurobiol 173:S13–S19
Derkacheva M, Liu S, Figueiredo D et al (2016) H2A deubiquitinases UBP12/13 are part of the Arabidopsis Polycomb group protein system. Nat Plant 2:16126
Doelling J, Yan N, Kurepa J et al (2001) The ubiquitin-specific protease UBP14 is essential for early embryo development in Arabidopsis thaliana. Plant J 27:393–405
Doelling J, Phillips A, Soyler-Ogretim G et al (2007) The ubiquitin-specific protease subfamily UBP3/UBP4 is essential for pollen development and transmission in Arabidopsis. Plant Physiol 145:801–813
Dreher K, Callis J (2007) Ubiquitin, hormones and biotic stress in plants. Annu Bot 99:787–822
Du L, Li N, Chen L et al (2014) The ubiquitin receptor DA1 regulates seed and organ size by modulating the stability of the ubiquitin-specific protease UBP15/SOD2 in Arabidopsis. Plant Cell 26:665–677
Ewan R, Pangestuti R, Thornber S et al (2011) Deubiquitinating enzymes AtUBP12 and AtUBP13 and their tobacco homologue NtUBP12 are negative regulators of plant immunity. New Phytol 191:92–106
Filimonenko M, Stuffers S, Raiborg C, Yamamoto A et al (2007) Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol 179:485–500
Fischer J (2003) Deubiquitinating enzymes: their roles in development, differentiation, and disease. Int Rev Cytol 229:43–72
Friedman JR, Nunnari J (2014) Mitochondrial form and function. Nature 505:335–343
Gegas VC, Nazari A, Griffiths S et al (2010) A genetic framework for grain size and shape variation in wheat. Plant Cell 22:1046–1056
Gilroy S, Jones DL (2000) Through form to function: root hair development and nutrient uptake. Trends Plant Sci 5:56–60
Glickman M, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428
Gonzalez N, De Bodt S, Sulpice R et al (2010) Increased leaf size: different means to an end. Plant Physiol 153:1261–1279
Haglund K, Dikic I (2005) Ubiquitylation and cell signaling. EMBO J 24:3353–3359
Harmer SL (2009) The circadian system in higher plants. Annu Rev Plant Biol 60:357–377
Hase Y, Trung K, Matsunaga T et al (2006) A mutation in the uvi4 gene promotes progression of endo-reduplication and confers increased tolerance towards ultraviolet B light. Plant J 46:317–326
Hasegawa P (2013) Sodium (Na+) homeostasis and salt tolerance of plants. Environ Exp Bot 92:19–31
Heath M (2000) Hypersensitive response-related death. Plant Mol Biol 44:321–334
Hepler P, Vidali L, Cheung A (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17:159–187
Hepler PK, Kunkel JG, Rounds CM et al (2012) Calcium entry into pollen tubes. Trends Plant Sci 17:32–38
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479
Heyman J, De Veylder L (2012) The anaphase-promoting complex/cyclosome in control of plant development. Mol Plant 5:1182–1194
Higashiyama T, Takeuchi H (2015) The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol 66:393–413
Hochstrasser M (1996) Ubiquitin-dependent protein degradation. Annu Rev Genet 30:405–439
Hu M, Li P, Li M et al (2002) Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell 111:1041–1054
Huang OW, Cochran AG (2013) Regulation of deubiquitinase proteolytic activity. Curr Opin Struct Biol 23:806–811
Ishida T, Kurata T, Okada K et al (2008) A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 59(1):365–386
Isono E, Nagel M (2014) Deubiquitylating enzymes and their emerging role in plant biology. Front Plant Sci 5:56
Jamai A, Salome P, Schilling S et al (2009) Arabidopsis photorespiratory serine hydroxymethyltransferase activity requires the mitochondrial accumulation of ferredoxin-dependent glutamate synthase. Plant Cell 21:595–606
Jung C, Muller AE (2009) Flowering time control and applications in plant breeding. Trends Plant Sci 14:563–573
Kanaoka MM, Higashiyama T (2015) Peptide signaling in pollen tube guidance. Curr Opin Plant Biol 28:127–136
Katsiarimpa A, Kalinowska K, Anzenberger F et al (2013) The deubiquitinating enzyme AMSH1 and the ESCRT-III subunit VPS2.1 are required for autophagic degradation in Arabidopsis. Plant Cell 25:2236–2252
Kerscher O, Felberbaum R, Hochstrasser M (2006) Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol 22:159–180
Kwak S, Schiefelbein J (2007) The role of the SCRAMBLED receptor-like kinase in patterning the Arabidopsis root epidermis. Dev Biol 302:118–131
Lafonplacette C, Kohler C (2014) Embryo and endosperm, partners in seed development. Curr Opin Plant Biol 17:64–69
Larson-Rabin Z, Li Z, Masson P et al (2009) FZR2/CCS52A1 expression is a determinant of endoreduplication and cell expansion in Arabidopsis. Plant Physiol 149:874–884
Lee HK, Cho SK, Son O, Xu Z, Hwang I et al (2009) Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell 21:622–641
Leishman M, Westoby M (1994) The role of seed size in seedling establishment in dry soil-conditions: experimental-evidence from semiarid species. J Ecol 82:249–258
Leitner J, Petrasek J, Tomanov K et al (2012) Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc Natl Acad Sci USA 109:8322–8327
Li Y, Zheng L, Corke F et al (2008) Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes Dev 22:1331–1336
Li WF, Perry PJ, Prafulla NN et al (2010) Ubiquitin-specific protease 14 (UBP14) is involved in root responses to phosphate deficiency in Arabidopsis. Mol Plant 3:212–223
Lim K, Lim GG (2011) K63-linked ubiquitination and neurodegeneration. Neurobiol Dis 43:9–16
Linkies A, Graeber K, Knight CA et al (2010) The evolution of seeds. New Phytol 186:817–831
Liu Y, Wang F, Zhang H et al (2008) Functional characterization of the Arabidopsis ubiquitin-specific protease gene family reveals specific role and redundancy of individual members in development. Plant J 55:844–856
Liu L, Cui F, Li Q, Yin B et al (2011) The endoplasmic reticulum-associated degradation is necessary for plant salt tolerance. Cell Res 21:957–969
Lopez-Bucio J, Cruz-Ramirez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6:280–287
Lu D, Lin W, Gao X et al (2011) Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332:1439–1442
Luisa I, Iniesto E, Rodriguez L et al (2014) Targeted degradation of abscisic acid receptors is mediated by the ubiquitin ligase substrate adaptor DDA1 in Arabidopsis. Plant Cell 26:712–728
Luo M, Luo M, Buzas D et al (2008) UBIQUITIN-SPECIFIC PROTEASE26 is required for seed development and the repression of PHERES1 in Arabidopsis. Genetics 180:229–236
Lyzenga WJ, Stone SL (2012) Abiotic stress tolerance mediated by protein ubiquitination. J Exp Bot 63:599–616
Ma Y, Szostkiewicz I, Korte A et al (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324:1064–1068
Makarevich G, Leroy O, Akinci U et al (2006) Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep 7:947–952
Marino D, Peeters N, Rivas S (2012) Ubiquitination during plant immune signaling. Plant Physiol 160:15–27
McClung C, Hsu M, Painter J et al (2000) Integrated temporal regulation of the photorespiratory pathway. Circadian regulation of two Arabidopsis genes encoding serine hydroxymethyltransferase. Plant Physio 123:381–392
Mcgouran JF, Gaertner SR, Altun M, Kramer HB et al (2013) Deubiquitinating enzyme specificity for ubiquitin chain topology profiled by di-ubiquitin activity probes. Chem Biol 20:1447–1455
Meinke DW (2003) Molecular genetics of plant embryogenesis. Annu Rev Plant Biol 46:369–394
Melaragno JE, Mehrotra BL, Coleman AW (1993) Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 5:1661–1668
Michaels S, Amasino R (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11:949–956
Miyakawa T, Fujita Y, Yamaguchishinozaki K et al (2013) Structure and function of abscisic acid receptors. Trends Plant Sci 18:259–266
Moon J, Parry G, Estelle M (2004) The ubiquitin-proteasome pathway and plant development. Plant Cell 16:3181–3195
Moon YK, Hong J, Cho Y et al (2009) Structure and expression of OsUBP6, an ubiquitin-specific protease 6 homolog in rice (Oryza sativa L.). Mol Cell 28:463–472
Moreno J, Martín R, Castresana C (2005) Arabidopsis SHMT1, a serine hydroxymethyltransferase that functions in the photorespiratory pathway influences resistance to biotic and abiotic stress. Plant J 41:451–463
Nijman SM, Lunavargas MP, Velds A et al (2005) A genomic and functional inventory of deubiquitinating enzymes. Cell 123:773–786
Nowack MK, Harashima H, Dissmeyer N et al (2012) Genetic framework of cyclin-dependent kinase function in Arabidopsis. Dev Cell 22:1030–1040
Pan R, Kaur N, Hu J (2014) The Arabidopsis mitochondrial membrane-bound ubiquitin protease UBP27 contributes to mitochondrial morphogenesis. Plant J 78:1047–1059
Park SY, Fung P, Nishimura N et al (2009) Abscisic acid inhibits type 2 C protein phosphatases via the PYR/PYL family of START proteins. Science 324:1068–1071
Paul MH (2003) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51:463–499
Peret B, Clement M, Nussaume L et al (2011) Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci 16:442–450
Pickart CM (2003) Mechanisms underlying ubiquitination. Annu Rev Biochem 70:503–533
Pickart C, Eddins M (2004) Ubiquitin: structures, functions, mechanisms. BBA Mol Cell Res 1695:55–72
Pien S, Fleury D, Mylne JS et al (2008) ARABIDOPSIS TRITHORAX1 dynamically regulates FLOWERING LOCUS C activation via histone 3 lysine 4 trimethylation. Plant Cell 20:580–588
Piper RC, Dikic I, Lukacs GL (2014) Ubiquitin-dependent sorting in endocytosis. CSH Perspect Biol 6:a016808
Qiu Q, Guo Y, Dietrich M et al (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA 99:8436–8441
Qu X, Jiang Y, Chang M et al (2015) Organization and regulation of the actin cytoskeleton in the pollen tube. Front Plant Sci 5:786
Quan R, Lin H, Mendoza I et al (2007) SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 19:1415–1431
Racioppi J, Dahlman D, Neukranz R (1981) Effects of L-Canavanine on arginine catabolism in Manduca-sexta (Sphingidae,Lepidoptera). Comp Biochem Physiol B 70:639–642
Raghavendra AS, Gonugunta VK, Christmann A et al (2010) ABA perception and signalling. Trends Plant Sci 15:395–401
Raonaik C, Chandler J, Mcardle B et al (2000) Ubiquitin-specific proteases from Arabidopsis thaliana: cloning of AtUBP5 and analysis of substrate specificity of AtUBP3, AtUBP4, and AtUBP5 using Escherichia coli in vivo and in vitro assays. Arch Biochem Biophys 379:198–208
Reyesturcu FE, Ventii KH, Wilkinson KD (2009) Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem 78:363–397
Rosenthal G (1991) The biochemical basis for the deleterious effects of L-canavanine. Phytochemistry 30:1055–1058
Rubio V, Linhares FR, Solano R et al (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Gene Dev 15:2122–2133
Rusten TE, Vaccari T, Lindmo K, Rodahl LM et al (2007) ESCRTs and Fab1 regulate distinct steps of autophagy. Curr Biol 17:1817–1825
Salazarhenao JE, Velezbermudez IC, Schmidt W (2016) The regulation and plasticity of root hair patterning and morphogenesis. Development 143:1848–1858
Sanchezcalderon L, Lopezbucio J, Chaconlopez A et al (2005) Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol 46:174–184
Schachtman DP, Shin R (2007) Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol 58:47–69
Schiefelbein J, Kwak S, Wieckowski Y et al (2009) The gene regulatory network for root epidermal cell-type pattern formation in Arabidopsis. J Exp Bot 60:1515–1521
Schmitz RJ, Tamada Y, Doyle MR et al (2009) Histone H2B deubiquitination is required for transcriptional activation of FLOWERING LOCUS C and for proper control of flowering in Arabidopsis. Plant Physiol 149:1196–1204
Searle N (2003) Physiology of flowering. Annu Rev Plant Biol 16:97–118
Sheldon CC, Burn JE, Perez P et al (1999) The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11:445–458
Shi H, Ishitani M, Kim C et al (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97:6896–6901
Smalle J, Vierstra RD (2004) The ubiquitin 26 S proteasome proteolytic pathway. Annu Rev Plant Biol 55:555–590
Somerville C, Ogren W (1981) Photorespiration-deficient Mutants of Arabidopsis thaliana lacking mitochondrial serine transhydroxymethylase activity. Plant Physiol 67:666–671
Sridhar V, Kapoor A, Zhang K et al (2007) Control of DNA methylation and heterochromatic silencing by histone H2B deubiquitination. Nature 447:735–U18
Steinhorst L, Kudla J (2013) Calcium: a central regulator of pollen germination and tube growth. BBA 1833:1573–1581
Suarezlopez P, Wheatley K, Robson F et al (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410:1116–1120
Sullivan ML, Callis J, Vierstra RD (1990) High performance liquid chromatography resolution of ubiquitin pathway enzymes from wheat germ. Plant Physiol 94:710–716
Taylor LP, Hepler PK (2003) Pollengermination and tube growth. Annu Rev Plant Biol 48:461–491
Thomma B, Nurnberger T, Joosten M (2011) Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23:4–15
Turck F, Fornara F, Coupland G (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59:573–594
Ulrich H (2012) Ubiquitin and SUMO in DNA repair at a glance. J Cell Sci 125:249–254
Urade R (2009) The endoplasmic reticulum stress signaling pathways in plants. Biofactors 35:326–331
Verma R, Aravind L, Oania R et al (2002) Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26 S proteasome. Science 298:611–615
Vierstra R (1996) Proteolysis in plants: mechanisms and functions. Plant Mol Biol 32:275–302
Voll L, Jamai A, Renne P et al (2006) The photorespiratory Arabidopsisshm1 mutant is deficient in SHM1. Plant Physiol 140:59–66
Wang F, Liu P, Zhang Q et al (2012) Phosphorylation and ubiquitination of dynamin-related proteins (AtDRP3A/3B) synergically regulate mitochondrial proliferation during mitosis. Plant J 72:43–56
Wilkinson KD (1997) Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J 11:1245–1256
Wilkinson KD (2000) Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin Cell Dev Biol 11:141–148
Xia T, Li N, Dumenil J et al (2013) The ubiquitin receptor DA1 interacts with the E3 ubiquitin ligase DA2 to regulate seed and organ size in Arabidopsis. Plant Cell 25:3347–3359
Xu Y, Jin W, Li N et al (2016) UBIQUITIN-SPECIFIC PROTEASE 14 interacts with ULTRAVIOLET-B INSENSITIVE 4 to regulate endoreduplication and cell and organ growth in Arabidopsis. Plant Cell 28:1200–1214
Yan N, Doelling J, Falbel T et al (2000) The ubiquitin-specific protease family from arabidopsis. AtUBP1 and 2 are required for the resistance to the amino acid analog canavanine. Plant Physiol 124:1828–1843
Zelazny E, Barberon M, Curie C, Vert G (2011) Ubiquitination of transporters at the forefront of plant nutrition. Plant Signal Behav 6:1597–1599
Zhang Y (2003) Transcriptional regulation by histone ubiquitination and deubiquitination. Gene Dev 17:2733–2740
Zhang X, Garreton V, Chua N (2005) The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Gene Dev 19:1532–1543
Zhao J, Zhou H, Zhang M et al (2016) Ubiquitin-specific protease24 negatively regulates abscisic acid signalling in Arabidopsis thaliana. Plant Cell Environ 39:427–440
Zhou H, Zhao J, Yang Y et al (2012) UBIQUITIN-SPECIFIC PROTEASE16 modulates salt tolerance in Arabidopsis by regulating Na+/H+antiport activity and serine hydroxymethyltransferase stability. Plant Cell 24:5106–5122
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zielke N, Querings S, Rottig C et al (2008) The anaphase-promoting complex/cyclosome (APC/C) is required for rereplication control in endoreplication cycles. Genes Dev 22:1690–1703
Zipfel C (2008) Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol 20:10–16
Acknowledgements
We sincerely apologize to those authors for not being able to cite their works in this review due to space limitation. We thank Dr. Yan Guo from China Agricultural University for critical reading of the manuscript and stimulating discussions. This work was supported by the National Natural Science Foundation of China (31600201 to H.Z.).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the discussion. JC illustrated the artwork. HZ wrote the article.
Corresponding author
Additional information
Accession numbers: Sequence data of AtUBPs related to this review can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: At2g32780 (AtUBP1), At1g04860 (AtUBP2), At4g39910 (AtUBP3), At2g22310 (AtUBP4), At2g40930 (AtUBP5), At1g51710 (AtUBP6), At3g21280 (AtUBP7), At5g22030 (AtUBP8), At4g10570 (AtUBP9), At4g10590 (AtUBP10), At1g32850 (AtUBP11), At5g06600 (AtUBP12), At3g11910 (AtUBP13), At3g20630 (AtUBP14), At1g17110 (AtUBP15), At4g24560 (AtUBP16), At5g65450 (AtUBP17), At4g31670 (AtUBP18), At2g24640 (AtUBP19), At4g17895 (AtUBP20), At5g46740 (AtUBP21), At5g10790 (AtUBP22), At5g57990 (AtUBP23), At4g30890 (AtUBP24), At3g14400 (AtUBP25), At3g49600 (AtUBP26), and At4g39370 (AtUBP27).
Rights and permissions
About this article
Cite this article
Zhou, H., Zhao, J., Cai, J. et al. UBIQUITIN-SPECIFIC PROTEASES function in plant development and stress responses. Plant Mol Biol 94, 565–576 (2017). https://doi.org/10.1007/s11103-017-0633-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-017-0633-5