Abstract
Key message
MdMYB16 forms homodimers and directly inhibits anthocyanin synthesis via its C-terminal EAR repressor. It weakened the inhibitory effect of MdMYB16 on anthocyanin synthesis when overexpressing MdbHLH33 in callus overexpressing MdMYB16. MdMYB16 could interact with MdbHLH33.
Abstract
Anthocyanins are strong antioxidants that play a key role in the prevention of cardiovascular disease, cancer, and diabetes. The germplasm of Malus sieversii f. neidzwetzkyana is important for the study of anthocyanin metabolism. To date, only limited studies have examined the negative regulatory mechanisms underlying anthocyanin synthesis in apple. Here, we analyzed the relationship between anthocyanin levels and MdMYB16 expression in mature Red Crisp 1–5 apple (M. domestica) fruit, generated an evolutionary tree, and identified an EAR suppression sequence and a bHLH binding motif of the MdMYB16 protein using protein sequence analyses. Overexpression of MdMYB16 or MdMYB16 without bHLH binding sequence (LBSMdMYB16) in red-fleshed callus inhibited MdUFGT and MdANS expression and anthocyanin synthesis. However, overexpression of MdMYB16 without the EAR sequence (LESMdMYB16) in red-fleshed callus had no inhibitory effect on anthocyanin. The yeast one-hybrid assay showed that MdMYB16 and LESMdMYB16 interacted the promoters of MdANS and MdUFGT, respectively. Yeast two-hybrid, pull-down, and bimolecular fluorescence complementation assays showed that MdMYB16 formed homodimers and interacted with MdbHLH33, however, the LBSMdMYB16 could not interact with MdbHLH33. We overexpressed MdbHLH33 in callus overexpressing MdMYB16 and found that it weakened the inhibitory effect of MdMYB16 on anthocyanin synthesis. Together, these results suggested that MdMYB16 and MdbHLH33 may be important part of the regulatory network controlling the anthocyanin biosynthetic pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flavonoid compounds occur widely in most plant species. In addition to their roles in coloring plant matter, acting as signal substances between plants and microbes, and as plant defensins, they also have anti-bacterial, anti-inflammatory, and anti-oxidation effects. Flavonoids have been shown to have a significant effect on the treatment of cardiovascular disease (Cook & Samman et al. 1996; Hollman, Katan et al. 1997; Boyer, Liu et al. 2004; Schaefer et al. 2008; Sun et al. 2013). Anthocyanin synthesis is a branch of the flavonoid biosynthetic pathway that is related to coloration in a variety of plant tissues and organs. Anthocyanin synthesis, which is induced by UV radiation, drought, cold, jasmonic acid, sugar, low nitrogen, and other environmental signals, involves multiple structural genes and transcription factors (Koes et al. 2005; Solfanelli et al. 2006; Bai et al. 2014; An et al. 2014).
The structural genes of the plant flavonoid biosynthetic pathway have been identified and include CHS, FLS, UFGT, and ANS among others (Chikako et al. 2002), with ANS and UFGT playing major roles in anthocyanin synthesis (Kondo et al. 2002; Kim et al. 2006). Anthocyanin biosynthesis is transcriptionally regulated and coordinately modulated by a conserved MBW regulatory complex. The role of MYB and bHLH family members in anthocyanin synthesis has been extensively studied in plant models (Koes et al. 2005; Ballester et al. 2010; Albert et al. 2011). Recently, significant research into fruit anthocyanins has also been undertaken. In grapevine, VvMYBA1 and VvMYBA2, which are homologs of Arabidopsis thaliana AtMYB75, AtMYB90, and AtMYB144, have been found to induce anthocyanin biosynthesis (Azuma et al. 2008). Additionally, homologs of these Arabidopsis TFs have been detected in peach, strawberry, and pear, including PpMYB10, FaMYB10, and PyMYB10, respectively (Wang et al. 2010; Feng et al. 2010). In apple, Takos et al. (2006) and Ban et al. (2007) found that MdMYB1 and MdMYBA were induced by light and were involved in regulating anthocyanin synthesis. Espley et al. (2007, 2009) determined that MdMYB10 regulates flesh anthocyanin synthesis and that its promoter had self-activating activity. Bai et al. (2014) determined that UV-B-induced MdCOL11 expression, thereby promoting anthocyanin synthesis (Bai et al. 2014). Taken together, these studies are indicative of the positive regulatory role of anthocyanin. This positive role, combined with the discovery of the EAR repressor, offers new perspectives into the negative regulation of anthocyanin biosynthesis.
The EAR transcriptional repressor plays an important role in regulating plant defenses and abiotic stress responses (Hiratsu et al. 2002; Kazan et al. 2006; Ciftci-Yilmaz et al. 2007). In addition to ethylene response factors (ERFs) and auxin response factors, other transcription factor family members, including MYB, ABI3/VP1, and MADS, all contain an EAR motif and have active suppressor functions (Preston et al. 2004; Tsukagoshi et al. 2007; Hill et al. 2008). AtMYB4, which was the first negative regulator identified in the Arabidopsis R2R3 class, was found to have a C-terminus pdLNL D/E Lxi G/S inhibition zone that inhibited the transcription of target genes through direct binding (Jin et al. 2000; Hemm et al. 2001). PhMYB4 was found that it has the similar function with AtMYB4, and it could repress the expression of C4H, indirectly controlling the balance of floral volatile benzenoid/phenylpropanoid (FVBP) production (Colquhoun et al. 2011). ZmMYB42 and CmMYB1 also have similar EAR suppression sequences, they inhibit the synthesis of phenylpropanoid and flavonoid, respectively. However, the functional data of ZmMYB42 and CmMYB1 are still lacking (Sonbol et al. 2009; Zhu et al. 2013).
The first anthocyanin biosynthesis-associated R2R3-MYB inhibitor to be discovered was FaMYB1. When FaMYB1 protein was heterologously expressed in tobacco it was found to inhibit anthocyanin accumulation in tobacco petals, and it also had the EAR at its C-Terminus(Aharoni et al. 2001). A petunia homologue, PhMYB27 was shown to act upon MBW complexes to repress anthocyanin synthesis (Albert et al. 2014) and similar modes of action had also been demonstrated for FaMYB1, VvMYBC2 and MtMYB2 (Paolocci et al. 2011; Jun et al. 2015; Cavallini et al. 2015).So these R2R3-MYB repressors that act upon MBW complexes differs from the R2R3-MYB repressors that directly bind the target genes (e.g., AtMYB4). MdMYB16 also had the EAR at its C-Terminus, and it co-transfected into tobacco with transcriptional activators MdMYB10 and MdbHLH3 could inhibit the leaf anthocyanin (Lin-Wang et al. 2011). However, the mechanism of inhibiting anthocyanin by MdMYB16 and the type of MdMYB16 belonging to AtMYB4-like or FaMYB1-like are not clear recently.
World-wide, cultivated apple (Malus domestica) is derived from the ancestral species M. sieversii and its red-fleshed variant (M. sieversii f. niedzwetzkyana). The rich genetic diversity contained in the germplasm of these varieties, therefore, represents an important resource for quality apple breeding (Chen et al. 2007; Velasco et al. 2010; Nocker et al. 2012). M. sieversii resources have, however, been severely damaged and are currently endangered. For this reason, our research group, while conducting research studies on genetic diversity in 2006, built hybrid segregating populations of “Fuji” ×Malus sieversii f. niedzwetzkyana. Furthermore, because of the high levels of anthocyanin and other healthy ingredients in M. sieversii f. niedzwetzkyana, the “functional apple” concept was proposed in 2014 (Chen et al. 2014). The hybrid offspring of Malus sieversii f. niedzwetzkyana have clearly separated red-fleshed, pink-fleshed, and white-fleshed traits. The research team conducted RNA-seq analysis of segregating populations with different flesh colors and screened differentially expressed genes associated with anthocyanin biosynthesis (Wang et al. 2015). However, the factors contributing to the differences in flesh coloration remained unclear. Characterizing this mechanism further may help to improve anthocyanin metabolism and provide a theoretical basis for functional apple breeding .
In the present study, we analyzed the expression of MdMYB16 in fruit with different flesh colors, cloned MdMYB16 of Red Crisp 1 apple and expressed it in prokaryotes, performed a phylogenetic analysis of 26 MYB transcription factors that were related to flavonoid biosynthesis, overexpressed MdMYB16 and MdbHLH33 in red callus, and verified the relationship of interaction between MdMYB16 and MdbHLH33 using yeast two-hybrid, pull-down, and bimolecular fluorescence complementation (BiFC) experiments. The results showed that MdMYB16 plays an inhibitory role through its own EAR inhibition sequence and forms homodimers. And MdMYB16 could interact with MdbHLH33.
Materials and methods
Plant materials and processing
In this study, we used plants of the apple strains Red Crisp 1–5 and Purple 3 that were first-generation hybrids bred from Malus sieversii f. neidzwetzkyana and M. domestica cv. Fuji. The Red Crisp 1–5 apples were picked from 5 different F1 hybrids of Malus sieversii f. neidzwetzkyana. The using materials were peel-out flesh during mature period. The flowering and maturation times of the six lines were basically the same. Red Crisp 1–5 were sampled at 125 days after flowering. We depended on firmness to measure the fruit maturity. Based on former results, the firmness of Red Crisp 1–5 apples was approximately 0.78 kg. The unit of firmness was kg measured by machine named TA.XT plus texture instrument (Stable Micro Systems). The samples were immediately frozen in liquid nitrogen and stored at −80 °C until use. Red-fleshed calli used in the experiments were induced from young leaves of the superior strain Purple 3, according to Ji et al. (2015). The callus was cultured in MS + 1 mg/L 6-Benzylaminopurine + 0.3 mg/L 1-naphthylacetic acid.
Anthocyanin extraction and absorbance measurements
Anthocyanin extraction was performed according to Jin et al. (2000) with minor modifications. Approximately 0.5 g of plant material was ground into a powder in liquid nitrogen and subjected to extraction in 20 mL of 1% (v/v) HCl-methanol at 4 °C in the dark for 24 h. After centrifugation at 12,000 rpm for 10 min, the absorbance of the supernatant was measured at 530 nm using a UV spectrophotometer.
Total RNA extraction and qRT-PCR
The plant RNA extraction kit, reverse transcription kit, and SYBR dyes were purchased from CICC Whole Formula (Beijing, China). The Bole CFX96 (Bio-Rad) system was used for PCR and the SYBR® Green system for real-time PCR according to the manufacturer’s instructions. Three replicates were prepared for each sample. Each 20-μL reaction consisted of 10 μL 2.5 × Real Master Mix/20 × SYBR Solution, 1 μL cDNA (50 ng μL−1), 1 μL each of stream and downstream primers (5 μmol L−1), and 7 μL of ddH2O. The reaction consisted of an initial denaturation at 95 °C for 30 s; followed by 45 cycles of denaturation at 95 °C for 5 s, annealing at 58 °C for 10 s, and extension at 72.0 °C for 30 s; then incubation at 65 °C for 20 s, dissolution from 55 to 95 °C, increasing by 0.5 °C/s; and, finally, termination of the reaction. MdActin was used as the internal control and was amplified simultaneously for each gene amplification. Ct values were read under default conditions and the 2−ΔΔCT method was used for data analysis (Kenneth et al. 2001).
MdMYB16 cloning, bioinformatic analyses
MdMYB16 (HM122617.1) CDS was amplified by PCR, products were separated by 1% agarose gel electrophoresis, and target bands were recovered. The isolated fragments were then connected to a PLB zero background vector (VT205) and sent to the Sangon Company (Shanghai, China) for sequencing. Phylogenetic analysis of related MYB transcription factors was conducted using MEGA5.0. The protein sequences of related MYB transcriptional repressors were compared using Clustal X.
Knockout of MdMYB16 inhibition sequence and bHLH binding sequenceby overlap PCR
Using the overlap PCR technique, the EAR suppression sequence of MdMYB16 (PDLNLDLQIS) was removed. A detailed description of the specific steps is included in Supplementary Fig. S1. The primers were designed as follows:
F1: 5′-ATGGGAAGATCTCCTTGCTG-3′.
R1: 5′-TGGCAGGGAGGGCACCTTTCCTGAACTGG-3′.
F2: 5′-AAAGGTGCCCTCCCTGCCAGCCTCA-3′.
R2: 5′-TCATTTCATCTCCAAGCTTCTG-3′.
Using the F1 and R1 primers the MdMYB16-1 fragment was amplified by PCR. Using the F2 and R2 primers, fragment MdMYB16-2 was amplified. Equal amounts of MdMYB16-1 and MdMYB16-2 were then mixed and used as a template for amplification with the F1 and R2 primers. The amplified fragment was identified as MdMYB16 in which the EAR suppression sequence was removed and was, therefore, named lost EAR sequence of MdMYB16 (LESMdMYB16).
Using the above method, the bHLH binding sequence of MdMYB16 (LIIKLHSLLG) was removed. The primers were designed as follows:
F1: 5′-ATGGGAAGATCTCCTTGCTG-3′.
R1: 5′-CATTTGTTCTCATCTTCTTCTTCAGTGAA-3′.
F2: 5′-AGAAGATGAGAACAAATGGTCTTTGATAGCTG-3′.
R2: 5′-TCATTTCATCTCCAAGCTTCTG-3′.
The amplified fragment was identified as MdMYB16 in which the bHLH binding sequence was removed and was, therefore, named lost bHLH binding sequence of MdMYB16 (LBSMdMYB16).
Red-fleshed callus transformation
The intact, LESMdMYB16 and LBSMdMYB16 CDS were ligated into the pRI101 vector containing the 35 S promoter and a GFP tag sequence to construct the 35S:MdMYB16-GFP, 35S:LESMdMYB16-GFP and 35S:LBSMdMYB16-GFP recombinant vectors, respectively (described in Supplementary Fig. S2a). The intact CDS of MdbHLH33 was transformed into the pCAMBIA1301 vector that also contains a 35S promoter and GFP tag sequence to generate the 35S:MdbHLH33-GFP recombinant vector (described in Supplementary Fig. S2b). The pRI101 vector confers kanamycin resistance in both prokaryotic and eukaryotic cells, and the pCAMBIA1301 vector confers kanamycin resistance in prokaryotic cells and hygromycin resistance in eukaryotic cells. The recombinant vector was transformed into Agrobacterium tumefaciens LBA4404. For transformation of the red callus, two-week-old calli grown in liquid media were co-cultured with A. tumefaciens LBA4404 carrying the 35S:MdMYB16-GFP or 35S:LESMdMYB16-GFP or 35S:LBSMdMYB16-GFP vectors. The calli were co-cultured on MS media containing 0.3 mg/L 1-naphthylacetic acid, 1 mg/L 6-Benzylaminopurine, and 7 g/L agar at 25 °C in the dark for 2 d. Subsequently, the callus was transferred onto MS media supplemented with 250 mg/L carbenicillin and 50 mg/L kanamycin for transgene selection. To obtain co-transfected MdMYB16 + MdbHLH33 or LBSMdMYB16 + MdbHLH33 callus, the same method was used, but the callus was transferred to MS media supplemented with 250 mg/L carbenicillin, 50 mg/L kanamycin, and 20 mg/L hygromycin for transgene selection.
Yeast one-hybrid (Y1H) analysis
Y1H assays were performed using yeast strain Y187 (Clontech) according to the manufacturers’ instructions. The MdMYB16 and LESMdMYB16 genes were cloned individually into the pGADT7 vector (described in Supplementary Fig. S2c). The promoters of MdANS and MdUFGT were inserted individually into the pHIS2 vector (described in Supplementary Fig. S2d). Different combinations were co-transformed into yeast Y187 and the interactions were examined on media lacking Trp, Leu, and His (SD/-Trp-Leu-His) with an optimal concentration of 3-AT.
Yeast two-hybrid (Y2H) analysis
The intact MdMYB16 and LBSMdMYB16 CDS were ligated into the pGBKT7 vector to construct the recombinant plasmids MdMYB16-BD and LBSMYB16-BD (described in Supplementary Fig. S2e). The intact CDS of MdbHLH33 and MdMYB16 was ligated into the pGADT7 vector to generate the MdbHLH33-AD and MdMYB16-AD recombinant plasmids (described in Supplementary Fig. S2c). These two recombinant plasmids were transformed into Y2H cells according to the instructions of the Yeastmaker™ Yeast Transformation System 2 Kit (Clontech). Initially, cells were cultured in selective media lacking Leu and Trp (-Leu/Trp, Clontech), and putative transformants were transferred to media lacking adenine (Ade), His, Leu, and Trp (-Ade/-His/-Leu/-Trp, Clontech). The substrate X-α-gal was added to media lacking four amino acids (-Ade/-His/-Leu/-Trp) for the detection of β-galactosidase activity.
BiFC assay
The intact MdMYB16 CDS was ligated into a pSPYNE vector containing the 35S promoter and an nYFP tag sequence and a pSPYCE vector containing the 35S promoter and a cYFP tag sequence to generate the 35S:MdMYB16-NYFP and 35S:MdMYB16-CYFP recombinant plasmids, respectively (described in Supplementary Fig. S2f, g). The MdbHLH33 CDS was ligated into a pSPYCE vector to construct the 35S:MdbHLH33-CYFP recombinant plasmid (described in Supplementary Fig. S2g). The recombinant plasmids were transformed into A. tumefaciens LBA4404. Approximately 30 mL of YEP + Kan (50 μg/mL) + Rif (50 μg/ml) culture media containing bacteria with different recombinant plasmids were cultured to an OD600 of 0.6. For each bacterial culture, a 15 mL aliquot was collected and added to centrifuge tubes, the cells were collected by centrifugation, and were then resuspended in 30 mL sterile distilled water supplemented with 100 μmol/L acetosyringone. Onion skin was immersed in the infection solution and cultured for 25–30 min. Upon removal, excess bacteria were absorbed with filter paper and the onion skin was cultured on MS solid medium at 28 °C in the dark for 1 to 2 days. The onion cells were later examined for YFP fluorescence signals using confocal microscopy.
Pull-down assays
The intact CDS of MdMYB16 was ligated into the pET32a and PGEX-4T-1 vectors while the intact CDS of MdbHLH33 was ligated into the PGEX-4T-1 vector (described in Supplementary Fig. S2h, i). The recombinant vectors were expressed in E. coli BL21 (DE3) to form the MdMYB16-HIS, MdMYB16-GST, and MdbHLH33-GST fusion proteins. The different combinations of mixed proteins were column purified using the His tag before the purified mixed-proteins were detected by western blotting with anti-HIS or anti-GST antibodies (Abmart).
Data analysis
We picked 20 fruits from every strain randomly and mixed them up to freeze samples. All the results were based on the average of three parallel experiments. The statistical analysis was performed with appropriate methods using Duncan’s new multiple range test. The significance tests were shown as i, ii, iii, iv. Completely different lowercase roman alphabet on the column chart indicated significant differences (P < 0.05).
Results
Fruit anthocyanin levels and associated transcription factors expression differed in the mature first-generation hybrid fruit of Malus sieversii f. neidzwetzkyana
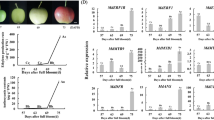
For the superior Red Crisp 1–5 first-generation Malus sieversii f. neidzwetzkyana hybrid strains flesh coloration and anthocyanin level differed significantly in mature fruit (Fig. 1a, c). The promoter region for MdMYB10 was amplified from five different cultivars. The agarose gel electrophoresis showed that their MdMYB10 promoters divided into two bands, ‘a’ and ‘b’ (Fig. 1b). Sequencing of ‘a’ and ‘b’ showed that the ‘a’ band had six repeat sequences of 23 bp, while the ‘b’ band had one (Fig. 1d), This indicated that the MdMYB10 promoters of Red Crisp 1–5 were all the R6R1 type. MdMYB10 expression in the Red Crisp 1 apples was higher than in Red Crisp 3 and 4, while the anthocyanin content in Red Crisp 1 was significantly lower than in Red Crisp 3 and 4 (Fig. 1c, e). However, in Red Crisp 1–5 apples, higher MdMYB16 expression was associated with lower anthocyanin levels and MdbHLH33 expression (Fig. 1c, e), suggesting that MdMYB16 and MdbHLH33 might involved of the anthocyanin biosynthesis in addition to MdMYB10.
Analysis of MdMYB10 promoter type, anthocyanin level, and associated transcription factor expression in the first-generation hybrid fruit of Malus sieversii f. niedzwetzkyana. The numbers 1, 2, 3, 4, and 5 in panels (a-c, e) represent the first-generation hybrid fruit, Red Crisp 1, 2, 3, 4, and 5. The significance tests are shown as i, ii, iii, iv.Completely different lowercase roman alphabet on the column chart indicates significant differences (P < 0.01). (a) Cross-sectional view of the mature fruit. (b) The promoter region for MdMYB10 was amplified from 5 different cultivars. (c) Anthocyanin level in mature fruit. (d) MdMYB10 promoter R6 and R1 sequence of mature fruit. (e) The relative expression of transcription factors MdMYB10 and MdMYB16 in mature fruit
Phylogenetic tree and sequence analyses
The results of phylogenetic analyses of related MYB transporters in the synthesis of different flavonoids is shown in Fig. 2a. Region 1 included related MYB transcription factors that promote anthocyanin biosynthesis (Liu et al. 2013). Region 2 was composed of related MYB transcription factors that promote procyanidin synthesis (An et al. 2014). Region 4 contained the MYB transcription factors that promote flavonol synthesis (Czemmel et al. 2009). Region 3 and 5 consisted of related MYB transcription factors that has the C2 repressor motif (Xu et al. 2014a, b; Zhu et al. 2013; Cavallini et al. 2015; Albert et al. 2014; Jun et al. 2015). However, region 3 and region 5 were separation, it indicated that these MYB repressors in the two regions are different. The region 3 might be FaMYB1-like, while the region 5 might be AtMYB4-like. MdMYB16 is within the AtMYB4 clade. Analysis of the protein sequence characteristics of MdMYB16 showed that MdMYB16 has the bHLH binding motif and EAR inhibition sequence.
The analysis of phylogenetic tree and amino acid sequence about MdMYB16 (a) Phylogenetic tree analysis of flavonoid biosynthesis-related MYB transcription factors in different species. (b) Multi-alignment of the amino acid sequences of partial MYB type transcription repressors. The NCBI accessions of MYB transcription factors are AtMYB11 (XM_002876634.1), AtMYB12 (NP_182268.1), MdMYB22 (DQ074470.1), VvMYBF1 (FJ948477.2), ZmMYB42 (NM_001112539.2), CmMYB1 (JF795917.1), MdMYB16 (HM122617.1), FaMYB1 (AF401220.1), AtMYB4 (AB005889.1), AtMYB7(NP_179263), AtMYB32(NP_195225), AtMYB3(AT1G22640), PhMYB4 (ADX33331), PhMYB27(AHX24372), VvMYBC2-L1(ABW34393), VvMYBC2-L3 (KM046932),AtMYBL2(NP_177259.1),MtMYB2(XP_003616388.1),AtMYB123 (NM_122946.2), MdMYB9 (DQ267900.1), MdMYB11 (DQ074463.1) AtMYB75 (NM_104541.3), FaMYB10 (EU155162.1), PpMYB10 (GU936492.1), MdMYB10 (EU518249.2), and PyMYB10 (HM585181.1)
MdMYB16, LESMdMYB16, and MdbHLH33 participate in anthocyanin biosynthesis pathways
Overexpression of MdMYB16 in the red-fleshed callus resulted in a change in callus coloration from red to yellow (Fig. 3a), a decrease in the expression of MdUFGT and MdANS and anthocyanin content (Fig. 3b, d). The red-fleshed callus overexpressing LESMdMYB16 remained red (Fig. 3a) despite a significant increase in the expression of MdMYB16 (Fig. 3d). In this callus, the expression levels of MdUFGT and MdANS, as well as the anthocyanin levels, remained essentially the same (Fig. 3b, d). Overexpressing MdbHLH33 in callus overexpressing MdMYB16 resulted in a coloration change from yellow to pink (Fig. 3a). In this callus, the expression levels of MdUFGT and MdANS and anthocyanin content was somewhat recovered (Fig. 3b, d). The results of western blotting are shown in Fig. 3c. The use of Anti-Actin showed that all calli had bands. Detection using anti-GFP showed that wild-type red-fleshed callus had no band. Callus overexpressing both MdMYB16 and LESMdMYB16 showed one band while callus simultaneously overexpressing both MdMYB16 and MdbHLH33 showed two bands.
Transgenic analysis of MdMYB16, LESMdMYB16, and MdbHLH33. Rc: red callus, MYB16-GFP: overexpressing MdMYB16 in red callus, MYB16-GFP + bHLH33-GFP: overexpressing MdbHLH33 in callus that overexpressing MdMYB16 LESMYB16-GFP: overexpression of MdMYB16 without the EAR sequence The significance tests are shown as i, ii, iii, iv.Completely different lowercase roman alphabet on the column chart indicates significant differences (P < 0.01). a Red calli and three types of transgenic calli. b Anthocyanin levels in four kinds of calli. c Western blot analysis of four kinds of calli using Anti-GFP and Anti-Actin d Expression of anthocyanin biosynthesis structural genes and overexpression of transcription factors in four kinds of calli
Y1H assays using the MdANS and MdUFGT promoters
To analyze the target DNA sequence of MdMYB16 using Y1H the 1692 bp MdANS and 1725 bp MdUFGT promoters were chosen (Fig. 4a). The pHIS2-MdANSPro and the pHIS2-MdUFGTPro vectors were respectively transformed into Y187 yeast strains that were cultivated in -T-H selective media containing different concentrations of 3-AT. Reduced yeast growth was observed with increasing 3-AT concentration, and yeast could not grow on -T-H selective media with 80 mM 3-AT (Fig. 4b). This suggested that 80 mM 3-AT could inhibit the expression of the reporter gene HIS3 under the control of the MdANS or MdUFGT promoters. Different combinations of pGADT7-MdMYB16 and pGADT7-LESMdMYB16 with pHIS2-MdANSPro and pHIS2-MdUFGTPro were respectively co-transformed into Y187 yeast strains and were successfully grown on -T-H-L selective media containing 80 mM 3-AT. Combinations of the untransformed pGADT7 control vector with pHIS2-MdANSPro and pHIS2-MdUFGTPro did not grow on this media (Fig. 4c). This suggests that MdMYB16 and LESMdMYB16 can interact with the promoters of MdANS and MdUFGT.
Yeast one-hybrid (Y1H) assays with the MdANS and MdUFGT promoters. a The 1692 bp long MdANS promoter and the 1725-bp long MdUFGT promoter chosen to analyze the target DNA sequence. b pHIS2-MdANSPro and pHIS2-MdUFGTPro were respectively transformed into Y187 yeast strains to screen different 3-AT concentrations for reporter gene inhibition. c MdMYB16 and LESMdMYB16 could interact with the promoters of MdANS and MdUFGT
MdMYB16 is capable of forming homodimers
The results of the BiFc analysis are shown in Fig. 5a. 35S:MdMYB16-NYFP and 35S:MdMYB16-CYFP were co-transformed into onion skin, and YFP fluorescence was observed by laser scanning confocal microscopy. Conversely, when 35S:NYFP was combined with 35S:MdMYB16-CYFP or 35S:MdMYB16-NYFP with 35S:CYFP fluorescence was not observed. The results of the Y2H assays are shown in Fig. 5b. MdMYB16-BD and MdMYB16-AD were co-transformed into Y2H yeast strains that grew on -T-L and -T-L-H-A selective media containing the substrate X-α-gal. Yeast co-transformed with MdMYB16-BD and the AD or MdMYB16-AD and the BD grew on -T-L selective media but not on -T-L-H-A selective media. The results of the His pull-down analysis are shown in Fig. 5c. MdMYB16-HIS, MdMYB16-GST with MdMYB16-HIS, and GST were purified from the columns using HIS tags. Western blotting determined that as MdMYB16-GST could be detected by Anti-GST, MdMYB16-GST could be pulled down by MdMYB16-His, whereas since GST could not be detected by Anti-GST, GST could not be pulled down by MdMYB16-His. In summary, MdMYB16 is capable of forming homodimers both in vivo and in vitro.
MdbHLH33 interacts with MdMYB16
The results of the BiFc analysis are shown in Fig. 6a. 35S:MdMYB16-NYFP and 35S:MdbHLH33-CYFP were co-transformed into onion skin, and YFP fluorescence was observed by laser scanning confocal microscopy. Conversely, when 35S:NYFP was combined with 35S:MdbHLH33-CYFP and 35S:MdMYB16-NYFP with 35S:CYFP no fluorescence was observed. The results of the Y2H assays are shown in Fig. 6b. MdMYB16-BD and MdbHLH33-AD were co-transformed into Y2H yeast strains that grew successfully on -TLHA and -TL selective media provided with the substrate X-α-gal. Conversely, yeast co-transformed with MdMYB16-BD and the AD or MdbHLH33-AD and the BD grew on -T-L selective media but not -T-L-H-A selective media. The results of the His pull-down analysis are shown in Fig. 6c. MdMYB16-His, MdbHLH33-GST with MdMYB16-His, and GST were purified from the columns using HIS tags. Western blot analysis indicated that as MdbHLH33-GST could be detected by Anti-GST, MdbHLH33-GST could be pulled down by MdMYB16-His, whereas since GST could not be detected by Anti-GST, GST could not be pulled down by MdMYB16-His. In summary, MdbHLH33 is capable of interacting with MdMYB16 both in vivo and in vitro. We, therefore, propose that MdbHLH33 competitively inhibits the formation of MdMYB16 homodimers or affects the stability of these MdMYB16 homodimers.
LBSMdMYB16 and MdbHLH33 participate in anthocyanin biosynthesis pathways
The callus overexpressing LBSMdMYB16 had the similar inhibitory effect on anthocyanin synthesis with the callus overexpressing MdMYB16 (Fig. 7a, b, d). Overexpressing MdbHLH33 in callus overexpressing LBSMdMYB16 resulted in a coloration change from yellow to light pink (Fig. 7a). However, in this callus, the expression levels of MdUFGT and MdANS and anthocyanin content was lower than those in the callus simultaneously overexpressing MdMYB16 and MdbHLH33 (Fig. 7a, c, d). The results of the Y2H assays are shown in Fig. 7b, the LBSMdMYB16 could not interact with the MdbHLH33.These results indicated that MdMYB16 without bHLH binding sequence could not influence the inhibitory effect of ownself on anthocyanin synthesis, however, it could influence the interaction bwtween MdMYB16 and MdbHLH33. Further analysis showed that although MdbHLH33 was knowed as a activator of anthocyanin synthesis, it was the interaction between MdbHLH33 and MdMYB16 that influenced the inhibitory effect of MdMYB16 on anthocyanin synthesis.
Transgenic analysis and Y2H assays of LBSMdMYB16 and MdbHLH33 Rc: red callus, MYB16-GFP: overexpressing MdMYB16 in red callus, LBSMYB16-GFP: overexpression of MdMYB16 without the bHLH binding sequence MYB16-GFP + bHLH33-GFP: overexpressing MdbHLH33 in callus that overexpressing MdMYB16 LBSMYB16-GFP + bHLH33-GFP: overexpressing MdbHLH33 in callus that overexpressing LBSMdMYB16. a Red calli and four types of transgenic calli. b Y2H analysis between LBSMdMYB16 and MdbHLH33. c Anthocyanin levels in five kinds of calli. d Expression of anthocyanin biosynthesis structural genes and overexpression of transcription factors in five kinds of calli
Discussion
Anthocyanin biosynthesis was regulated by MBW complexes, among which MYB proteins that include activators and repressors were widely studied. The current research shows that MYB repressors may have two types, one acted upon MBW complexes (e.g., Fa-MYB1; Aharoni et al. 2001; Paolocci et al. 2011), the other directly binded the target genes (e.g., At-MYB4; Jin et al. 2000). This study showed that MdMYB16 is within the AtMYB4 clade, and could bind the promoter of MdANS and MdUFGT, thereby directly inhibited anthocyanin biosynthesis via the EAR sequence. Additionally, we also found that MdMYB16 could form homodimers and interact with MdbHLH33.
MdMYB16 might play a important role in color differences among five Red Crisp first-generation hybrids
Espley et al. (2007, 2009) found that fruit anthocyanin biosynthesis is mainly regulated by MdMYB10, the promoter of which has self-activation characteristics and can appear in two forms, namely R6 and R1, depending on the number of repeats it contains. They also showed that the MdMYB10 promoter type in red-fleshed apple was R6R6, while the MdMYB10 promoter type in white-fleshed apple was R1R1. So it might be the different MdMYB10 promoter type that cause the difference of anthocyanin content. In our study, the MdMYB10 promoter type in five Red Crisp first-generation hybrids was all R6R1. Therefore, their anthocyanin content should be consistent. However, the significant differences were observed in the flesh color of Red Crisp apple (Fig. 1a), and the expression of MdMYB10 in each line was not consistent with the anthocyanin levels (Fig. 1c, e). This suggested that there might be other transcription factors in addition to MdMYB10 involved of the anthocyanin biosynthesis. Lin-Wang et al. (2011) found that MdMYB16/17/111 could weaken the activation of MYB10-bHLH3 transcriptional activation complex acting on the DFR promoter. They also showed that MdMYB16 is within the AtMYB4 clade of repressors by phylogenetic tree analysis. However, it was not known how MdMYB16 inhibit anthocyanin synthesis. Therefore, we took MdMYB16 as an important candidate gene in our paper. Analysis of MdMYB16 expression in fruit of the five Red Crisp lines showed that fruit with high anthocyanin levels had low MdMYB16 expression levels, while fruit with low anthocyanin levels had high MdMYB16 expression levels (Fig. 1c, e). Further study showed that overexpressing MdMYB16 in red callus showed that it could suppress both the expression of anthocyanin structural genes and the accumulation of anthocyanins (Fig. 3a, b, d). So, except for MdMYB10, we think MdMYB16 might play a important role in color differences among five Red Crisp first-generation hybrids.
MdMYB16 could directly inhibit anthocyanin biosynthesis via the EAR sequence
The EAR motif was a common feature of EAR-type transcriptional repressors, with changes in the amino acid residues within the EAR motif resulting in a reduction or loss of suppression (Ohta et al. 2001; Tiwari et al. 2001, 2004). Several studies of MYB proteins that has the EAR sequence had a great progress. AtMYB4 and PhMYB4 were found that they can repress the expression of C4H (Jin et al. 2000; Hemm et al. 2001; Colquhoun et al. 2011). FaMYB1, PhMYB27, VvMYBC2 and MtMYB2 were found that they can act upon MBW complexes (Paolocci et al. 2011; Albert et al. 2014; Jun et al. 2015; Cavallini et al. 2015). So, these MYB proteins were divided into two types of FaMYB1-like and AtMYB4-like by phylogenetic tree analysis (Jun et al. 2015). In our study, MdMYB16 is within the AtMYB4 clade (Fig. 2a). Overexpressing MdMYB16 in red callus showed that it could suppress both the expression of anthocyanin structural genes and the accumulation of anthocyanins (Fig. 3a, b, d). The YIH assay showed that MdMYB16 could interact with the promoters of MdANS and MdUFGT (Fig. 4). So MdMYB16 could directly inhibit anthocyanin biosynthesis by repressing the expression of MdANS and MdUFGT.
The Arabidopsis full-length AtERF3 and the EAR motif peptide at its C-terminus inhibits the binding of AtERF5 to the GCC box in plants. However, this inhibitory activity is lost when the amino acid residue D is substituted with A in the AtERF3 EAR motif (LDLNLAP) (Ohta et al. 2001). Overexpression of ZAT7 genes in Arabidopsis results in increased salt stress resistance, while mutations or deletions in the EAR motif of ZAT7 proteins reduces this increased resistance in transgenic plants (Ciftci-Yilmaz et al. 2007). SUPERMAN is a transcription factor that has been associated with organ development and contains an EAR motif at its C-terminus. When the EAR motif is absent, the ectopic expression of SUPERMAN results in a similar phenotype to the loss-of-function mutant superman gene (Hiratsu et al. 2002). Recent studies on the structural and functional aspects of EAR-type transcriptional repression have developed a new suppression tool in the form of chimeric repressor gene-silencing technology. The EAR motif from an EAR-type transcriptional repressor was added to the C-terminus of the activator and fused as a chimeric protein that in turn can be transformed into a highly efficient negative regulator that can be introduced into plants using transgenic methods for the specific and efficient inhibition of the expression of a target gene (Hiratsu et al. 2003; Mitsuda et al. 2006; Kam et al. 2008). In the present study, we analyzed the characteristics of the MdMYB16 protein sequence and determined that a 10-amino acid EAR suppression sequence (PDLNLDLQIS) was present at the C-terminus (Fig. 2b). We removed the EAR sequence of MdMYB16 using overlapping PCR and overexpressed the resulting MdMYB16 gene without the EAR motif (LESMdMYB16) in red callus. This showed that the expression of anthocyanin structural genes and anthocyanin levels in the red callus overexpressing LESMdMYB16 were essentially the same as in the wild-type red-fleshed callus (Fig. 3a, b, d). The YIH assay showed that MdMYB16 and LESMdMYB16 could each interact with the promoters of MdANS and MdUFGT, suggesting that the MdMYB16 EAR sequence did not affect the interaction between MdMYB16 and MdANS and MdUFGT. Therefore, we concluded that the inhibitory role of MdMYB16 is achieved by the EAR sequence at the C-terminus.
MdMYB16 could interact with MdbHLH33 and form homodimers
The interaction between MYB and bHLH transcription factors is crucial for the establishment of a regulatory complex controlling the late enzymatic steps of the anthocyanin (Xu et al. 2014a, b). In the present study, we present a new interaction between MYB and bHLH proteins. MdMYB16 had the bHLH binding motif and could interact with MdbHLH33 (Figs. 2b, 6), the relationship of which was similar to the relationship between AtMYB4 and AtbHLH (Zimmermann et al. 2004). Overexpression of MdbHLH33 in calli overexpressing MdMYB16 resulted in greater expression of the anthocyanin structural genes, and greater anthocyanin levels, when compared with the calli only overexpressing MdMYB16 (Fig. 3a, b, d). Further study showed that the expression levels of MdUFGT and MdANS and anthocyanin content in the callus simultaneously overexpressing LBSMdMYB16 and MdbHLH33 was lower than those in the callus simultaneously overexpressing MdMYB16 and MdbHLH33 (Fig. 7a, c, d). And the LBSMdMYB16 could not interact with the MdbHLH33 (Fig. 7b). This indicated that it was the interaction between MdbHLH33 and MdMYB16 that influenced the the inhibitory effect of MdMYB16 on anthocyanin synthesis. Additionally, we also found that MdMYB16 could form homodimers (Fig. 5), we thought the discoveries about MYB–MYB dimerization were very interesting and might help explain the operatation of this class of transcription factor normally. On the basis of these results, we speculated that MdMYB16 could form a large number of stable homodimers by overexpressing MdMYB16 in red callus, and it could directly inhibit anthocyanin biosynthesis via the EAR sequence. However, overexpression of MdbHLH33 knowed as a activator of anthocyanin biosynthesis in callus with up-regulated MdMYB16 resulted in an interaction between MdMYB16 and MdbHLH33, and then affected MdMYB16 represser complex, thereby ultimately weakened the inhibitory effect of MdMYB16 on anthocyanins. However, this speculation required further validation, and my research provided a basis for the new regulatory model.
Conclusion
In the present study, we used transgenic approaches and protein interactions to examine the anthocyanin synthesis pathway genes MdMYB16 and MdbHLH33. A putative regulatory network of anthocyanin biosynthesis controlled by MdMYB16 and MdbHLH33 transcription factors was showed in Fig. 8. The results showed that MdMYB16 could directly inhibit anthocyanin biosynthesis via the C-terminal EAR repressor. We also found that MdMYB16 interacted with MdbHLH33 and could form homodimer with itself. These results suggested that MdMYB16 and MdbHLH33 may be important part of the regulatory network controlling the anthocyanin biosynthetic pathway.
A putative regulatory network of anthocyanin biosynthesis controlled by MdMYB16 and MdbHLH33 transcription factors in apple fruit. a MdANS and MdUFGT could control anthocyanin biosynthesis in red callus. b MdMYB16 could form a large number of stable homodimers by overexpressing MdMYB16 in red callus, and it could directly inhibit anthocyanin biosynthesis via the EAR sequence. c Overexpression of MdbHLH33 in callus with up-regulated MdMYB16 resulted in an interaction between MdMYB16 and MdbHLH33, and then affected MdMYB16 represser complex, thereby ultimately weakened the inhibitory effect of MdMYB16 on anthocyanins
Abbreviations
- CHS:
-
Chalone synthase
- FLS:
-
Flavonol synthase
- DFR:
-
Dihydroflavonol reductase
- C4H:
-
Cinnamate 4-hydroxylase
- ANS:
-
Anthocyanin synthase
- UFGT:
-
UDP-glucose:flavonoid 3- glucosyltransferase
- EAR:
-
ERF-associated amphiphilic repression
- GFP:
-
Green fluorescent protein
- GST:
-
Glutathione S-transferase
- His:
-
Histidine
- Anti:
-
Antibody
- MS:
-
Murashige and Skoog
- -T(-Trp):
-
No tryptophan
- -L(-Leu):
-
No leucine
- -H(-His):
-
No histidine
- -A(-Ade):
-
No adenine
- AD:
-
Activation domain
- BD:
-
Binding domain
- CDS:
-
Coding DNA sequence
References
Aharoni A, De vos C, Wein M, Sun Z, Greco R, Kroon A, Mol JN, O’connell AP (2001) The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J 28(3):319–332
Albert NW, Lewis DH, Zhang H, Schwinn KE, Jameson PE & Davies KM (2011) Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J 65:771–784
Albert NW, Davies KM, Lewis DH, Zhang H, Montefiori M, Brendolis C, Boase MR, Ngo H, Jameson PE, Schwinn KE (2014) A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell 26:962–980
An XH, Tian Y, Chen KQ, Liu XJ, Liu DD, Xie XB, Cheng CG, Cong PH, Hao YJ (2014) MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol 56(4):650–662
Azuma A, Kobayashi S, Mitani N, Shiraishi M, Yamada M, Ueno T, Kono A, Yakushiji H, Koshita Y (2008) Genomic and genetic analysis of Myb-related genes that regulate anthocyanin biosynthesis in grape berry skin. Theor Appl Genet 117:1009–1019
Bai SL, Saito A, Honda C, Hatsuyama Y, Ito A, Moriguchi T (2014) An apple B-box protein, MdCOL11, is involved in UV-B- and temperature-induced anthocyanin biosynthesis. Planta 240:1051–1062
Ballester AR, Molthoff J, de Vos R, te Lintel HB, Orzaez D, Fernandezmoreno JP, Tripodi P, Grandillo S, Martin C, Heldens J, Ykema M, Granell A, Bovy A (2010) Biochemical molecular analysis of pink tomatoes, deregulated expression of the gene encoding transcription factor SlMYB12 leads to pink tomato fruit color. Plant Physiol 152:71–84
Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T (2007) Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol 48:958–970
Boyer J, Liu RH (2004) Apple phytochemicals and their health benefits. Nutr J 3:5
Cavallini E, Matus JT, Finezzo L, Zenoni S, Loyola R, Guzzo F, Schlechter R, Ageorges A, Arce-Johnson P, Tornielli GB (2015) The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYB C2 repressors in grapevine. Plant Physiol 167:1448–1470. doi:10.1104/pp.114.256172
Chen X, Feng T, Zhang Y, He T, Feng J, Zhang C (2007) Genetic diversity of volatile components in xinjiang wild apple (Malus sieversii). J Genetic Genom 34(2):171–179
Chen XS, Zhang J, Liu DL, Ji XH, Zhang ZY, Zhang R, Mao ZQ, Zhang YM, Wang LX, Li M. 2014. Genetic Variation of F1 population between Malus sieversii f. neidzwetzkyana and apple varieties and evaluation on fruit characters of functional apple excellent strains. Sci Agric Sin 47(11), 2193–2204
Chikako H, Nobuhiro K, Masato W, Satoru K, Shozo K, Junichi S, Zilian Z, Tomomi T, Takaya M (2002) Anthocyanin biosynthetic gene are coordinately expressed during red coloration in apple skin. Plant Physiol Biochem 40:955–962
Ciftci-Yilmaz S, Morsy MR, Song LH, Coutu A, Krizek BA, Lewis MW, Warren D, Cushman J, Connolly EL, Mittler R (2007) The EAR-motif of the Cys2/His2-type zinc finger protein Zat7 plays a key role in the defense response of Arabidopsis to salinity stress. J Biol Chem 282:9260–9268
Colquhoun TA, Kim JY, Wedde AE, Levin LA, Schmitt KC, Schuurink RC, Clark DG (2011) PhMYB4 fine-tunes the floral volatile signature of Petunia x hybrida through PhC4H. J Exp Bot 62:1133–1143
Cook NC, Samman S (1996) Flavonoids-Chemistry, metabolism, cardiop rotective effects, and dietary sources. J Nutr Biochem 7:66–76
Czemmel S, Stracke R, Weisshaar B, Cordon N, Harris NN, Walker AR, Robinson SP, Bogs J (2009) The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol fynthesis in feveloping Grape berries. Plant Physiol 151:1513–1530
Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC (2007) Red colouration in apple fruit is due to the activity of the MYB transcription factor MdMYB10. Plant J 49:414–427
Espley RV, Brendolise C, Chagné D, Kutty-Amma S, Green S, Volz R, Putterill J, Schouten HJ, Gardiner SE, Hellens RP, Allan AC (2009) Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell 21:168–183
Feng SQ, Wang YL, Song Y, Xu YT, Chen XS (2010) Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta 232:245–255
Hemm MR, Herrmann KM, Chapple C (2001) AtMYB4:A transcription factor general in the battle against UV. Trends Plant Sci 6(4):135–136
Hill K, Wang H, Perry SE. (2008). A transcriptional repression motif in the MADS factor AGL15 is involved in recruitment of histone deacetylase complex components. Plant J 53(1), 172–185
Hiratsu K, Ohta M, Matsui K, Ohme-Takagi M (2002) The SUPERMAN protein is an active repressor whose carboxy-terminal repression domain is required for the development of normal flowers. FEBS Lett 514(2):351–354
Hiratsu K, Kyoko M, Koyama T, Ohme-Takagi M (2003) Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J 34(5):733–739
Hollman P, Katan M (1997) Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother 51:305–310
Ji XH, Wang YT, Zhang R, Wu SJ, An MM, Li M, Wang CZ, Chen XL, Zhang YM, Chen XS (2015) Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f.niedzwetzkyana). Plant Cell Tiss Organ Cult 120:325–337
Jin HL, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C (2000) Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J 19:6150–6161. doi:10.1093/emboj/19.22.6150
Jun JY, Liu CG, Xiao XR, Dixon RA (2015) The Transcriptional repressor MYB2 regulates both spatial and temporal patterns of proanthocyandin and anthocyanin pigmentation in Medicago truncatula. Plant Cell. doi:10.1105/tpc.15.00476
Kam J, Gresshoff PM, Shorter R, Xue GP (2008) The Q-type C2H2 zinc finger subfamily of transcription factors in Triticum aestivum is predominantly expressed in roots and enriched with members containing an EAR repressor motif and responsive to drought stress. Plant Mol Biol 67(3):305–322
Kazan K (2006) Negative regulation of defence and stress genes by EAR-motif-containing repressors. Trends Plant Sci 11(3):109–112
Kenneth JL, Thomas DS (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2—ΔΔCT method. Methods 25:402–408
Kim SY, Lee JR, Kim SR (2006) Characterization of an Apple anthocyanidin synthase gene in transgenic Tobacco. Plants. J Plant Biol 49(4):326–330
Koes R, Verweij W, Quattrocchio F (2005) Flavonoids, a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 10:236–242
Kondo S, Hiraoka K, Kobayashi S, Honda C, Terahara N (2002) Changes in the expression of anthocyanin biosynthetic genes during apple development. J Am Soc Hortic Sci 127:971–976
Liu XF, Li F, Yin XR, Xu CJ, Chen KS (2013) Recent advances in the transcriptional regulation of anthocyanin biosynthesis. Acta Hortic Sinica 40(11):2295–2306
Lin-Wang K, Micheletti D, Palmer J, Volz R, Lozano L, Espley R, Hellens RP, Chagnè D, Rowan DD, Troggio M, Iglesias I, Allan AC (2011) High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant Cell Environ 34(7):1176–1190
Mitsuda N, Todaka D, Nakashima K, Yamaguchi-Shinozaki K, Ohme-Takagi M (2006) Efficient production of male and female sterile plants by expression of a chimeric repressor in Arabidopsis and rice. Plant Biotechnol J 4:325–332
Nocker SV, Berry G, Najdowski J, Michelutti R, Luffman M, Forsline P, Alsmairat N, Beaudry R, Muraleedharan GN, Ordidge M (2012) Genetic diversity of red-fleshed apples (Malus). Euphytica 185:281–293
Ohta M, Matsui K, Shinshi H, Ohme-Takagi M (2001) Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13:1959–1968
Paolocci F, Robbins MP, Passeri V, Hauck B, Morris P, Rubini A, Arcioni S, Damiani F (2011) The strawberry transcription factor FaMYB1 inhibits the biosynthesis of proanthocyanidins in Lotus corniculatus leaves. J Exp Bot 62(3):1189–1200
Preston J, Wheeler J, Heazlewood J, Li SF, Parish RW. 2004. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J 40(6), 979–995
Schaefer HM, McGraw K, Catoni C (2008) Birds use fruit colour as honest signal of dietary antioxidant rewards. Funct Ecol 22(2):303–310
Solfanelli C, Bartolini S, Vitagliano C, Lorenzi R (2006) Immunolocalization and quantification of IAA after self- and freepollination in Olea europaea L. Sci Hortic 110(4):345–351
Sonbol FM, Fornale S, Capellades M, Encina A, Tourino S, Torres JS, Rovira P, Ruel K, Puigdomenech P, Rigau J, Caparros-Ruiz D (2009) The maize ZmMYB42 represses the phenylpropanoid pathway and affects the cell wall structure, composition and degradability in Arabidopsis thaliana. Plant Mol Biol 70:283–296
Sun CD, Huang HZ, Xu CJ, Li X, Chen KS (2013) Biological activities of extracts from Chinese bayberry(Myrica rubra Sieb. et Zucc): a review. Plant Foods Hum Nutr 68(2):97–106
Takos AM, Jaffé FW, Jacob SR, Bogs J, Robinson SP, Walker AR (2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142(3):1216–1232
Tiwari SB, Wang XJ, Hagen G, Guilfoyle TJ (2001) Aux/IAA proteins are active repressors and their stability and activity are modulated by auxin. Plant Cell 13:2809–2822
Tiwari SB, Hagen G, Guilfoyle TJ (2004) Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16:533–543
Tsukagoshi H, Morikami A, Nakamura K. 2007. Two B3 domain transcriptional repressors prevent sugar-inducible expression of seed maturation genes in Arabidopsis seedlings. Proc Nat Acad Sci USA 104(7), 2543–2547
Velasco R et al (2010) The genome of the domesticated apple (Malus domestica Borkh). Nat Genet 42:833–839
Wang LK, Bolitho K, Grafton K, Kortstee A, Karunairetnam S, McGhie TK, Espley RV, Hellens RP, Allan AC (2010) An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol 10:50
Wang N, Zheng Y, Duan NB, Zhang ZY, Ji XH, Jiang SH, Sun SS, Yang L, Bai Y, Fei ZJ, Chen XS. (2015). Comparative Transcriptomes Analysis of Red and White-Fleshed Apples in an F1 Populationof Malus sieversii f. niedzwetzkyana Crossed with M. domestica ‘Fuji’. PLoS ONE. 10(7):e0133468
Xu F, Ning Y, Zhang W, Liao Y, Li L, Cheng H, Cheng S (2014a) An R2R3-MYB transcription factor as a negative regulator of the flavonoid biosynthesis pathway in Ginkgo biloba. Funct Integr Genom 14(1):177–189
Xu W, Grain D, Bobet S, Le Gourrierec J, Thévenin J, Kelemen Z, Lepiniec L, Dubos C (2014b) Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB-bHLHWDR complexes and their targets in Arabidopsis seed. New Phytol 202:132–144
Zhu L, Shan H, Chen SM, Jiang JF, Gu CS, Zhou GQ, Chen Y, Song AP, Chen FD.2013. The Heterologous expression of the Chrysanthemum R2R3-MYB transcription factor CmMYB1 alters lignin composition and represses flavonoid synthesis in Arabidopsis thaliana. PLoS ONE 8(6), e65680
Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF (2004) Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J 40:22–34
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31572091) and National Key Research and Development Project of China (2016YFC0501505).
Author contributions
XC and HX: conceived and designed the experiments. HX and NW: performed the experiments. HX: analyzed the data. JL, CQ, YW, SJ, NL, ZZ: contributed reagents/materials/analysis tools. HX and XC: wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, H., Wang, N., Liu, J. et al. The molecular mechanism underlying anthocyanin metabolism in apple using the MdMYB16 and MdbHLH33 genes. Plant Mol Biol 94, 149–165 (2017). https://doi.org/10.1007/s11103-017-0601-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-017-0601-0