Abstract
The survival and reproduction of plants depend on their ability to cope with a wide range of daily and seasonal environmental fluctuations during their life cycle. Phytohormones are plant growth regulators that are involved in almost every aspect of growth and development as well as plant adaptation to myriad abiotic and biotic conditions. The circadian clock, an endogenous and cell-autonomous biological timekeeper that produces rhythmic outputs with close to 24-h rhythms, provides an adaptive advantage by synchronizing plant physiological and metabolic processes to the external environment. The circadian clock regulates phytohormone biosynthesis and signaling pathways to generate daily rhythms in hormone activity that fine-tune a range of plant processes, enhancing adaptation to local conditions. This review explores our current understanding of the interplay between the circadian clock and hormone signaling pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As sessile organisms, plants spend their entire life cycle in the same place that they germinated. This, along with their poikilothermic nature, forces them to adapt to a variety of abiotic and biotic stresses that change both on short- and long-term time scales. Persistent challenges such as drought, shade, cold, and the changing seasons are dealt with in part by the impressive developmental and physiological plasticity of plants (de Jong and Leyser 2012). Hormone signaling pathways have long been known to play key roles in plant responses to such long-term environmental challenges. Daily environmental fluctuations also present plants with significant difficulties. For example, day/night cycles cause huge alterations not only in light levels but also in water availability; plants undergo profound daily changes in their metabolism to cope with fluctuations in these essential resources (Farre and Weise 2012; Muller et al. 2014). The circadian clock plays a central role in plant adaptations to daily and even seasonal changes in the environment. It is therefore perhaps not surprising that multiple connections between the clock and hormone pathways have recently been revealed. In this review, we will focus on studies demonstrating circadian modulation of hormone levels and physiological pathways controlled by hormones. We will also discuss evidence that hormone signaling may feed back to influence the circadian network.
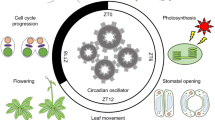
Circadian clocks are found in most eukaryotes and some prokaryotes. They are cell-autonomous biological timekeepers that generate roughly 24-h rhythms in many metabolic and physiological processes (Greenham and McClung 2015; Hsu and Harmer 2014). Daily rhythms can be diel, observed when there are regular rhythmic inputs such as daily light and dark cycles, or circadian, persisting in the absence of rhythmic environmental cues. It has been demonstrated in plants, bacteria, and mammals that circadian clocks that run with a period matched to that of external environmental cycles provide a competitive advantage (Dodd et al. 2005; Ouyang et al. 1998; Spoelstra et al. 2016), presumably by allowing organisms to correctly anticipate regular changes in the environment including alterations in temperature, light, and humidity. The circadian system can be generalized as consisting of input or entrainment pathways, the central clock or oscillator, and output pathways. Inputs such as light perceived by plant receptors entrain the central oscillator to generate precisely-phased rhythmic outputs, such as the release of volatiles timed to attract appropriate pollinators and enhanced resistance to cold at night (Greenham and McClung 2015; Yakir et al. 2007). The plant circadian clock generates daily and even seasonal rhythms in many physiological processes including stomatal opening, leaf movement, hypocotyl elongation, photosynthesis and carbon metabolism, resistance to abiotic and biotic stresses, and flowering time (Angelmann and Johnsson 1998; Farre 2012; Hsu and Harmer 2014; Muller et al. 2014; Song et al. 2015; Webb 1998; Yakir et al. 2007).
In addition to generating obvious daily rhythms, the circadian clock plays a more subtle role in the regulation of plant physiology. Many signaling pathways are modulated by the clock so that they are differentially active at different times of the day or night in a process known as “gating”. For example, plants treated with auxin (indole-3-acetic acid; IAA) at night are more responsive than plants treated with the same auxin concentration during the day (Covington and Harmer 2007; Went and Thimann 1937). Similar gating of responses to environmental cues such as light and temperature have also been reported (Adams and Carre 2011). It is thought that circadian gating may help plants distinguish between random fluctuations in the environment and longer-term alterations.
The plant circadian clock
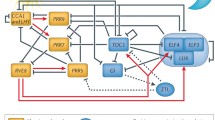
The plant circadian clock is the most complex yet reported in any organism and consists of a highly interconnected network of transcription factors that regulate each other’s expression (Fig. 1). Here we present a brief overview of our current understanding of the plant circadian clock. Readers are directed to recent excellent reviews and references therein for more details about the clock machinery (Hsu and Harmer 2014; McClung 2014). The closely-related, morning-expressed MYB-like transcription factors CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) and the evening expressed TIMING OF CAB EXPRESSION 1 (TOC1/PRR1) reciprocally repress each other’s expression. The repression of CCA1 and LHY by TOC1 requires the CCA1 HIKING EXPEDITION (CHE) gene. The morning-phased CCA1 and LHY proteins repress expression of the “evening complex” (EC) components LUX ARRHYTHMO (LUX), EARLY FLOWERING 3 (ELF3), and ELF4, while the afternoon-phased MYB-like transcription factor REVEILLE8 (RVE8) activates their expression. Another double-negative feedback loop exists between CCA1 and LHY and the day-phased transcription factors PSEUDO RESPONSE REGULATOR9 (PRR9), PRR7, and PRR5 (Adams et al. 2015; Fogelmark and Troein 2014). In addition to repressing CCA1 and LHY expression, these PRRs repress expression of RVE8 (Fig. 1).
Brief overview of the plant circadian clock. Three different size ovals represent levels of the indicated proteins. Arrows and perpendicular bars indicate activation and repression, respectively. For simplicity, the morning-expressed MYB-like transcription factor LATE ELONGATED HYPOCOTYL (LHY), which functions semi-redundantly with its homolog CCA1, is not shown. For more details about the clock machinery, see recent reviews (Adams et al. 2015; Hsu and Harmer 2014; McClung 2014)
In addition to regulating expression of other oscillator components, the transcription factors that make up the plant clock regulate expression of thousands of output genes. Genome-wide studies carried out with RNA extracted from intact seedlings suggest that about 30 % of expressed genes are clock regulated (Covington et al. 2008; Hsu and Harmer 2012; Michael et al. 2008b), although the fraction of the transcriptome that is clock regulated in some but not all cell types is likely considerably higher (Endo et al. 2014). Intriguingly, genes regulated by the hormones abscisic acid (ABA), brassinosteroids (BR), cytokinins (CK), ethylene (ET), gibberellins (GAs), IAA, jasmonates (JAs), and salicylic acid (SA) are more likely to be clock-regulated than expected by chance (Covington and Harmer 2007; Covington et al. 2008; Dodd et al. 2007; Mizuno and Yamashino 2008). Our recent analysis with a more complete list of cycling genes (Hsu and Harmer 2012) than used in previous studies reveals that between 35 and 46 % of hormone related genes are also clock regulated in Arabidopsis, significantly more than the 29 % expected by chance (Fig. 2). Recent chromatin immunoprecipitation studies demonstrated that CCA1, TOC1, and the PRRs bind to the promoters of hundreds of genes (Huang et al. 2012; Liu et al. 2013, 2016b; Nagel et al. 2015; Nakamichi et al. 2012). Interestingly, more of these putative direct targets of the circadian clock machinery are regulated by plant hormones than expected by chance (Fig. 3). Additional genome-wide analyses suggest functional links between clock components and plant hormone pathways. For example, more than one-third of the likely direct targets of PRR7 also contain ABA-responsive elements in their upstream regions; the functional relevance of this finding is supported by the reduction of ABA-induced gene expression in plants overexpressing PRR7 (Liu et al. 2013). Thus the circadian clock machinery has been implicated in direct control of genes involved in hormone signaling.
The percentages of clock-regulated genes (Hsu and Harmer 2012) that are also regulated by individual phytohormones (Blanco et al. 2009; Nemhauser et al. 2006; Schenk et al. 2000) are plotted. Asterisks indicate statistically significant circadian enrichment over the 29 % circadian regulation expected by chance (Fisher’s exact test; p < 0.05)
The percentages of CCA1, TOC1, PRR9, PRR7, and PRR5 target genes (as identified by chromatin immunoprecipitation (Huang et al. 2012; Liu et al. 2013, 2016b; Nagel et al. 2015; Nakamichi et al. 2012) that are regulated by individual phytohormones (Blanco et al. 2009; Nemhauser et al. 2006; Schenk et al. 2000). Asterisks indicate statistically significant enrichment of phytohormone-regulated genes among all identified clock protein target genes (Fisher’s exact test; p < 0.05)
Daily rhythms in hormone levels
Clock regulation of hormone signaling occurs at additional levels as well. It has long been noted that levels of many phytohormones oscillate over 24-h day/night cycles. For example, diel variations in ethylene levels have been demonstrated in bean, cotton, sorghum, Arabidopsis, rice, low-elevation longstalk starwort, red goosefoot, and Kalanchoe daigremontiana (Emery et al. 1994; Finlayson et al. 1998; Kapuya and Hall 1977; Lee et al. 1981; Lipe and Morgan 1973; Machackova et al. 1997; Morgan et al. 1990; Thain et al. 2004). In many species, ethylene production has been reported to persist in constant conditions and can thus be classified as circadian regulated (Finlayson et al. 1998; Jasoni et al. 2000; Morgan et al. 1990; Thain et al. 2004). However, in other species daily oscillations do not persist in constant conditions (Machackova et al. 1997) or are even absent in all conditions tested (Emery et al. 1994).
Diel oscillations in the growth-related hormones IAA, GAs, CKs and BRs have been reported in multiple species. Diel changes in IAA levels have been observed in leaves of Coffea arabica and tobacco, with peak levels in the middle of the day (Janardhan et al. 1973; Novakova et al. 2005). Similar diel oscillations were reported in the tropical tree West Indian locust (Velho do Amaral et al. 2012) and in red goosefoot (Krekule et al. 1985), but with peak IAA levels occurring at night. Circadian regulation of free IAA levels has been demonstrated in Arabidopsis and Chenopodium rubrum (Jouve et al. 1999; Pavlova and Krekule 1984), but with peak levels at the end of the subjective day and midday, respectively. Interestingly, the cycling patterns of expression of many IAA biosynthetic and signaling genes are highly conserved across poplar, rice, and Arabidopsis (Filichkin et al. 2011).
Levels of some but not all GAs have been reported to peak at the end of the day in spinach and sorghum (Foster and Morgan 1995; Lee et al. 1998; Talon et al. 1991), at the beginning of the day in pea (Stavang et al. 2005), and to show no significant daily variation in begonia (Myster et al. 1997). CK levels showed diel cycling in tobacco leaves, with peak levels at midday (Bancos et al. 2002; Novakova et al. 2005) while in pineapple levels were reported to peak near dawn in shoots but in the middle of the night in roots (Freschi et al. 2009). Finally, in Arabidopsis and tobacco, BR and CK levels were reported to show diel regulation with peak levels at midday (Bancos et al. 2002; Novakova et al. 2005).
Stress and defense-related hormones undergo diel oscillations as well. Levels of SA and JA are clock regulated in Arabidopsis, with peak accumulation in the middle of the subjective night and in the middle of subjective day, respectively (Goodspeed et al. 2012). Diurnal rhythms of JA have been reported in roots but not leaves of Nicotiana attenuata (Kim et al. 2011); however, in this plant JA levels peak at night. Similar variations in the timing of ABA oscillations have been reported. While ABA levels oscillate in poplar (Barta and Loreta 2006), field-grown pearl millet (Henson et al. 1982), Arabidopsis (Lee et al. 2006), and Arbutus unedo (Burschka et al. 1983) with peak levels around midday, ABA levels in soybean are circadian regulated with peak levels occurring at night (Lecoq et al. 1983). Finally, in tobacco leaves, ABA levels showed a complex pattern with two peaks during the day and a higher peak at the beginning of the dark phase (Novakova et al. 2005).
In summary, diel and circadian regulation of hormone levels is widespread in plants, but species- and tissue-specific variation is considerable. Thus there are undoubtedly many ways in which the circadian system interacts with hormone metabolic pathways. We will discuss a few below.
Circadian regulation of genes that control hormone levels
Genome-wide transcriptome studies have revealed that expression of many genes that encode hormone biosynthetic enzymes is clock regulated. For example, many genes that function in the synthesis of isoprenoids, precursors of the hormones ABA, BR, CK, and GA (Vranova et al. 2013), are clock controlled. In Arabidopsis, the circadian clock regulates at least 50 % of the genes encoding key enzymes of the mevalonate (MVA) and the methylerythritol phosphate (MEP) pathways leading to isoprenoid synthesis (Fig. 4). Key genes in the MEP pathway have been shown to be targets of the central clock proteins CCA1 and LHY (Pokhilko et al. 2015). Interestingly, the conversion of 3-hydroxy-3-methylglutaryl-coenzyme A to mevalonate by 3-hydroxy-3-methylglutaryl-CoA reductase is also clock regulated in mammals (Shapiro and Rodwell 1969).
Circadian regulation of many phytohormone biosynthesis enzymes in Arabidopsis. a The times of peak expression of clock-regulated phytohormone biosynthesis genes. Light red early subjective day, dark red late subjective day, light blue early subjective night, dark blue late subjective night. b Overview of the major phytohormone biosynthesis pathways and the enzymes involved. Black metabolites, blue enzymes, red enzymes with clock regulated gene expression. MVA—mevalonate, MEP—methylerythritol phosphate, ABA—abscisic acid, BR—brassinosteroids, CK—Cytokinins, ET—ethylene, GA—Gibberellins, IAA—indole-3-acetic acid, JA—Jasmonates, and SA—salicylic acid. More details are found at http://biocyc.org/ARA/NEW-IMAGE?object=Plant-Hormone-Biosynthesis and within the following references (Dempsey et al. 2011; Gupta and Chakrabarty 2013; Mano and Nemoto 2012; Ruiz-Sola and Rodriguez-Concepcion 2012; Vranova et al. 2013; Wang et al. 2002; Wasternack and Hause 2013; Xu et al. 2013; Zhao and Li 2012)
Downstream of the MVA and MEP pathways, the carotenoid biosynthesis pathway supplies precursors for the biosynthesis of ABA. Circadian clock regulation of many of the genes encoding enzymes in this pathway (Fig. 4) has been demonstrated in both Arabidopsis and maize (Covington et al. 2008; Khan et al. 2010). Diel regulation of genes involved in ABA synthesis has also been shown in species such as tomato (Thompson et al. 2000) and the perennial desert plant Rhazya stricta (Yates et al. 2014). Studies in Arabidopis plants deficient for the circadian clock components PRR5, 7, and 9 have shown that these pseudo-response regulator-like proteins negatively regulate the expression of both genes involved in the ABA biosynthetic pathway and ABA levels (Fukushima et al. 2009). This regulation may be direct, as PRR7 directly binds to the promoter region of ABA DEFICIENT 1 (ABA1), which encodes a zeaxanthin epoxidase involved in ABA biosynthesis (Liu et al. 2013). Another mechanism controlling daily levels of active ABA may be via the diel regulation of AtBG1, a β-glucosidase that releases active hormone from glucose-conjugated, inactive ABA (Lee et al. 2006).
GAs are also major hormones generated from isoprenoid precursors. At least in diel conditions, many GA biosynthetic genes show daily rhythms in Arabidopsis, pea, potato, and maize (Carrera et al. 1999; Garcia-Martinez and Gil 2002; Hisamatsu et al. 2005; Khan et al. 2010). In Arabidopsis, expression levels of the clock-regulated gene AtGA20ox1 are increased in toc1 mutants (Blazquez et al. 2002). Similarly, expression levels of several GA biosynthesic genes and levels of active GAs are increased in barley mutant for the clock gene ELF3 (Boden et al. 2014), further implicating the circadian clock in regulation of GA biosynthesis. The clock may also be involved in the inactivation of active GAs: mRNA levels of all six Arabidopsis GA2ox genes, which encode enzymes that catabolize active GAs, exhibited diel rhythms, with GA2ox1 and possibly GA2ox2 also being circadian regulated (Zhao et al. 2007).
Less has been published on mechanisms underlying diel and circadian regulation of CK and BR levels, two other types of hormones generated from isoprenoid precursors. However, the expression of two BR-biosynthetic genes has been reported to be under circadian control in Arabidopsis (Bancos et al. 2002).
The most abundant auxin in plants is IAA. In land plants it is thought to be primarily derived from tryptophan via the action of the tryptophan aminotransferase/flavin monooxygenase (YUCCA) pathway, although tryptophan-independent biosynthetic pathways have also been proposed (Yue et al. 2014). In rice, at least one member of each paralogous set of genes from each of the six reactions in tryptophan biosynthetic pathway is under strong diel regulation (Dharmawardhana et al. 2013). A number of homologous genes are also circadian regulated in Arabidopsis (Fig. 4). One mechanism by which the clock regulates free auxin levels is through the circadian-regulated MYB-like transcription factor RVE1. RVE1 directly promotes the expression of the auxin biosynthetic gene YUCCA8 (YUC8) and thus increases free auxin production during the day (Rawat et al. 2009). Several transcripts encoding GH3 enzymes, which join auxin to amino acids to produce inactive conjugates, are also clock-regulated (Covington et al. 2008; Khan et al. 2010), suggesting an additional mechanism by which daily rhythms in free auxin levels may be generated.
Although ET emissions have long been recognized as clock controlled, mechanisms underlying this regulation are elusive. Under typical conditions, ACC synthase (ACS) is thought to be the rate-limiting step for ET synthesis, and in Arabidopsis transcript level of ACS8 shows circadian rhythm of expression with a peak phase similar to that of ET production. However, plants mutant for ACS8 do not exhibit altered ethylene rhythms (Thain et al. 2004), indicating other biosynthetic components are under clock control. Under some conditions ACC oxidase can be the rate-limiting step in ethylene synthesis (Rieu et al. 2005), and two genes encoding putative ACC oxidase enzymes are clock regulated with a phase similar to that of ACS8 (Covington et al. 2008; Khan et al. 2010). It is therefore possible that these enzymes might act with ACS8 to generate rhythms in ethylene production. Diel cycling of genes predicted to encode homologs of the ethylene receptors ETHYLENE RESPONSE SENSOR1 and ETHYLENE INSENSITIVE1 has been reported in Japanese cedar (Nose and Watanabe 2014), suggesting the clock may gate ethylene signaling in addition to regulating ethylene production.
As described above, circadian regulation of JA and SA levels has been reported in both Arabidopsis and other plants. In Arabidopsis, the clock protein CCA1 has been shown to bind to the promoter of the JA biosynthetic gene LIPOXYGENASE2 (Nagel et al. 2015). It has been proposed that similar regulation of the JA biosynthesis gene LIPOXYGENASE3 also occurs in Nicotiana attenuata (Kim et al. 2011). More is known about daily regulation of SA levels in Arabidopsis. ISOCHORISMATE SYNTHASE1 (ICS1) encodes an enzyme essential for SA biosynthesis (Wildermuth et al. 2001) and its expression is clock-regulated. The clock protein CHE directly, and perhaps also indirectly, regulates ICS1 expression and is required for daily rhythms in SA levels (Zheng et al. 2015). The clock may also regulate SA signaling via additional mechanisms: CCA1 has been implicated in the regulation of expression of the phosphate transporter gene PHT4;1, a negative regulator of plant defenses that acts genetically upstream of SA signaling (Wang et al. 2014).
In summary, the circadian clock has been implicated in control of most major plant hormones and therefore by extension most physiological events. Below, we discuss recent findings regarding joint clock and hormone regulation of two important processes, plant growth and plant defense.
The roles of hormones and the clock in growth regulation
Plant growth is a complex process controlled by many environmental and endogenous signals including major roles for the phytohormones IAA and GAs. Daily rhythms in stem and leaf growth are observed in multiple species and at least in dicots are generated by the circadian clock (Ruts et al. 2012). The mechanisms underlying these rhythms have been best studied in the Arabidopsis hypocotyl. Clock and environmental regulation of hypocotyl elongation is mediated in part via the transcription factors PHYTOCHROME INTERACTING FACTOR (PIF) 4 and 5 (Dowson-Day and Millar 1999; Nozue et al. 2007). Daily rhythms in PIF4/5 expression and thus hypocotyl elongation are generated by the evening complex, ELF3, ELF4, and LUX (Nusinow et al. 2011). Additional regulation may be provided by PRR7 and PRR5, which also bind to the promoters of PIF4 and PIF5 (Franklin et al. 2011; Liu et al. 2013; Nakamichi et al. 2012).
A number of studies have linked PIF4 and 5 to the control of IAA and GA signaling (de Lucas et al. 2008; Koini et al. 2009; Kunihiro et al. 2011; Nozue et al. 2011) and biosynthesis (Filo et al. 2015; Franklin et al. 2011; Hornitschek et al. 2012). A simple model for regulation of daily growth patterns can be generated from the following results: PIF4 and PIF5 are required for expression of key GA biosynthetic enzymes (Filo et al. 2015) and plants deficient for GA production exhibit large reductions in rhythmic growth (Nozue et al. 2011). Expression of GA receptors is clock controlled and plant responsiveness to GA is accordingly gated by the clock (Arana et al. 2011). Together, these data suggest that daily hypocotyl growth rhythms are driven by the PIF-dependent rhythmic production of GA combined with circadian gating of GA perception.
However, the full story is much more complex. PIF function is modulated by other hormone signaling pathways: a BR-dependent kinase phosphorylates PIF4 and promotes its degradation (Bernardo-Garcia et al. 2014) while the ability of multiple PIF proteins to bind to DNA is inhibited by their binding to DELLA proteins, negative regulators of GA signaling that are themselves degraded in response to GA (de Lucas et al. 2008; Feng et al. 2008). PIF4 transactivation activity is also inhibited upon binding to the clock protein ELF3 (Nieto et al. 2015), providing another layer of clock regulation on PIF function. PIFs have also been implicated in auxin signaling. PIF4 and 5 regulate auxin biosynthesis (Franklin et al. 2011; Hornitschek et al. 2012) and PIF4 and 5 modulate plant sensitivity to auxin (Nozue et al. 2011). Finally, auxin- (but not GA-) responsive genes are overrepresented among those misexpressed in pif4 pif5 seedlings (Nozue et al. 2011), suggesting that PIFs may play a more important role in growth control via auxin signaling than the GA pathway.
PIF-independent clock control of plant growth has also been reported. Circadian rhythms in leaf growth persist in plants mutant for PIF4 and 5 (Dornbusch et al. 2014). Since daily rhythms in floral stem elongation require IAA (Jouve et al. 1999) and plant growth and transcriptional responses to IAA are gated by the clock (Covington and Harmer 2007), clock regulation of auxin signaling may play a role in PIF-independent growth rhythms. Indeed, the clock-regulated transcription factor RVE1 promotes hypocotyl growth by increasing free auxin levels; this is independent of PIF4 and PIF5 function (Rawat et al. 2009). However, further complexity is suggested by a genome-wide transcriptome study implicating daily rhythms in ABA and BR signaling, in addition to rhythms in IAA and GA signaling, in daily rhythms in growth (Michael et al. 2008a). Therefore diel and circadian regulation of plant growth likely involves a complex network of hormone signaling pathways that are modulated at many steps.
The roles of hormones and the clock in defense responses
Plants are subjected to various biotic stresses throughout their sedentary life cycle. In general, SA and JA are recognized as the major defense hormones with SA being essential for the immune response against biotrophic pathogens and JA helping defend against necrotrophic pathogens and herbivorous insects. The other phytohormones act as modulators of the plant immune signaling network (Pieterse et al. 2012). The roles of hormonal signaling pathways may vary depending on the plant and the type of the threat (Kunkel and Brooks 2002; Lund et al. 1998; Thomma et al. 2001).
Not surprisingly, defense responses are diel and circadian regulated. Susceptibility of plants to bacteria, oomycetes, fungi, and chewing insects has been shown to be clock regulated (Bhardwaj et al. 2011; Goodspeed et al. 2012; Hevia et al. 2015; Ingle et al. 2015; Wang et al. 2011). Even after harvest, the circadian clock regulates pest resistance and plant nutritional value (Goodspeed et al. 2013). Many hormone pathways and mechanisms have been implicated in clock modulation of plant defense, even to the same pathogen. For example, while plant defense responses to mechanical infiltration of the bacterial pathogen Pseudomonas syringae into leaves are maximal in the morning, defense responses are maximal in the evening when these bacteria are simply sprayed on plants (Korneli et al. 2014; Zhang et al. 2013). These distinct phases of peak resistance are likely due to circadian regulation of both stomatal aperture and downstream defense signaling pathways. Regulation of stomatal aperture is perturbed in plants mutant for the clock genes CCA1 and LHY (Dodd et al. 2005; Shin et al. 2012; Zhang et al. 2013), which regulate expression of GLYCINE-RICH RNA-BINDING PROTEIN7 (GRP7) (also known as COLD AND CIRCADIAN REGULATED 2 [CCR2]) (Zhang et al. 2013), a protein shown to promote stomatal closure (Kim et al. 2008). Notably, GRP7 also promotes translation of FLAGELLIN SENSITIVE 2, a receptor for bacterial flagellin (Nicaise et al. 2013), demonstrating the complexity of clock regulation of defense signaling. The clock genes PRR7, TIC, ELF3 and the clock output gene PATHOGEN AND CIRCADIAN CLOCK CONTROLLED 1 have also been implicated in regulation of stomatal aperture (Kinoshita et al. 2011; Korneli et al. 2014; Liu et al. 2013; Mir et al. 2013).
Roles for JA in circadian-driven variation in non-stomatal dependent defense pathways have been demonstrated. Circadian-driven variation in susceptibility to the fungus Botrytis cinerea requires a functional JA signaling pathway (Ingle et al. 2015), as do daily rhythms in resistance to cabbage looper (Goodspeed et al. 2012). Expression of the JA receptor CORONATINE INSENSITIVE1 is clock regulated, as is expression of the transcription factor MYC2, a positive regulator of JA signaling. In addition, the clock-associated protein TIC interacts with MYC2 and is required for daily variation in JA-mediated defense responses (Shin et al. 2012). Studies demonstrating the functional importance of circadian regulation in SA-mediated defense pathways have not yet been published, and in fact one report suggests that CCA1 and LHY modulation of defense responses is largely SA-independent (Zhang et al. 2013).
Most studies to date have focused on the role of the plant circadian clock in daily rhythms of plant susceptibility to pathogens and pests. However, studies on Botrytis cinerea (Hevia et al. 2015) and chewing insects (Goodspeed et al. 2013) have shown that circadian regulation of pathogen and pest physiology helps determine the outcome of their interactions with plants. Future work on how circadian clocks act in both plants and pathogens to modulate defense responses is likely to be of great interest.
Roles for hormones in regulation of the circadian clock
In animals, it has long been recognized that circadian rhythms are generated in diverse organs but that rhythms at the organismal level are coordinated via a ‘master clock’ in the brain that entrains ‘slave oscillators’ in peripheral organs. This coordination is achieved by multiple mechanisms including daily rhythms in body temperature, metabolism, and hormone levels (Mohawk et al. 2012). Although some work suggests at most only weak coupling between clocks in individual plant cells (Fukuda et al. 2007; Shimizu et al. 2015; Thain et al. 2000; Wenden et al. 2012), other studies suggest there may be significant interactions between clocks in different plant tissues (Endo et al. 2014; James et al. 2008; Takahashi et al. 2015). An obvious question is therefore whether hormone pathways can entrain the plant clock and help coordinate rhythms between far-flung organs such as roots and shoots.
A number of studies have found that application of exogenous hormones can affect clock function in plants. Although treatment with ET, SA, auxins, or GAs have been reported to have little or no effect on clock pace (Covington and Harmer 2007; Hanano et al. 2006), more significant changes in phase and/or period were reported after treatment with ABA, CKs, or BR (Hanano et al. 2006; Salome et al. 2006; Zheng et al. 2006). SA treatment did not affect clock phase or period (Hanano et al. 2006; Zhang et al. 2013; Zhou et al. 2015) but has been suggested to reinforce circadian robustness (Zhou et al. 2015). However, flg22, a peptide activator of basal defense pathways, has been reported to shorten clock pace (Zhang et al. 2013) via an unknown mechanism. Consistent with the ability of exogenous hormones to alter clock pace, mutation of genes that act in hormone signaling pathways can affect free-running period (Hanano et al. 2006; Salome et al. 2006; Zheng et al. 2006).
However, there are inconsistencies in the literature that complicate interpretation of these findings. For example, ABA treatment has been variously reported to cause either modest or significant period shortening (Lee et al. 2016; Liu et al. 2013) or period lengthening (Hanano et al. 2006). Such discrepancies may be due to differences in hormone concentration or formulation and the clock reporter gene examined: different concentrations of a CK can evoke either period shortening or period lengthening (Salome et al. 2006), and the periodicity of different circadian reporter genes may be oppositely affected by treatment with the same hormone (Hanano et al. 2006). Moreover, many of the above studies used high concentrations of hormones over prolonged periods of times, calling into question the physiological relevance of the observed effects on clock function. Finally, the authors of one study concluded that the effects of mutation of hormone-related genes on clock function were likely due to an unknown mode of action independent of hormone signaling (Salome et al. 2006). Thus our current understanding of the role of plant hormones in control of clock function remains incomplete.
In addition to changes in hormone levels, daily cycles in plant metabolites have been suggested to coordinate clock function in disparate organs (Haydon et al. 2013; James et al. 2008). In support of this hypothesis, acute treatment of plants with sucrose during the day was shown to cause phase advances while acute treatment at night caused phase delays (Haydon et al. 2013). Such time-of-day-dependent effects of sucrose on circadian phase are consistent with it acting as an endogenous regulator of the circadian system, making fixed carbon a strong candidate as a global coordinator of clock function. Similar studies on the acute effects of hormones on clock phase will be of great interest and may help reveal whether they normally coordinate clock function in disparate organs, as for example between the shoot apex and roots (Takahashi et al. 2015).
Conclusions and future perspectives
It is now clear that the circadian system has a pervasive effect on many, and perhaps all, hormone signaling pathways. A deeper understanding of the physiological significance of the connections between circadian and hormone signaling networks will require experiments carefully designed to deal with the biological complexity of these interactions. One complicating factor is the multifactorial nature of clock modulation of hormone signaling pathways, with circadian control of a single pathway occurring at levels ranging from hormone synthesis to signal reception and processing. In addition, significant differences in clock control of hormone signaling have been observed between species and even among organs within a single plant, limiting our ability to make generalizations based on single studies. Finally, clock regulation of multiple hormone pathways with partially overlapping physiological activities further complicates the picture. However, the development of new techniques, for example those enabling precise genome editing (Liu et al. 2016a) and the isolation of specific cell types (Wang et al. 2012), is providing us with powerful new tools that will ultimately allow a better understanding of how the intricate interplay between clock and hormone signaling networks enhances plant adaptation to a continually changing environment.
References
Adams S, Carre IA (2011) Downstream of the plant circadian clock: output pathways for the control of physiology and development. Essays Biochem 49:53–69. doi:10.1042/BSE0490053
Adams S, Manfield I, Stockley P, Carre IA (2015) Revised morning loops of the Arabidopsis circadian clock based on analyses of direct regulatory interactions. PLoS One 10:e0143943. doi:10.1371/journal.pone.0143943
Angelmann W, Johnsson A (1998) Rhythms in organ movement. In: Lumsden PJ, Millar AJ (eds) Biological Rhythms and photoperiodism in plants, 1st edn. BIOS Scientific, Abingdon, pp 35–50
Arana MV, Marin-de la Rosa N, Maloof JN, Blazquez MA, Alabadi D (2011) Circadian oscillation of gibberellin signaling in Arabidopsis. Proc Natl Acad Sci USA 108:9292–9297. doi:10.1073/pnas.1101050108
Bancos S, Nomura T, Sato T, Molnar G, Bishop GJ, Koncz C, Yokota T et al (2002) Regulation of transcript levels of the Arabidopsis cytochrome p450 genes involved in brassinosteroid biosynthesis. Plant Physiol 130:504–513. doi:10.1104/pp.005439
Barta C, Loreta F (2006) The relationship between the methyl-erythritol phosphate pathway leading to emission of volatile isopreniods and abscisic acid content in leaves. Plant Physiol 141:1676–1683
Bernardo-Garcia S, de Lucas M, Martinez C, Espinosa-Ruiz A, Daviere JM, Prat S (2014) BR-dependent phosphorylation modulates PIF4 transcriptional activity and shapes diurnal hypocotyl growth. Genes Dev 28:1681–1694. doi:10.1101/gad.243675.114
Bhardwaj V, Meier S, Petersen LN, Ingle RA, Roden LC (2011) Defence responses of Arabidopsis thaliana to infection by Pseudomonas syringae are regulated by the circadian clock. PLoS One 6:e26968. doi:10.1371/journal.pone.0026968
Blanco F, Salinas P, Cecchini NM, Jordana X, Van Hummelen P, Alvarez ME, Holuigue L (2009) Early genomic responses to salicylic acid in Arabidopsis. Plant Mol Biol 70:79–102. doi:10.1007/s11103-009-9458-1
Blazquez MA, Trenor M, Weigel D (2002) Independent control of gibberellin biosynthesis and flowering time by the circadian clock in Arabidopsis. Plant Physiol 130:1770–1775. doi:10.1104/pp.007625
Boden SA, Weiss D, Ross JJ, Davies NW, Trevaskis B, Chandler PM, Swain SM (2014) EARLY FLOWERING3 regulates flowering in spring barley by mediating gibberellin production and FLOWERING LOCUS T expression. Plant Cell 26:1557–1569. doi:10.1105/tpc.114.123794
Burschka C, Tenhunen JD, Hartung W (1983) Diurnal variations in abscisic acid content and stomatal response to applied abscisic acid in leaves of irrigated and non-irrigated Arbutus unedo plants under naturally fluctuating environmental conditions. Oecologia 58:128–131
Carrera E, Jackson SD, Prat S (1999) Feedback control and diurnal regulation of gibberellin 20-oxidase transcript levels in potato. Plant Physiol 119:765–774
Covington MF, Harmer SL (2007) The circadian clock regulates auxin signaling and responses in Arabidopsis. PLoS Biol 5:e222. doi:10.1371/journal.pbio.0050222
Covington MF, Maloof JN, Straume M, Kay SA, Harmer SL (2008) Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol 9:R130. doi:10.1186/gb-2008-9-8-r130
de Jong M, Leyser O (2012) Developmental plasticity in plants. Cold Spring Harb Symp Quant Biol 77:63–73. doi:10.1101/sqb.2012.77.014720
de Lucas M, Daviere JM, Rodriguez-Falcon M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C et al (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451:480–484. doi:10.1038/nature06520
Dempsey DA, Vlot AC, Wildermuth MC, Klessig DF (2011) Salicylic acid biosynthesis and metabolism. The Arabidopsis Book 9:e0156. doi:10.1199/tab.0156
Dharmawardhana P, Ren L, Amarasinghe V, Monaco M, Thomason J, Ravenscroft D, McCouch S et al (2013) A genome scale metabolic network for rice and accompanying analysis of tryptophan, auxin and serotonin biosynthesis regulation under biotic stress. Rice 6:15. doi:10.1186/1939-8433-6-15
Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM et al (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309:630–633. doi:10.1126/science.1115581
Dodd AN, Gardner MJ, Hotta CT, Hubbard KE, Dalchau N, Love J, Assie JM et al (2007) The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science 318:1789–1792
Dornbusch T, Michaud O, Xenarios I, Fankhauser C (2014) Differentially phased leaf growth and movements in Arabidopsis depend on coordinated circadian and light regulation. Plant Cell 26:3911–3921. doi:10.1105/tpc.114.129031
Dowson-Day MJ, Millar AJ (1999) Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. Plant J 17:63–71
Emery RJN, Reid DM, Chinnappa CC (1994) Phenotypic plasticity of stem eiongation in two ecotypes of Stellaria longipes: the role of ethylene and response to wind. Plant Cell Environ 17:691–700
Endo M, Shimizu H, Nohales MA, Araki T, Kay SA (2014) Tissue-specific clocks in Arabidopsis show asymmetric coupling. Nature 515:419–422. doi:10.1038/nature13919
Farre EM (2012) The regulation of plant growth by the circadian clock. Plant Biol 14:401–410. doi:10.1111/j.1438-8677.2011.00548.x
Farre EM, Weise SE (2012) The interactions between the circadian clock and primary metabolism. Curr Opin Plant Biol 15:293–300. doi:10.1016/j.pbi.2012.01.013
Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L et al (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451:475–479. doi:10.1038/nature06448
Filichkin SA, Breton G, Priest HD, Dharmawardhana P, Jaiswal P, Fox SE, Michael TP et al (2011) Global profiling of rice and poplar transcriptomes highlights key conserved circadian-controlled pathways and cis-regulatory modules. PLoS One 6:e16907. doi:10.1371/journal.pone.0016907
Filo J, Wu A, Eliason E, Richardson T, Thines BC, Harmon FG (2015) Gibberellin driven growth in elf3 mutants requires PIF4 and PIF5. Plant Signal Behav 10:e992707. doi:10.4161/15592324.2014.992707
Finlayson SA, Lee IJ, Morgan PW (1998) Phytochrome B and the regulation of circadian ethylene production in sorghum. Plant Physiol 116:17–25
Fogelmark K, Troein C (2014) Rethinking transcriptional activation in the Arabidopsis circadian clock. PLoS Comput Biol 10:e1003705. doi:10.1371/journal.pcbi.1003705
Foster KR, Morgan PW (1995) Genetic regulation of development in Sorghum bicolor (IX. The ma3R allele disrupts diurnal control of gibberellin biosynthesis). Plant Physiol 108:337–343
Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, Gu C, Ye S et al (2011) Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci USA 108:20231–20235. doi:10.1073/pnas.1110682108
Freschi L, Nievola CC, Rodrigues MA, Domingues DS, Van Sluys MA, Mercier H (2009) Thermoperiod affects the diurnal cycle of nitrate reductase expression and activity in pineapple plants by modulating the endogenous levels of cytokinins. Physiol Plantarum 137:201–212. doi:10.1111/j.1399-3054.2009.01283.x
Fukuda H, Nakamichi N, Hisatsune M, Murase H, Mizuno T (2007) Synchronization of plant circadian oscillators with a phase delay effect of the vein network. Phys Rev Lett 99:098102. doi:10.1103/PhysRevLett.99.098102
Fukushima A, Kusano M, Nakamichi N, Kobayashi M, Hayashi N, Sakakibara H, Mizuno T et al (2009) Impact of clock-associated Arabidopsis pseudo-response regulators in metabolic coordination. Proc Natl Acad Sci USA 106:7251–7256. doi:10.1073/pnas.0900952106
Garcia-Martinez JL, Gil J (2002) Light regulation of gibberellin biosynthesis and mode of action. J Plant Growth Regul 20:354–368
Goodspeed D, Chehab EW, Min-Venditti A, Braam J, Covington MF (2012) Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc Natl Acad Sci USA 109:4674–4677. doi:10.1073/pnas.1116368109
Goodspeed D, Liu JD, Chehab EW, Sheng Z, Francisco M, Kliebenstein DJ, Braam J (2013) Postharvest circadian entrainment enhances crop pest resistance and phytochemical cycling. Curr Biol 23:1235–1241. doi:10.1016/j.cub.2013.05.034
Greenham K, McClung CR (2015) Integrating circadian dynamics with physiological processes in plants. Nat Rev Genet 16:598–610. doi:10.1038/nrg3976
Gupta R, Chakrabarty SK (2013) Gibberellic acid in plant: still a mystery unresolved. Plant Signal Behav. doi:10.4161/psb.25504
Hanano S, Domagalska MA, Nagy F, Davis SJ (2006) Multiple phytohormones influence distinct parameters of the plant circadian clock. Genes Cells Devot Mol Cell Mech 11:1381–1392. doi:10.1111/j.1365-2443.2006.01026.x
Haydon MJ, Mielczarek O, Robertson FC, Hubbard KE, Webb AA (2013) Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 502:689–692. doi:10.1038/nature12603
Henson IE, Alagarswamy G, Mahalakshmi V, Bidinger FR (1982) Diurnal changes in endogenous abscisic acid in leaves of pearl millet (Pennisetum americanum (L.) Leeke) under field conditions. J Exp Bot 33:416–425
Hevia MA, Canessa P, Muller-Esparza H, Larrondo LF (2015) A circadian oscillator in the fungus Botrytis cinerea regulates virulence when infecting Arabidopsis thaliana. Proc Natl Acad Sci USA 112:8744–8749. doi:10.1073/pnas.1508432112
Hisamatsu T, King RW, Helliwell CA, Koshioka M (2005) The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiol 138:1106–1116. doi:10.1104/pp.104.059055
Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, Lopez-Vidriero I, Franco-Zorrilla JM et al (2012) Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J 71:699–711. doi:10.1111/j.1365-313X.2012.05033.x
Hsu PY, Harmer SL (2012) Circadian phase has profound effects on differential expression analysis. PLoS One 7:e49853. doi:10.1371/journal.pone.0049853
Hsu PY, Harmer SL (2014) Wheels within wheels: the plant circadian system. Trends Plant Sci 19:240–249. doi:10.1016/j.tplants.2013.11.007
Huang W, Perez-Garcia P, Pokhilko A, Millar AJ, Antoshechkin I, Riechmann JL, Mas P (2012) Mapping the core of the Arabidopsis circadian clock defines the network structure of the oscillator. Science 336:75–79. doi:10.1126/science.1219075
Ingle RA, Stoker C, Stone W, Adams N, Smith R, Grant M, Carre I et al (2015) Jasmonate signalling drives time-of-day differences in susceptibility of Arabidopsis to the fungal pathogen Botrytis cinerea. Plant J 84:937–948. doi:10.1111/tpj.13050
James AB, Monreal JA, Nimmo GA, Kelly CL, Herzyk P, Jenkins GI, Nimmo HG (2008) The circadian clock in Arabidopsis roots is a simplified slave version of the clock in shoots. Science 322:1832–1835. doi:10.1126/science.1161403
Janardhan KV, Vasudeva N, Gopel NH (1973) Diurnal variation of endogenous auxin in arabica coffee leaves. J Plant Crops 1:93–95
Jasoni RL, Cothren JT, Morgan PW, Sohan DE (2000) Circadian ethylene production in cotton. Plant Growth Regul 00:1–7
Jouve L, Gaspar T, Kevers C, Greppin H, Degli Agosti R (1999) Involvement of indole-3-acetic acid in the circadian growth of the first internode of Arabidopsis. Planta 209:136–142
Kapuya JL, Hall MA (1977) Diurnal variations in endogenous ethylene levels in plants. New Phytol 79:233–237
Khan S, Rowe SC, Harmon FG (2010) Coordination of the maize transcriptome by a conserved circadian clock. BMC Plant Biol 10:126. doi:10.1186/1471-2229-10-126
Kim JS, Jung HJ, Lee HJ, Kim KA, Goh CH, Woo Y, Oh SH et al (2008) Glycine-rich RNA-binding protein 7 affects abiotic stress responses by regulating stomata opening and closing in Arabidopsis thaliana. Plant J 55:455–466. doi:10.1111/j.1365-313X.2008.03518.x
Kim SG, Yon F, Gaquerel E, Gulati J, Baldwin IT (2011) Tissue specific diurnal rhythms of metabolites and their regulation during herbivore attack in a native tobacco, Nicotiana attenuata. PloS One 6:e26214. doi:10.1371/journal.pone.0026214
Kinoshita T, Ono N, Hayashi Y, Morimoto S, Nakamura S, Soda M, Kato Y et al (2011) FLOWERING LOCUS T regulates stomatal opening. Curr Biol 21:1232–1238. doi:10.1016/j.cub.2011.06.025
Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA (2009) High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol 19:408–413. doi:10.1016/j.cub.2009.01.046
Korneli C, Danisman S, Staiger D (2014) Differential control of pre-invasive and post-invasive antibacterial defense by the Arabidopsis circadian clock. Plant Cell Physiol 55:1613–1622. doi:10.1093/pcp/pcu092
Krekule J, Pavlova L, Souckova D, Machackova I (1985) Auxin in flowering of short-day and long-day Chenopodium species. Biol Plantarum 27:310–317
Kunihiro A, Yamashino T, Nakamichi N, Niwa Y, Nakanishi H, Mizuno T (2011) Phytochrome-interacting factor 4 and 5 (PIF4 and PIF5) activate the homeobox ATHB2 and auxin-inducible IAA29 genes in the coincidence mechanism underlying photoperiodic control of plant growth of Arabidopsis thaliana. Plant Cell Physiol 52:1315–1329. doi:10.1093/pcp/pcr076
Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5:325–331
Lecoq C, Koukkari WL, Brenner ML (1983) Rhythmic changes in abscisic acid (ABA) content of soybean leaves. Plant Physiol 72:52
Lee MH, Seo SW, Ota Y (1981) Diurnal variation in ethylene evolution in leaf and panicle. JPN J Crop Sci 50:396–400
Lee IJ, Foster KR, Morgan PW (1998) Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol 116:1003–1011
Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I et al (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126:1109–1120. doi:10.1016/j.cell.2006.07.034
Lee HG, Mas P, Seo PJ (2016) MYB96 shapes the circadian gating of ABA signaling in Arabidopsis. Sci Rep 6:17754. doi:10.1038/srep17754
Lipe JA, Morgan PW (1973) Ethylene, a regulator of young fruit abscission. Plant Physiol 51:949–953
Liu T, Carlsson J, Takeuchi T, Newton L, Farre EM (2013) Direct regulation of abiotic responses by the Arabidopsis circadian clock component PRR7. Plant J 76:101–114. doi:10.1111/tpj.12276
Liu D, Hu R, Palla KJ, Tuskan GA, Yang X (2016a) Advances and perspectives on the use of CRISPR/Cas9 systems in plant genomics research. Curr Opin Plant Biol 30:70–77. doi:10.1016/j.pbi.2016.01.007
Liu TL, Newton L, Liu MJ, Shiu SH, Farre EM (2016b) A G-box-like motif is necessary for transcriptional regulation by circadian pseudo-response regulators in Arabidopsis. Plant Physiol 170:528–539. doi:10.1104/pp.15.01562
Lund ST, Stall RE, Klee HJ (1998) Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 10:371–382
Machackova I, Chauvaux N, Dewitte W, Van Onckelen H (1997) Diurnal fluctuations in ethylene formation in Chenopodium rubrum. Plant Physiol 113:981–985
Mano Y, Nemoto K (2012) The pathway of auxin biosynthesis in plants. J Exp Bot 63:2853–2872. doi:10.1093/jxb/ers091
McClung CR (2014) Wheels within wheels: new transcriptional feedback loops in the Arabidopsis circadian clock. F1000Prime Rep 6:2. doi:10.12703/P6-2
Michael TP, Breton G, Hazen SP, Priest H, Mockler TC, Kay SA, Chory J (2008a) A morning-specific phytohormone gene expression program underlying rhythmic plant growth. PLoS Biol 6:e225. doi:10.1371/journal.pbio.0060225
Michael TP, Mockler TC, Breton G, McEntee C, Byer A, Trout JD, Hazen SP et al (2008b) Network discovery pipeline elucidates conserved time-of-day-specific cis-regulatory modules. PLoS Genet 4:e14. doi:10.1371/journal.pgen.0040014
Mir R, Hernandez ML, Abou-Mansour E, Martinez-Rivas JM, Mauch F, Metraux JP, Leon J (2013) Pathogen and Circadian Controlled 1 (PCC1) regulates polar lipid content, ABA-related responses, and pathogen defence in Arabidopsis thaliana. J Exp Bot 64:3385–3395. doi:10.1093/jxb/ert177
Mizuno T, Yamashino T (2008) Comparative transcriptome of diurnally oscillating genes and hormone-responsive genes in Arabidopsis thaliana: insight into circadian clock-controlled daily responses to common ambient stresses in plants. Plant Cell Physiol 49:481–487. doi:10.1093/pcp/pcn008
Mohawk JA, Green CB, Takahashi JS (2012) Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35:445–462. doi:10.1146/annurev-neuro-060909-153128
Morgan PW, He CJ, De Greef JA, De Proft MP (1990) Does water deficit stress promote ethylene synthesis by intact plants? Plant Physiol 94:1616–1624
Muller LM, von Korff M, Davis SJ (2014) Connections between circadian clocks and carbon metabolism reveal species-specific effects on growth control. J Exp Bot 65:2915–2923. doi:10.1093/jxb/eru117
Myster J, Junttila O, Lindgard B, Moe R (1997) Temperature alternations and the influence of gibberellins and indoleacetic acid on elongation growth and fowering of Begonia x hiemalis Fotsch. Plant Growth Regul 21:135–144
Nagel DH, Doherty CJ, Pruneda-Paz JL, Schmitz RJ, Ecker JR, Kay SA (2015) Genome-wide identification of CCA1 targets uncovers an expanded clock network in Arabidopsis. Proc Natl Acad Sci USA. doi:10.1073/pnas.1513609112
Nakamichi N, Kiba T, Kamioka M, Suzuki T, Yamashino T, Higashiyama T, Sakakibara H et al (2012) Transcriptional repressor PRR5 directly regulates clock-output pathways. Proc Natl Acad Sci USA 109:17123–17128. doi:10.1073/pnas.1205156109
Nemhauser JL, Hong F, Chory J (2006) Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126:467–475. doi:10.1016/j.cell.2006.05.050
Nicaise V, Joe A, Jeong BR, Korneli C, Boutrot F, Westedt I, Staiger D et al (2013) Pseudomonas HopU1 modulates plant immune receptor levels by blocking the interaction of their mRNAs with GRP7. EMBO J 32:701–712. doi:10.1038/emboj.2013.15
Nieto C, Lopez-Salmeron V, Daviere JM, Prat S (2015) ELF3-PIF4 interaction regulates plant growth independently of the Evening Complex. Curr Biol 25:187–193. doi:10.1016/j.cub.2014.10.070
Nose M, Watanabe A (2014) Clock genes and diurnal transcriptome dynamics in summer and winter in the gymnosperm Japanese cedar (Cryptomeria japonica (L.f.) D.Don). BMC Plant Biol 14:308. doi:10.1186/s12870-014-0308-1
Novakova M, Motyka V, Dobrev PI, Malbeck J, Gaudinova A, Vankova R (2005) Diurnal variation of cytokinin, auxin and abscisic acid levels in tobacco leaves. J Exp Bot 56:2877–2883. doi:10.1093/jxb/eri282
Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN (2007) Rhythmic growth explained by coincidence between internal and external cues. Nature 448:358–361. doi:10.1038/nature05946
Nozue K, Harmer SL, Maloof JN (2011) Genomic analysis of circadian clock-, light-, and growth-correlated genes reveals PHYTOCHROME-INTERACTING FACTOR5 as a modulator of auxin signaling in Arabidopsis. Plant Physiol 156:357–372. doi:10.1104/pp.111.172684
Nusinow DA, Helfer A, Hamilton EE, King JJ, Imaizumi T, Schultz TF, Farre EM et al (2011) The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475:398–402. doi:10.1038/nature10182
Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH (1998) Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci USA 95:8660–8664
Pavlova L, Krekule J (1984) Fluctuation of free IAA under inductive and non-inductive photoperiods in Chenopodium rubrum. Plant Growth Regul 2:91–98
Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:489–521. doi:10.1146/annurev-cellbio-092910-154055
Pokhilko A, Bou-Torrent J, Pulido P, Rodriguez-Concepcion M, Ebenhoh O (2015) Mathematical modelling of the diurnal regulation of the MEP pathway in Arabidopsis. New Phytol 206:1075–1085. doi:10.1111/nph.13258
Rawat R, Schwartz J, Jones MA, Sairanen I, Cheng Y, Andersson CR, Zhao Y et al (2009) REVEILLE1, a Myb-like transcription factor, integrates the circadian clock and auxin pathways. Proc Natl Acad Sci USA 106:16883–16888. doi:10.1073/pnas.0813035106
Rieu I, Cristescu SM, Harren FJ, Huibers W, Voesenek LA, Mariani C, Vriezen WH (2005) RP-ACS1, a flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of Rumex palustris, is involved in rhythmic ethylene production. J Exp Bot 56:841–849. doi:10.1093/jxb/eri078
Ruiz-Sola MA, Rodriguez-Concepcion M (2012) Carotenoid biosynthesis in Arabidopsis: a colorful pathway. The Arabidopsis Book 10:e0158. doi:10.1199/tab.0158
Ruts T, Matsubara S, Wiese-Klinkenberg A, Walter A (2012) Diel patterns of leaf and root growth: endogenous rhythmicity or environmental response? J Exp Bot 63:3339–3351. doi:10.1093/jxb/err334
Salome PA, To JP, Kieber JJ, McClung CR (2006) Arabidopsis response regulators ARR3 and ARR4 play cytokinin-independent roles in the control of circadian period. Plant Cell 18:55–69. doi:10.1105/tpc.105.037994
Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97:11655–11660. doi:10.1073/pnas.97.21.11655
Shapiro DJ, Rodwell VW (1969) Diurnal variation and cholesterol regulation of hepatic HMG-CoA reductase activity. Biochem Biophys Res Commun 37:867–872
Shimizu H, Katayama K, Koto T, Torii K, Araki T, Endo M (2015) Decentralized circadian clocks process thermal and photoperiodic cues in specific tissues. Nat Plants. doi:10.1038/nplants.2015.163
Shin J, Heidrich K, Sanchez-Villarreal A, Parker JE, Davis SJ (2012) TIME FOR COFFEE represses accumulation of the MYC2 transcription factor to provide time-of-day regulation of jasmonate signaling in Arabidopsis. Plant Cell 24:2470–2482. doi:10.1105/tpc.111.095430
Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T (2015) Photoperiodic flowering: time measurement mechanisms in leaves. Annu Rev Plant Biol 66:441–464. doi:10.1146/annurev-arplant-043014-115555
Spoelstra K, Wikelski M, Daan S, Loudon AS, Hau M (2016) Natural selection against a circadian clock gene mutation in mice. Proc Natl Acad Sci USA 113:686–691. doi:10.1073/pnas.1516442113
Stavang JA, Lindgard B, Erntsen A, Lid SE, Moe R, Olsen JE (2005) Thermoperiodic stem elongation involves transcriptional regulation of gibberellin deactivation in pea. Plant Physiol 138:2344–2353. doi:10.1104/pp.105.063149
Takahashi N, Hirata Y, Aihara K, Mas P (2015) A hierarchical multi-oscillator network orchestrates the Arabidopsis circadian system. Cell 163:148–159. doi:10.1016/j.cell.2015.08.062
Talon M, Zeevaart JA, Gage DA (1991) Identification of gibberellins in spinach and effects of light and darkness on their levels. Plant Physiol 97:1521–1526
Thain SC, Hall A, Millar AJ (2000) Functional independence of circadian clocks that regulate plant gene expression. Curr Biol 10:951–956
Thain SC, Vandenbussche F, Laarhoven LJ, Dowson-Day MJ, Wang ZY, Tobin EM, Harren FJ et al (2004) Circadian rhythms of ethylene emission in Arabidopsis. Plant Physiol 136:3751–3761. doi:10.1104/pp.104.042523
Thomma BPHJ, Penninckx IAMA, Cammue BPA, Broekaert WF (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13:63–68
Thompson AJ, Jackson AC, Parker RA, Morpeth DR, Burbidge A, Taylor IB (2000) Abscisic acid biosynthesis in tomato: regulation of zeaxanthin epoxidase and 9-cis-epoxycarotenoid dioxygenase mRNAs by light/dark cycles, water stress and abscisic acid. Plant Mol Biol 42:833–845
Velho do Amaral LI, Santo HP, Rossatto DR, Buckeridge MS (2012) Diurnal changes in storage carbohydrate metabolism in cotyledons of the tropical tree Hymenaea courbaril L. (Leguminosae). Braz J Bot 35:347–355
Vranova E, Coman D, Gruissem W (2013) Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol 64:665–700. doi:10.1146/annurev-arplant-050312-120116
Wang KL, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14(Suppl):S131–S151
Wang W, Barnaby JY, Tada Y, Li H, Tor M, Caldelari D, Lee DU et al (2011) Timing of plant immune responses by a central circadian regulator. Nature 470:110–114. doi:10.1038/nature09766
Wang D, Mills ES, Deal RB (2012) Technologies for systems-level analysis of specific cell types in plants. Plant Sci 197:21–29. doi:10.1016/j.plantsci.2012.08.012
Wang G, Zhang C, Battle S, Lu H (2014) The phosphate transporter PHT4;1 is a salicylic acid regulator likely controlled by the circadian clock protein CCA1. Front Plant Sci 5:701. doi:10.3389/fpls.2014.00701
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058. doi:10.1093/aob/mct067
Webb AAR (1998) Stomatal rhythms. In: Lumsden PJ, Millar AJ (eds) Biological rhythms and photoperiodism in plants. BIOS Scientific Publishers, Oxford, pp 69–79
Wenden B, Toner DL, Hodge SK, Grima R, Millar AJ (2012) Spontaneous spatiotemporal waves of gene expression from biological clocks in the leaf. Proc Natl Acad Sci USA 109:6757–6762. doi:10.1073/pnas.1118814109
Went FW, Thimann KV (1937) Phytohormones. The Macmillan Company, New York
Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414:562–565. doi:10.1038/35107108
Xu ZY, Kim DH, Hwang I (2013) ABA homeostasis and signaling involving multiple subcellular compartments and multiple receptors. Plant Cell Rep 32:807–813. doi:10.1007/s00299-013-1396-3
Yakir E, Hilman D, Harir Y, Green RM (2007) Regulation of output from the plant circadian clock. FEBS J 274:335–345. doi:10.1111/j.1742-4658.2006.05616.x
Yates SA, Chernukhin I, Alvarez-Fernandez R, Bechtold U, Baeshen M, Baeshen N, Mutwakil MZ et al (2014) The temporal foliar transcriptome of the perennial C3 desert plant Rhazya stricta in its natural environment. BMC Plant Biol 14:2. doi:10.1186/1471-2229-14-2
Yue J, Hu X, Huang J (2014) Origin of plant auxin biosynthesis. Trends Plant Sci 19:764–770. doi:10.1016/j.tplants.2014.07.004
Zhang C, Xie Q, Anderson RG, Ng G, Seitz NC, Peterson T, McClung CR et al (2013) Crosstalk between the circadian clock and innate immunity in Arabidopsis. PLoS Pathog 9:e1003370. doi:10.1371/journal.ppat.1003370
Zhao B, Li J (2012) Regulation of brassinosteroid biosynthesis and inactivation. J Integr Plant Biol 54:746–759. doi:10.1111/j.1744-7909.2012.01168.x
Zhao X, Yu X, Foo E, Symons GM, Lopez J, Bendehakkalu KT, Xiang J et al (2007) A study of gibberellin homeostasis and cryptochrome-mediated blue light inhibition of hypocotyl elongation. Plant Physiol 145:106–118. doi:10.1104/pp.107.099838
Zheng B, Deng Y, Mu J, Ji Z, Xiang T, Niu Q-W, Chua N-H et al (2006) Cytokinin affects circadian-clock oscillation in a phytochrome B- and Arabidopsis response regulator 4-dependent manner. Physiol Plant 127:277–292
Zheng XY, Zhou M, Yoo H, Pruneda-Paz JL, Spivey NW, Kay SA, Dong X (2015) Spatial and temporal regulation of biosynthesis of the plant immune signal salicylic acid. Proc Natl Acad Sci USA 112:9166–9173. doi:10.1073/pnas.1511182112
Zhou M, Wang W, Karapetyan S, Mwimba M, Marques J, Buchler NE, Dong X (2015) Redox rhythm reinforces the circadian clock to gate immune response. Nature 523:472–476. doi:10.1038/nature14449
Acknowledgments
Work in the Harmer laboratory is supported by Grants R01 GM 069418 from the National Institutes of Health and IOS 1238040 from the National Science Foundation.
Author contribution
H.S.A and S.L.H both contributed to the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atamian, H.S., Harmer, S.L. Circadian regulation of hormone signaling and plant physiology. Plant Mol Biol 91, 691–702 (2016). https://doi.org/10.1007/s11103-016-0477-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-016-0477-4