Abstract
Post-translational modifications namely ubiquitination, phosphorylation, methylation and acetylation play distinct roles in regulating the growth and development of plants. Among these, the ubiquitination regulates the abundance, activities, subcellular compartmentalization and trafficking of regulatory proteins involved in diverse developmental as well as stress-responsive processes. The ubiquitin–proteasome system (UPS) involves five essential components namely ubiquitin, ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), ubiquitin ligase (E3) and the intact 26S proteasome. The E3 ubiquitin ligase is the major component of UPS that recognizes and tethers poly-ubiquitins on the target proteins. Owing to its specificity of substrate recognition, the E3 ubiquitin ligase contributes not only to the proteome plasticity of the cell but also regulates the plant’s response to environmental cues. In this context, the review summarizes the components involved in UPS and elaborates the role of E3 ubiquitin ligase in biotic and abiotic stress responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Being sessile, plants are continually challenged by several biotic and abiotic stresses, and the severity of these stresses are projected to increase with the change in global climatic conditions. To counteract, plants have also evolved several sophisticated physiological as well as molecular mechanisms that act in a coordinated fashion conferring tolerance characteristic to the plant [48, 50, 72]. Amidst these, the morphological and physiological barriers have been extensively studied [4, 7, 48, 50, 72]; however, the molecular aspects of defense response majorly remain elusive [8, 18, 19, 24, 38, 72]. Ubiquitination is one such process which was initially perceived as a process of selective protein degradation; however, recent studies have highlighted the role of protein ubiquitination in dormancy and germination, tissue and organelle development, self-incompatibility, and biotic and abiotic stress response [1, 54, 64, 66, 71].
Post-translational modifications namely ubiquitination, phosphorylation, methylation and acetylation play distinct roles in regulating the growth and development of all the eukaryotic species. These processes are also reported to interact with each other to form complex cross-talk networks [68]. Among these, the ubiquitination regulates the abundance, activities, subcellular compartmentalization and trafficking of regulatory proteins involved in diverse developmental as well as stress-responsive processes [1, 54, 64, 66, 71]. The central component of UPS is the ubiquitin molecule (Ub) which is transferred to target protein via ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3). Further, the intact 26S proteasome is involved in the identification and degradation of polyubiquitinylated target protein [64]. Ubiquitin is the central component of UPS and is the highly conserved protein consisting of ~ 76 amino acids [1, 64]. The E1 activates the inactive ubiquitin, and the activated ubiquitin is transferred onto E2. The E3 mediates the further transfer of the activated ubiquitin onto the target protein. This transfer could either be direct [homology to the E6-associated protein C-terminus (HECT)-type E3s] or indirect [really interesting new gene (RING), U-box and Cullin-based types E3s], which leads to the degradation of polyubiquitinated-target protein by the 26S proteasome [42, 64].
In plants, the gene families encoding the core components of UPS have been extensively studied. Arabidopsis thaliana contains two E1 genes, thirty-seven E2 genes and more than 1300 E3-related genes [22, 29, 65, 76]. The relatively higher number of E3 genes is justified by their roles in maintaining substrate specificity [64], and thus, several studies were conducted to delineate the roles of E3 ubiquitin ligases [14, 20, 23, 26, 35, 46, 55, 77, 79]. Among these, several reports highlight their involvement in biotic and abiotic stress responses of plants [2, 12, 23, 34, 37, 49, 55, 63, 80]. Given this, the present review summarizes the components involved in UPS and elaborates the role of E3 ubiquitin ligases in biotic and abiotic stress responses.
The components of ubiquitin–proteasome system
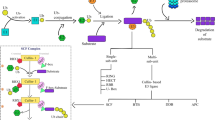
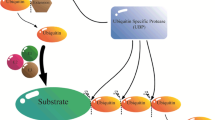
The ubiquitination process involves three distinct classes of enzymes namely E1, E2 and E3 ligase. The ubiquitin-activating enzyme (UBA;E1) activates the ubiquitin in the presence of ATP. The cysteine residue of E1 is utilized for the formation of thioester-linked intermediate E1-ubiquitin (E1-Ub). The ubiquitin-conjugating enzyme (UBC;E2) interacts with E1-Ub followed by the transfer of activated Ub to an active cysteine residue of E2, forming a thioester-linked E2-Ub intermediate. Subsequently, the Ub is transferred to the target protein by ubiquitin ligase (E3) [76]. The E3 interacts with both the target protein and E2-Ub to establish an isopeptide bond between the glycine residue at the C terminal of Ub and lysine residue of the target [76]. The process is repeated several times to generate polyubiquitinated target proteins, and it is reported that a minimum of four Ub molecules is required to develop the polyubiquitin chain that activates proteasomal degradation (Fig. 1) [17, 73]. The ubiquitin gene family of A. thaliana contains fourteen members belonging to three gene types namely, polyubiquitin genes, ubiquitin-like genes and ubiquitin extension genes [10]. The polyubiquitin and ubiquitin-like genes possess tandem repeats of the 228-bp ubiquitin coding region [10]. Post-translation, the Ub monomers are generated through proteolytic cleavage [75]. Each Ub contains seven lysine residues at different positions; Lys6, Lys11, Lys27, Lys29, Lys33, Lys48, and Lys63, and all these lysine residues are capable of forming structurally different polyubiquitin chains [26, 27]. The fate of modified protein depends on the topological attachment of polyubiquitin chain. The chain with Lys48 linkage is targeted for degradation by the 26S proteasome, whereas the ones having Lys63-linked ubiquitin chains are targeted for endocytosis and intracellular trafficking [53]. The monoubiquitinated target proteins are destined for vesicle sorting [47].
The schematic representation of ubiquitin modification cycle. Ubiquitination of a substrate protein happens through sequential cascade of enzymes. Initially ubiquitin-activating enzyme (E1) forms a thioester linkage with ubiquitin with the expense of an ATP molecule. Through a transesterification reaction ubiquitin is transferred to ubiquitin conjugating enzyme (E2). The E3 ligase associates with E2-Ub complex. The ubiquitin protein is transferred to lysine residue of the substrate from E2-Ub complex. Ub can be attached to one another via one of its seven lysine residues. The site of attachment of Ub determines its fate, polyubiquitin chains originating from Lys48, Lys63 and monoubiquitin are destined for proteosomal degradation, vesicular transport and DNA repair, respectively. The deubiquitination enzymes transforms the polyubiquitin chain to monomeric inactive ubiquitin to be used in next round of ubiquitination process. Finally, the substrate is degraded by 26S proteasome complex
The ubiquitin-activating enzyme (E1) regulates the rate of ubiquitination, and given its importance, the genes encoding for E1 are extensively studied. In yeast, E1 is encoded by a single gene, whereas two E1 genes were reported to be present in A. thaliana and tobacco [22, 70]. However, three genes were identified in wheat, and in soybean, a maximum of four genes was reported [82]. The size of E1 proteins ranges from 110–125 kDa, and these proteins possess four conserved domains [22]. First is the adenylation domain that contains two ThiF-homology motifs, followed by the catalytic cysteine domain (which possesses the first and second catalytic cysteine half-domains denoted as FCCH and SCCH, respectively). The third is a four-helix bundle (4HB) followed by the C-terminal ubiquitin-fold domain (UFD) which determines the specificity of E1 towards E2 [82].
Similar to E1, the E2 enzymes also possess a highly conserved region of ~ 140–150 amino acids called the ubiquitin-conjugating (UBC) domain. The UBC domain contains the cysteinyl residue of the active site [29]. Besides 37 E2-encoding genes of A. thaliana, the UBC domain was also found to be present in two RUB-conjugating enzymes (AtRCE1 and AtRCE2) and a SUMO-conjugating enzyme (AtSCE1) [29]. E2 enzymes were initially inferred to be involved in Ub transit; however, recent studies have also identified their role in regulating the polyubiquitination process [82]. E2 activates chain elongation and also governs the topology of chain formation [78], thus determining the fate of the target proteins.
Unlike E1 and E2 proteins, the E3 ubiquitin ligases form the largest and highly diverse group of proteins. The presence of large number of E3 ligases in A. thaliana accounts for ~ 5% of the genome [64]. E3 ubiquitin ligases are classified into single-polypeptide proteins and multi-subunit complexes. The former group includes the HECT-, RING- and U-box-domain containing E3s [15]. The HECT-type E3 possesses a ~ 350 amino acid HECT domain at its C-terminal which mediates the covalent transfer of Ub from E2 to the target protein. In contrary to HECT proteins, the RING and U-box type E3 ligases interact noncovalently with the E2-Ub through a conserved RING- or U-box domain [15]. The RING and U-box type E3 ligase thus facilitate the transfer of Ub to the target protein through zinc-chelating domain and hydrogen bonds/salt bridges, respectively [15, 67]. Structurally, RING and U-box ligases are similar where the conserved Zn-coordinating residues are absent in U-box domain of the latter. Among the HECT-, RING- and U-box-domain containing E3s, the RING-type E3 ligases are capable of forming complex multi-subunits E3s, such as Skp1-Cullin-F-box (SCF), the anaphase-promoting complex/cyclosome (APC/C) and the Cullin-Elongin-BC-VHL (CBC VHL)-type E3 ligases [5, 15, 33, 67].
Among the gene families encoding for different E3 ligases, HECT-type E3 ligase family is the smallest having seven and nineteen genes in A. thaliana and soybean, respectively [6, 16, 64, 82]. In the case of RING-, U-box and F-box gene families, the numbers ranged from 469, 64 and 694 in A. thaliana and 760, 124 and 472 in soybean, respectively [64, 82]. In case of rice, 77 and 687 genes encoded for U-box and F-box gene families [18, 82]. Integrated computational pipelines and advanced bioinformatics tools play a systematic role in the identification of gene families, and several genome-wide studies are being undertaken in sequenced crops that reveal the characteristic features of corresponding gene family members. In this context, similar studies should also be performed to identify the genes encoding core components of the ubiquitin system in crop plants which will further enable their functional characterization towards delineating the potential role for ubiquitination in stress endurance.
Role of E3 ligase in plant defense against biotic stresses
The involvement of UPS in stress perception and activating downstream responses is well reported [21]. The UPS suppress the stress signalling pathway during the absence of stress, thus eliminating the negative regulators of signalling responses. Also, UPS functions as a positive regulator depending on the nature of the biotic stress (Table 1) [9, 13, 19, 30, 63, 74]. Several studies have underlined the differential expression of RING-type genes during treatment with different elicitors [reviewed by 40]. Tobacco plants treated with fungal Avr9 effector showed enhanced expression of an ATL gene, AVR9/CF-9-RAPIDLY ELICITED132 (NtACRE132) [57]. Three different E3 ligases namely NtACRE132, NtACRE276 and NtACRE189 were identified through cDNA-AFLP analysis of Avr9 treated Cf9 tobacco cell cultures. Reduced Avr4/Cf4- or Avr9/Cf9-induced hypersensitive response were observed in NtACRE189 and NtACRE276 silenced background, which highlights the role of E3 ligases as a positive regulator of Cf4- and Cf9-mediated cell death and resistance against fungus infection [57]. Similarly, induced expression of AtATL2, AtATL6, AtATL9, SlATL6 and OsEL5 were observed during elicitor treatment in A. thaliana, Solanum lycopersicum and Oryza sativa, respectively [45, 60, 62, 69, 74]. A loss-of-function mutant of AtATL9 (atl9), an active E3 ubiquitin ligase resulted in susceptibility to Golovinomyces cichoracearum [40].
In parsley, rapid induction in the expression of two RING-type E3 ligase genes namely CMPG1 and CMPG2 was observed during treatment with Pep25 oligopeptide elicitor. The CMPG1 and CMPG2 consist of N-terminal RING domain (31–96 amino acids) and C-terminal leucine-rich repeats [28], and in A. thaliana, these proteins were reported to have roles in conferring tolerance to pathogen infection [25]. In tobacco, upregulation of ubiquitin-activating enzymes, NtE1A and NtE1B during tobacco mosaic virus (TMV) and tomato mosaic virus (ToMV) infection suggested the involvement of ubiquitination in plant-virus interaction [70]. Higher expression of UPS component genes during Tomato leaf curl New Delhi virus infection in tomato suggested the possible involvement of UPS in defense response [58]. Functional characterization of SlRPT4, a component of 19S regulatory particle showed its involvement in activating hypersensitive response and programmed cell death, thus restricting the viral spread [32, 58].
In tobacco, a RING-type E3 ligase, NtRFP1 has been shown to positively regulate Tomato yellow leaf curl China virus resistance. NtRFP1 interacts with viral βC1 protein and initiates degradation of the viral protein. The NtRFP1 overexpressing plants showed reduced symptoms after virus infection. However, NtRFP1 silenced plants showed significant symptom development [63]. Similarly, another RING-type E3 ligase, NtRING1 has been shown to restrict pathogen spread by enhancing HR response against elicitin, Ralstonia solanacearum and TMV in tobacco [20].
A lesion mimic rice mutant spotted leaf11 (spl11) identified from an ethyl methanesulfonate–mutagenized indica cultivar IR68 showed enhanced, non-race-specific resistance to blast (Magnaporthe grisea) and blight (Xanthomonas oryzae pv oryzae) diseases [81]. Further analysis showed that Spl11 gene encodes a novel protein with both a U-box domain and six armadillo (ARM) repeats, and the point-mutation in spl11 results in premature termination of translation. In vitro ubiquitination assay showed that the SPL11 protein has E3 ligase activity which requires the U-box domain [81]; and altogether, the study suggested the role of UPS in regulating cell death and defense. In A. thaliana, MIEL1, a RING-type E3 ligase is a negative regulator of defense response [39]. MIEL1 ubiquitinates a defense-activating transcription factor, Myb30. The proteasomal degradation of Myb30 results in the attenuation of defense and HR responses [39]. In normal conditions, Myb30 activates the biosynthesis of very long chain fatty acids (VLCFAs) that confers resistance to the pathogen [56].
BOI1 (Botrytis Susceptible1 Interactor) is a RING-type E3 ligase that interacts with an R2R3MYB transcription factor, BOS1 leading to its ubiquitination in A. thaliana [37, 43]. BOI1 RNAi lines showed enhanced susceptibility to Botrytis cinerea; however, BOI1 RNAi plants overexpressing BOS1 showed higher resistance to the pathogen [37, 43]. Another RING-type E3 ligase, EIRP1 of wild grapevine (Vitis pseudoreticulata) interacts with the transcription factor, VpWRKY11. This transcription factor activates the expression of JA-responsive genes that negatively regulate resistance to fungal infection [80]. Therefore, degradation of VpWRKY11 by EIRP1 results in lower JA-responsive signaling, followed by resistance to fungus [80]. These observations highlight the involvement of ubiquitin ligases in modulating the immune responses. Further, the involvement of UPS components in all the defense processes from pathogen perception to defense signalling during both pathogen- and effector-triggered immune responses demonstrate the versatile roles of UPS in biotic stress response. However, the targets of numerous defense-responsive E3 ligases are unknown, and therefore, the identification and functional characterization of these targets are imperative.
Role of E3 ligase in abiotic stress responses
In contrary to biotic stresses, the role of E3 ligases has been largely shown in abiotic stresses. Dehydration-responsive Element Binding Protein 2A (DREB2A) is a class of transcription factors that are involved in dehydration stress response. In A. thaliana, two RING-type E3 ligases namely DRIP1 and DRIP2 were shown to affect the accumulation of DREB2A protein, thereby negatively regulating the plant’s response to drought [55]. Arabidopsis drip1drip2 double mutants showed enhanced tolerance to drought stress as compared to wild-type plants underlines the DRIP1/DRIP2-mediated DREB2A ubiquitination and degradation in normal conditions. However, the degradation of DREB2A is inhibited during stress which results in the transcriptional activation of drought-responsive genes [59].
Another E3 ubiquitin ligase, Rma1H1 was initially characterized as a dehydration-responsive gene in Capsicum annuum [31, 51]. Overexpression of Rma1H1 resulted in enhanced drought tolerance in A. thaliana [31]. This protein targets a plasma membrane-localized aquaporin, PIP2 that helps in symplastic water transport leading to a negative impact on plants during water stress [3]. The protoplast co-transformed with PIP2;1 and Rma1H1 showed a significant reduction of PIP2;1 [3]. The reduction of PIP2;1 protein level via ubiquitinylation was validated with the addition of proteasomal inhibitor in the initial co-transformation experiment, which highlighted the role of Rma1H1 in proteasomal degradation of PIP2;1 [31]. In rice, OsDIS1 is a RING-type E3 ligase that negatively regulates a drought-responsive gene, OsNEK6 which has positive roles in drought tolerance [49].
Similar to RING-type E3 ligases, the U-box domain containing proteins (PUB) were also shown to be involved in abiotic stress responses. In A. thaliana, overexpression of two PUB genes, PUB22 and PUB23 conferred susceptibility to salinity and drought stresses [12]. Further downstream experiments revealed that PUB22 and PUB23 interact with RPN6 and RPN12a, respectively. RPN6 and RPN12a are the components of proteasome complex, and their ubiquitination disrupts the 26S proteasome cascade, thus making the plant vulnerable to environmental cues [12]. Similarly, the PUB genes, PUB46 and PUB48 have shown to play roles in drought stress response [11].
Inducer of CBF expression 1 (ICE1) regulates the transcription of a cold-responsive transcription factor, CBF3/DREB1A that subsequently controls the expression of numerous cold-responsive genes [2]. In A. thaliana, high expression of osmotically responsive gene 1 (HOS1) is a RING-type E3 ligase that interacts with ICE1 during cold stress and subsequent ubiquitination of ICE1 results in the attenuation of cold stress response [2, 14]. Another RING-type E3 ligase of rice, Oryza sativa heat and cold-induced 1 (OsHCI1) has been shown to positively regulate heat and cold stresses by ubiquitinating six target proteins including 20S proteasome subunit α7 (OsPSA7), periplasmic beta-glucosidase (OsBGLU1), ethylene-responsive protein (OsbHLH065), glycine-rich cell-wall structural protein (OsGRP1), peroxidase (OsPOX1), and 14-3-3 protein (Os14-3-3) [34]. Overexpression of OsHCI1 has shown to confer enhanced tolerance to heat and cold stresses in transgenic A. thaliana plants [34].
In A. thaliana, NLA (Nitrogen Limitation Adaptation) gene has been reported to encode for a RING-type E3 ligase that regulates the adaptability of plants to limited nitrogen conditions [44]. This protein interacts with ubiquitin conjugase 8 (AtUBC8), a negative regulator of the nitrogen limitation sensing and signalling pathway. NLA mutants showed premature senescence phenotype, and during nitrogen-limiting conditions, the mutant lines failed to develop any response that is required for the survival of the plants [52]. The ratio of nitrogen to glucose is vital for development post germination. Plant growth halts at a higher level of glucose than nitrogen, but a slight increase in nitrogen level recovers the plant growth [36, 41]. In A. thaliana, it has been shown that a RING-type E3 ligase, Carbon–Nitrogen Insensitive 1-dominant (CNI1) is required for the C/N response during the early post-germinative growth of seedlings [61].
In addition, several other RING-type E3 ligases namely Salt- and Drought-Induced Ring Finger 1 (SDIR1), RING Zinc Finger 1 (RZF1), ABA-insensitive RING protein 1 (AIRP), Keep On Going (KEG), Ring finger protein with Microtubule-Targeting domain 1 (RMT1), Salt-Induced RING Finger Protein 1 (SIRP1) and Stress-related Ring Finger Protein 1 (SRFP1) were reported as either positive or negative regulators in response to several abiotic stresses. However, no much information is available on the precise regulatory roles of these proteins at molecular and biochemical levels. The major drawback is the non-availability of information about the target proteins. Also, the lack of efficient screening systems for the target substrates is a shortcoming. Thus, high throughput screening approaches are to be developed and introduced in this study for efficient screening of target proteins.
Conclusions
Plants inadvertently encounter biotic and abiotic stresses throughout their lifecycle, and this has enabled the plants to develop several sophisticated yet complex defense mechanisms. One such defense response is equipped via the UPS. Increasing evidence indicates that UPS could be a versatile tool which confers adaptability to diverse stressors. To date, very few E3 ligases have been identified to have roles in biotic as well as abiotic stresses. Although the expression profiles of these proteins during stress and non-stress conditions are available, but not much information about the target proteins of these ligases. Compared to the reports available in the animal system, relatively fewer studies have been performed on this aspect in the plant system, and much of the mechanistic part remains elusive. The knowledge on the timing and the site of ubiquitination requires a detailed study for gaining further insights. Not all the substrate proteins are destined for proteasomal degradation since some lead to vesicular transport and DNA repair functions; however, no much information is available on the mechanistic as well as biological perspective. Altogether, UPS remains an interesting yet challenging field to explore and gain a better understanding of their molecular as well as biological functions in stress responses.
References
Adams EHS, Spoel SH. The ubiquitin–proteasome system as a transcriptional regulator of plant immunity. J Exp Bot. 2018;69:4529–37.
Adler G, Konrad Z, Zamir L, Mishra AK, Raveh D, Bar-Zvi D. The Arabidopsis paralogs, PUB46 and PUB48, encoding U-box E3 ubiquitin ligases, are essential for plant response to drought stress. BMC Plant Biol. 2017;17:8.
Alexandersson E, Fraysse L, Sjovall-Larsen S, Gustavsson S, Fellert M, Karlsson M, et al. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol. 2005;59:469–84.
Aragon W, Reina-Pinto JJ, Serrano M. The intimate talk between plants and microorganisms at the leaf surface. J Exp Bot. 2017;68:5339–50.
Aravind L, Koonin EV. The U box is a modified RING finger—a common domain in ubiquitination. Curr Biol. 2000;10:R132–4.
Azevedo C, Santos-Rosa MJ, Shirasu K. The U-box protein family in plants. Trends Plant Sci. 2001;6:354–8.
Blanc C, Coluccia F, L’Haridon F, Torres M, Ortiz-Berrocal M, Stahl E, et al. The cuticle mutant eca2 modifies plant defense responses to biotrophic and necrotrophic pathogens and herbivory insects. Mol Plant Microbe Interact. 2018;31:344–55.
Bonas U, Lahaye T. Plant disease resistance triggered by pathogen-derived molecules: refined models of specific recognition. Curr Opin Microbiol. 2002;5:44–50.
Boyes DC, Nam J, Dangl JL. The Arabidopsis thaliana RPM1 disease resistance gene product is a peripheral plasma membrane protein that is degraded coincident with the hypersensitive response. Proc Natl Acad Sci USA. 1998;95:15849–54.
Callis J, Carpenter T, Sun CW, Vierstra RD. Structure and evolution of genes encoding polyubiquitin and ubiquitin-like proteins in Arabidopsis thaliana ecotype Columbia. Genetics. 1995;139:921–39.
Chinnusamy V. ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003;17:1043–54.
Cho SK, Ryu MY, Song C, Kwak JM, Kim WT. Arabidopsis PUB22 and PUB23 are homologous U-Box E3 ubiquitin ligases that play combinatory roles in response to drought stress. Plant Cell. 2008;20:1899–914.
Christensen AH, Sharrock RA, Quail PH. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol. 1992;18:675–89.
Dong CH, Agarwal M, Zhang Y, Xie Q, Zhu JK. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc Natl Acad Sci USA. 2006;103:8281–6.
Downes B, Vierstra RD. Post-translational regulation in plants employing a diverse set of polypeptide tags. Biochem Soc Trans. 2005;33:393–9.
Downes BP, Stupar RM, Gingerich DJ, Vierstra RD. The HECT ubiquitin–protein ligase (UPL) family in Arabidopsis: UPL3 has a specific role in trichome development. Plant J. 2003;35:729–42.
Dreher K, Callis J. Ubiquitin, hormones and biotic stress in plants. Ann Bot. 2007;99:787–822.
Freemont PS. The RING finger. A novel protein sequence motif related to the zinc finger. Ann N Y Acad Sci. 1993;684:174–92.
Genschik P, Parmentier Y, Durr A, Marbach J, Criqui MC, Jamet E, et al. Ubiquitin genes are differentially regulated in protoplast-derived cultures of Nicotiana sylvestris and in response to various stresses. Plant Mol Biol. 1992;20:897–910.
Ghannam A, Jacques A, de Ruffray P, Kauffmann S. NtRING1, putative RING-finger E3 ligase protein, is a positive regulator of the early stages of elicitin-induced HR in tobacco. Plant Cell Rep. 2016;35:415–28.
Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–9.
Hatfield PM, Gosink MM, Carpenter TB, Vierstra RD. The ubiquitin-activating enzyme (E1) gene family in Arabidopsis thaliana. Plant J. 1997;11:213–26.
He F, Wang HL, Li HG, Su Y, Li S, Yang Y, et al. PeCHYR1, a ubiquitin E3 ligase from Populus euphratica, enhances drought tolerance via ABA-induced stomatal closure by ROS production in Populus. Plant Biotechnol J. 2018;16:1514–28.
Heath MC. Hypersensitive response-related death. Plant Mol Biol. 2000;44:321–34.
Heise A, Lippok B, Kirsch C, Hahlbrock K. Two immediate-early pathogen-responsive members of the AtCMPG gene family in Arabidopsis thaliana and the W-box-containing elicitor-response element of AtCMPG1. Proc Natl Acad Sci USA. 2002;99:9049–54.
Kim HT, Kim KP, Lledias F, Kisselev AF, Scaglione KM, Skowyra D, et al. Certain pairs of ubiquitin-conjugating enzymes (E2s) and ubiquitin–protein ligases (E3s) synthesize nondegradable forked ubiquitin chains containing all possible isopeptide linkages. J Biol Chem. 2007;282:17375–86.
Kirkpatrick DS, Hathaway NA, Hanna J, Elsasser S, Rush J, Finley D, et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nat Cell Biol. 2006;8:700–10.
Kirsch C, Logemann E, Lippok B, Schmelzer E, Hahlbrock K. A highly specific pathogen-responsive promoter element from the immediate-early activated CMPG1 gene in Petroselinum crispum. Plant J. 2001;26:217–27.
Kraft E, Stone SL, Ma L, Su N, Gao Y, Lau OS, Deng XW, Callis J. Genome analysis and functional characterization of the E2 and RING-type E3 ligase ubiquitination enzymes of Arabidopsis. Plant Physiol. 2005;139(4):1597–611.
Kurepa J, Smalle JA. Structure, function and regulation of plant proteasomes. Biochimie. 2008;90:324–35.
Lee HK, Cho SK, Son O, Xu Z, Hwang I, Kim WT. Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell. 2009;21:622–41.
Lee JC, Peter ME. Regulation of apoptosis by ubiquitination. Immunol Rev. 2003;193:39–47.
Li W, Bengtson MH, Ulbrich A, Matsuda A, Reddy VA, Orth A, et al. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS ONE. 2008;3:e1487.
Lim SD, Cho HY, Park YC, Ham DJ, Lee JK, Jang CS. The rice RING finger E3 ligase, OsHCI1, drives nuclear export of multiple substrate proteins and its heterogeneous overexpression enhances acquired thermotolerance. J Exp Bot. 2013;64:2899–914.
Lim SD, Hwang JG, Jung CG, Hwang SG, Moon JC, Jang CS. Comprehensive analysis of the rice RING E3 ligase family reveals their functional diversity in response to abiotic stress. DNA Res. 2013;20:299–314.
Lopez-Molina L, Mongrand S, Chua NH. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA. 2001;98:4782–7.
Luo H, Laluk K, Lai Z, Veronese P, Song F, Mengiste T. The Arabidopsis Botrytis Susceptible1 Interactor defines a subclass of RING E3 ligases that regulate pathogen and stress responses. Plant Physiol. 2010;154:1766–82.
Mandal A, Mishra AK, Dulani P, Muthamilarasan M, Shweta S, Prasad M. Identification, characterization, expression profiling, and virus-induced gene silencing of armadillo repeat-containing proteins in tomato suggest their involvement in tomato leaf curl New Delhi virus resistance. Funct Integr Genomics. 2018;18:101–11.
Marino D, Froidure S, Canonne J, Ben Khaled S, Khafif M, Pouzet C, et al. Arabidopsis ubiquitin ligase MIEL1 mediates degradation of the transcription factor MYB30 weakening plant defence. Nature Commun. 2013;4:1476.
Marino D, Peeters N, Rivas S. Ubiquitination during plant immune signaling. Plant Physiol. 2012;160:15–27.
Martin T. Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon: nitrogen availability. Plant Physiol. 2002;128:472–81.
Mazzucotelli E, Belloni S, Marone D, De Leonardis A, Guerra D, Di Fonzo N, et al. The E3 ubiquitin ligase gene family in plants: regulation by degradation. Curr Genomics. 2006;7:509–22.
Mengiste T, Chen X, Salmeron J, Dietrich R. The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell. 2003;15:2551–65.
Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM. Nitrate transport and signalling. J Exp Bot. 2007;58:2297–306.
Molnar G, Bancos S, Nagy F, Szekeres M. Characterisation of BRH1, a brassinosteroid-responsive RING-H2 gene from Arabidopsis thaliana. Planta. 2002;215:127–33.
Mudgil Y, Shiu SH, Stone SL, Salt JN, Goring DR. A large complement of the predicted Arabidopsis ARM repeat proteins are members of the U-box E3 ubiquitin ligase family. Plant Physiol. 2004;134:59–66.
Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–5.
Muthamilarasan M, Prasad M. Plant innate immunity: an updated insight into defense mechanism. J Biosci. 2013;38:433–49.
Ning Y, Jantasuriyarat C, Zhao Q, Zhang H, Chen S, Liu J, et al. The SINA E3 ligase OsDIS1 negatively regulates drought response in rice. Plant Physiol. 2011;157:242–55.
Nurnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev. 2004;198:249–66.
Park J-A, Cho SK, Kim JE, Chung HS, Hong J-P, Hwang B, et al. Isolation of cDNAs differentially expressed in response to drought stress and characterization of the Ca-LEAL1 gene encoding a new family of atypical LEA-like protein homologue in hot pepper (Capsicum annuum L. cv. Pukang). Plant Sci. 2003;165:471–81.
Peng M, Hannam C, Gu H, Bi Y-M, Rothstein SJ. A mutation in NLA, which encodes a RING-type ubiquitin ligase, disrupts the adaptability of Arabidopsis to nitrogen limitation. Plant J. 2007;50:320–37.
Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–6.
Pokhilko A, Ramos JA, Holtan H, Maszle DR, Khanna R, Millar AJ. Ubiquitin ligase switch in plant photomorphogenesis: a hypothesis. J Theor Biol. 2011;270:31–41.
Qin F, Sakuma Y, Tran LS, Maruyama K, Kidokoro S, Fujita Y, et al. Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress-responsive gene expression. Plant Cell. 2008;20:1693–707.
Raffaele S, Vailleau F, Leger A, Joubes J, Miersch O, Huard C, et al. A MYB transcription factor regulates very-long-chain fatty acid biosynthesis for activation of the hypersensitive cell death response in Arabidopsis. Plant Cell. 2008;20:752–67.
Rowland O, Ludwig AA, Merrick CJ, Baillieul F, Tracy FE, Durrant WE, et al. Functional analysis of Avr9/Cf-9 rapidly elicited genes identifies a protein kinase, ACIK1, that is essential for full Cf-9-dependent disease resistance in tomato. Plant Cell. 2005;17:295–310.
Sahu PP, Sharma N, Puranik S, Chakraborty S, Prasad M. Tomato 26S Proteasome subunit RPT4a regulates ToLCNDV transcription and activates hypersensitive response in tomato. Sci Rep. 2016;6:270–8.
Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc Natl Acad Sci USA. 2006;103:18822–7.
Salinas-Mondragon RE, Garciduenas-Pina C, Guzman P. Early elicitor induction in members of a novel multigene family coding for highly related RING-H2 proteins in Arabidopsis thaliana. Plant Mol Biol. 1999;40:579–90.
Sato T, Maekawa S, Yasuda S, Sonoda Y, Katoh E, Ichikawa T, et al. CNI1/ATL31, a RING-type ubiquitin ligase that functions in the carbon/nitrogen response for growth phase transition in Arabidopsis seedlings. Plant J. 2009;60:852–64.
Serrano M, Guzman P. Isolation and gene expression analysis of Arabidopsis thaliana mutants with constitutive expression of ATL2, an early elicitor-response RING-H2 zinc-finger gene. Genetics. 2004;167:919–29.
Shen Q, Hu T, Bao M, Cao L, Zhang H, Song F, et al. Tobacco RING E3 ligase NtRFP1 mediates ubiquitination and proteasomal degradation of a geminivirus-encoded betaC1. Mol Plant. 2016;9:911–25.
Shu K, Yang W. E3 ubiquitin ligases: ubiquitous actors in plant development and abiotic stress responses. Plant Cell Physiol. 2017;58:1461–76.
Smalle J, Vierstra RD. The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol. 2004;55:555–90.
Sonoda Y, Sako K, Maki Y, Yamazaki N, Yamamoto H, Ikeda A, et al. Regulation of leaf organ size by the Arabidopsis RPT2a 19S proteasome subunit. Plant J. 2009;60:68–78.
Stone SL, Hauksdottir H, Troy A, Herschleb J, Kraft E, Callis J. Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol. 2005;137:13–30.
Sun CW, Callis J. Independent modulation of Arabidopsis thaliana polyubiquitin mRNAs in different organs and in response to environmental changes. Plant J. 1997;11:1017–27.
Takai R, Matsuda N, Nakano A, Hasegawa K, Akimoto C, Shibuya N, et al. EL5, a rice N-acetylchitooligosaccharide elicitor-responsive RING-H2 finger protein, is a ubiquitin ligase which functions in vitro in co-operation with an elicitor-responsive ubiquitin-conjugating enzyme, OsUBC5b. Plant J. 2002;30:447–55.
Takizawa M, Goto A, Watanabe Y. The tobacco ubiquitin-activating enzymes NtE1A and NtE1B are induced by tobacco mosaic virus, wounding and stress hormones. Mol Cells. 2005;19:228–31.
Thomann A, Brukhin V, Dieterle M, Gheyeselinck J, Vantard M, Grossniklaus U, et al. Arabidopsis CUL3A and CUL3B genes are essential for normal embryogenesis. Plant J. 2005;43:437–48.
Thordal-Christensen H. Fresh insights into processes of nonhost resistance. Curr Opin Plant Biol. 2003;6:351–7.
Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102.
Trujillo M, Shirasu K. Ubiquitination in plant immunity. Curr Opin Plant Biol. 2010;13:402–8.
Vierstra RD. Proteolysis in plants: mechanisms and functions. Plant Mol Biol. 1996;32:275–302.
Vierstra RD. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol. 2009;10:385–97.
Wiborg J, O’Shea C, Skriver K. Biochemical function of typical and variant Arabidopsis thaliana U-box E3 ubiquitin–protein ligases. Biochem J. 2008;413:447–57.
Windheim M, Peggie M, Cohen P. Two different classes of E2 ubiquitin-conjugating enzymes are required for the mono-ubiquitination of proteins and elongation by polyubiquitin chains with a specific topology. Biochem J. 2008;409:723–9.
Yee D, Goring DR. The diversity of plant U-box E3 ubiquitin ligases: from upstream activators to downstream target substrates. J Exp Bot. 2009;60:1109–21.
Yu Y, Xu W, Wang J, Wang L, Yao W, Yang Y, et al. The Chinese wild grapevine (Vitis pseudoreticulata) E3 ubiquitin ligase Erysiphe necator-induced RING finger protein 1 (EIRP1) activates plant defense responses by inducing proteolysis of the VpWRKY11 transcription factor. New Phytol. 2013;200:834–46.
Zeng LR, Qu S, Bordeos A, Yang C, Baraoidan M, Yan H, et al. Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell. 2004;16:2795–808.
Zhang C, Song L, Choudhary MK, Zhou B, Sun G, Broderick K, Gieslet L, Zeng L. Genome-wide analysis of genes encoding core components of the ubiquitin system in soybean (Glycine max) reveals a potential role for ubiquitination in host immunity against soybean cyst nematode. BMC Plant Biol. 2018;18:149.
Acknowledgments
Authors’ work in this area is financially supported by the Department of Biotechnology, Ministry of Science and Technology, Govt. of India (Project No.: BT/PR8357/PBD/16/1033/2013). AM acknowledges the NPDF award from DST-SERB, Govt. of India. MM acknowledges the DST INSPIRE Faculty Award from DST, Govt. of India. The authors are thankful to DBT-eLibrary Consortium (DeLCON) for providing access to the e-resources.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to the memory of Profs AK Sharma and Archana Sharma.
Rights and permissions
About this article
Cite this article
Mandal, A., Sharma, N., Muthamilarasan, M. et al. Ubiquitination: a tool for plant adaptation to changing environments. Nucleus 61, 253–260 (2018). https://doi.org/10.1007/s13237-018-0255-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-018-0255-6